Effect of Bout Length on Gait Measures in People with and without Parkinson’s Disease during Daily Life
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Daily Life Gait Data Collection
2.3. Measures of Gait
2.4. Statistical Analysis
3. Results
3.1. Group Characteristics and Adherence
3.2. Frequency of Gait Bout Lengths over a Week of Daily Life
3.3. Gait Measures as a Function of Gait Bout Length
3.4. ICC and AUC of Gait Measures as a Function of Gait Bout Length
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Gait Measure | Term/Component of Bout Length | Estimates | Std. Error | t | p |
|---|---|---|---|---|---|
| Gait Speed (meters/second) | Intercept | 0.892 | 0.025 | 35.549 | 0.000 |
| Linear | 0.271 | 0.028 | 9.629 | 0.000 | |
| Quadratic | −0.089 | 0.024 | −3.701 | 0.000 | |
| PD: Intercept | −0.128 | 0.033 | −3.916 | 0.000 | |
| PD: Linear | 0.058 | 0.037 | 1.570 | 0.116 | |
| PD: Quadratic | 0.088 | 0.031 | 2.808 | 0.005 | |
| Stride Length (meters) | Intercept | 1.056 | 0.031 | 34.286 | 0.000 |
| Linear | 0.237 | 0.026 | 9.111 | 0.000 | |
| Quadratic | −0.059 | 0.023 | −2.559 | 0.010 | |
| PD: Intercept | −0.166 | 0.040 | −4.144 | 0.000 | |
| PD: Linear | 0.095 | 0.034 | 2.790 | 0.005 | |
| PD: Quadratic | 0.061 | 0.030 | 2.014 | 0.044 | |
| Pitch at Initial Contact (degrees) | Intercept | −19.025 | 0.958 | −19.857 | 0.000 |
| Linear | −5.829 | 0.909 | −6.412 | 0.000 | |
| Quadratic | 1.915 | 0.743 | 2.577 | 0.010 | |
| PD: Intercept | 5.157 | 1.246 | 4.140 | 0.000 | |
| PD: Linear | −3.744 | 1.189 | −3.148 | 0.002 | |
| PD: Quadratic | −2.059 | 0.974 | −2.115 | 0.034 | |
| Double Support (%) | Intercept | 24.097 | 0.651 | 37.002 | 0.000 |
| Linear | −4.585 | 0.478 | −9.586 | 0.000 | |
| Quadratic | 1.969 | 0.563 | 3.500 | 0.000 | |
| PD: Intercept | 1.441 | 0.847 | 1.702 | 0.089 | |
| PD: Linear | −1.046 | 0.626 | −1.670 | 0.095 | |
| PD: Quadratic | −0.739 | 0.735 | −1.006 | 0.314 | |
| Cadence (strides/minute) | Intercept | 49.988 | 0.914 | 54.719 | 0.000 |
| Linear | 4.596 | 0.927 | 4.959 | 0.000 | |
| Quadratic | −2.882 | 0.577 | −4.994 | 0.000 | |
| PD: Intercept | 1.440 | 1.188 | 1.212 | 0.225 | |
| PD: Linear | −2.608 | 1.209 | −2.158 | 0.031 | |
| PD: Quadratic | 2.412 | 0.756 | 3.191 | 0.001 | |
| Swing Duration (seconds) | Intercept | 0.467 | 0.007 | 62.463 | 0.000 |
| Linear | −0.022 | 0.008 | −2.966 | 0.003 | |
| Quadratic | 0.016 | 0.004 | 4.205 | 0.000 | |
| PD: Intercept | −0.020 | 0.010 | −2.039 | 0.041 | |
| PD: Linear | 0.027 | 0.010 | 2.706 | 0.007 | |
| PD: Quadratic | −0.022 | 0.005 | −4.387 | 0.000 |
References
- Nutt, J.G.; Marsden, C.D.; Thompson, P.D. Human walking and higher-level gait disorders, particularly in the elderly. Neurology 1993, 43, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.H.; Van De Warrenburg, B.P.; Giladi, N.; Bloem, B.R. Neurological gait disorders in elderly people: Clinical approach and classification. Lancet Neurol. 2007, 6, 63–74. [Google Scholar] [CrossRef]
- Mancini, M.; Nutt, J.G.; Horak, F.B. Balance Dysfunction in Parkinson’s Disease: Basic Mechanisms to Clinical Management, 1st ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Peterson, D.S.; Horak, F.B. Neural Control of Walking in People with Parkinsonism. Physiology 2016, 31, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Lord, S.; Galna, B.; Rochester, L. Moving forward on gait measurement: Toward a more refined approach. Mov. Disord. 2013, 28, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Del Din, S.; Elshehabi, M.; Galna, B.; Hobert, M.A.; Warmerdam, E.; Sünkel, U.; Brockmann, K.; Metzger, F.; Hansen, C.; Berg, D.; et al. Gait analysis with wearables predicts conversion to Parkinson disease. Ann. Neurol. 2019, 86, 357–367. [Google Scholar] [CrossRef]
- Curtze, C.J.; Nutt, G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’ s Disease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- Hale, L.; Pal, J.; Becker, I. Measuring Free-Living Physical Activity in Adults With and Without Neurologic Dysfunction with a Triaxial Accelerometer. Arch. Phys. Med. Rehabil. 2008, 89, 1765–1771. [Google Scholar] [CrossRef]
- Chastin, S.F.; Baker, K.; Jones, D.; Burn, D.J.; Granat, M.; Rochester, L. The pattern of habitual sedentary behavior is different in advanced Parkinson’s disease. Mov. Disord. 2010, 25, 2114–2120. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Galna, B.; Lord, S.; Rochester, L. Free-living gait characteristics in ageing and Parkinson’s disease: Impact of environment and ambulatory bout length. J. Neuroeng. Rehabil. 2016, 13, 46. [Google Scholar] [CrossRef] [Green Version]
- Mancini, M.; Schlueter, H.; El-Gohary, M.; Mattek, N.; Duncan, C.; Kaye, J.; Horak, F.B. Continuous Monitoring of Turning Mobility and Its Association to Falls and Cognitive Function: A Pilot Study. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2016, 71, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernad-Elazari, H.; Herman, T.; Mirelman, A.; Gazit, E.; Giladi, N.; Hausdorff, J. Objective characterization of daily living transitions in patients with Parkinson’s disease using a single body-fixed sensor. J. Neurol. 2016, 263, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- De Lima, A.L.S.; Hahn, T.; Evers, L.J.W.; De Vries, N.M.; Cohen, E.; Afek, M.; Bataille, L.; Daeschler, M.; Claes, K.; Boroojerdi, B.; et al. Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS ONE 2017, 12, e0189161. [Google Scholar]
- Adams, J.L.; Dinesh, K.; Xiong, M.; Tarolli, C.G.; Sharma, S.; Sheth, N.; Aranyosi, A.; Zhu, W.; Goldenthal, S.; Biglan, K.M.; et al. Multiple Wearable Sensors in Parkinson and Huntington Disease Individuals: A Pilot Study in Clinic and at Home. Digit. Biomarkers 2017, 1, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Lipsmeier, F.; Taylor, K.I.; Msc, T.K.; Wolf, D.; Scotland, A.; Schjodt-Eriksen, J.; Cheng, W.-Y.; Fernandez-Garcia, I.; Siebourg-Polster, J.; Jin, L.; et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov. Disord. 2018, 33, 1287–1297. [Google Scholar] [CrossRef]
- Arora, S.; Baig, F.; Lo, C.; Barber, T.R.; Lawton, M.; Zhan, A.; Rolinski, M.; Ruffmann, C.; Klein, J.C.; Rumbold, J.; et al. Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder. controls, and PD. Neurology 2018, 91, e1528–e1538. [Google Scholar] [CrossRef] [Green Version]
- Zhan, A.; Mohan, S.; Tarolli, C.; Schneider, R.B.; Adams, J.L.; Sharma, S.; Terzis, A. Using smartphones and machine learning to quantify Parkinson disease severity the mobile Parkinson disease score. JAMA Neurol. 2018, 75, 876–880. [Google Scholar] [CrossRef]
- Mancini, M.; Weiss, A.; Herman, T.; Hausdorff, J.M. Turn Around Freezing: Community-Living Turning Behavior in People with Parkinson’s Disease. Front. Neurol. 2018, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.V.; McNames, J.; Mancini, M.; Carlson-Kuhta, P.; Nutt, J.G.; El-Gohary, M.; Lapidus, J.A.; Horak, F.B.; Curtze, C. Digital Biomarkers of Mobility in Parkinson’s Disease During Daily Living. J. Park. Dis. 2020. [Google Scholar] [CrossRef]
- Weiss, A.; Sharifi, S.; Plotnik, M.; Van Vugt, J.P.P.; Giladi, N.; Hausdorff, J.M. Toward Automated, At-Home Assessment of Mobility Among Patients with Parkinson Disease, Using a Body-Worn Accelerometer. Neurorehabilit. Neural Repair 2011, 25, 810–818. [Google Scholar] [CrossRef]
- Shah, V.V.; McNames, J.; Mancini, M.; Carlson-Kuhta, P.; Spain, R.I.; Nutt, J.G.; Horak, F.B. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living. J. Neurol. 2020, 267, 1188–1196. [Google Scholar] [CrossRef]
- Cavanaugh, J.T.; Ellis, T.D.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Dibble, L.E. Capturing ambulatory activity decline in parkinson’s disease. J. Neurol. Phys. Ther. 2012, 36, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, S.; Godfrey, A.; Galna, B.; Mhiripiri, D.; Burn, D.J.; Rochester, L. Ambulatory activity in incident Parkinson’s: More than meets the eye? J. Neurol. 2013, 260, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Brozgol, M.; Dorfman, M.; Herman, T.; Shema, S.; Giladi, N.; Hausdorff, J.M. Does the Evaluation of Gait Quality During Daily Life Provide Insight Into Fall Risk? A Novel Approach Using 3-Day Accelerometer Recordings. Neurorehabilit. Neural Repair 2013, 27, 742–752. [Google Scholar] [CrossRef]
- Weiss, A.; Herman, T.; Giladi, N.; Hausdorff, J.M. Objective Assessment of Fall Risk in Parkinson’s Disease Using a Body-Fixed Sensor Worn for 3 Days. PLoS ONE 2014, 9, e96675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gohary, M.; Pearson, S.; McNames, J.; Mancini, M.; Horak, F.; Mellone, S.; Chiari, L. Continuous Monitoring of Turning in Patients with Movement Disability. Sensors 2013, 14, 356–369. [Google Scholar] [CrossRef] [Green Version]
- Wallén, M.B.; Jonsson, E.; Nero, H.; Hagströmer, M. Levels and Patterns of Physical Activity and Sedentary Behavior in Elderly People With Mild to Moderate Parkinson Disease. Phys. Ther. 2015, 95, 1135–1141. [Google Scholar] [CrossRef]
- Mancini, M.; El-Gohary, M.; Pearson, S.; McNames, J.; Schlueter, H.; Nutt, J.G.; King, L.A.; Horak, F.B. Continuous monitoring of turning in Parkinson’s disease: Rehabilitation potential. NeuroRehabilitation 2015, 37, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Rovini, E.; Maremmani, C.; Cavallo, F.; Mirabella, G. How Wearable Sensors Can Support Parkinson’ s Disease Diagnosis and Treatment: A Systematic Review. Front. Neurosci. 2017, 11, 555. [Google Scholar] [CrossRef]
- Block, V.A.J.; Pitsch, E.; Tahir, P.; Cree, B.A.C.; Allen, D.D.; Gelfand, J.M. Remote Physical Activity Monitoring in Neurological Disease: A Systematic Review. PLoS ONE 2016, 11, e0154335. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Mazzà, C.; Lord, S.; Rochester, L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov. Disord. 2016, 31, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, E.; Hausdorff, J.; Atrsaei, A.; Zhou, Y.; Mirelman, A.; Aminian, K.; Espay, A.J.; Hansen, C.; Evers, L.J.W.; Keller, A.; et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020, 19, 462–470. [Google Scholar] [CrossRef]
- Hillel, I.; Gazit, E.; Nieuwboer, A.; Avanzino, L.; Rochester, L.; Cereatti, A.; Del Din, S. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur. Rev. Aging Phys. Act. 2019, 16, 1–12. [Google Scholar] [CrossRef]
- Barbosa, A.F.; Chen, J.; Freitag, F.; Valente, D.; Souza, C.D.O.; Voos, M.C.; Chien, H.F. Gait, posture and cognition in Parkinson’ s disease. Dement Neuropsychol. 2016, 10, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Orendurff, M.S. How humans walk: Bout duration, steps per bout, and rest duration. J. Rehabilitation Res. Dev. 2008, 45, 1077–1090. [Google Scholar] [CrossRef]
- Barry, G.; Galna, B.; Lord, S.; Rochester, L.; Godfrey, A. Defining ambulatory bouts in free-living activity: Impact of brief stationary periods on bout metrics. Gait Posture 2015, 42, 594–597. [Google Scholar] [CrossRef] [Green Version]
- Hickey, A.; Del Din, S.; Rochester, L.; Godfrey, A. Detecting free-living steps and walking bouts: Validating an algorithm for macro gait analysis. Physiol. Meas. 2016, 38, N1–N15. [Google Scholar] [CrossRef]
- Shah, V.V.; McNames, J.; Harker, G.; Curtze, C.; Carlson-Kuhta, P.; Spain, R.I.; El-Gohary, M.; Mancini, M.; Horak, F.B. Does gait bout definition influence the ability to discriminate gait quality between people with and without multiple sclerosis during daily life? Gait Posture 2020. (Under review). [Google Scholar]
- Galperin, I.; Hillel, I.; Del Din, S.; Bekkers, E.M.; Nieuwboer, A.; Abbruzzese, G.; Della Croce, U. Associations between daily-living physical activity and laboratory-based assessments of motor severity in patients with falls and Parkinson’ s disease. Park. Relat. Disord. 2018, 62, 85–90. [Google Scholar] [CrossRef]
- Del Din, S.; Galna, B.; Godfrey, A.; Bekkers, E.M.; Pelosin, E.; Nieuwhof, F.; Rochester, L. Analysis of Free-Living Gait in Older Adults With and Without Parkinson’ s Disease and With and without a History of Falls: Identifying Generic and Disease-Specific Characteristics. J. Gerontol. Ser. A 2017, 74, 500–506. [Google Scholar] [CrossRef]
- Van Schooten, K.S.; Rispens, S.M.; Elders, P.J.; Van Dieën, J.H.; Pijnappels, M. Toward ambulatory balance assessment: Estimating variability and stability from short bouts of gait. Gait Posture 2014, 39, 695–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodie, M.A.; Lord, S.R.; Coppens, M.J.; Annegarn, J.; Delbaere, K.; Brodie, M.A. Eight-Week Remote Monitoring Using a Freely Worn Device Reveals Unstable Gait Patterns in Older Fallers. IEEE Trans. Biomed. Eng. 2015, 62, 2588–2594. [Google Scholar] [CrossRef] [PubMed]
- Van Schooten, K.S.; Pijnappels, M.; Rispens, S.M.; Elders, P.J.M.; Lips, P.; Daffertshofer, A.; Beek, P.J.; Van Dieën, J.H. Daily-Life Gait Quality as Predictor of Falls in Older People: A 1-Year Prospective Cohort Study. PLoS ONE 2016, 11, e015862. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Herman, T.; Giladi, N.; Hausdorff, J. New evidence for gait abnormalities among Parkinson’s disease patients who suffer from freezing of gait: Insights using a body-fixed sensor worn for 3 days. J. Neural Transm. 2014, 122, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Storm, F.; Nair, K.; Clarke, A.J.; Van Der Meulen, J.M.; Mazzà, C. Free-living and laboratory gait characteristics in patients with multiple sclerosis. PLoS ONE 2018, 13, e0196463. [Google Scholar] [CrossRef] [Green Version]
- Mueller, A.; Hoefling, H.A.; Muaremi, A.; Praestgaard, J.; Walsh, L.; Bunte, O.; Huber, R.M.; Fürmetz, J.; Keppler, A.M.; Schieker, M.; et al. Continuous Digital Monitoring of Walking Speed in Frail Elderly Patients: Noninterventional Validation Study and Longitudinal Clinical Trial. JMIR Mhealth Uhealth 2019, 7, e15191. [Google Scholar] [CrossRef]
- Cheng, W.-Y.; Lipsmeier, F.; Scotland, A.; Creagh, A.; Kilchenmann, T.; Jin, L.; Schjodt-Eriksen, J.; Wolf, D.; Zhang-Schaerer, Y.-P.; García, I.F.; et al. Smartphone-based continuous mobility monitoring of Parkinsons disease patients reveals impacts of ambulatory bout length on gait features. In Proceedings of the IEEE Life Sciences Conference (LSC); Institute of Electrical and Electronics Engineers (IEEE), Sydney, NSW, Australia, 13–15 December 2017; pp. 166–169. [Google Scholar]
- Crenna, P.; Frigo, C.; Giovannini, P.; Piccolo, I. The Initiation of Gait in Parkinson’s Disease; No. 5; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Rosin, R.; Topka, H.; Dichgans, J. Gait initiation in parkinson’s disease. Mov. Disord. 1997, 12, 682–690. [Google Scholar] [CrossRef]
- Dudman, J.T.; Krakauer, J.W. The basal ganglia: From motor commands to the control of vigor. Curr. Opin. Neurobiol. 2016, 37, 158–166. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- King, M.M.L.; Mancini, M.; Salarian, A.; Holmstrom, L.; McNames, J.; Horak, F.B. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J. Bioeng. Biomed. Sci. 2013, 7. [Google Scholar] [CrossRef]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, R.; Stuart, S.; McBarron, G.; Fino, P.C.; Mancini, M.; Curtze, C. Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol. Meas. 2019, 40, 095003. [Google Scholar] [CrossRef] [PubMed]
- Rennie, L.; Löfgren, N.; Moe-nilssen, R.; Opheim, A.; Dietrichs, E.; Franzén, E. The reliability of gait variability measures for individuals with Parkinson’ s disease and healthy older adults—The effect of gait speed. Gait Posture 2018, 62, 505–509. [Google Scholar] [CrossRef]
- Riva, F.; Bisi, M.; Stagni, R. Gait variability and stability measures: Minimum number of strides and within-session reliability. Comput. Biol. Med. 2014, 50, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Carcreff, L.; Gerber, C.N.; Paraschiv-Ionescu, A.; De Coulon, G.; Newman, C.J.; Aminian, K.; Armand, S. Comparison of gait characteristics between clinical and daily life settings in children with cerebral palsy. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Mirman, D. Growth Curve Analysis and Visualization Using R; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Shrout, P.E.; Fleiss, J.L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Brodie, M.; Coppens, M.J.; Ejupi, A.; Gschwind, Y.J.; Annegarn, J.; Schoene, D.; Wieching, R.; Lord, S.R.; Delbaere, K. Comparison between clinical gait and daily-life gait assessments of fall risk in older people. Geriatr. Gerontol. Int. 2017, 17, 2274–2282. [Google Scholar] [CrossRef]
- Murray, M.P.; Kory, R.C.; Clarkson, B.H.; Sepic, S.B. Comparison of free and fast speed walking patterns of normal men. Am. J. Phys. Med. Rehabil. 1966, 45, 8–24. [Google Scholar] [CrossRef]
- Cho, C.; Kunin, M.; Kudo, K.; Osaki, Y.; Olanow, C.W.; Cohen, B.; Raphan, T. Frequency-Velocity Mismatch: A Fundamental Abnormality in Parkinsonian. Gait. J. Neurophysiol. 2010, 103, 1478–1489. [Google Scholar] [CrossRef]
- Horak, F.B.; Frank, J.; Nutt, J. Effects of dopamine on postural control in parkinsonian subjects: Scaling, set, and tone. J. Neurophysiol. 1996, 75, 2380–2396. [Google Scholar] [CrossRef] [PubMed]
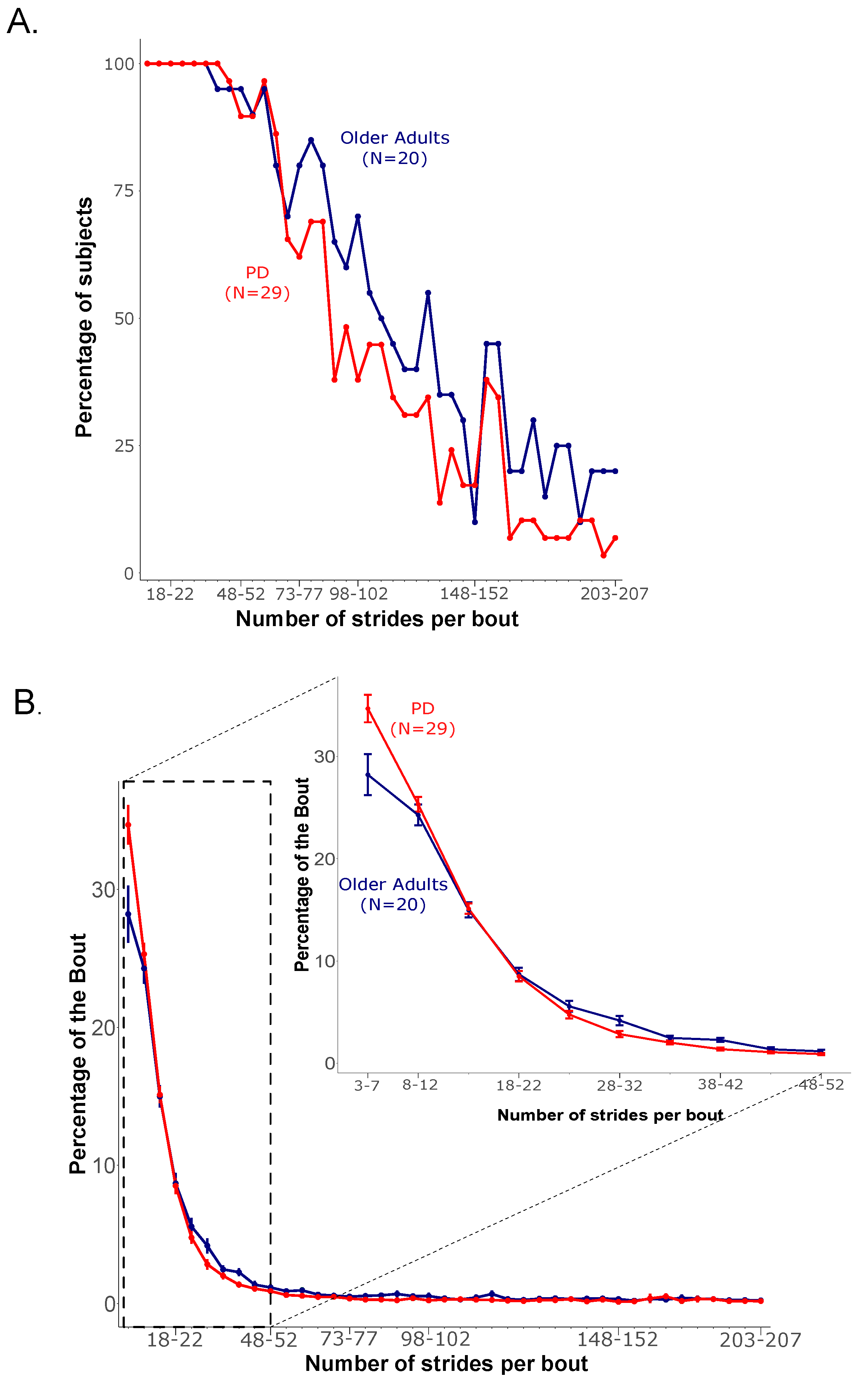

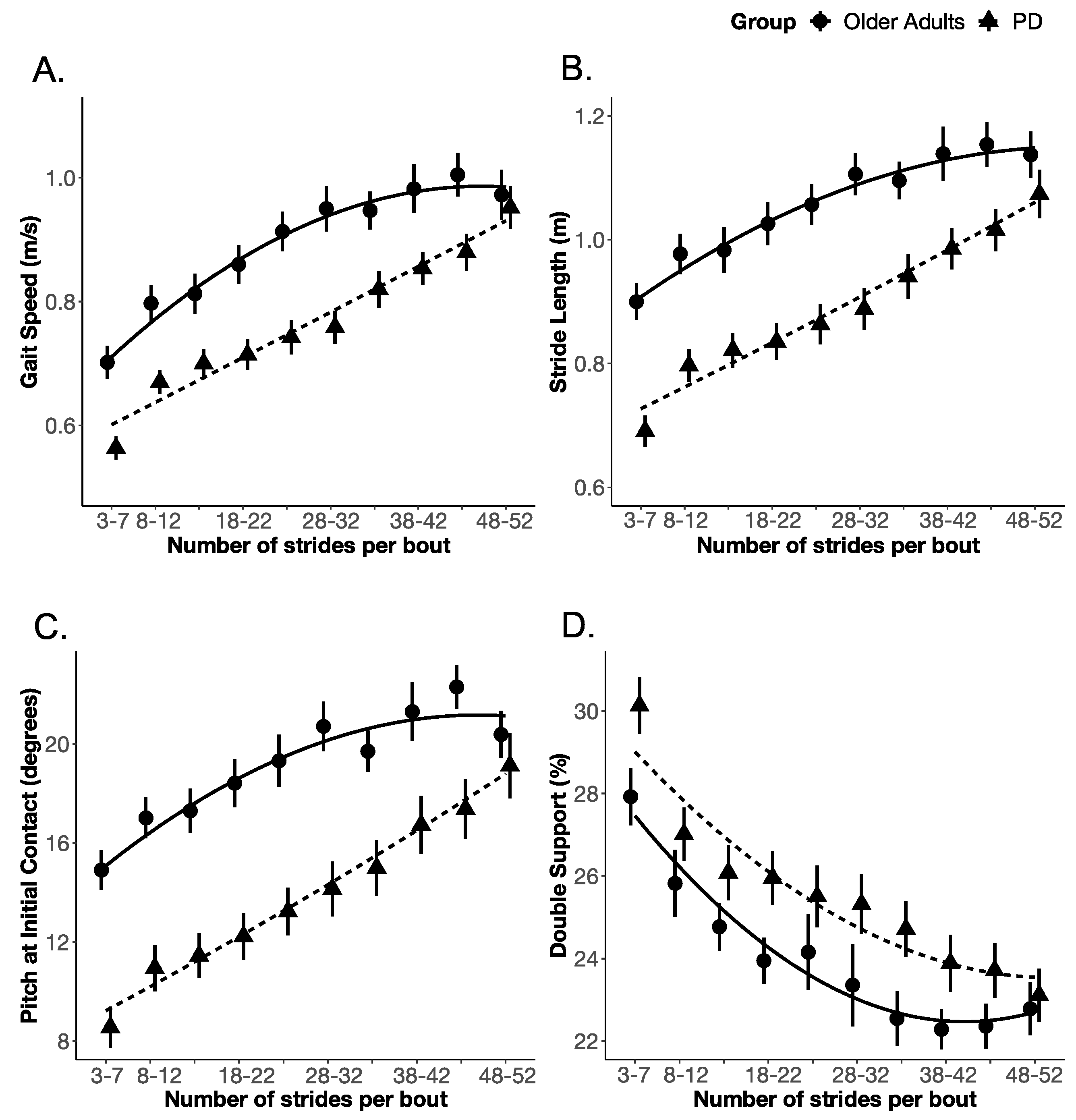

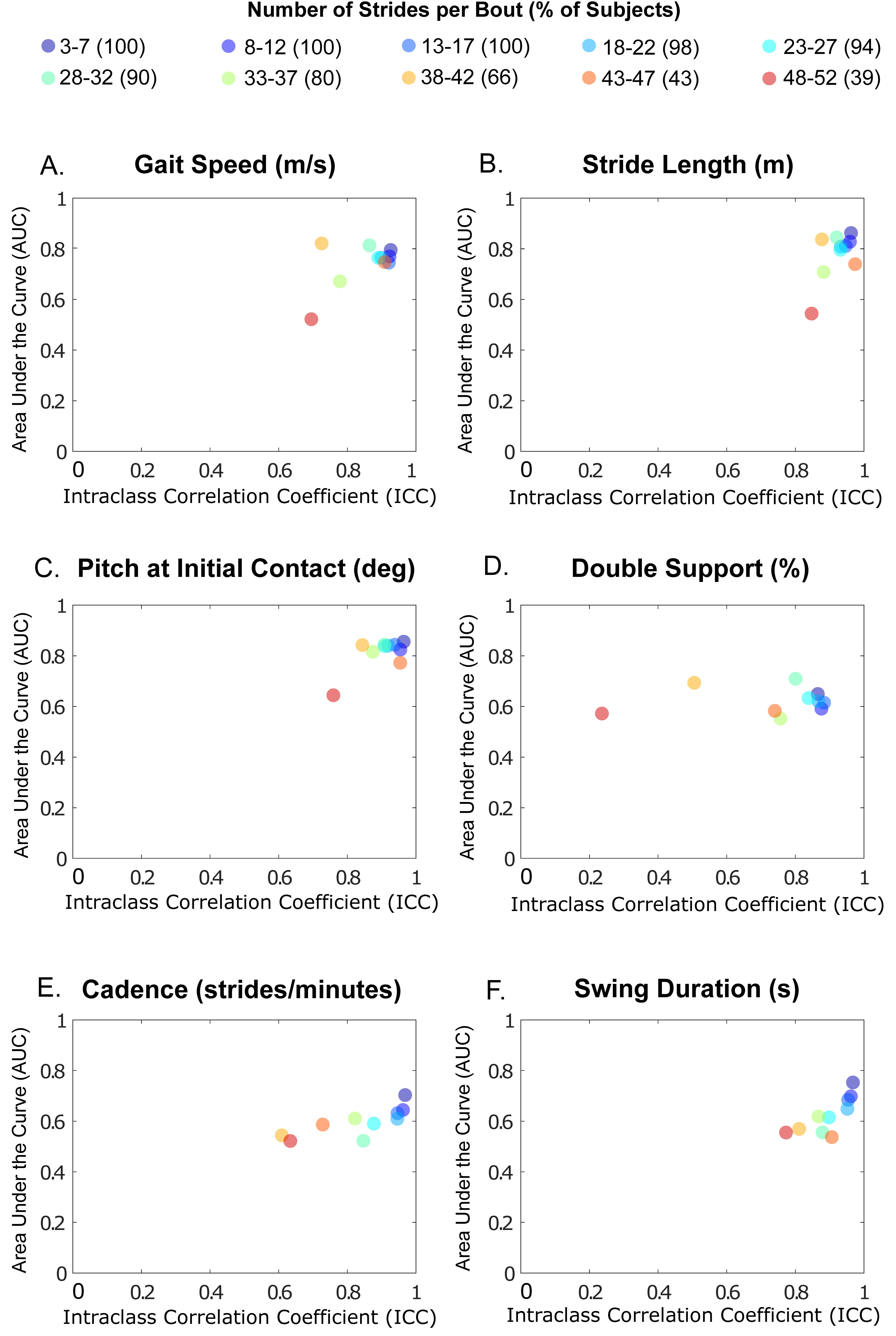
| OA (N = 20) | PD (N = 29) | p | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 66.85 | 7.16 | 67.66 | 5.27 | 0.45 |
| Height (m) | 1.70 | 0.18 | 1.71 | 0.13 | 0.32 |
| Weight (kg) | 74.61 | 9.38 | 75.95 | 12.74 | 0.91 |
| Male/Female (#) | 12/8 | 17/12 | |||
| Bouts/hour (#) | 22.37 | 1.95 | 20.72 | 1.30 | 0.68 |
| Turns/hour (#) | 102.89 | 10.96 | 77.67 | 7.59 | 0.03 |
| Gait Measure | Term/Component of Bout Length | Goodness of Fit (Log Likelihood) | χ2 (1) | p |
|---|---|---|---|---|
| Gait Speed (meters/second) | Intercept | 418.596 | 11.015 | 0.001 |
| Linear | 419.489 | 1.785 | 0.182 | |
| Quadratic | 423.150 | 7.323 | 0.007 | |
| Stride Length (meters) | Intercept | 432.732 | 13.695 | <0.001 |
| Linear | 436.061 | 6.656 | 0.010 | |
| Quadratic | 438.011 | 3.901 | 0.048 | |
| Pitch at Initial Contact (degrees) | Intercept | −1184.259 | 14.521 | <0.001 |
| Linear | −1180.753 | 7.012 | 0.008 | |
| Quadratic | −1178.601 | 4.304 | 0.038 | |
| Double Support (%) | Intercept | −1061.506 | 0.975 | 0.324 |
| Linear | −1059.899 | 3.214 | 0.073 | |
| Quadratic | −1059.399 | 0.999 | 0.317 | |
| Cadence (strides/minute) | Intercept | −1106.360 | 1.132 | 0.287 |
| Linear | −1103.814 | 5.091 | 0.024 | |
| Quadratic | −1099.274 | 9.081 | 0.003 | |
| Swing Duration (seconds) | Intercept | 1206.537 | 1.677 | 0.195 |
| Linear | 1210.184 | 7.294 | 0.007 | |
| Quadratic | 1217.958 | 15.547 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, V.V.; McNames, J.; Harker, G.; Mancini, M.; Carlson-Kuhta, P.; Nutt, J.G.; El-Gohary, M.; Curtze, C.; Horak, F.B. Effect of Bout Length on Gait Measures in People with and without Parkinson’s Disease during Daily Life. Sensors 2020, 20, 5769. https://doi.org/10.3390/s20205769
Shah VV, McNames J, Harker G, Mancini M, Carlson-Kuhta P, Nutt JG, El-Gohary M, Curtze C, Horak FB. Effect of Bout Length on Gait Measures in People with and without Parkinson’s Disease during Daily Life. Sensors. 2020; 20(20):5769. https://doi.org/10.3390/s20205769
Chicago/Turabian StyleShah, Vrutangkumar V., James McNames, Graham Harker, Martina Mancini, Patricia Carlson-Kuhta, John G. Nutt, Mahmoud El-Gohary, Carolin Curtze, and Fay B. Horak. 2020. "Effect of Bout Length on Gait Measures in People with and without Parkinson’s Disease during Daily Life" Sensors 20, no. 20: 5769. https://doi.org/10.3390/s20205769




_Carlson-Kuhta.jpg)


