Performance Enhancement of a Quartz Tuning Fork Sensor Using a Cellulose Nanocrystal-Reinforced Nanoporous Polymer Fiber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning of the PMMA and CNC/PMMA Fibers Under a Different RH
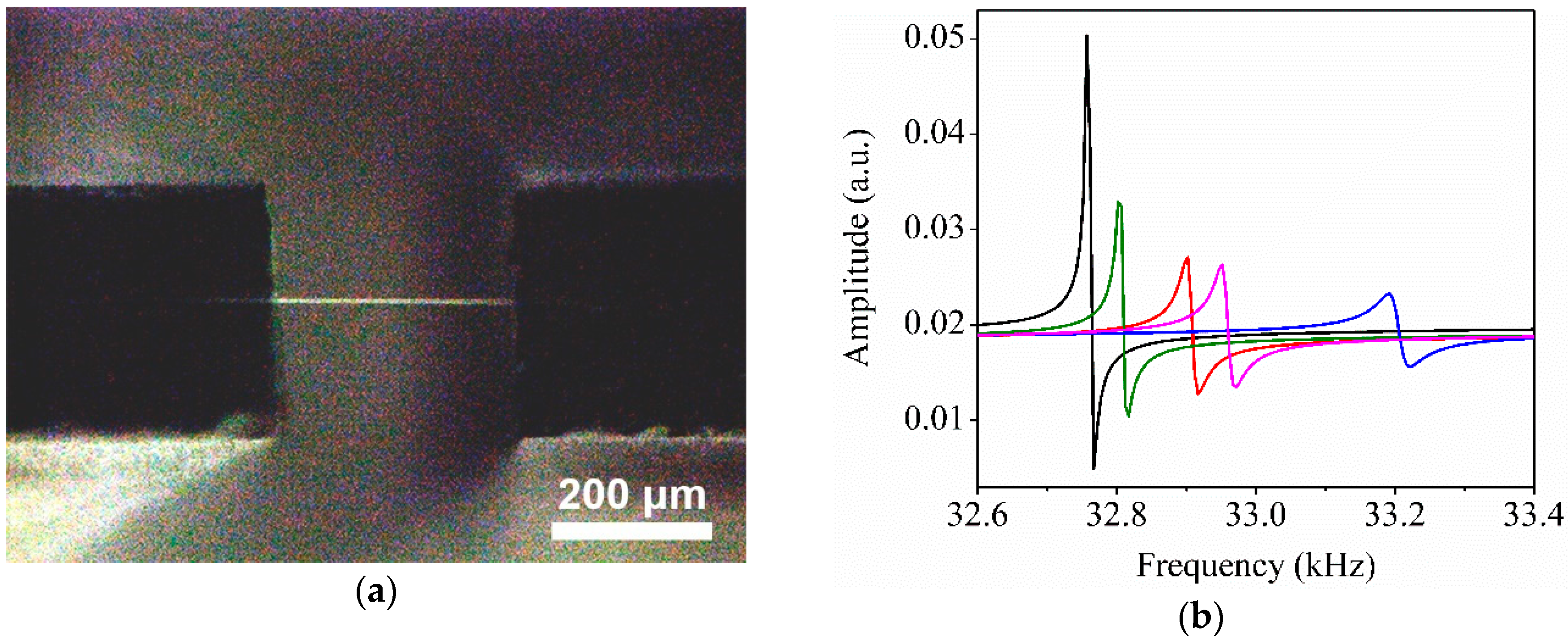
2.3. QTF Measurements
3. Results and Discussion
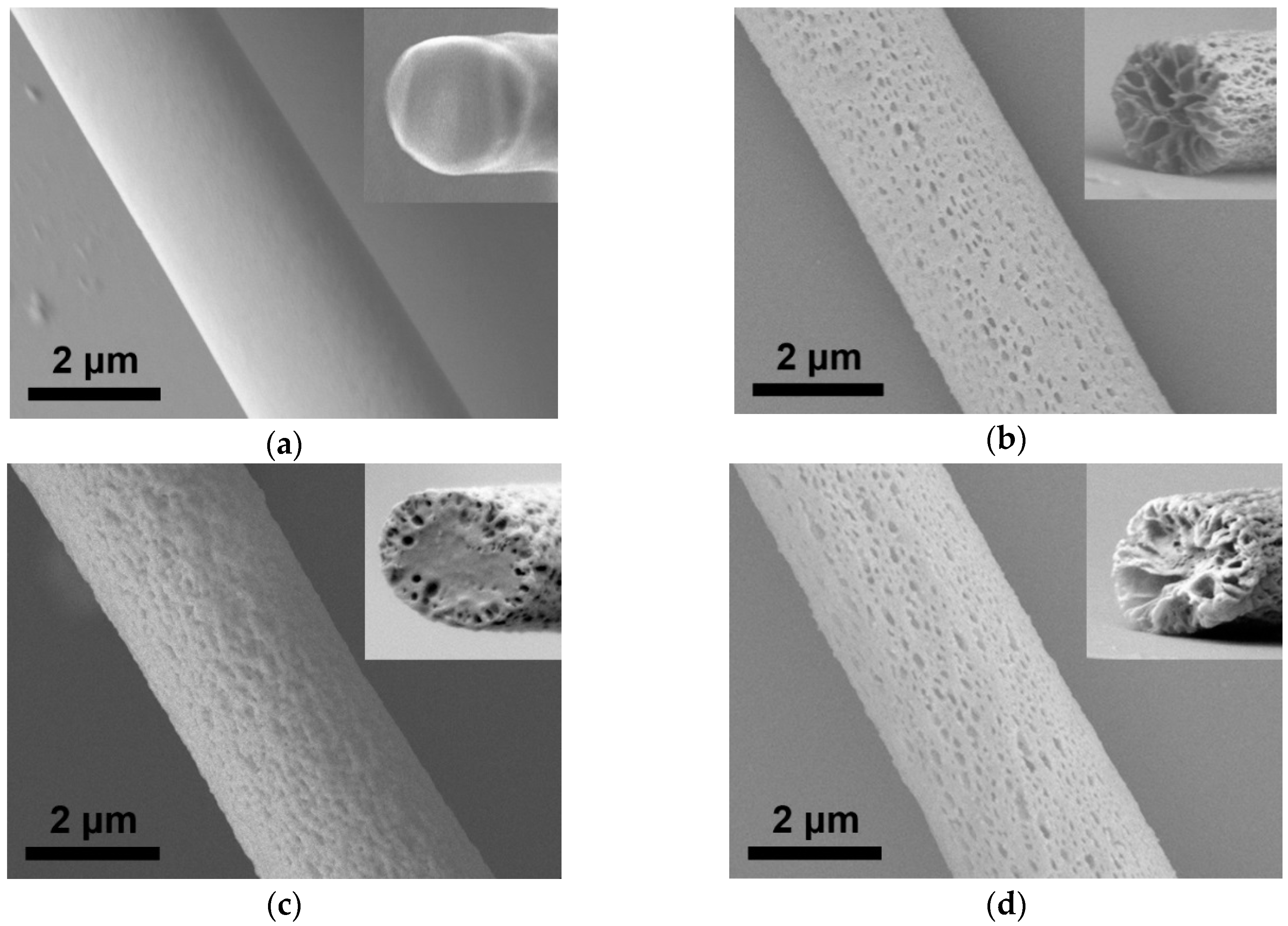
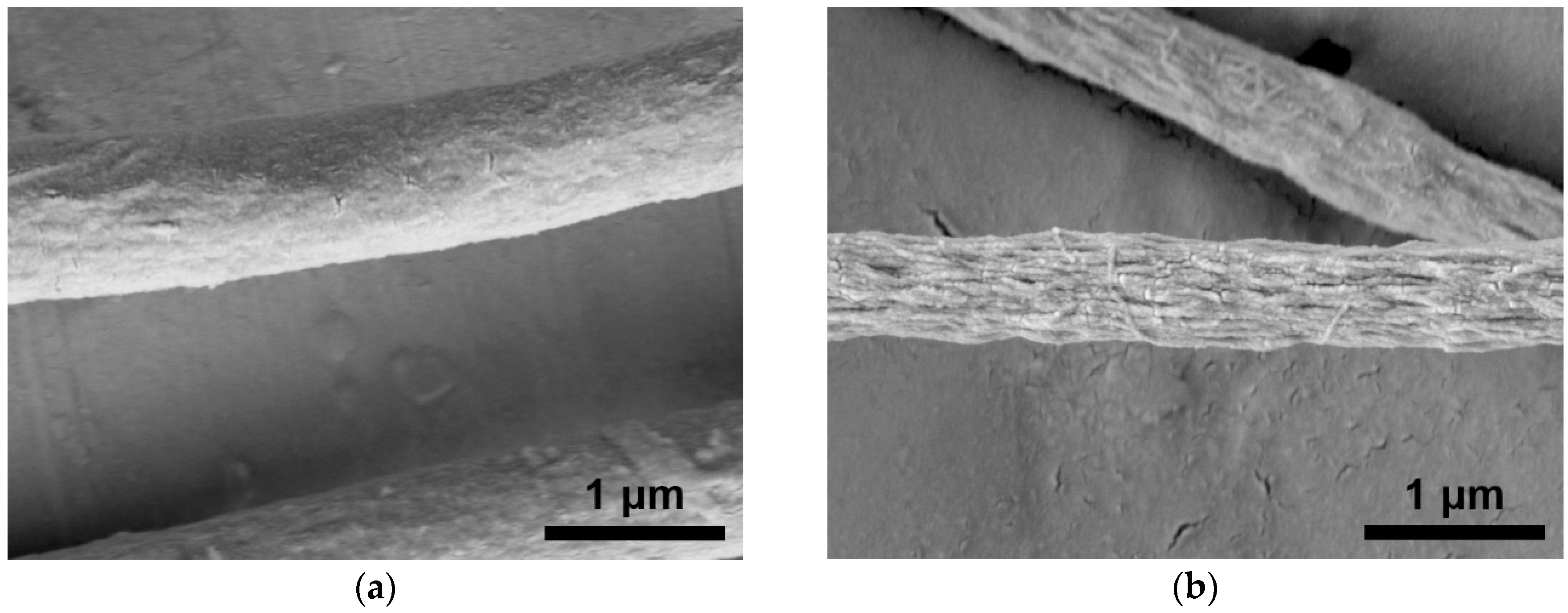
3.1. Characterization of Electrospun PMMA and CNC/PMMA Fibers
3.2. Effect of CNCs on Reinforcement of the PMMA Fibers
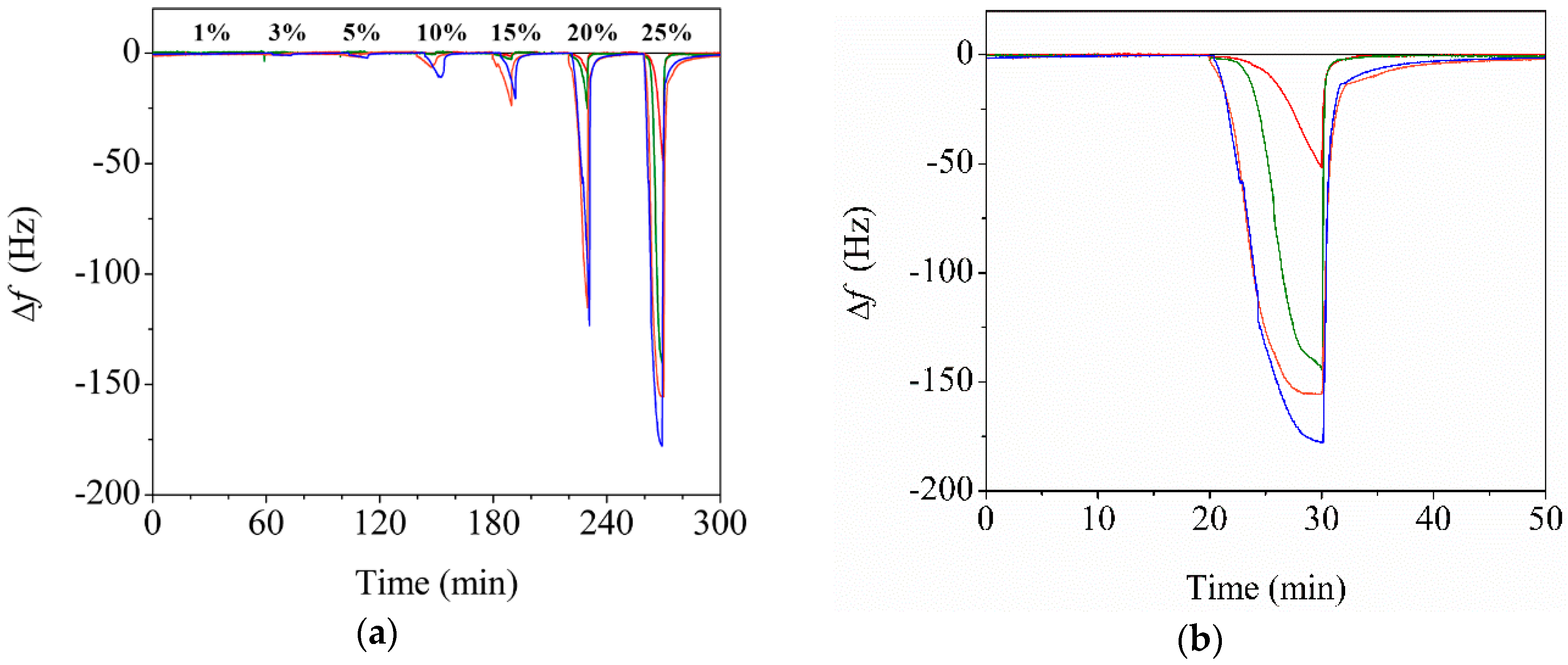
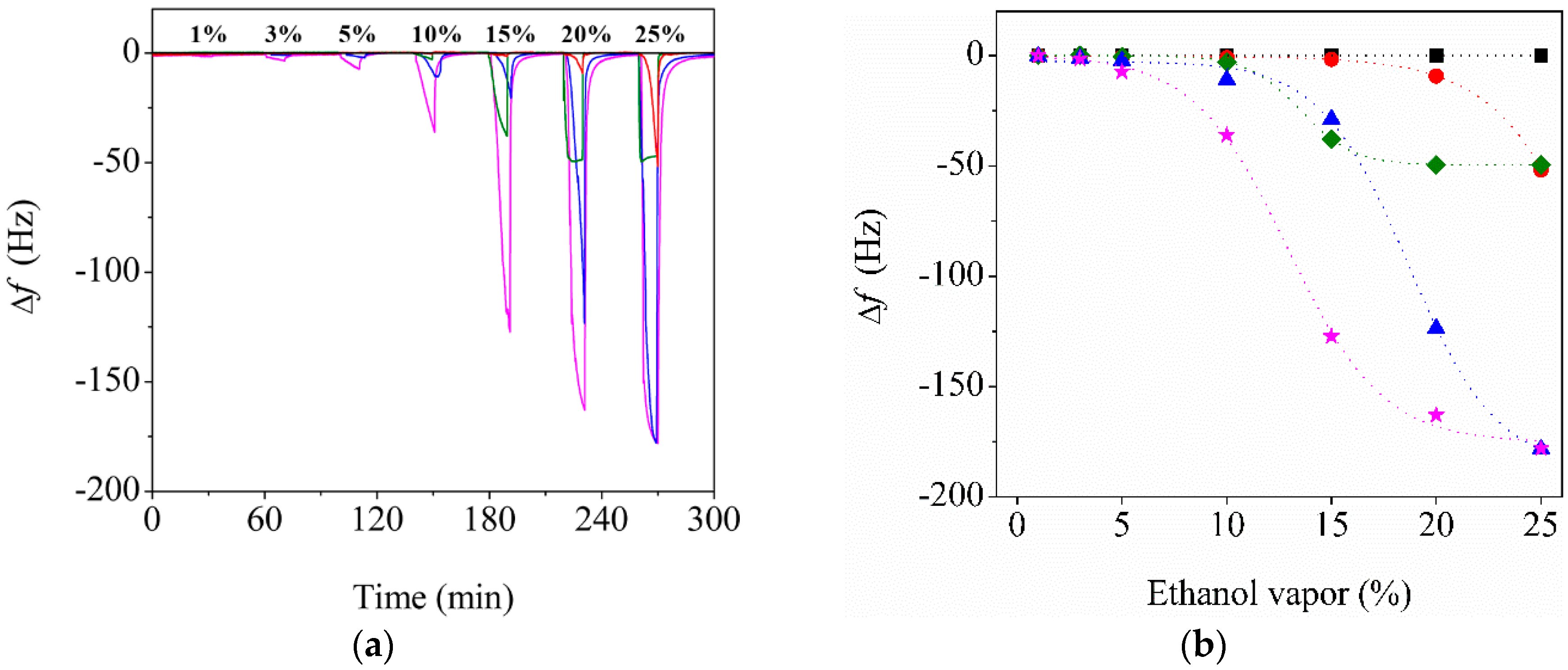
3.3. Effect of CNC Contents on the Sensor Performance
3.4. Enhanced Performance of the Reinforced Nanoporous Fiber-Mount QTF
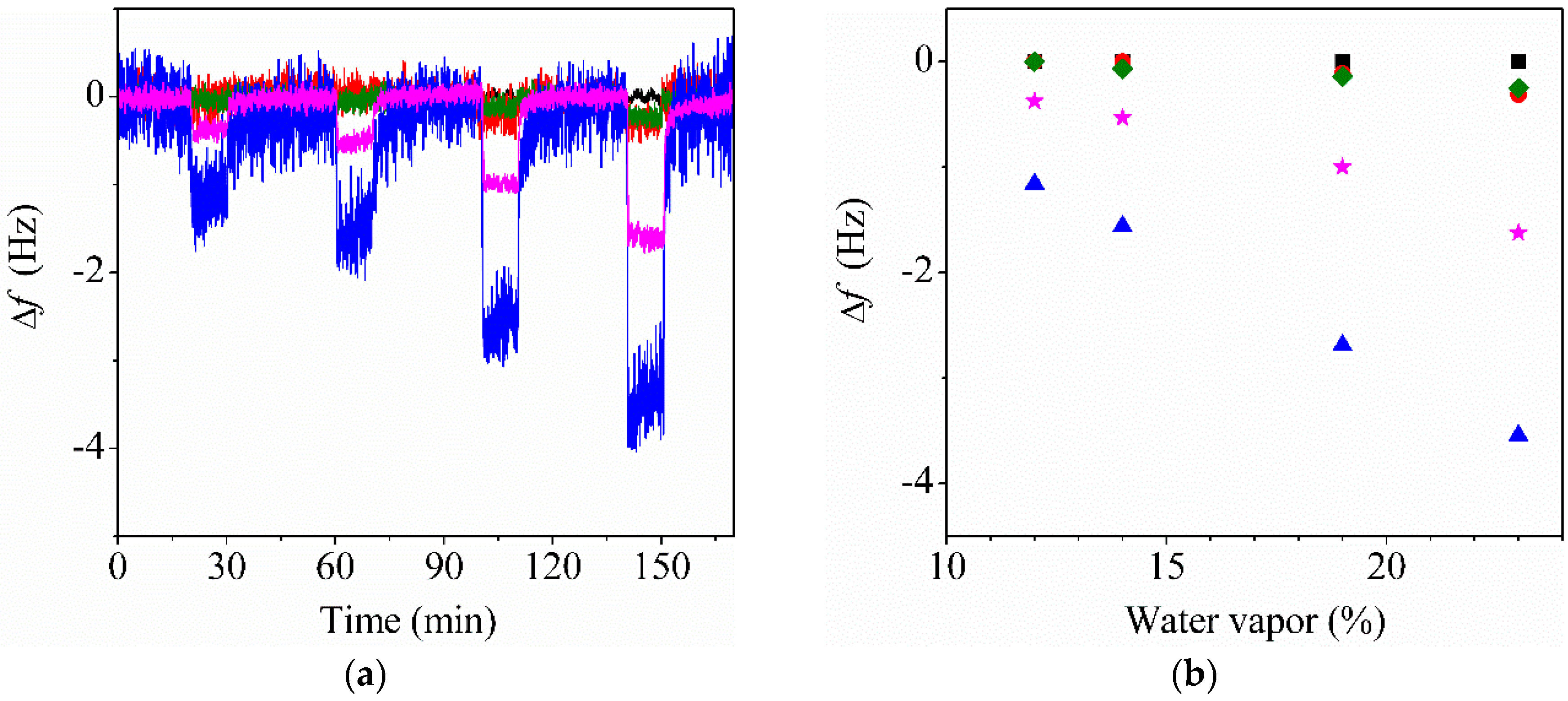
3.5. Water Sensing Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Levit, N.; Pestov, D.; Tepper, G. High surface area polymer coatings for SAW-based chemical sensor applications. Sens. Actuators B Chem. 2002, 82, 241–249. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.A.; Leclerc, M. Optical sensors based on hybrid aptamer/conjugated polymer complexes. J. Am. Chem. Soc. 2004, 126, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kameoka, J.; Czaplewski, D.A.; Craighead, H.G. Polymeric nanowire chemical sensor. Nano Lett. 2004, 4, 671–675. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, J.; Ma, D.F.; Hui, S.C.; Liu, Y.L.; Yao, W. Preparation of gold-poly (vinyl pyrrolidone) core-shell nanocomposites and their humidity-sensing properties. J. Appl. Polym. Sci. 2011, 121, 1685–1690. [Google Scholar] [CrossRef]
- Hu, J.; Qu, H.; Chang, Y.; Pang, W.; Zhang, Q.; Liu, J.; Duan, X. Miniaturized polymer coated film bulk acoustic wave resonator sensor array for quantitative gas chromatographic analysis. Sens. Actuators B Chem. 2018, 274, 419–426. [Google Scholar] [CrossRef]
- Wisser, F.M.; Grothe, J.; Kaskel, S. Nanoporous polymers as highly sensitive functional material in chemiresistive gas sensors. Sens. Actuators B Chem. 2016, 223, 166–171. [Google Scholar] [CrossRef]
- Boussaad, S.; Tao, N.J. Polymer wire chemical sensor using a microfabricated tuning fork. Nano Lett. 2003, 3, 1173–1176. [Google Scholar] [CrossRef]
- Guan, S. Frequency encoding of resonant mass sensors for chemical vapor detection. Anal. Chem. 2003, 75, 4551–4557. [Google Scholar] [CrossRef]
- Ren, M.; Forzani, E.S.; Tao, N. Chemical sensor based on microfabricated wristwatch tuning forks. Anal. Chem. 2005, 77, 2700–2707. [Google Scholar] [CrossRef]
- Hu, J.; Qu, H.; Chang, Y.; Pang, W.; Zhang, Q.; Liu, J.; Duan, X. Real-time ozone detection based on a microfabricated quartz crystal tuning fork sensor. Sensors 2009, 9, 5655–5663. [Google Scholar] [CrossRef]
- Iglesias, R.A.; Tsow, F.; Wang, R.; Forzani, E.S.; Tao, N. Hybrid separation and detection device for analysis of benzene, toluene, ethylbenzene, and xylenes in complex samples. Anal. Chem. 2009, 81, 8930–8935. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Dickert, F.L. An Overview of High Frequency Acoustic Sensors—QCMs, SAWs and FBARs—Chemical and Biochemical Applications. Sensors 2019, 19, 4395. [Google Scholar] [CrossRef]
- Yun, M.; Lee, S.; Yim, C.; Jung, N.; Thundat, T.; Jeon, S. Suspended polymer nanobridge on a quartz resonator. Appl. Phys. Lett. 2013, 103, 053109. [Google Scholar] [CrossRef]
- Kim, W.; Yun, M.; Lee, S.; Jeon, S. Enhanced sensitivity of quartz tuning fork sensors using electrospun polymer wires. RSC Adv. 2016, 6, 31131–31134. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, W.; Yoon, H.; Jeon, S. Performance Enhancement of a Microfabricated Resonator Using Electrospun Nanoporous Polymer Wire. ACS Sens. 2017, 2, 1355–1358. [Google Scholar] [CrossRef]
- Lee, S.; Yun, M.; Jeon, S. A tunable microresonator sensor based on a photocrosslinking polymer microwire. Appl. Phys. Lett. 2014, 104, 053506. [Google Scholar] [CrossRef]
- Yun, M.; Lee, E.; Cho, K.; Jeon, S. Enhanced sensitivity of a microfabricated resonator using a graphene-polystyrene bilayer membrane. Appl. Phys. Lett. 2014, 105, 073116. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Renneckar, S.; Zink-Sharp, A.; Esker, A.R.; Johnson, R.K.; Glasser, W.G. Novel Methods for Interfacial Modification of Cellulose-Reinforced Composites. In Cellulose Nanocomposites; Kristiina, O., Mohini, S., Eds.; American Chemical Society: Washington, DC, USA, 2006; Volume 938, pp. 78–96. [Google Scholar] [CrossRef]
- Sturcova, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef]
- Bae, H.S.; Haider, A.; Selim, K.K.; Kang, D.Y.; Kim, E.J.; Kang, I.K. Fabrication of highly porous PMMA electrospun fibers and their application in the removal of phenol and iodine. J. Polym. Res. 2013, 20, 158. [Google Scholar] [CrossRef]
- Dayal, P.; Liu, J.; Kumar, S.; Kyu, T. Experimental and theoretical investigations of porous structure formation in electrospun fibers. Macromolecules 2007, 40, 7689–7694. [Google Scholar] [CrossRef]
- Hou, H.; Ge, J.J.; Zeng, J.; Li, Q.; Reneker, D.H.; Greiner, A.; Cheng, S.Z. Electrospun polyacrylonitrile nanofibers containing a high concentration of well-aligned multiwall carbon nanotubes. Chem. Mater. 2005, 17, 967–973. [Google Scholar] [CrossRef]
- Zhou, C.; Chu, R.; Wu, R.; Wu, Q. Electrospun polyethylene oxide/cellulose nanocrystal composite nanofibrous mats with homogeneous and heterogeneous microstructures. Biomacromolecules 2011, 12, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, T.; Zhou, X.; Tjiu, W.C.; Hou, H. Electrospinning fabrication of high strength and toughness polyimide nanofiber membranes containing multiwalled carbon nanotubes. J. Phys. Chem. B 2009, 113, 9741–9748. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strawhecker, K.E.; Snyder, J.F.; Orlicki, J.A.; Reiner, R.S.; Rudie, A.W. Cellulose nanocrystals as a reinforcing material for electrospun poly(methyl methacrylate) fibers: Formation, properties and nanomechanical characterization. Carbohydr. Polym. 2012, 87, 2488–2495. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced Mechanical Properties of Graphene-Based Poly (vinyl alcohol) Composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Bal, S.; Samal, S.S. Carbon nanotube reinforced polymer composites—A state of the art. Bull. Mater. Sci. 2007, 30, 379. [Google Scholar] [CrossRef]
- Zimmermann, T.; Pohler, E.; Geiger, T. Cellulose fibrils for polymer reinforcement. Adv. Eng. Mater. 2004, 6, 754–761. [Google Scholar] [CrossRef]
- Rai, A.; Tsow, F.; Nassirpour, S.; Bankers, J.; Spinatsch, M.; He, M.P.; Forzania, E.S.; Tao, N.J. Selective Detection of Sulfur Derivatives Using Microfabricated Tuning Fork-Based Sensors. Sens. Actuators B Chem. 2009, 140, 490–499. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.; Park, E.; Jeon, S. Performance Enhancement of a Quartz Tuning Fork Sensor Using a Cellulose Nanocrystal-Reinforced Nanoporous Polymer Fiber. Sensors 2020, 20, 437. https://doi.org/10.3390/s20020437
Kim W, Park E, Jeon S. Performance Enhancement of a Quartz Tuning Fork Sensor Using a Cellulose Nanocrystal-Reinforced Nanoporous Polymer Fiber. Sensors. 2020; 20(2):437. https://doi.org/10.3390/s20020437
Chicago/Turabian StyleKim, Wuseok, Eunjin Park, and Sangmin Jeon. 2020. "Performance Enhancement of a Quartz Tuning Fork Sensor Using a Cellulose Nanocrystal-Reinforced Nanoporous Polymer Fiber" Sensors 20, no. 2: 437. https://doi.org/10.3390/s20020437
APA StyleKim, W., Park, E., & Jeon, S. (2020). Performance Enhancement of a Quartz Tuning Fork Sensor Using a Cellulose Nanocrystal-Reinforced Nanoporous Polymer Fiber. Sensors, 20(2), 437. https://doi.org/10.3390/s20020437




