Ethylene Sensor-Enabled Dynamic Monitoring and Multi-Strategies Control for Quality Management of Fruit Cold Chain Logistics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Conceptual Framework
2.1.1. Cold Chain Logistics
2.1.2. Ethylene Mechanism
2.1.3. Dynamic Monitoring
2.1.4. Multi-Strategies Control
2.2. Mechanism Analysis
- ➣
- Control Layer: Fruit genes control all life activities and trait expression of fruits. Some genes proteins related to ethylene synthesis, including ETR1, CTR1, EIN2, and EIN3 [21]. These proteins could respond to different ethylene levels inside the fruit, controlling the ethylene biosynthesis.
- ➣
- Biosynthesis Layer: Under the stimulation of above control proteins, the methionine is converted into S-adenosylmethionine, which is subsequently converted to ethylene by means of enzymes ACS, and ACO [11,19,20]. The biosynthesized ethylene itself also strongly regulates the expression and activity of ACS and ACO, thereby achieving the feedback regulation [18,21].
- ➣
- Physiological Metabolism Layer: Endogenous ethylene regulates a series of physiological and metabolic activities of fruits, including respiration, transpiration, hydrolysis, and oxidation [22]. For example, for climacteric fruits, the ethylene content rises rapidly during the maturity stage, the respiration rate of the fruit also increases almost exponentially.
- ➣
- Quality Layer: These physiological and metabolic activities of fruits directly affect the fruit quality, including firmness, SSC, pH, chromatic aberration, etc [23]. For example, the respiration of the fruit consumes the starch and converts it to fructose, thus resulting in a decrease of firmness and the increase of pH.
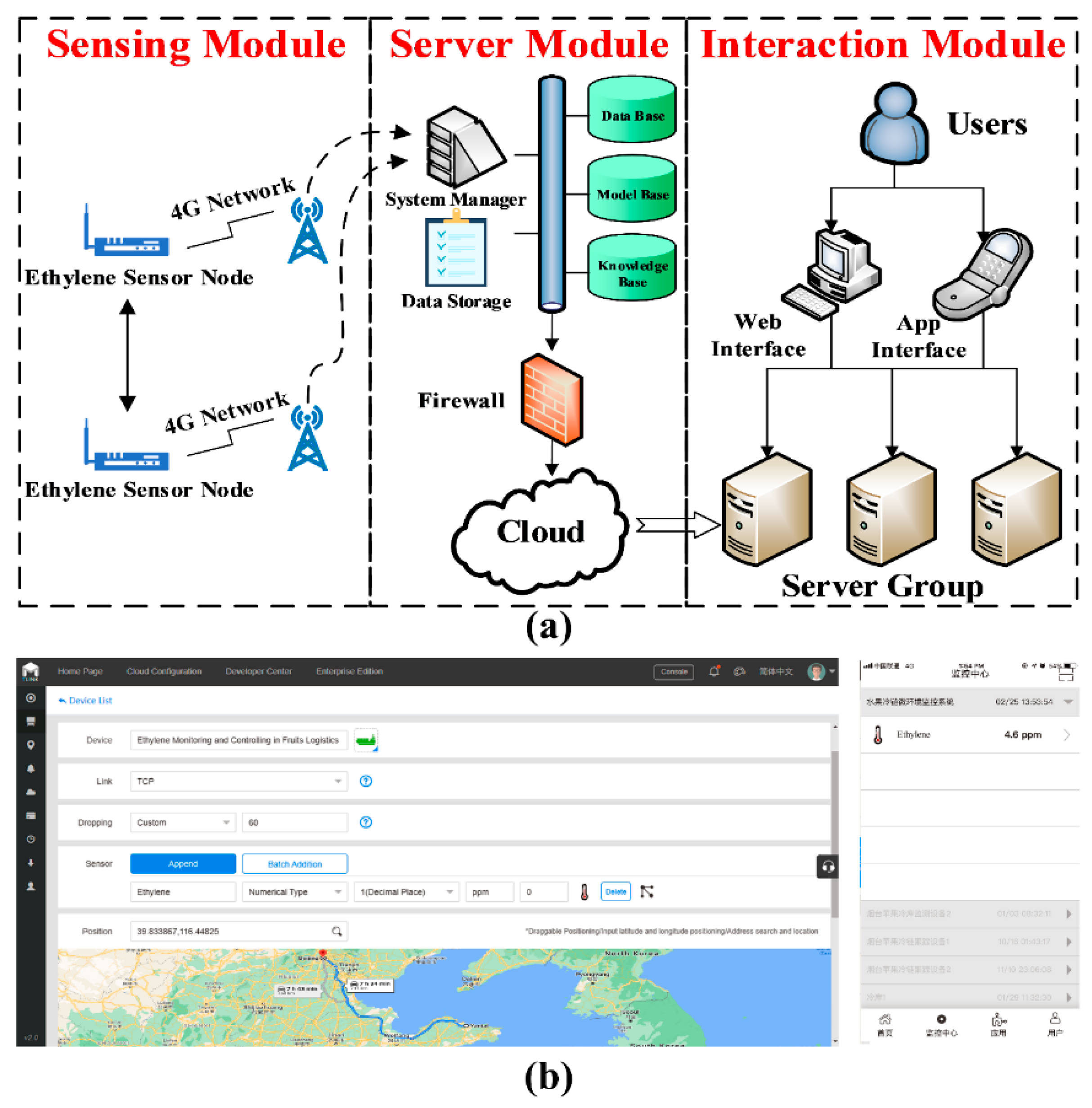
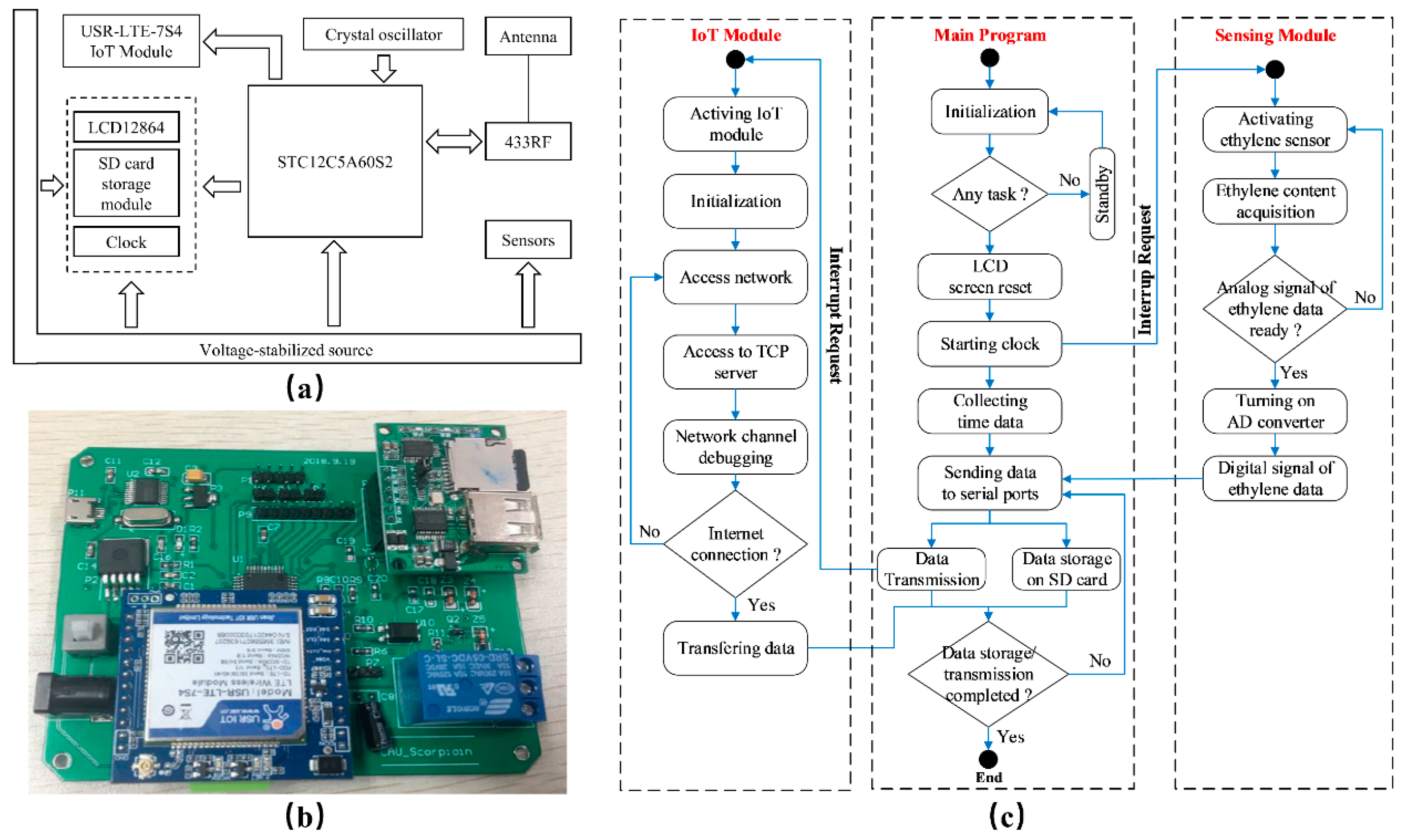
2.3. Ethylene Dynamic Monitoring
2.3.1. Design of Ethylene Dynamic Monitoring
2.3.2. Implementation of Ethylene Dynamic Monitoring
2.4. Design and Methods of Experiment
2.4.1. Static Calibration Experiment of Ethylene Sensor
2.4.2. Dynamic Response Experiment of Ethylene Sensor
2.4.3. Verification Experiment
2.4.4. Quality Parameters Determination Methods
- (1)
- Firmness. The firmness was measured by the FHT-05 Firmness Tester (produced by Landtek Co., Ltd., Guangzhou, China). Fifteen experimental samples were randomly selected for measurement, and two symmetrical parts were taken for each fruit and then averaged.
- (2)
- Soluble solids contents (SSC). Fifteen experimental samples were randomly selected, the juice was squeezed with gauze, and the soluble solids content was determined using an ATAGO PAL-1 digital refractometer (manufactured by ATAGO Company, Guangzhou, China).
- (3)
- pH. The pH was determined with a pH digital acid meter (produced by Testo SE & Co. KgaA, Shanghai, China). Fifteen experimental samples were randomly selected, and the average value was obtained after two determinations.
- (4)
- Weight loss rate. The weight loss rate was determined by the weighing method [24], the calculation formula was as follows:where, —weight loss rate; —origin weight; —final weight.
- (5)
- Rotting rate. Rotten fruit means that there is at least one lesion on the surface of the fruit or leakage of juice, softening, shrinkage, or rot of the fruit [25]. The calculation formula was as follow:where, —the weight of rotten fruit; —total weight of fruits; ε-the rotting rate.
- (6)
- Chromatic aberration. The chromatic aberration was measured by a CR-410 chromatic aberration analyzer (produced by Konica Minolta Co., Ltd., Japan, Tokyo). The final value of the L*, a*, and b* were obtained by averaged three measurement results. The total chromatic aberration ΔE was calculated by the following formula [26]:
3. Results and Discussion
3.1. Characteristic Analysis of Ethylene Dynamic Monitoring
- ➣
- S0 stage: The S0 stage was the zero-response stage of the ethylene sensor in the air, also known as the baseline value. From the response curve, it could be seen that the response value of the ethylene dynamic monitoring was 0 ppm in the air, which was consistent with the actual ethylene concentration in the atmosphere.
- ➣
- S1 stage: The S1 stage was the fast response stage. When the 20 ppm of standard calibration ethylene gas (mixture of with ethylene and nitrogen) was added into the gas chamber at a rate of 500 mL/min through the flow controller, the ethylene gas sensor responded quickly to ethylene gas and reached 90% of the maximum response value after 18 s, which was called response time.
- ➣
- S2 stage: The S2 stage was called the stable response stage, namely the ethylene concentration in the gas chamber had reached a stable state. From the time-domain curve, it could be seen that the ethylene dynamic monitoring reached the maximum response value (20.40 ppm) at about 600 s, and then kept the dynamic stability at 20.13 ppm. Therefore, it could be considered that the ethylene sensor showed a good dynamic response.
- ➣
- S3 stage: The S3 stage was the fast recovery stage. In this stage, the air chamber cover of the experimental device was opened to make the ethylene sensor fully expose to the air. From the time-domain curve, it could be seen that the value of the ethylene sensor decreased rapidly to 30% of the maximum response value during the 58 s.
- ➣
- S4 stage: The S4 phase was a slow recovery stage. At this time, the value of the ethylene dynamic monitoring dropped slowly. It took about 100 s to fully recover to the baseline value. The possible reason may be that there was still a small amount of ethylene gas around the sensor, which had a limited impact on the ethylene sensor (influence of residual gas on maximum ethylene sensor response was less than 1%).
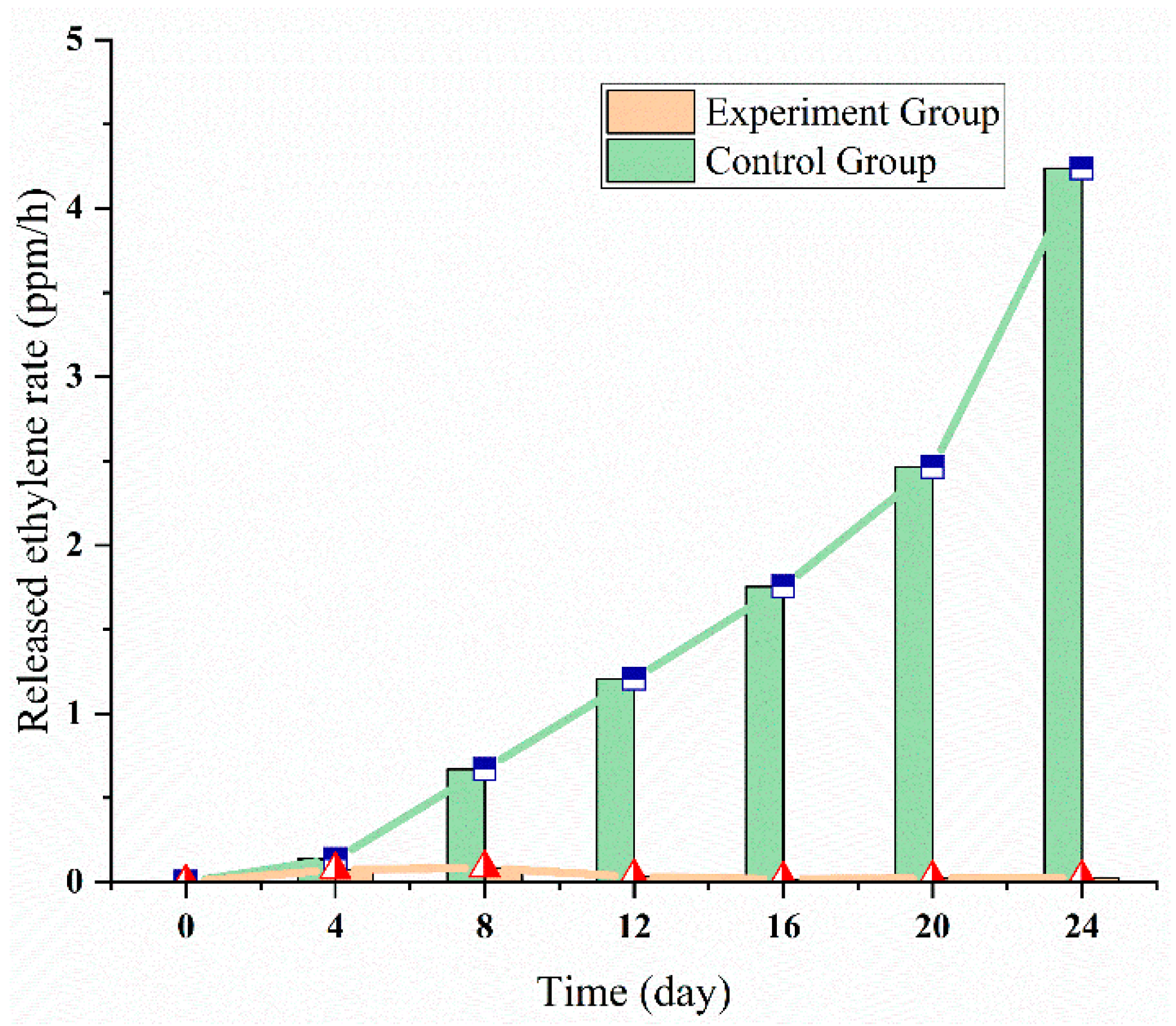
3.2. Ethylene Multi-Strategies Control: Low Temperature
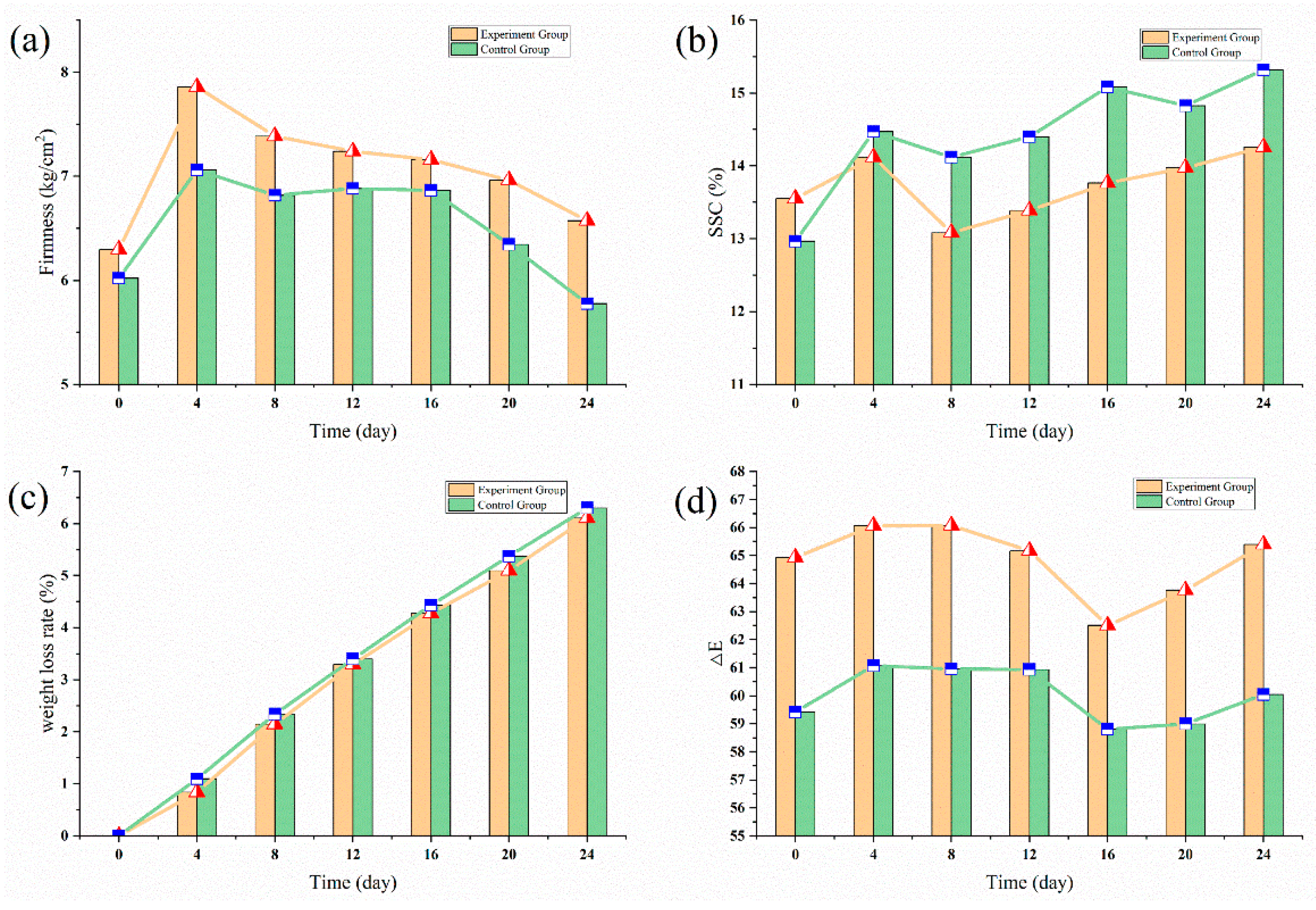
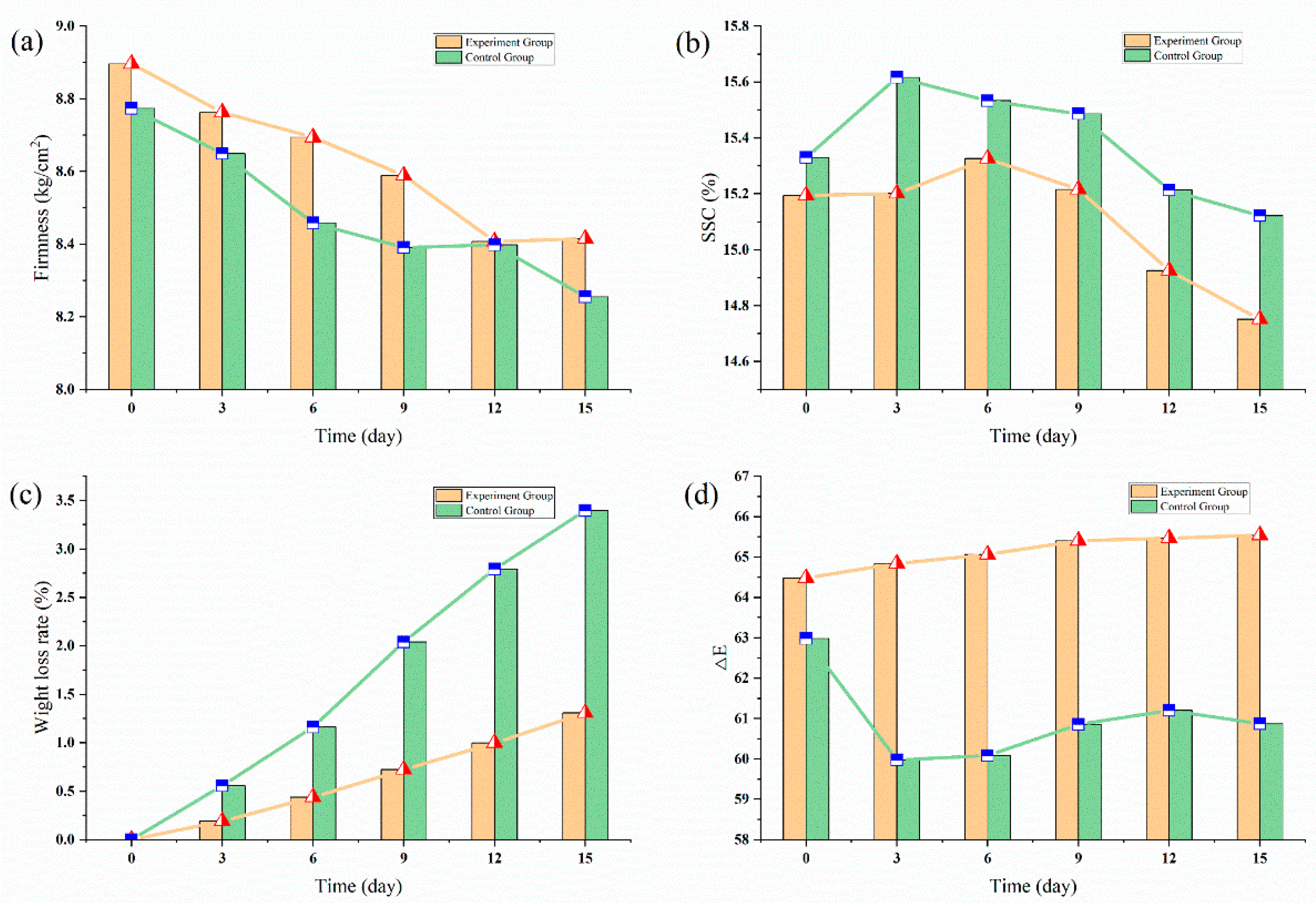
3.2.1. Blueberry Experiment
3.2.2. Sweet Cherries Experiment
3.3. Ethylene Multi-Strategies Control: 1-MCP
3.4. Ethylene Multi-Strategies Control: Modified Atmosphere Package (20% CO2)
3.5. Analysis of Multi-Strategies Ethylene Control
3.5.1. Strategy I—Adding Ethylene for Climacteric Fruits
3.5.2. Strategy II—Reducing Ethylene for Climacteric Fruits
- ➣
- Low temperature (such as cold chain transportation or cold storage) reduces the metabolic activities of fruits, thereby reducing ethylene biosynthesis and quality decay. This is currently the most widely used technical means, with the advantages of high efficiency and easy operation. However, the cold chain trucks or large cold storage may require a huge investment.
- ➣
- Turning on the ethylene scrubbers could quickly and reliably reduce the ethylene concentration in the fruit’s microenvironment. Besides, the exhaust fans for ethylene scrubbers are convenient to integrate with the ethylene dynamic monitoring. This method is suitable for both storage and logistics processes.
- ➣
- Spraying ethylene inhibitors. It is demonstrated that some ethylene inhibitors could antagonize with ethylene [33,34]. 1-Methylcyclopropene (1-MCP) is a popular ethylene inhibitor that prevents ethylene biosynthesis and furthering ethylene-dependent responses (including ripening and senescence of fruit tissues) by preventing ethylene to bind to the receptor [35,36,37,38,39]. Another ethylene inhibitor is the aminoethoxyvinylglycine (AVG), which inhibits the synthesis of ethylene by inhibiting the synthesis of ACC [40,41,42];
- ➣
- Ethylene adsorbent, such as activated carbon, molecular sieve, potassium permanganate, and ethanol, could absorb the microenvironment ethylene gas. These adsorbents also easily integrate with fruit packages, thereby reducing ethylene contents in fruit’s independent packages.
- ➣
- The microenvironment gas composition could be controlled with modified atmosphere packaging (MAP) for slowing down the quality decay and inhibiting the production of ethylene [43]. For example, low O2 and high CO2 in the microenvironment of fruit could inhibit ethylene biosynthesis and respiration, thereby delaying the ripening of fruit [2].
3.5.3. Strategy III—For Non-Climacteric Fruits
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, X.; Wolff, D.J.T.; Genetics, A. Ethylene production, shelf-life and evidence of RFLP polymorphisms linked to ethylene genes in melon (Cucumis melo L.). Theor. Appl. Genet. 2000, 101, 613–624. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—An overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Huč, A.; Vidrih, R.; Trebar, M. Determination of Pears Ripening Stages Based on Electrochemical Ethylene Sensor. IEEE Sens. J. 2020, 1. [Google Scholar] [CrossRef]
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Xing, S.; Ma, Y.; Wang, X.J.F. Multi-Sensors Enabled Dynamic Monitoring and Quality Assessment System (DMQAS) of Sweet Cherry in Express Logistics. Foods 2020, 9, 602. [Google Scholar] [CrossRef]
- Palou, L.; Crisosto, C.H.; Garner, D.; Basinal, L.M. Effect of continuous exposure to exogenous ethylene during cold storage on postharvest decay development and quality attributes of stone fruits and table grapes. Postharvest Biol. Technol. 2003, 27, 243–254. [Google Scholar] [CrossRef]
- Mayuoni, L.; Tietel, Z.; Patil, B.S.; Porat, R. Does ethylene degreening affect internal quality of citrus fruit? Postharvest Biol. Technol. 2011, 62, 50–58. [Google Scholar] [CrossRef]
- Yuan, R.; Li, J. Effect of sprayable 1-MCP, AVG, and NAA on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Delicious’ apples. HortScience 2008, 43, 1454–1460. [Google Scholar] [CrossRef] [Green Version]
- Blanke, M. Reducing ethylene levels along the food supply chain: A key to reducing food waste? J. Sci. Food Agric. 2014, 94, 2357–2361. [Google Scholar] [CrossRef]
- Knee, M. Effects of temperature and daminozide on the induction of ethylene synthesis in two varieties of apple. J. Plant Growth Regul. 1988, 7, 111–119. [Google Scholar] [CrossRef]
- Antunes, M.; Pateraki, I.; Kanellis, A.; Sfakiotakis, E. Differential effects of low-temperature inhibition on the propylene induced autocatalysis of ethylene production, respiration and ripening of ‘Hayward’kiwifruit. J. Hortic. Sci. Biotechnol. 2000, 75, 575–580. [Google Scholar] [CrossRef]
- Lü, E.; Lu, H.; Yang, Z.; Liu, Y. Research Status and Prospects in Fruits and Vegetables Fresh-keeping with Controlled Atmosphere Transportation Technologies. J. Agric. Mech. Res. 2010, 32, 225–228. [Google Scholar]
- Feng, L.; Me, H.; Xie, C.; Wu, H.; Zhao, F.; Fu, T.; Zhang., X. Study on Low Temperature Modified Atmosphere and Plasma Preservation Technology for Tender Shoots and Tender Leaves of Acanthopanax senticosus. For. By-Prod. Spec. China 2014, 26–29. [Google Scholar] [CrossRef]
- Jiang, G.; Sun, Z.; Shen, H.; Zhang, Y. Progress in research on role of low temperature in storage techniques for fresh-cut fruits and vegetables. Beverage Ind. 2011, 14, 15–17. [Google Scholar]
- Jiber, Y.; Harroud, H.; Karmouch, A. Precision agriculture monitoring framework based on WSN. In Proceedings of the 2011 7th International Wireless Communications and Mobile Computing Conference, Istanbul, Turkey, 4–8 July 2011; pp. 2015–2020. [Google Scholar]
- Feng, H.; Wang, W.; Chen, B.; Zhang, X.J.I.A. Evaluation on frozen shellfish quality by blockchain based multi-sensors monitoring and SVM algorithm during cold storage. IEEE Access 2020, 8, 54361–54370. [Google Scholar] [CrossRef]
- Cristescu, S.M.; Mandon, J.; Arslanov, D.; De Pessemier, J.; Hermans, C.; Harren, F.J. Current methods for detecting ethylene in plants. Ann. Bot. 2013, 111, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.G.; Yu, J.Q.; Han, P.L.; Xie, X.B.; Sun, C.H.; Zhang, Q.Y.; Wang, J.H.; Hao, Y.J. The regulatory module Md PUB 29-Mdb HLH 3 connects ethylene biosynthesis with fruit quality in apple. New Phytol. 2019, 221, 1966–1982. [Google Scholar] [CrossRef]
- Adams, D.O.; Yang, S.F. Ethylene the gaseous plant hormone: Mechanism and regulation of biosynthesis. Trends Biochem. Sci. 1981, 6, 161–164. [Google Scholar] [CrossRef]
- Fluhr, R.; Mattoo, A.K.; Dilley, D.R. Ethylene—Biosynthesis and perception. Crit. Rev. Plant Sci. 1996, 15, 479–523. [Google Scholar]
- Wang, K.L.-C.; Li, H.; Ecker, J. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Duan, Z.; Yuan, Z.; Li, X.; Si, W.; Liu, B.; Zhang, Y.; Jiang, Y.; Tai, H. High performance ethylene sensor based on palladium-loaded tin oxide: Application in fruit quality detection. Chin. Chem. Lett. 2020, 31, 2045–2049. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, M.; Liu, M.; Su, D.; Chen, J.; Gao, Y.; Bouzayen, M.; Li, Z. The Molecular Regulation of Ethylene in Fruit Ripening. Small Methods 2020, 4, 1900485. [Google Scholar] [CrossRef]
- Shibo, Y. Guidance for Physical and Chemical testing Methods of Foods; Peking University Press: Beijing, China, 1991; pp. 346–351. [Google Scholar]
- Meng, X.; Jiang, A.; Hu, W.; Tian, M.; Liu, C. Effects of plastic box modified atmosphere storage on the physiological and biochemical changes of postharvest blueberry fruits. Sci. Technol. Food Ind. 2011, 10, 379–383. [Google Scholar]
- Fangdong, L.; Yuxia, W.; Liquan, K.; Fuxing, Z.; Qingtian, S.; Xu, Z.; Yanju, L. Effects of Harvesting Period on the Quality of Meizao Cherry Fruit. Yantai Fruit Tree 2018, 2, 9–11. [Google Scholar]
- Leone, A.; Distante, C.; Ancona, N.; Persaud, K.; Stella, E.; Siciliano, P. A powerful method for feature extraction and compression of electronic nose responses. Sens. Actuators B Chem. 2005, 105, 378–392. [Google Scholar] [CrossRef]
- Zheng, H.; Jun, W. Studies on Signal Feature Extraction and Sensor Optimization of an Electronic Nose. Chin. J. Sens. Actuators 2006, 3, 606–610. [Google Scholar]
- Wen, A.; Guangfen, W.; Chunying, Y. Analysis of feature parameters of gas sensors. J. Transduct. Technol. 2012, 25. [Google Scholar] [CrossRef]
- Guo, X.-Z.; Chen, J.-Y.; Gu, H.; Cheng, D.-W.; Zhang, W.-Y.; Yang, Z. The Secure Application of Ethephon on Fruits and Vegetables Production. Hubei Agric. Sci. 2018, 57, 5–8. [Google Scholar]
- Kader, A.A. Postharvest Technology of Horticultural Crops; University of California Agriculture and Natural Resources: Richmond, CA, USA, 2002; Volume 3311. [Google Scholar]
- Pongprasert, N.; Srilaong, V.; Sugaya, S. An alternative technique using ethylene micro-bubble technology to accelerate the ripening of banana fruit. Sci. Hortic 2020, 272, 109566. [Google Scholar] [CrossRef]
- Linkies, A.; Leubner-Metzger, G. Beyond gibberellins and abscisic acid: How ethylene and jasmonates control seed germination. Plant Cell Rep. 2012, 31, 253–270. [Google Scholar] [CrossRef]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 181–195. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Golding, J.; Shearer, D.; Wyllie, S.; McGlasson, W. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol. Technol. 1998, 14, 87–98. [Google Scholar] [CrossRef]
- Cai, C.; Chen, K.; Xu, W.; Zhang, W.; Li, X.; Ferguson, I. Effect of 1-MCP on postharvest quality of loquat fruit. Postharvest Biol. Technol. 2006, 40, 155–162. [Google Scholar] [CrossRef]
- Khan, A.S.; Singh, Z. 1-MCP regulates ethylene biosynthesis and fruit softening during ripening of ‘Tegan Blue’plum. Postharvest Biol. Technol. 2007, 43, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Wei, X.; Lü, E.; Wang, Y.; Deng, Z.J.F.C. Ripening behavior and quality of 1-MCP treated d’Anjou pears during controlled atmosphere storage. Food Control 2020, 117, 107364. [Google Scholar] [CrossRef]
- Yuan, R.; Carbaugh, D.H. Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Golden Supreme’and ‘Golden Delicious’ apples. HortScience 2007, 42, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Sigal-Escalada, V. Interactions of AVG, MCP and heat treatment on apple fruit ripening and quality after harvest and cold storage. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2006. [Google Scholar]
- Bregoli, A.M.; Scaramagli, S.; Costa, G.; Sabatini, E.; Ziosi, V.; Biondi, S.; Torrigiani, P. Peach (Prunus persica) fruit ripening: Aminoethoxyvinylglycine (AVG) and exogenous polyamines affect ethylene emission and flesh firmness. Physiol. Plant. 2002, 114, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Singh, Z. 1-Methylcyclopropene application and modified atmosphere packaging affect ethylene biosynthesis, fruit softening, and quality of ‘Tegan Blue’Japanese plum during cold storage. J. Am. Soc. Hortic. Sci. 2008, 133, 290–299. [Google Scholar] [CrossRef]
- Chervin, C.; El-Kereamy, A.; Roustan, J.-P.; Latché, A.; Lamon, J.; Bouzayen, M. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 2004, 167, 1301–1305. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, E.; Huberman, M.; Goren, R. Probing the role of endogenous ethylene in the degreening of citrus fruit with ethylene antagonists. Plant Growth Regul. 1993, 12, 325–329. [Google Scholar] [CrossRef]
- Trainotti, L.; Pavanello, A.; Casadoro, G. Different ethylene receptors show an increased expression during the ripening of strawberries: Does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J. Exp. Bot. 2005, 56, 2037–2046. [Google Scholar] [CrossRef] [Green Version]
- Flores, F.; El Yahyaoui, F.; De Billerbeck, G.; Romojaro, F.; Latche, A.; Bouzayen, M.; Pech, J.C.; Ambid, C. Role of ethylene in the biosynthetic pathway of aliphatic ester aroma volatiles in Charentais Cantaloupe melons. J. Exp. Bot. 2002, 53, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]




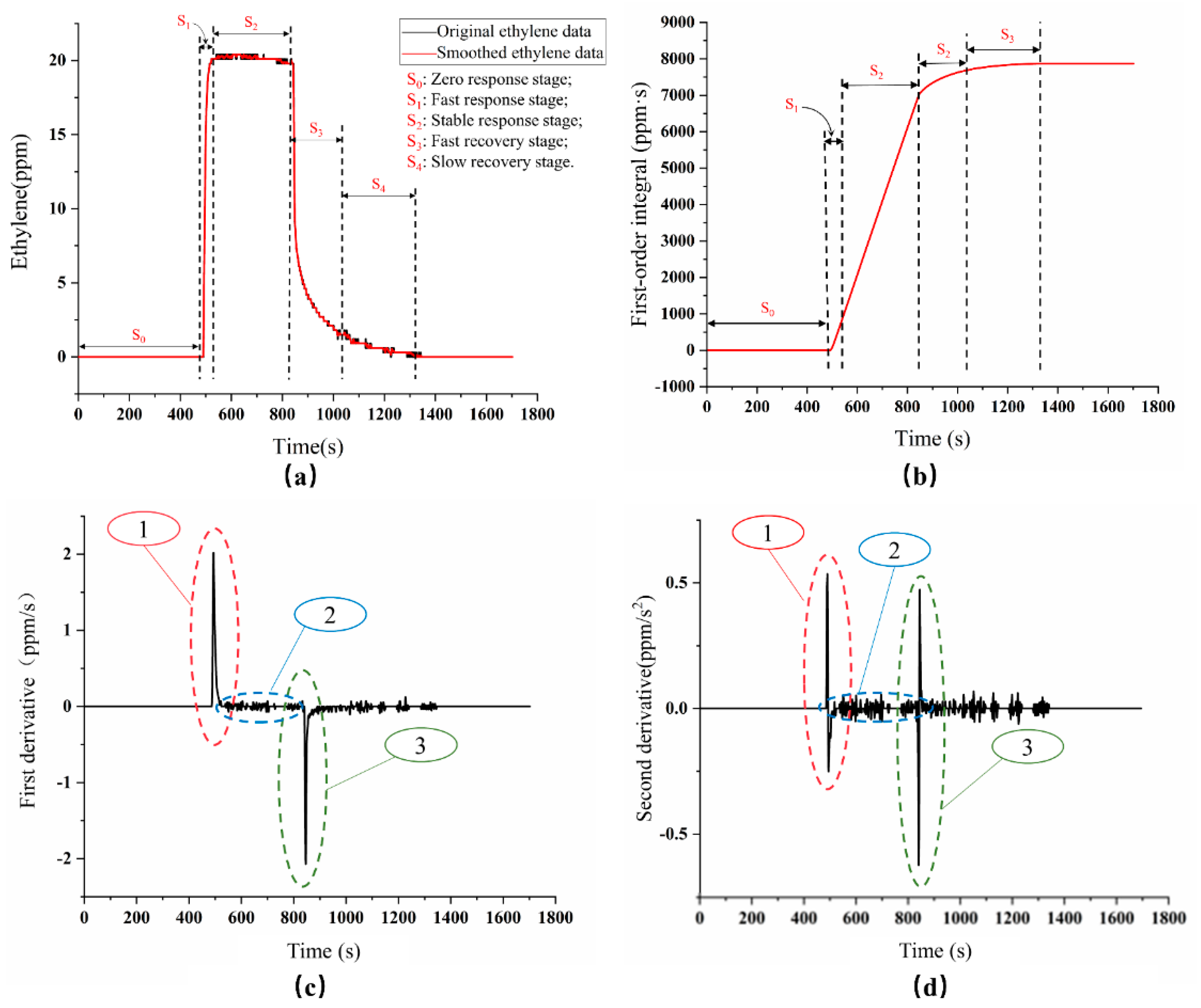



| Type | No | Characteristic Parameters | Description | Value |
|---|---|---|---|---|
| Time-domain curve (Dynamic response curve) | 1 | Xa (ppm) | Baseline value in air | 0 |
| 2 | Xg (ppm) | Stable response value | 20.13 | |
| 3 | Xm (ppm) | Maximum response value | 20.40 | |
| 4 | Xrecair (ppm) | Final recovery value | 0.03 | |
| 5 | Tres (s) | Response time | 18 | |
| 6 | Trec (s) | Recovery time | 158 | |
| 7 | TDres (s) | Duration from gas entry to Dres | 6 | |
| 8 | TDrec (s) | Duration from gas out to Drec | 9 | |
| 9 | TDresx (s) | Duration from gas entry to Dresx | 2 | |
| 10 | TDresn (s) | Duration from gas entry to Dresn | 12 | |
| 11 | TDrecx (s) | Duration from gas out to Drecx | 3 | |
| 12 | TDrecn (s) | Duration from gas out to Drecn | 60 | |
| First-order derivative | 12 | Dres (ppm/s) | The maximum value of the first-order derivative in the response phase | 2.3986 |
| 13 | Drec (ppm/s) | The maximum value of the first-order derivative in the recovery phase | −2.4043 | |
| Second-order derivative | 14 | Dresx (ppm/s2) | Maximum value of the second-order derivative in the response phase | 0.8586 |
| 15 | Dresn (ppm/s2) | The minimum value of the second-order derivative in the response phase | −0.0236 | |
| 16 | Drecx (ppm/s2) | The maximum value of the second-order derivative in the recovery phase | −0.9979 | |
| 17 | Drecn (ppm/s2) | The minimum value of the second-order derivative in the recovery phase | −0.0064 | |
| First-order integral | 18 | IntT (ppm·s) | Signal integration in the period from gas entry to gas out | 6963.72 |
| 19 | IntPres (ppm·s) | Signal integration in the period from gas entry to Tres | 475.96 | |
| 20 | IntPrec (ppm·s) | Signal integration in the period from gas entry to Trec | 818.23 |
| Gas content | Temperature | Firmness | SSC | pH | Rotting Rate |
|---|---|---|---|---|---|
| Ethylene | 0 °C | −0.96 | 0.38 | 0.81 | 0.80 |
| 5 °C | −0.98 | 0.12 | 0.57 | 0.86 | |
| 22 °C | −0.99 | −0.51 | −0.12 | 0.92 |
| Gas Content | Group | Firmness | SSC | pH | ΔE |
|---|---|---|---|---|---|
| Ethylene | I | −0.39274 | −0.71885 | 0.691314 | −0.33042 |
| II | −0.29544 | −0.65403 | 0.770447 | −0.22301 | |
| III | −0.12128 | −0.78234 | 0.90137 | −0.18586 |
| No | Control Strategy | Control Methods | Applicable Scenario | Typical Fruits | Applications | Main Advantages | Limitations |
|---|---|---|---|---|---|---|---|
| I | Adding ethylene | Fumigated with ethylene gas | Climacteric fruits that need to be ripened | Banana Mango Kiwi fruit | Storage | Automatically control. Direct action. | Complex devices, Costly |
| Application of ethephon (ETH) | Storage/ Logistics | High Efficiency, Low-cost User-friendly. | Ethephon residual risk, Safety usage (strong acidity), Strict temperature control | ||||
| Dipped in ethylene microbubble | Storage | Convenient, Easy to carry out | Not Found Yet | ||||
| II | Reducing ethylene | Low temperature | Climacteric fruits that need to inhibit ripen | Blueberry Peach Pear | Storage/ Logistics | High efficiency, User-convenient, Safe and eco-friendly. | Costly equipment |
| Ventilation treatment | Storage/ Logistics | Easy operation, Automatic control | Limited effect | ||||
| Ethylene inhibitor (1-MCP or AVG) | Storage | High efficiency, Safe and eco-friendly, Prevention of chilling injury. | High concentration treatment may accelerate the deterioration of fruit quality | ||||
| Ethylene adsorbent | Storage/ Logistics | High efficiency, Less affected by temperature | Easy desorption, Poor adsorption capacity, Food safety risk | ||||
| Modified atmosphere packaging (MAP) | Logistics | Flexible packaging, Controllable gas composition | High cost | ||||
| III | Adding ethylene | Application of ethephon (ETH) | Non-climacteric fruits | Cherries Strawberry Citrus | Storage/Logistics | Convenient User-friendly | Ethephon residual risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, X.; Fu, D.; Vidrih, R.; Zhang, X. Ethylene Sensor-Enabled Dynamic Monitoring and Multi-Strategies Control for Quality Management of Fruit Cold Chain Logistics. Sensors 2020, 20, 5830. https://doi.org/10.3390/s20205830
Wang X, Li X, Fu D, Vidrih R, Zhang X. Ethylene Sensor-Enabled Dynamic Monitoring and Multi-Strategies Control for Quality Management of Fruit Cold Chain Logistics. Sensors. 2020; 20(20):5830. https://doi.org/10.3390/s20205830
Chicago/Turabian StyleWang, Xuepei, Xinwu Li, Daqi Fu, Rajko Vidrih, and Xiaoshuan Zhang. 2020. "Ethylene Sensor-Enabled Dynamic Monitoring and Multi-Strategies Control for Quality Management of Fruit Cold Chain Logistics" Sensors 20, no. 20: 5830. https://doi.org/10.3390/s20205830
APA StyleWang, X., Li, X., Fu, D., Vidrih, R., & Zhang, X. (2020). Ethylene Sensor-Enabled Dynamic Monitoring and Multi-Strategies Control for Quality Management of Fruit Cold Chain Logistics. Sensors, 20(20), 5830. https://doi.org/10.3390/s20205830






