A Facile Fabrication of a Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO
Abstract
:1. Introduction
2. Experimental
2.1. Materials
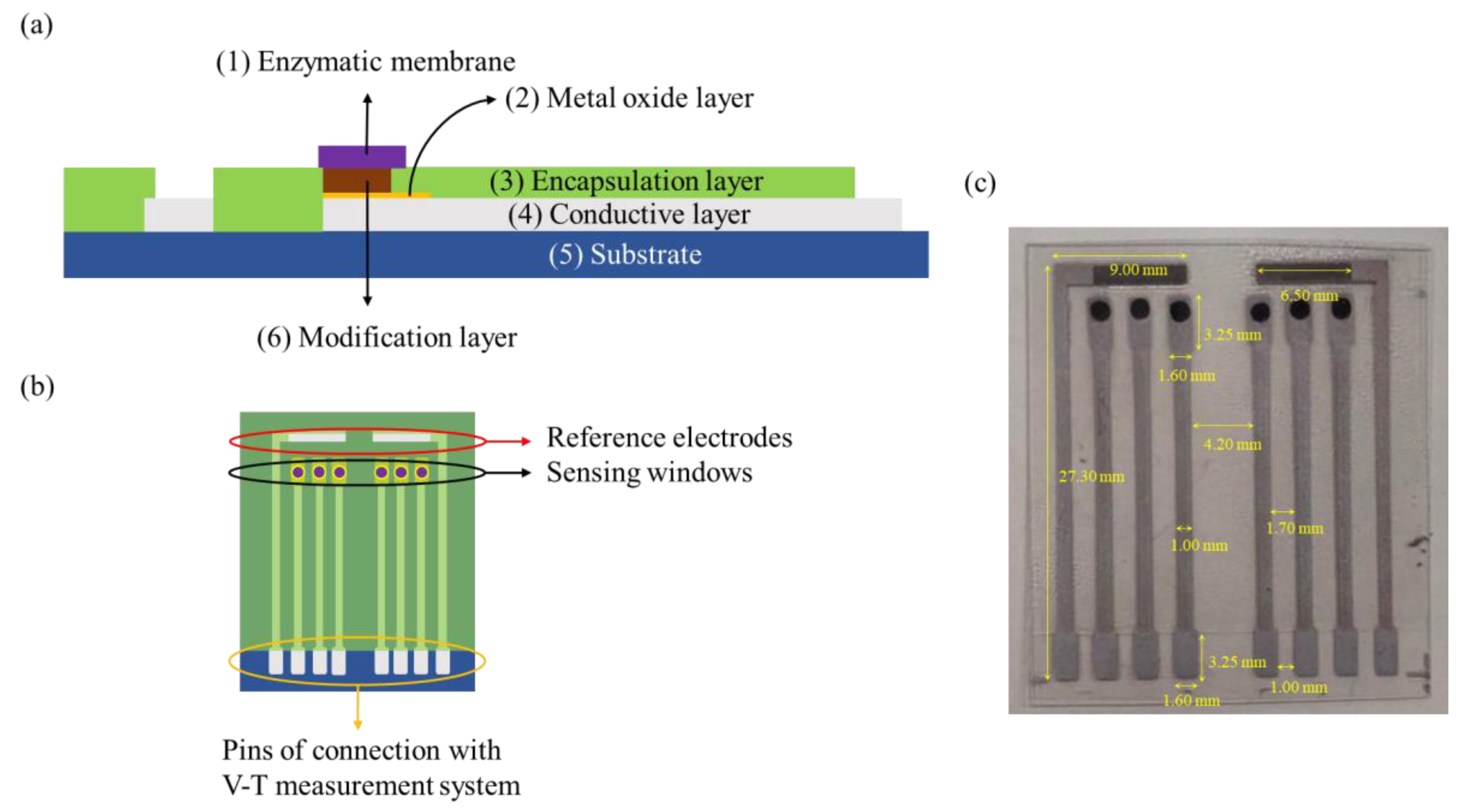
2.2. Deposition of Sensing Membranes and Preparation of Electrodes
2.3. Synthesis of Graphene Oxide and Modification of the Sensing Membranes
2.4. Immobilization of Nafion-GOx Sensing Membranes
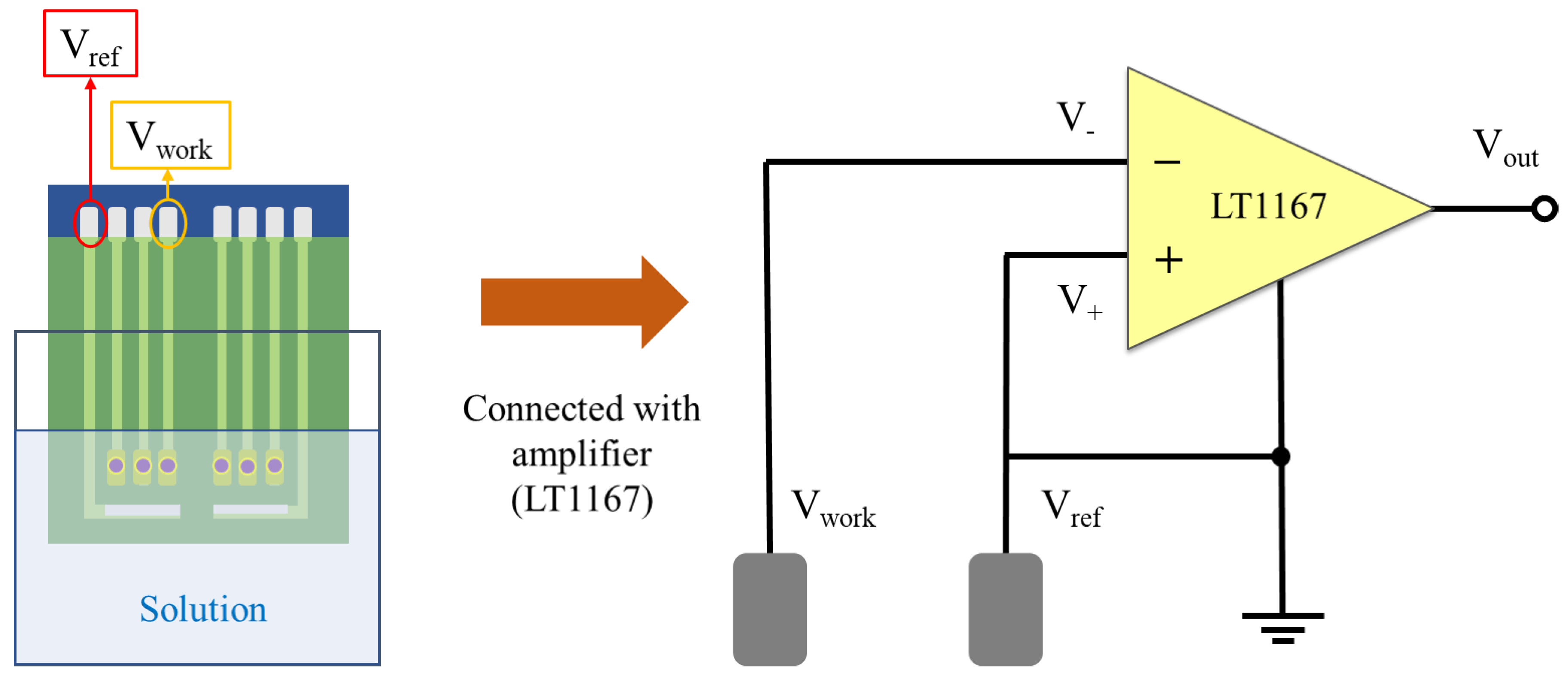
2.5. Voltage–Time Measurement System
2.6. Characterization of Materials
2.7. Analysis of Electrochemical Impedance
3. Results and Discussion
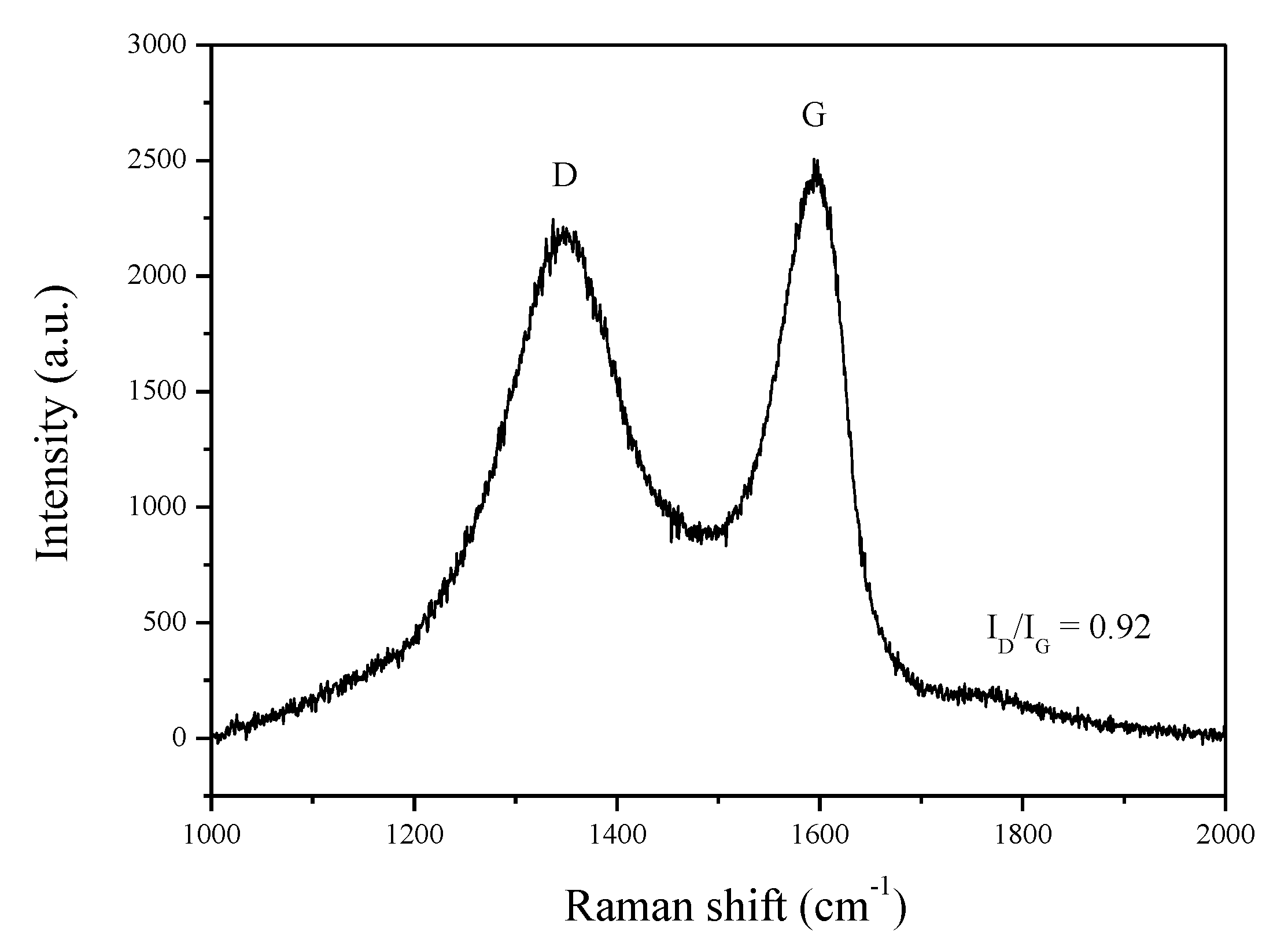
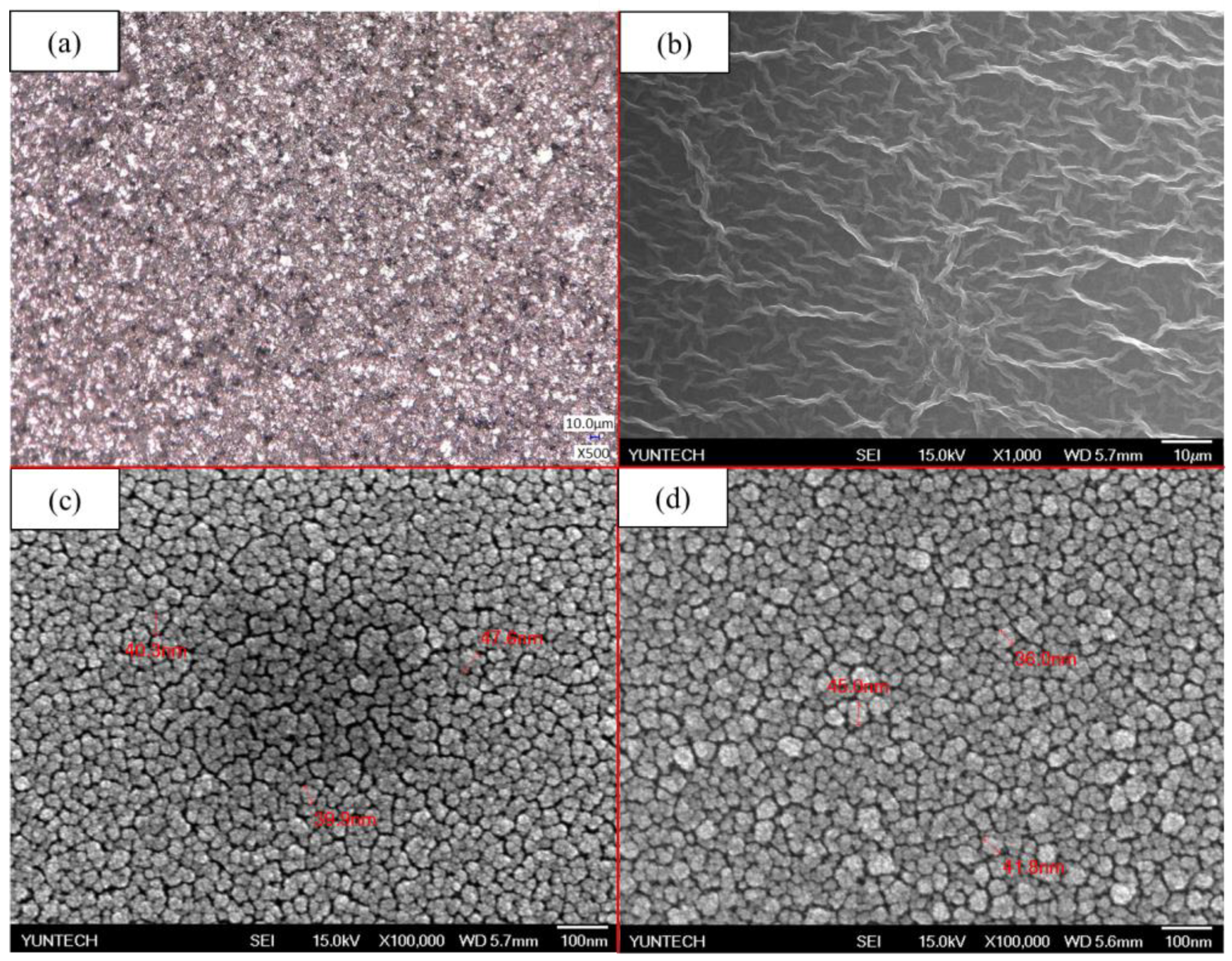
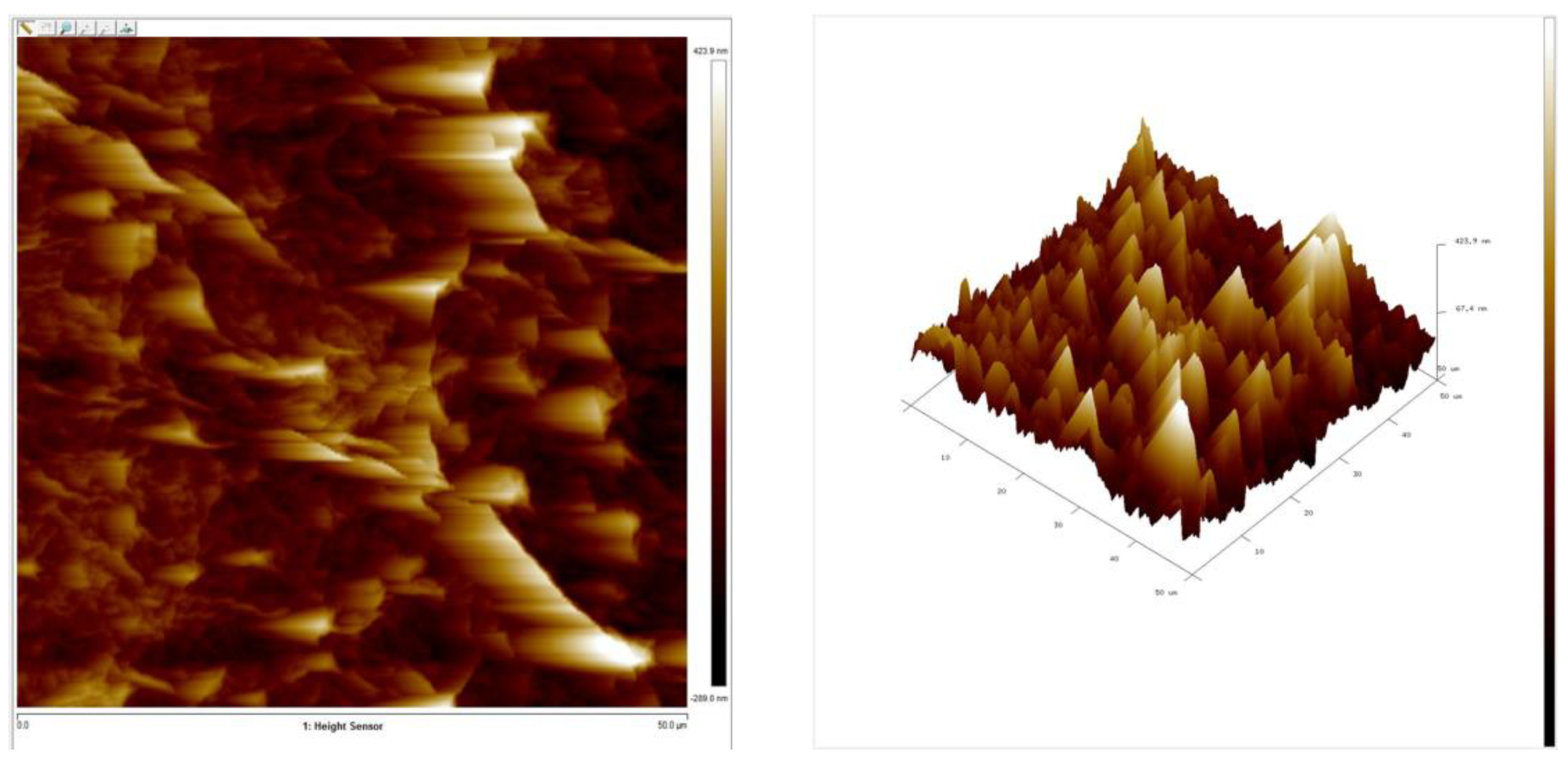
3.1. Raman Spectroscopy of GO and Morphology of Membrane
3.2. Average Sensitivity, Linearity, LOD, and Response Time of Potentiometric Arrayed Glucose Biosensors
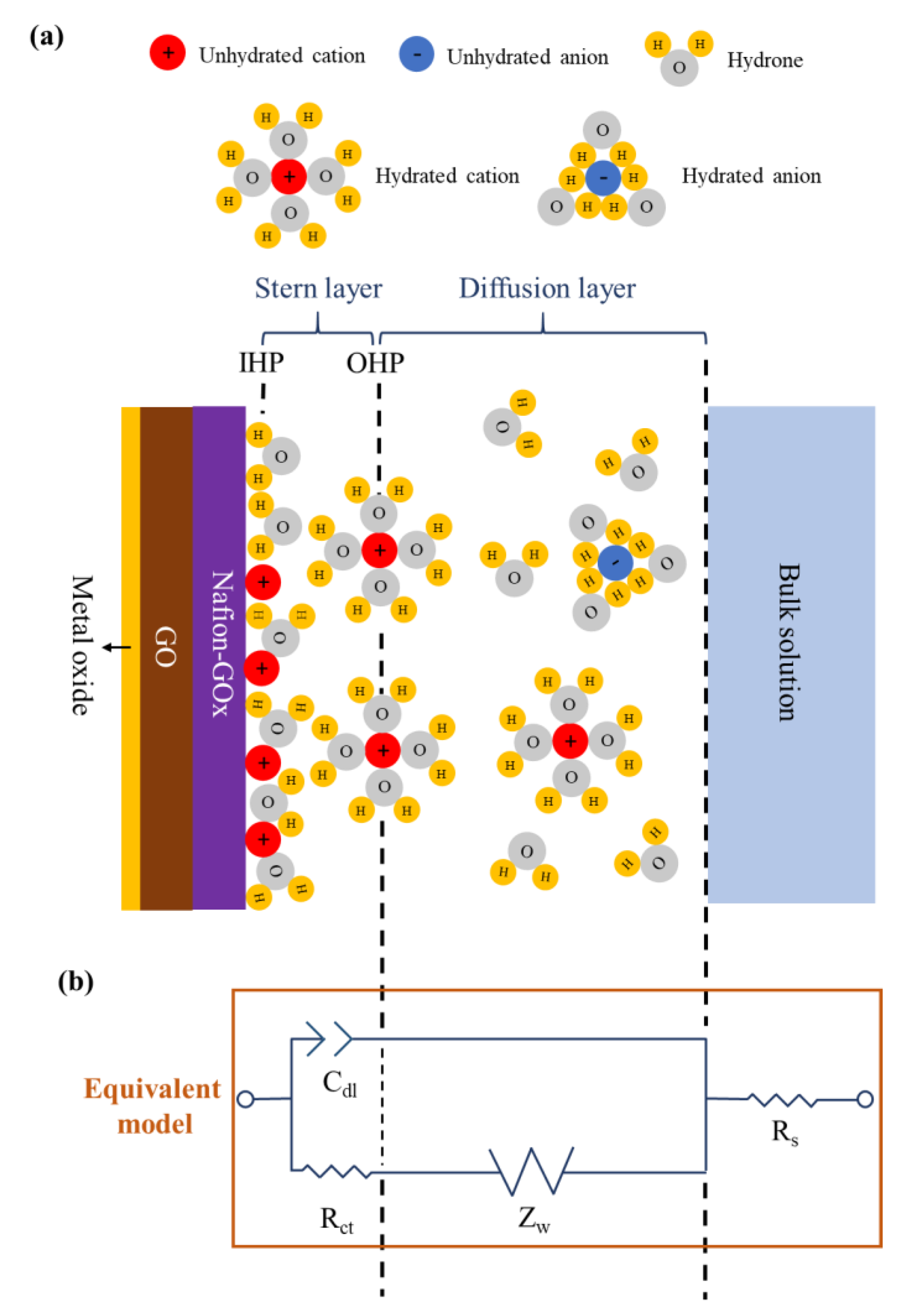
3.3. Electrochemical Impedance Spectroscopy
3.4. Hysteresis of Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO
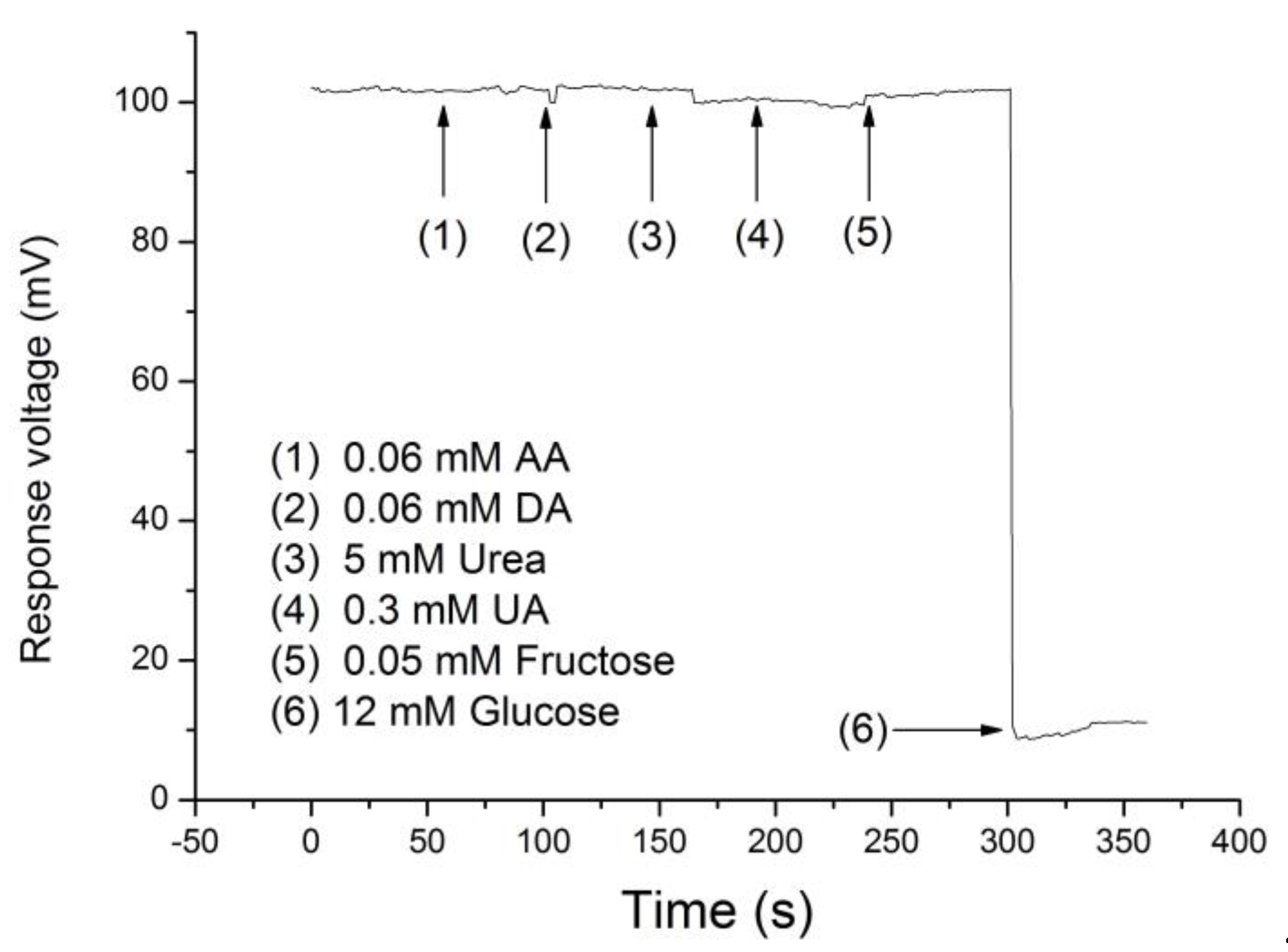
3.5. Anti-Interference Ability of Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO
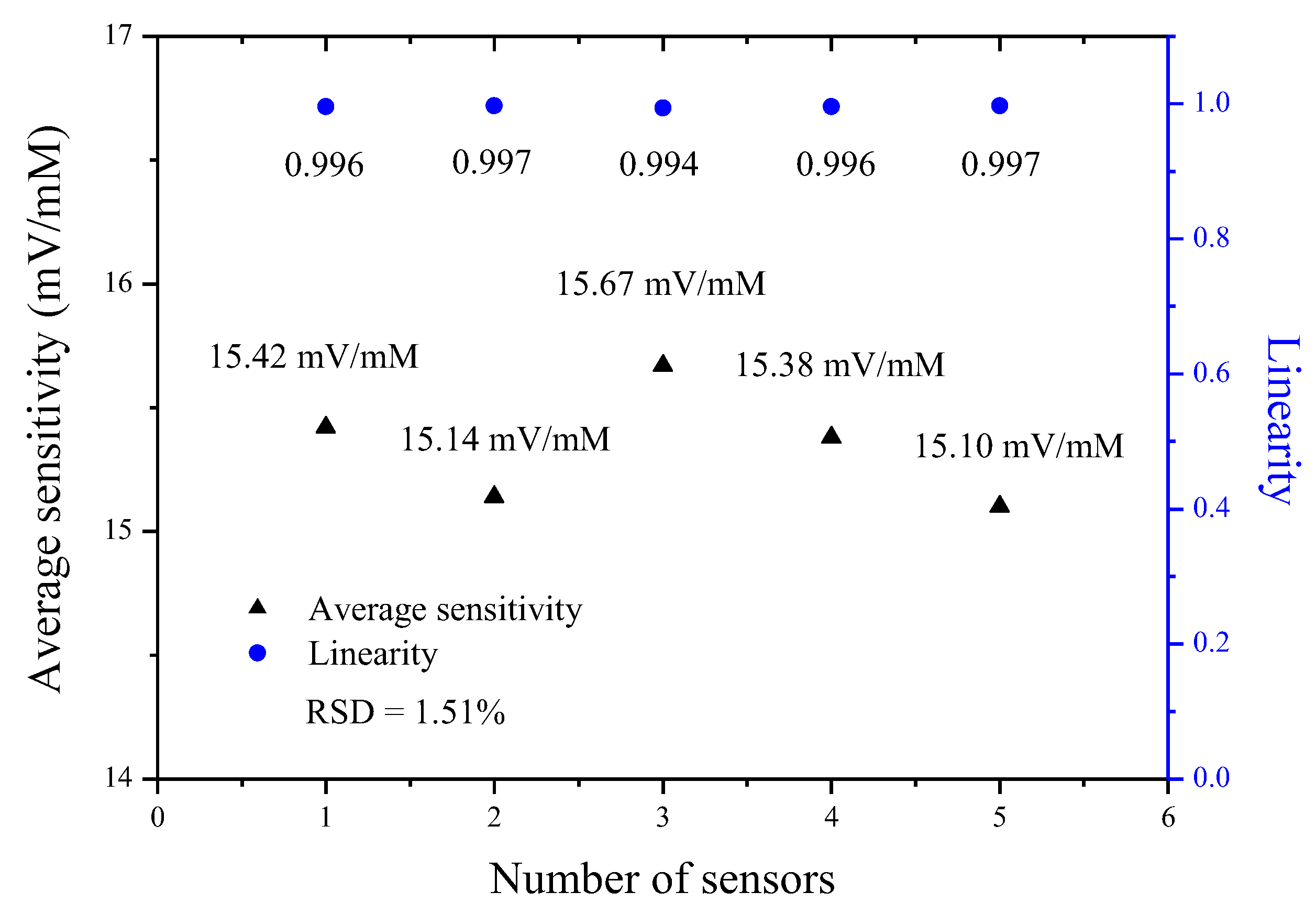
3.6. Reproducibility of Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO
3.7. Lifetime of Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO
3.8. Comparisons of Glucose Biosensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vashist, S.K.; Zhenga, D.; Al-Rubeaan, K.; Luonge, J.H.T.; Sheu, F.S. Technology behind commercial devices for blood glucose monitoring in diabetes management: A review. Anal. Chim. Acta 2011, 703, 124–136. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diabetes Care 2008, 31, 12–54.
- Available online: https://doi.org/10.2337/dc14-S014 (accessed on 21 January 2019).
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Iliadis, A.A. Properties of high sensitivity ZnO surface acoustic wave sensors on SiO2/(1 0 0) Si substrates. Solid-State Electron. 2008, 52, 1710–1716. [Google Scholar] [CrossRef]
- Wei, A.; Sun, X.W.; Wang, J.X.; Lei, Y.; Cai, X.P.; Li, C.M.; Dong, Z.L.; Huang, W. Enzymatic glucose biosensor based on ZnO nanorod array grown by hydrothermal decomposition. Appl. Phys. Lett. 2006, 89, 123902. [Google Scholar] [CrossRef]
- Pazur, J.H.; Kleppe, K. The oxidation of glucose and related compounds by glucose oxidase from aspergillus niger. Biochemistry 1964, 3, 578–583. [Google Scholar] [CrossRef]
- Gupta, V. ZnO based third generation biosensor. Thin Solid Films 2010, 519, 1141–1144. [Google Scholar] [CrossRef]
- Ali, S.M.U.; Nur, O.; Willander, M.; Danielsson, B. A fast and sensitive potentiometric glucose microsensor based on glucose oxidase coated ZnO nanowires grown on a thin silver wire. Sens. Actuators B Chem. 2010, 145, 869–874. [Google Scholar]
- Fulati, A.; Ali, S.M.U.; Asif, M.H.; Alvia, N.H.; Willander, M.; Brännmark, C.; Strålfors, P.; Börjesson, S.I.; Elinder, F.; Danielssond, B. An intracellular glucose biosensor based on nanoflake ZnO. Sens. Actuators B Chem. 2010, 150, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Wahab, H.A.; Salama, A.A.; Saeid, A.A.E.; Willander, M.; Nur, O.; Battisha, I.K. Zinc oxide nano-rods based glucose biosensor devices fabrication. Results Phys. 2018, 9, 809–814. [Google Scholar] [CrossRef]
- Zhao, J.; Mu, F.; Qin, L.; Jia, X.; Yang, C. Synthesis and characterization of MgO/ZnO composite nanosheets for biosensor. Mater. Chem. Phys. 2015, 166, 176–181. [Google Scholar] [CrossRef]
- Chu, X.; Zhu, X.; Dong, Y.; Chen, T.; Ye, M.; Sun, W. An amperometric glucose biosensor based on the immobilization of glucose oxidase on the platinum electrode modified with NiO doped ZnO nanorods. J. Electroanal. Chem. 2012, 676, 20–26. [Google Scholar] [CrossRef]
- Fidal, V.T.; Chandra, T.S. Effect of neutral red incorporation on Al-doped ZnO thin membranes and its bio-electrochemical interaction with NAD+/NADP+ dependent enzymes. Enzym. Microb. Technol. 2018, 116, 57–63. [Google Scholar]
- Janotti, A.; Van De Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Shakti, N.; Gupta, P. Structural and optical properties of Al & Li doped ZnO nanorods. Mater. Today Proc. 2018, 5, 10149–10153. [Google Scholar]
- Fidal, V.T.K.P.; Inguva, S.; Krishnamurthy, S.; Marsili, E.; Mosnier, J.P.; Chandra, T.S. Mediator-free interaction of glucose oxidase, as model enzyme for immobilization, with Al-doped and undoped ZnO thin membranes laser-deposited on polycarbonate supports. Enzym. Microb. Technol. 2017, 96, 67–74. [Google Scholar]
- Wilkins, E.; Atanasov, P.; Muggenburg, B.A. Integrated implantable device for long-term glucose monitoring. Biosens. Bioelectron. 1995, 10, 485–494. [Google Scholar] [CrossRef]
- Ghosh, J.; Ghosh, R.; Giri, P.K. Tuning the visible photoluminescence in Al doped ZnO thin membrane and its application in label-free glucose detection. Sens. Actuators. B Chem. 2018, 254, 681–689. [Google Scholar] [CrossRef]
- Khan, M.S.; Misra, S.K.; Dighe, K.; Wang, Z.; Schwartz-Duval, A.S.; Sar, D.; Pan, D. Electrically-receptive and thermally-responsive paper-based sensor chip for rapid detection of bacterial cells. Biosens. Bioelectron. 2018, 110, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.T. Progress in utilisation of graphene for electrochemical biosensors. Biosens. Bioelectron. 2018, 106, 149–178. [Google Scholar] [CrossRef]
- Park, C.S.; Yoon, H.; Kwon, O.S. Graphene-based nanoelectronic biosensors. J. Ind. Eng. Chem. 2016, 38, 13–22. [Google Scholar] [CrossRef]
- Justino, C.I.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Pumera, M. Graphene in biosensing. Mater. Today. 2011, 14, 308–315. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Li, D.W.; Karpuzov, D.; Long, Y.T. Electrocatalytic oxidation of NADH on graphene oxide and reduced graphene oxide modified screen-printed electrode. Int. J. Electrochem. Sci. 2011, 6, 819–829. [Google Scholar]
- Shi, J.; Zhang, H.; Snyder, A.; Wang, M.; Xie, J.; Porterfield, D.M.; Stanciu, L.A. An aqueous media based approach for the preparation of a biosensor platform composed of graphene oxide and Pt-black. Biosens. Bioelectron. 2012, 38, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhu, W.; Zhang, J.; Jiang, J.; Shen, G.; Yu, R. A label-free amplified fluorescence DNA detection based on isothermal circular strand-displacement polymerization reaction and graphene oxide. Analyst 2013, 138, 3616–3620. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Lü, X.; Qin, C.; Li, C.; Ye, M. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Derkus, B. Applying the miniaturization technologies for biosensor design. Biosens. Bioelectron. 2016, 79, 901–913. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S. The Research of Integrating the Differential Reference Electrode as Well as Magnetic Beads and Graphene Modified in Arrayed Flexible IGZO Glucose Biosensor Based on Microfluidic Framework and the Fabrication of Multifunctional Enzyme Real-Time Sensing System. Master’s Thesis, National Yunlin University of Science and Technology, Douliou, Taiwan, June 2016. [Google Scholar]
- Chou, J.C.; Lin, C.Y.; Liao, Y.H.; Chen, J.T.; Tsai, Y.L.; Chen, J.L.; Chou, H.T. Data fusion and fault diagnosis for flexible arrayed pH sensor measurement system based on LabVIEW. IEEE Sens. J. 2014, 14, 1405–1411. [Google Scholar] [CrossRef]
- Huang, Y.; Xue, Y.; Zeng, J.; Li, S.; Wang, Z.; Dong, C.; Li, G.; Liang, J.; Zhou, Z. Non-enzymatic electrochemical hydrogen peroxide biosensor based on reduction graphene oxide-persimmon tannin-platinum nanocomposite. Mater. Sci. Eng. C. 2018, 92, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.; Dresselhaus, G.; Hofmann, M. Raman spectroscopy as a probe of graphene and carbon nanotubes. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 366, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Wu, S.; Wang, Y.; Wu, L.; Yuan, P.; Wang, X. Self-assembly of mildly reduced graphene oxide monolayer for enhanced Raman scattering. J. Solid State Chem. 2016, 237, 57–63. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Nanocages-augmented aligned polyaniline nanowires as unique platform for electrochemical non-enzymatic glucose biosensor. Appl. Catal. 2016, 517, 21–29. [Google Scholar] [CrossRef]
- Sardarinejad, A.; Maurya, D.K.; Alameh, K. The effects of sensing electrode thickness on ruthenium oxide thin-film pH sensor. Sens. Actuators A Phys. 2014, 214, 15–19. [Google Scholar] [CrossRef]
- Rajaković, L.V.; Marković, D.D.; Rajaković-Ognjanović, V.N.; Antanasijević, D.Z. Review: The approaches for estimation of limit of detection for ICP-MS trace analysis of arsenic. Talanta 2012, 102, 79–87. [Google Scholar] [CrossRef]
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Schwartz-Duval, A.S.; Misra, S.K.; Pan, D. Electrochemical-digital immunosensor with enhanced sensitivity for detecting human salivary glucocorticoid hormone. Analyst 2018, 144, 1448–1457. [Google Scholar] [CrossRef]
- Khuna, K.; Ibupoto, Z.H.; Lu, J.; AlSalhi, M.S.; Atif, M.; Ansari, A.A.; Willander, M.; Hsieh, J.H. Potentiometric glucose sensor based on the glucose oxidase immobilized ironferrite magnetic particle/chitosan composite modified gold coated glass electrode. Sens. Actuators B Chem. 2012, 173, 698–703. [Google Scholar] [CrossRef]
- Muñoz, J.; Montes, R.; Baeza, M. Trends in electrochemical impedance spectroscopy involving nanocomposite transducers: Characterization, architecture surface and bio-sensing. TrAC Trends Anal. Chem. 2017, 97, 201–215. [Google Scholar] [CrossRef]
- Pajkossy, T.; Jurczakowski, R. Electrochemical impedance spectroscopy in interfacial studies. Curr. Opin. Electrochem. 2017, 1, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Usui, S. Electrical double-layer interaction between oppositely charged dissimilar oxide surfaces with charge regulation and Stern–Grahame layers. J. Colloid Interface Sci. 2008, 320, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bajpai, R.; Misra, D.S.; McLaughlin, J.A.; Roy, S.S.; Soin, N. Graphene oxide for electrochemical sensing applications. J. Mater. Chem. 2011, 21, 14725–14731. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, J.; Chen, Z.; Xu, C.; Wang, Y.; Yao, C. A novel bienzyme glucose biosensor based on three-layer Au–Fe3O4@SiO2 magnetic nanocomposite. Sens. Actuators B Chem. 2011, 159, 220–228. [Google Scholar] [CrossRef]
- Wang, A.-J.; Li, Y.-F.; Li, Z.-H.; Feng, J.-J.; Sun, Y.-L.; Chen, J.-R. Amperometric glucose sensor based on enhanced catalytic reduction of oxygen using glucose oxidase adsorbed onto core-shell Fe3O4@silica@Au magnetic nanoparticles. Mater. Sci. Eng. C 2012, 32, 1640–1647. [Google Scholar] [CrossRef]
- Bousse, L.; Van Den Vlekkert, H.; De Rooij, N.F. Hysteresis in Al2O3-gate ISFETs. Sens. Actuators B 1990, 2, 103–110. [Google Scholar] [CrossRef]
- Chou, J.C.; Chen, J.S.; Huang, M.S.; Liao, Y.H.; Lai, C.H.; Wu, T.Y.; Yan, S.J. The Characteristic Analysis of IGZO/Al pH Sensor and Glucose Biosensor in Static and Dynamic Measurements. IEEE Sens. J. 2016, 16, 8509–8516. [Google Scholar] [CrossRef]
- Deng, H.; Liang Teo, A.K.; Gao, Z. An interference-free glucose biosensor based on a novel low potential redox polymer mediator. Sens. Actuators B Chem. 2014, 191, 522–528. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Moline, A.N. Fabrication of ultra-thin polypyrrole–glucose oxidase membrane from supporting electrolyte-free monomer solution for potentiometric biosensing of glucose. Biosens. Bioelectron. 2001, 16, 133–139. [Google Scholar] [CrossRef]
- Theâvenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Gong, W.; Wang, L.; Chen, Z. Enhanced amperometric response of a glucose oxidase and horseradish peroxidase based bienzyme glucose biosensor modified with a film of polymerized toluidine blue containing reduced graphene oxide. Microchim. Acta 2015, 182, 1949–1956. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Wang, Y.; Du, H.; Xia, C.; Tian, F. Glucose biosensor based on glucose oxidase immobilized on unhybridized titanium dioxide nanotube arrays. Microchim. Acta 2013, 181, 381–387. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, C.; Zhang, J.; Bai, W. Potentiometric glucose biosensor based on core–shell Fe3O4–enzyme–polypyrrole nanoparticles. Biosens. Bioelectron. 2014, 51, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Çiftci, H.; Tamer, U.; Teker, M. Şen; Pekmez, N. Özçiçek An enzyme free potentiometric detection of glucose based on a conducting polymer poly (3-aminophenyl boronic acid-co-3-octylthiophene). Electrochim. Acta 2013, 90, 358–365. [Google Scholar] [CrossRef]
- Ngeontae, W.; Janrungroatsakul, W.; Maneewattanapinyo, P.; Ekgasit, S.; Aeungmaitrepirom, W.; Tuntulani, T. Novel potentiometric approach in glucose biosensor using silver nanoparticles as redox marker. Sens. Actuators B Chem. 2009, 137, 320–326. [Google Scholar] [CrossRef]
- Widayani, S.; Yanti, T.; Wungu, T.D.K. Suprijadi Preliminary Study of Molecularly Imprinted Polymer-based Potentiometric Sensor for Glucose. Procedia Eng. 2017, 170, 84–87. [Google Scholar] [CrossRef]

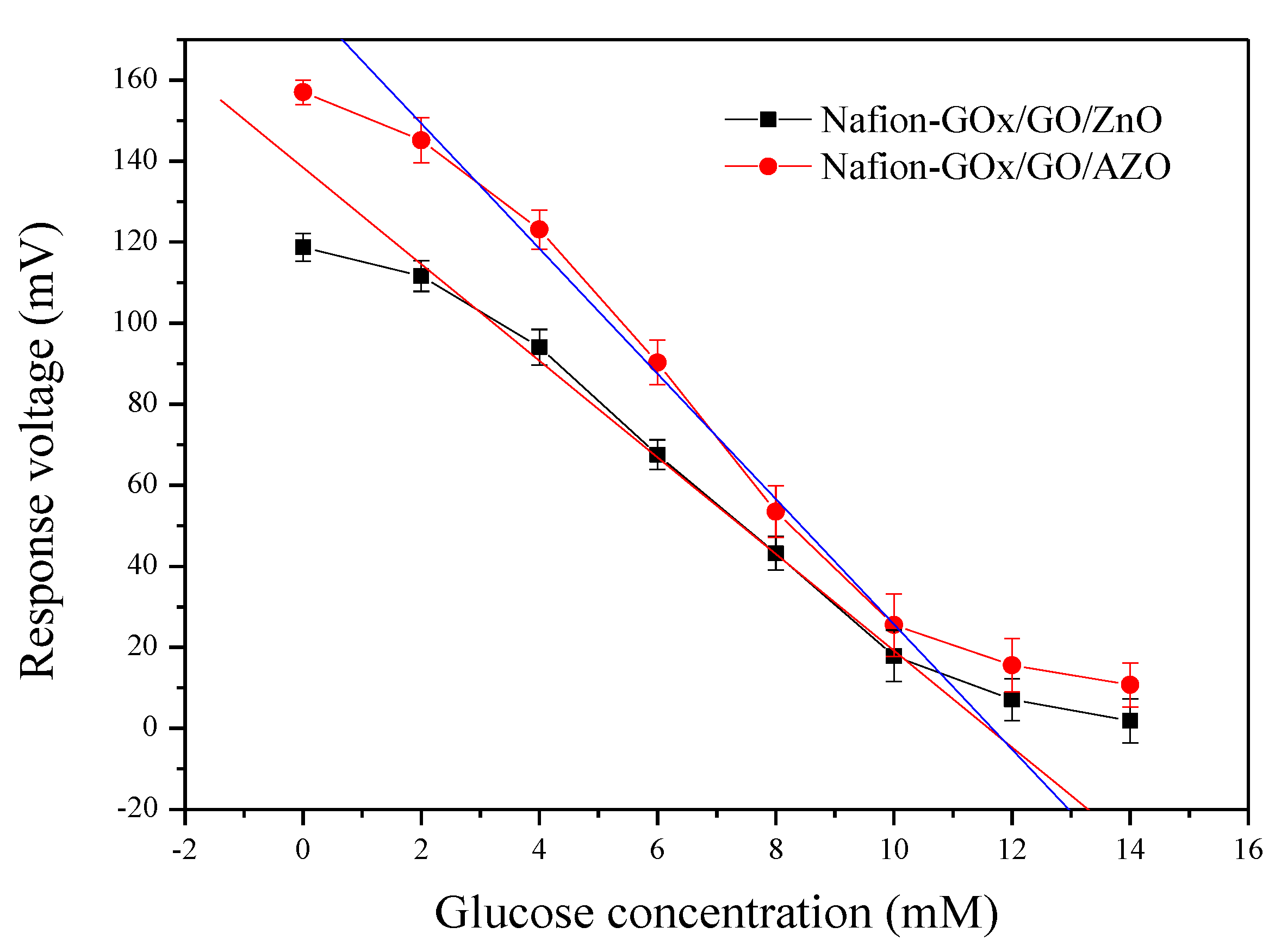



| Membrane | Glucose Concentration (mM) | Response Voltage (Mean ± SD, mV) | Average Sensitivity (mV/mM) | Linearity |
|---|---|---|---|---|
| Nafion-GOx/GO/ZnO | 0 | 118.73 ± 1.76 | 11.92 | 0.998 |
| 2 | 111.64 ± 3.77 | |||
| 4 | 94.10 ± 4.36 | |||
| 6 | 67.54 ± 3.68 | |||
| 8 | 43.20 ± 4.16 | |||
| 10 | 17.88 ± 6.36 | |||
| 12 | 7.13 ± 5.17 | |||
| 14 | 1.83 ± 5.45 | |||
| Nafion-GOx/GO/AZO | 0 | 157.02 ± 2.01 | 15.44 | 0.997 |
| 2 | 145.13 ± 5.57 | |||
| 4 | 123.12 ± 4.80 | |||
| 6 | 90.32 ± 5.47 | |||
| 8 | 53.49 ± 6.38 | |||
| 10 | 25.50 ± 7.71 | |||
| 12 | 15.56 ± 6.58 | |||
| 14 | 10.72 ± 5.49 |
| Membrane | Solution | Rct (Ω) |
|---|---|---|
| ZnO | PBS | 1.17 × 104 |
| AZO | PBS | 5.72 × 103 |
| GO/ZnO | PBS | 2.99 × 103 |
| GO/AZO | PBS | 1.83 × 103 |
| Nafion-GOx/GO/ZnO | PBS (5 mM glucose) | 3.53 × 103 |
| Nafion-GOx/GO/AZO | PBS (5 mM glucose) | 2.31 × 103 |
| Number of Sensors | Glucose Concentration (mM) | Response Voltage (Mean ± SD, mV) | Average Sensitivity (mV/mM) | Linearity |
|---|---|---|---|---|
| 1 | 2 | 146.64 ± 3.77 | 15.42 | 0.996 |
| 4 | 121.10 ± 4.36 | |||
| 6 | 91.54 ± 3.68 | |||
| 8 | 50.20 ± 4.16 | |||
| 10 | 27.88 ± 6.36 | |||
| 2 | 2 | 145.13 ± 4.57 | 15.14 | 0.997 |
| 4 | 123.12 ± 4.80 | |||
| 6 | 90.31 ± 5.47 | |||
| 8 | 53.49 ± 6.38 | |||
| 10 | 28.50 ± 5.71 | |||
| 3 | 2 | 142.87 ± 5.46 | 15.67 | 0.994 |
| 4 | 125.10 ± 4.92 | |||
| 6 | 89.35 ± 5.16 | |||
| 8 | 51.34 ± 6.01 | |||
| 10 | 23.07 ± 6.38 | |||
| 4 | 2 | 145.25 ± 4.25 | 15.38 | 0.996 |
| 4 | 124.69 ± 5.28 | |||
| 6 | 92.10 ± 6.16 | |||
| 8 | 53.46 ± 5.74 | |||
| 10 | 27.03 ± 5.95 | |||
| 5 | 2 | 144.51 ± 6.34 | 15.10 | 0.997 |
| 4 | 119.39 ± 4.31 | |||
| 6 | 89.37 ± 5.84 | |||
| 8 | 50.86 ± 3.17 | |||
| 10 | 27.79 ± 5.06 |
| Electrode | Linear Range | Sensitivity | Linearity | Response Time | LOD | Ref. |
|---|---|---|---|---|---|---|
| Nafion-GOx/GO/AZO/Ag | 2 mM to 10 mM | 15.44 mV/mM | 0.997 | 26 s | 1.89 mM | This study |
| BSA-Nafion-GOx/ZnO NWs/Ag | 0.5–1000 μM | 35 mV/decade | N/A | 1–4 s | N/A | [9] 2010 |
| BSA-GOx/ZnO NFs/Al | 500 nM to 10 mM | 65.2 mV/decade | 0.990 | 4 s | N/A | [10] 2010 |
| GOx/ZnO NRs/Ag | 1 µM to 10mM | 2.51 mV/decade | 0.980 | N/A | N/A | [11] 2018 |
| Fe3O4-GOx-Ppy/MGCE | 0.5 μM t 34 mM | 19.4 mV/decade | 0.998 | 6 s | 0.3 μM | [56] 2014 |
| PAPBAOT/GCE | 5–50 mM | 0.58 mV/mM | 0.992 | 200 s | 0.5 mM | [57] 2013 |
| AgNPs-GOx/Ag-ISE | 0.1–3 mM | 8.62 mV/decade | N/A | N/A | 10 μM | [58] 2009 |
| MIP-based on MAA | 0.02–7 mM | 43.7 mV/mM | 0.980 | N/A | N/A | [59] 2017 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, J.-C.; Lin, S.-H.; Lai, T.-Y.; Kuo, P.-Y.; Lai, C.-H.; Nien, Y.-H.; Su, T.-Y. A Facile Fabrication of a Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO. Sensors 2020, 20, 964. https://doi.org/10.3390/s20040964
Chou J-C, Lin S-H, Lai T-Y, Kuo P-Y, Lai C-H, Nien Y-H, Su T-Y. A Facile Fabrication of a Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO. Sensors. 2020; 20(4):964. https://doi.org/10.3390/s20040964
Chicago/Turabian StyleChou, Jung-Chuan, Si-Hong Lin, Tsu-Yang Lai, Po-Yu Kuo, Chih-Hsien Lai, Yu-Hsun Nien, and Tzu-Yu Su. 2020. "A Facile Fabrication of a Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO" Sensors 20, no. 4: 964. https://doi.org/10.3390/s20040964
APA StyleChou, J.-C., Lin, S.-H., Lai, T.-Y., Kuo, P.-Y., Lai, C.-H., Nien, Y.-H., & Su, T.-Y. (2020). A Facile Fabrication of a Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO. Sensors, 20(4), 964. https://doi.org/10.3390/s20040964








