1. Introduction
The investigation of brain functions with noninvasive methods such as functional magnetic resonance imaging (fMRI), magnetencephalography (MEG), electroencephalography (EEG), near-infrared spectroscopy (NIRS), and transcranial magnetic stimulation (TMS) is typically limited to laboratory settings. This is because the systems are very large and cannot be moved, and head movements must be avoided (fMRI, MEG), or head and body movements generate large movement artefacts. Within the last decade, there has been a rapidly increasing interest in investigating brain functions in ecological settings (e.g., cognitive or neuropsychological processes in interaction with a natural environment or in a social context; and movement analysis and motor learning), which require portable systems [
1,
2,
3,
4,
5,
6]. Especially in the field of movement research/motor learning, laboratory settings are strongly limiting, because only simple finger or hand or arm movements can be investigated, and it is questioned whether the results of these studies can be transferred to complex movements [
7,
8,
9]. In principle, NIRS and EEG are suited for measures in moving subjects, because the sensors are small and fixed to the head (rather than the head being fixed to the sensor), and the necessary electronics and recording devices can be built small enough. NIRS, similar to fMRI, measures the hemodynamic response related to specific brain processes with a low temporal resolution. Spatially, it is restricted to cortical layers close to the skull. Ref. [
10] used a modified NIRS system successfully in a cognitive task when subjects were walking around. Ref. [
11] developed a miniaturised wearable functional NIRS system and tested it when subjects performed a left hand gripping task (i) sitting still on a bicycle, (ii) pedaling indoor on a stationary training bicycle, and (iii) during outdoor bicycle riding. The event-related data showed that the task was performed successfully and comparably in all three conditions, whereas data loss was highest in real cycling (about 35%) but much lower in indoor cycling (7.5%) and under rest (5%).
Event-related potential (ERP) measures, which are the focus of this paper, have the well-known limited spatial resolution but a high temporal resolution, which is essential to analyse complex movements: for example, the time course of feedback and feedforward processing in visuomotor learning [
12,
13]. Conventional EEG systems are very sensitive to mechanical (cable and electrode movements) and physiological (electromyogram (EMG) of head and neck muscles, and sweating) movement artefacts [
14]. If cognitive processes in a moving subject rather than the movement itself are the focus of interest, data preprocessing algorithms informed by the behavioural movement data can be used to clean the EEG data from movement-related (neuronal and artefactual) activity [
15,
16]. If the motor-related activity is of interest, advanced data preprocessing algorithms such as independent component analysis (ICA) [
17] can be used to correct such artefacts. However, a study measuring ERPs during walking and running on a treadmill showed that data loss was very high (on average 130 of 248 EEG channel signals remained), even using a system with active electrodes that are considerably less prone to artefacts than conventional passive electrodes [
18,
19]. Furthermore, the usability of conventional EEG systems such as the one used in these studies is limited for measures with moving subjects. In movement tasks with only marginal head movements, such as cycling on an ergometer, these laboratory systems combined with ICA-based artefact correction can be applied successfully for EEG measures [
20]. Ref. [
21] found in a high-intensive cycling exercise an increase in spectral power when the athletes were fatigued. If spectral changes of higher EEG frequencies (alpha to gamma) are the focus of interest, such as in ref. [
21], artefacts directly coupled to movement execution are outside of this frequency range, because movement frequencies are considerably lower. However, the harmonics of these movement frequencies may occur, which have to be considered. With fully moving subjects, in contrast, artefacts are more difficult to handle. In a cocktail party study (including eating, drinking, chatting, etc.) with ten subjects wearing self-made (noncommercial) wireless EEG headsets, about 40% of the data were lost due to artefacts in contrast to 4% in two laboratory studies [
22]. Despite this high data loss, these studies revealed valuable ERP [
18,
19] or spectral EEG [
22] results. However, especially in movement research, the method of choice is to avoid the generation of mechanical artefacts beforehand by technical modifications. This approach was used in the four pilot studies reported in this paper that tested the suitability of different technical solutions to measure ERPs during ergometer rowing (
Figure 1). Especially for analysing motor-related brain activity with ERPs, rowing is well suited. It is a cyclic movement with a high number of repetitions, and the degrees of freedom of the movement are limited by the biomechanical constraints of the equipment (boat and scull/oar, or ergometer). Furthermore, in contrast to e.g., cycling or kayaking, the rowing movement is composed of different (more or less) distinct movement elements, and the movement frequency is lower (20–40 strokes/min vs. 60–120 revolutions/min in cycling). Finally, the biomechanical data (dynamics and kinematics of the movement, and boat and scull/oar movement), which are partly necessary for ERP analysis, can be measured meanwhile with relative ease.
The most critical question before pilot Study 1 (see below) was whether movement artefacts distort the EEG data completely or if there are systematic artefacts that can be controlled and either (partly) excluded or corrected in offline analysis. To test this question, a reliability check was made. A standard visual oddball task was applied in a rest condition (without movement) and during ergometer rowing. Similar—not movement-related—ERP activities during rest and during rowing would show that ERPs can be measured reliably in a moving subject. The second methodological question—concerning the analysis of motor behavior with ERPs—is: Can movement-related artefacts be identified and separated from motor-related neuronal activity? As an indicator for a reliable measure of motor-related activity, at least a motor potential (MP), a negative activity related to force output, should be expected. Ref. [
23] showed in a study that used isometric elbow flexions that the amplitude of the MP (labeled motor-related cortical potential (MRCP) in this study) correlates very high with EMG activity (r > 0.8) and the generated muscle force (r = 0.95). Furthermore, in an fMRI study, a high correlation between isometric force (using a hand-grip dynamometer) and activity in the primary motor cortex was found [
24]. The MP/MRCP must be generated by different generators because it can already be evoked when a muscle activation is only imagined. This should originate in the supplementary motor area (SMA) [
25]. A second part of the MP is thought to be related to the control of muscle activation by the primary motor cortex.
The purpose of this series of four consecutive pilot studies was as follows: first, to test if ERPs can in principle be measured during an athletic movement pattern such as rowing; second, to explore the limitations due to movement artefacts; and third, to test if movement artefacts can be reduced. Methodologically, the impact of physical and physiological artefact sources was investigated by comparing ERP waveforms measured at rest and during movement. For the existing data material, this physical approach is the method of choice compared to a statistical analysis of parametrised abstract data derived from the original data. Study outlines: In pilot Study 1 (conducted in 2005), a small, purpose-built 20-channel system was used with a preamplifier connected to the head and electrodes with shortened and fixed cables mounted to a standard electrode cap. Since this approach was only partly successful, the second approach (Study 2) was performed. This experiment involved the use of a system that was available in our lab at that time (2008) with active electrodes with built-in preamplifiers, and the amplifier was worn in a backpack. In pilot Study 3 (2013), as the newest improvement in EEG technology, a small head-mounted EEG system with wireless data transmission was combined with the electrodes and cap used in Study 1. In Study 4 (2018), finally, the headset used in Study 3 was combined with active electrodes, and movement intensity (force and speed) was systematically varied to investigate the influence of these factors on data quality.
5. Pilot Study 4
5.1. Introduction
Under combining the technical advantages of Study 2 (active electrodes) and Study 3 (mobile headset), Study 4 followed two aims. (i) First, can movement artefacts be reduced when the passive electrodes of Study 3 are replaced by active electrodes, and can movement-related ERPs be measured in sufficient quality then? This would be the optimal result. (ii) If the first aim is not achieved, how is the signal quality of standard VEPs affected by movement dynamics (force output) and movement kinematics (movement speed)? That is, is there a trade-off between signal quality and movement intensity in the measurement of ERPs in e.g., cognitive tasks in moving subjects.
5.2. Materials and Methods
The Emotiv system used in Study 3 was combined with eight active electrodes (EasyCap active), which were provided by EasyCap GmbH (
www.easycap.de) together with electrode caps (EasyCap). In contrast to the older version of the Acticap electrodes used in Study 2, these electrodes are smaller and very flat, and should therefore be less susceptible to tilting movements generated by inertial forces. After pretests, electrode cables were shortened, fixed to the cap, and connected to the headset. Power for the electrodes was provided by a 9 V battery attached to the connector (
Figure 8). The electrodes were fixed with adaptors and a highly viscous conductive paste (Elefix). The active electrodes were placed at positions Cz, FCz, C4’, C3’, O1, left and right mastoid (TP9, TP10), focusing the measure of motor-related activity and the VEP. The active reference electrode was placed at AFz for different reasons: (i) it could be controlled by generating eye blinks if all electrodes worked properly; (ii) sections with eye blink and vertical eye movement artefacts could be detected and removed from the EEG data; (iii) a mastoid reference is largely affected by EMG artefacts generated by neck and head muscles; and (iv) the spatial distance to occipital sites is the largest and allows measuring larger VEPs. A disposable baby-ECG electrode, which served as ground/DRL (driven right leg), was placed at the right anterior temple. The original passive ground electrode of the EasyCap-active system was used as a signal electrode and placed at Oz. Of course, it is unusual to combine a passive electrode with an active reference electrode, and it was not certain whether it would work, because the amplifier would saturate if the impedances are very different. However, this electrode always provided a clear EEG signal, and no saturation effects were observed in any measure. Therefore, the quality of the VEP measured with the active electrode at O1 could be directly compared with the VEP measured with the passive electrode at Oz. Signal quality was controlled with the Emotiv Testbench recording software. The recording parameters (A-D rate 128 Hz, bandpass filter 0.2–45 Hz) of the ergometer, its measuring equipment, and the oddball task were the same as in Study 3 except for two modifications. The condition with the 60 deviant stimuli of the previous studies was removed because the VEPs did not differ from the standard checkerboard task. Therefore, the number of trials of the latter was increased from 120 to 180. Secondly, because no VEP signal was obtained from one subject, a second recording session was conducted at a later time (with a nearly identical rowing performance). To ensure that the visual stimulation was observed, the target stimulus (cross) was replaced by a lower number (27–33) of pictures of different airplanes. This was done because the oddball task was repeated six times instead of two times in the previous studies, and therefore, more salient stimuli were used. In this Study 4, the target condition of the oddball task was only used to control performance.
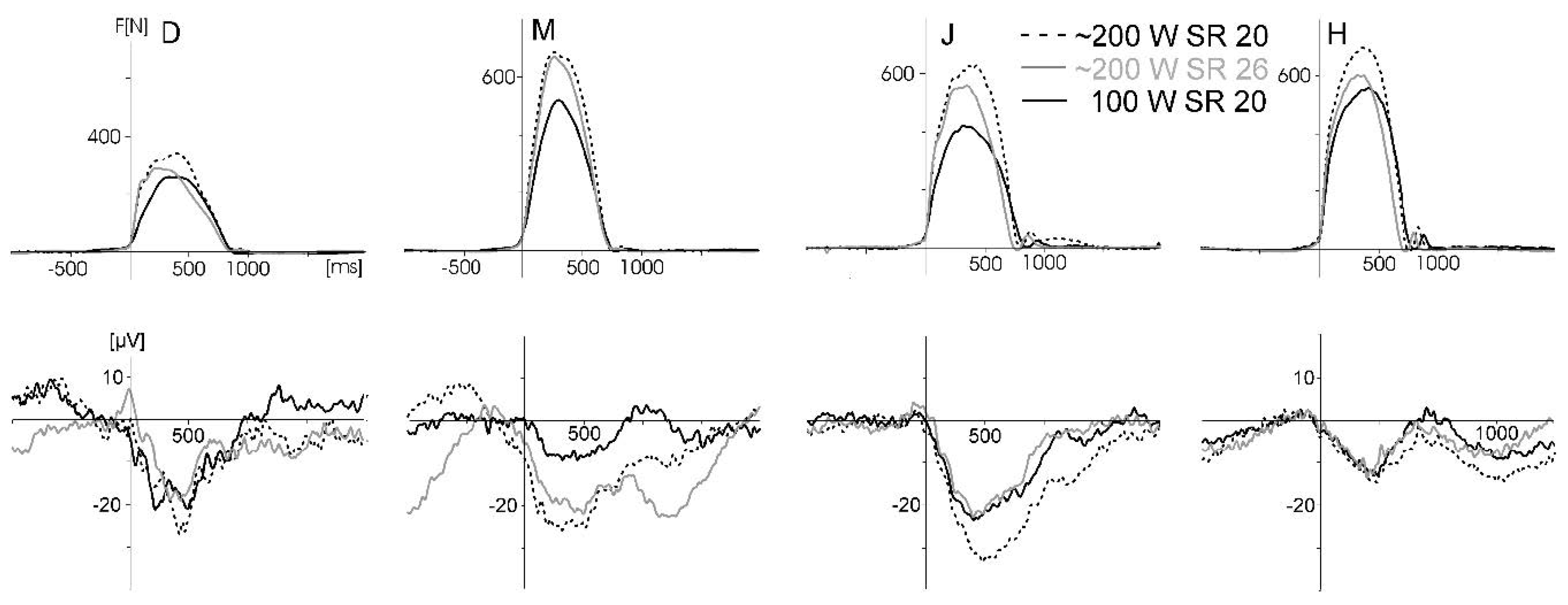
The same four male subjects as in Study 3 (aged 19, 23, 26, and 59 years, height 180–190 cm, weight 80–84 kg) performed six experimental conditions of about 4 min duration comparable to Study 3: (i) rowing with lower force output and lower stroke rate; (ii) lower force output, higher stroke rate; (ii) higher force output, lower stroke rate; (iv) higher force output, higher stroke rate; (v) visual stimulation in rest (without rowing); and (vi) rowing with arm pull only (details are provided in the legend of
Figure 9). Power and stroke rates could be controlled with the performance monitor of the ergometer and were close to the instruction. The rowing power ranged from recreational (condition i) to long-distance (e.g., 10 km) racing (condition iv). The visual stimulation was applied in all six conditions. For comparison, one subject repeated all six conditions with the cap used in Study 3 with 14 passive electrodes.
Data processing was performed as in Study 3, using Vision Analyzer 2.1 (20 Hz lowpass filtering, segment lengths 1 s for VEPs, 3 s movement related, 2 s condition 6). VEPs were analysed at electrode sites O1 and Oz (referenced to Cz as in Study 3). The number of included trials (total 180) was on average 138 (O1) and 140 (Oz) (range 88–176). Movement-related activity was analysed relative to force onset and referenced to AFz. The number of rowing strokes was on average (across subjects and conditions) 104 (range 84–117). The number of included trials (sites C3, C4, Cz, FCz) was on average 63 (range 20–117). Excluded trials were mainly contaminated by ocular artefacts. Due to the low number of channels, an ICA-based artefact correction was not performed. The number of included trials in the movement-related condition was lower as in the VEP condition because of the longer segments (3 s vs. 1 s). One recording (subject D) was made on a hot August day. This resulted in large sweating artefacts, starting in condition three. Therefore, for the VEP analysis, the data of all recordings were filtered with a 1-Hz/24 dB highpass. This filtering did not visibly affect the morphology of the VEP waveform. For the analysis of the movement-related activity, no additional highpass filtering was made because at a stroke rate of 20/min movement, the frequency is about 0.33 Hz.
5.3. Results
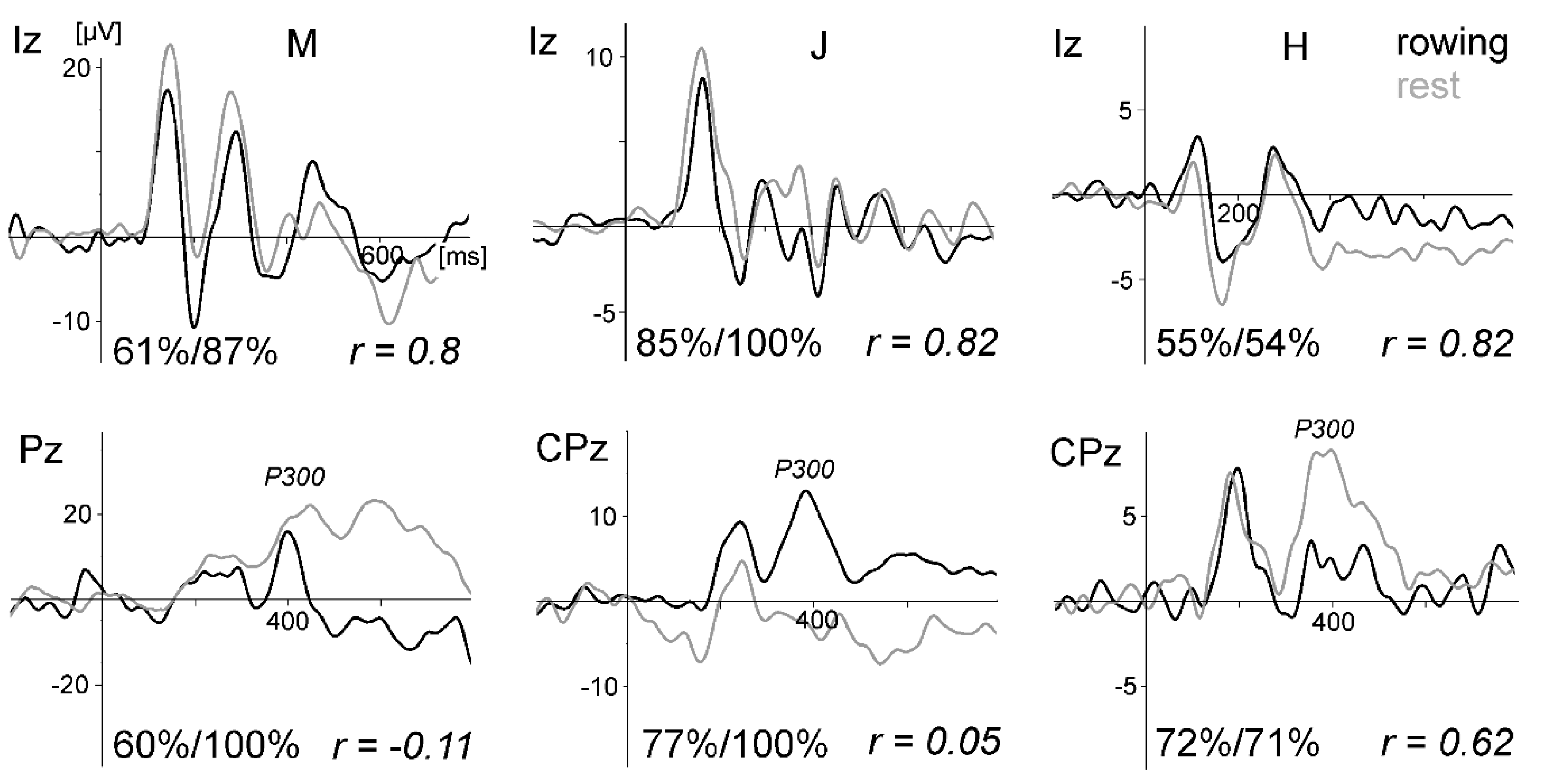
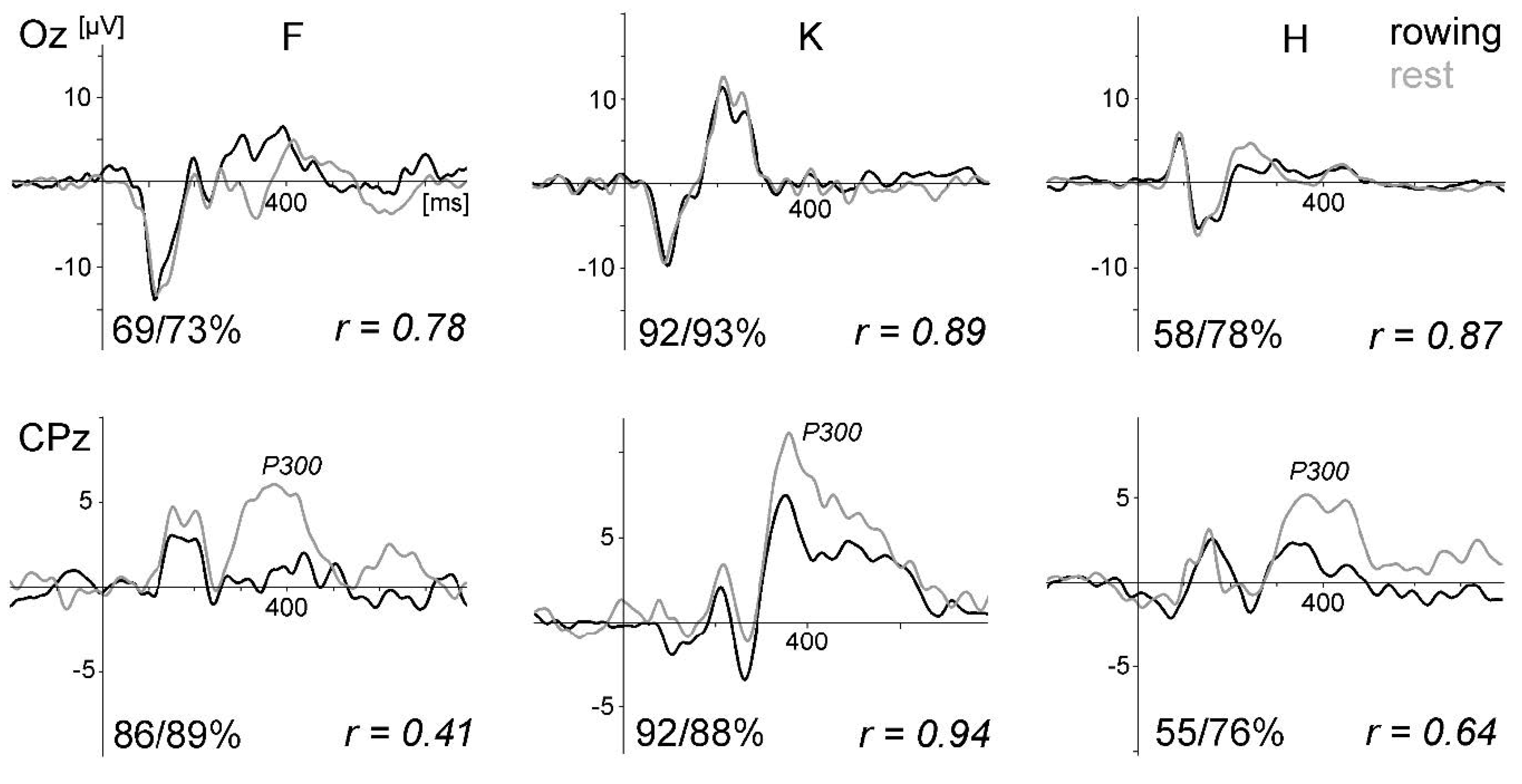
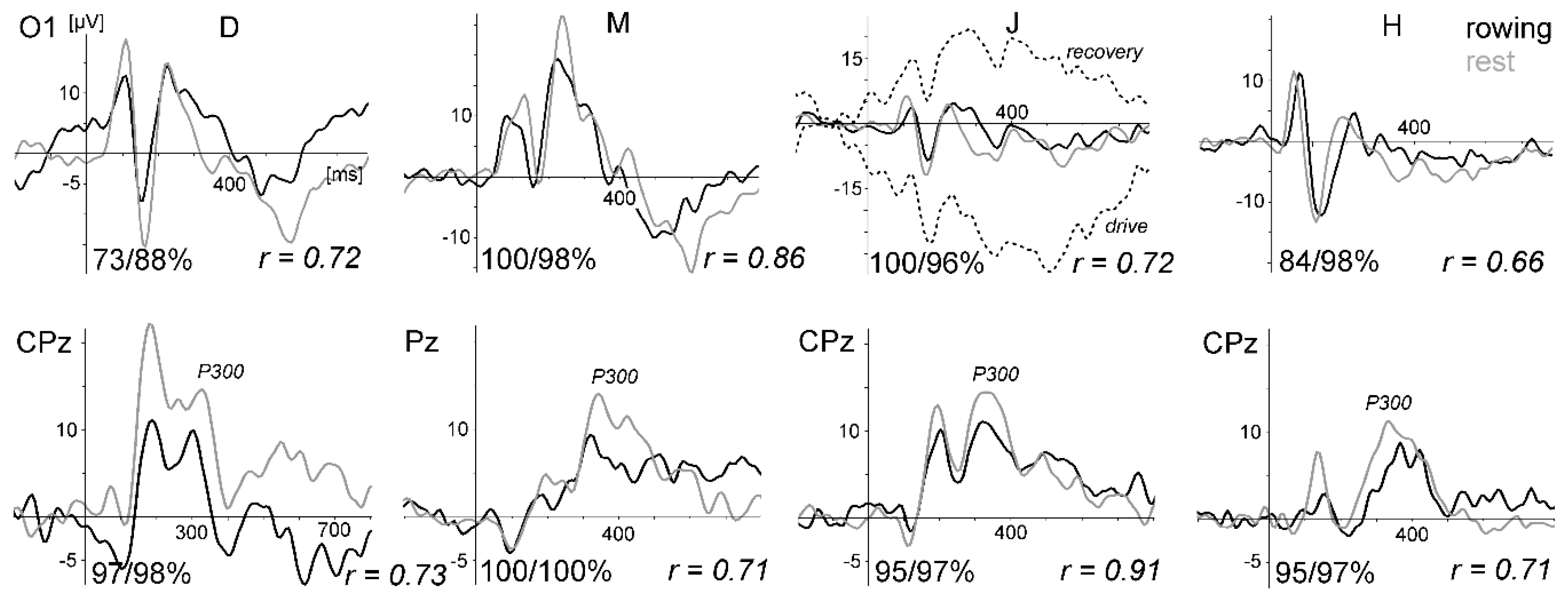
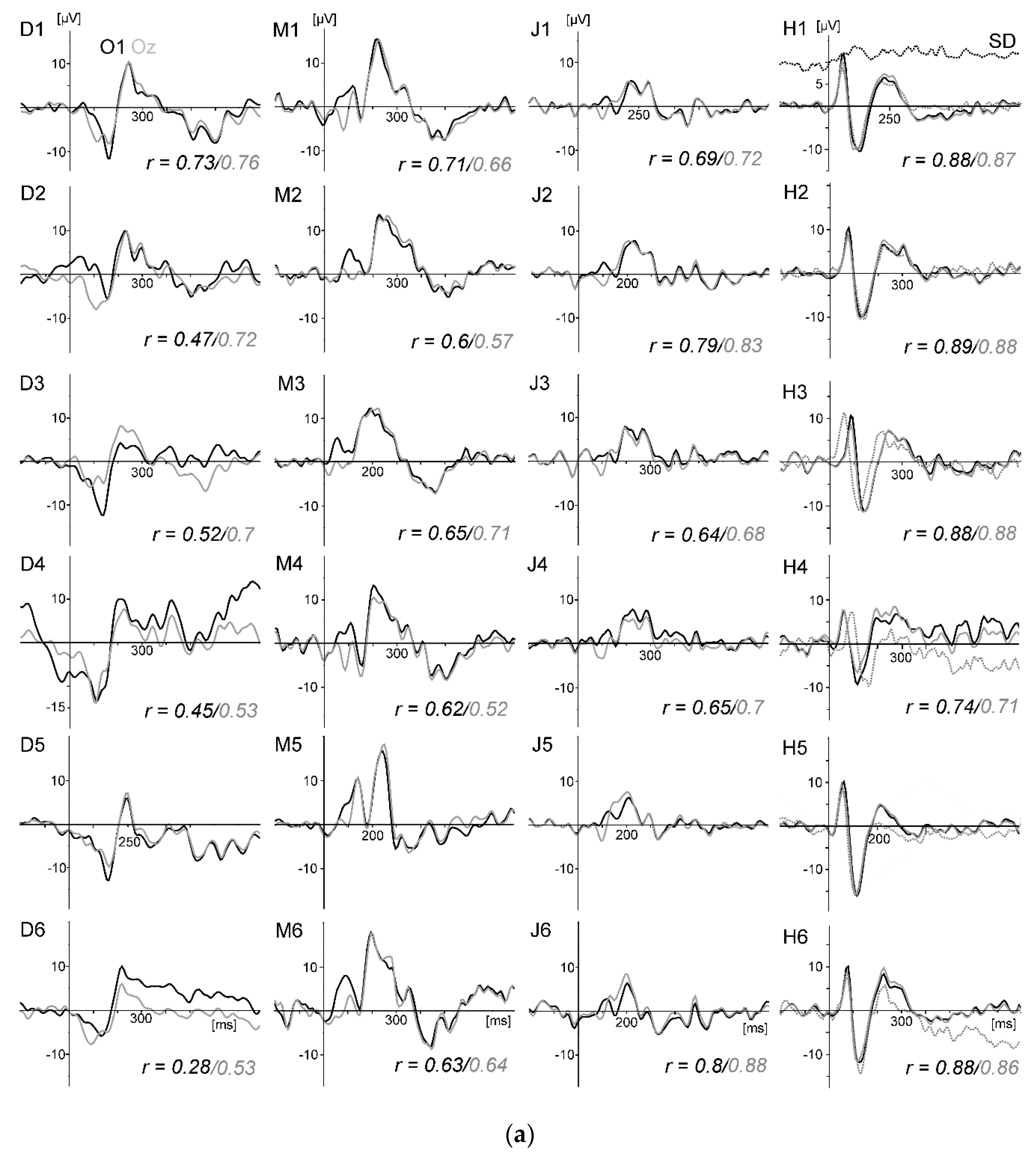
Figure 10a displays the VEPs generated by the standard stimulation (checkerboard) of the oddball task for all four subjects and conditions. For direct comparison, the VEPs measured at active electrode O1 and passive electrode Oz are superimposed. As in the previous studies, the VEPs were similar between rowing and rest, and the intraindividual differences were much smaller than the interindividual differences. Remarkably, the VEPs measured at O1 and Oz were highly congruent.
Figure 10b displays the peak differences (maxima–minima in the interval 50 to 300 ms) of the VEP waveforms (mean of all subjects) for the six conditions. The SNR revealed the highest values in condition five (rest) and the lowest in condition four (
Figure 10c).
A statistical analysis was applied to these two parameters. For identifying within-differences between the six conditions and the two electrodes, we performed multilevel analyses with two within-factors conditions and electrodes and included random intercepts into the models. As a multiple comparison adjustment when performing pairwise comparisons of the conditions, Bonferroni was used. The results of the multilevel analyses were generated using SAS/STAT software, Version 9.4 of the SAS System.
For the peak differences, no statistical difference was found. For the SNR, the test of the main effect for each condition resulted in F (5,38) = 10.73, p < 0.0001; for the electrode effect, the test was not significant (F (1,38) = 0.60, p = 0.4415). After Bonferroni adjustment, the SNR differed significantly between condition five (rest) and all rowing conditions (p values between 0.032 and < 0.0001). Rowing condition four (highest power) differed additionally from condition one (p = 0.0236).
Figure 9 displays the motor-related activity at Cz. The waveforms show a systematic modulation by force output (and probably force-related artefacts) but not by movement speed (stroke rate). However, the large interindividual differences are physiologically less plausible.
The SNR data for these motor-related activities revealed no statistical difference. However, as indicated by the about ten times higher SNR values in comparison to the VEP waveform, the computation of the SNR could be misleading if an average waveform is dominated by a large and systematic artefact. Nevertheless, these data show that the quality of the ERP is lower when movement intensity increases, as is indicated in the VEP waveforms as well.
In summary, as shown in the previous studies, standard VEPs can be measured reliably during rowing, whereas signal quality decreases when movement intensity increases. The differences between the VEP measured at active electrode O1 and passive electrode Oz are marginal to small. This shows that active electrodes may not reduce movement artefacts further when electrode cables are shortened and fixated and a head-mounted amplifier is used. Consequently, the motor-related activity is still distorted by remaining artefacts.
6. General Discussion
The present pilot studies tested the practicability of four different EEG acquisition systems for ERP measures in moving subjects (a summary is given in
Table 1). Technically, including the modifications, the used systems were suited to measure ERPs in moving subjects in contrast to conventional laboratory EEG hardware with passive electrodes. The advantages of the used systems were the head-mounted amplifier (studies 1, 3, and 4) and the active electrodes (studies 2 and 4), both methods reducing cable movement artefacts. However, as recently shown by ref. [
37], increasing cable sway leads to a decrease in SNR, even when active electrodes are used; that is, to shorten and fix cables is essential. Meanwhile, a further advantage of available systems with low weight and small dimensions for movement research (research on moving subjects) is either wireless data transmission or storing the data on an SD card in the device itself.
The data from fourteen single case measures from six different subjects revealed for a standard paradigm (visual oddball task) comparable intraindividual ERPs during rowing and during rest (non-movement condition) in all cases, despite remaining artefacts in the data. EEG parameters and ERP waveforms are genetically determined and show generally a broad range of interindividual differences, but they are also remarkably stable over time in adult subjects [
28,
38]. This fact (although probably not well known) strongly supports the reliability of the data, because the intraindividual differences were much smaller than the interindividual differences. Higher intraindividual differences instead would indicate that the ERP pattern is largely distorted by movement artefacts.
Artefacts were visibly larger in the raw data of the rowing condition(s) and quantified by considerably lower SNR values. The use of the SNR to quantify signal quality could be further extended. Computing the SNR from subsets of the data may inform about the minimum number of trials necessary for an experiment and may allow comparisons between different movement classes. Furthermore, because SNR is the relation between the power of the averaged ERP waveform and the power of the underlying EEG (see
Appendix A), these two values may be helpful to identify if the ERP or the EEG is mainly affected by artefacts.
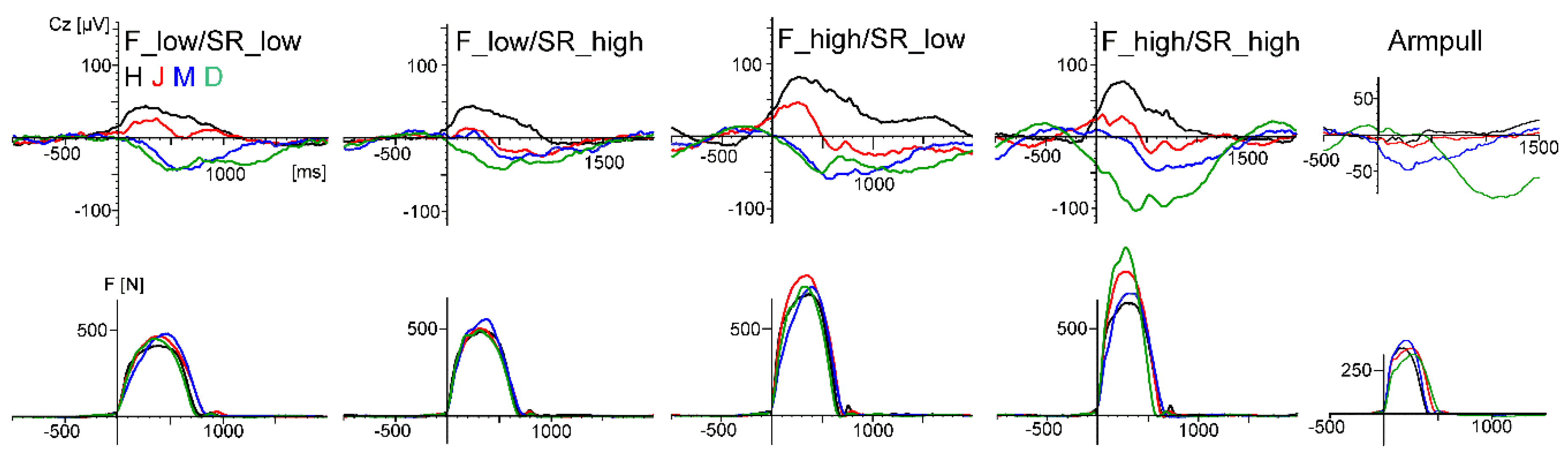
Whereas the VEPs were quite similar, the P300 was smaller during rowing than during rest (studies 1–3). This may be partly due to a habituation effect (the rest condition was always performed before the rowing condition), as recently suggested by ref. [
39] as well, who used also an oddball task to compare ERP measures during indoor cycling with a resting condition. Another contribution to this effect was probably that multiple task demands reduce the P300 (e.g., as reviewed by ref. [
40]). Here, attention is divided by counting the targets on the one hand and performing the rowing movement and the monitoring of stroke rate and power output on the other hand, which may be demanding for nonskilled rowers. Similar results with about a 30% smaller P300 amplitude during walking compared to sitting still (in counterbalanced order) were reported by ref. [
33,
41], who also suggested that the different task demands were the reason for this result. However, it has to be emphasised that the aim of the present pilot studies was not to investigate cognitive processes in rowing; instead, these robust ERP components (VEPs, P300) were measured for methodological reasons—that is, to compare ERP data quality during rowing and rest in a repeated measure design.
The positive results of the oddball task, including the high intensive rowing conditions of study 4, are promising for the investigation of brain functions in naturally behaving subjects outside the laboratory: for example, in cognition research, brain–computer interface (BCI) applications, ambulatory assessment, and others. Since rowing is a very athletic sport and therefore a source of large movement-related artefacts, ERP measures with less motor activity such as walking around [
33], walking or jogging slowly on a treadmill [
15,
18,
19], or cycling when pedaling slowly at a subaerobic level [
39] can easily be obtained using suitable equipment. Technically, non-movement related ERPs can be measured when active electrodes are combined with laboratory recording systems [
3,
4,
15,
18,
19,
21,
39] or when passive electrodes are combined with head-mounted recording systems [
5,
22,
33]. The reported analysis of movement-related activities in walking/slow jogging [
19] or cycling were related to spectral analysis of the EEG, which is easier to assess than ERPs. Further related studies are reviewed by ref. [
20].
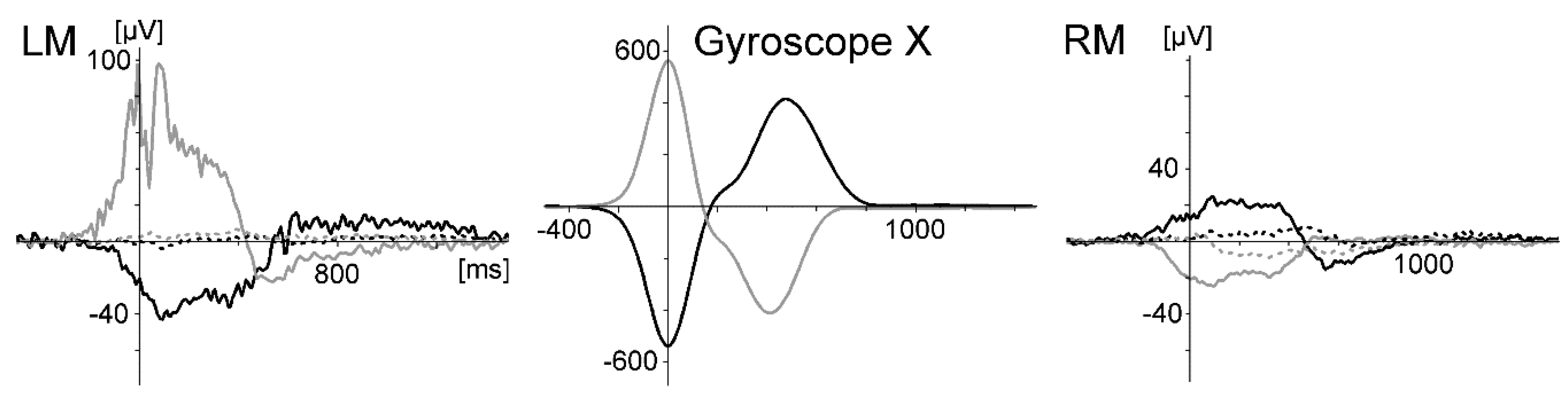
Due to the nature of ecological settings, more limitations compared to laboratory settings have to be accepted. The placement of the stimulation monitor besides the ergometer and the oscillating viewing distance may be regarded as critical. Alternatives might be the use of a head-up display or an acoustical stimulation. However, the gyroscope data in studies 3 and 4 revealed only small head movements in the oddball task during rowing; therefore, it can be concluded that this was comparable in Study 1 and 2, because the placement of the monitor was the same. Eye movements were marginal or not present in the high-density recording of Study 2 but could be monitored at frontal sites in the other studies as well.
The (slight) differences in hardware settings, filter settings, and electrode positions were acceptable because reliability was assessed by the within-subject comparisons for each study separately.
The small sample size of these four pilot studies may be seen as critical from a cognitive neuroscience or psychological point of view. In those studies, mainly complex processes are investigated, which may differ between participants, will not be present in all participants, or may interfere with other factors. This will require a larger sample size and a statistical analysis to show if the found effects are random or if the probability is high that the effects are real. In contrast, the present studies used a salient visual stimulation with a full-screen reversed checkerboard. This stimulation reliably evoked a robust electrophysiological (sensory) response that cannot be suppressed when the stimulation is observed. The measure of VEPs is a valid test for the functioning of the visual system and a well-established tool for clinical diagnostics in neurology and ophthalmology [
42]. Furthermore, a recent study proposed, based on ERPs measured in an oddball task, an ERP–EEG-based authentication system as an efficient biometric tool due to its convincing results [
43]. In studies investigating higher cognitive processes (e.g., semantic processing) where an ERP component such as the N400, which is sensitive to these processes cannot be observed, researchers can use the VEP as a tool to check if the participants paid attention to the presented visual stimuli. This VEP, which was dominated by a negative and a positive peak in the interval between 100 and 170 ms, was obtained during rowing and rest in all fourteen measures, and it was therefore a valuable tool to compare signal quality between rowing and rest on an individual level.
The second and more challenging aim of the four studies was to test if motor-related activity could be measured. This approach is unique for these pilot studies, as the cited studies in the introduction aimed to measure cognitive processing in movement conditions, not the movement itself. Although motor potentials during the drive phase of the rowing movement, modulated by force output, were indicated (cf.
Figure 6 and
Figure 9), in all four studies, large movement-related artefacts occurred, which distorted motor-related activity. These artefacts can be identified at electrode sites apart from the pre- and primary motor cortex. In this context, it has to be considered that artefacts originating from the reference electrode will affect the other electrodes. As the classical mastoid reference captures EMG activity of head and neck muscles, reference electrode positions less affected by this EMG activity, as well as the activity of cortical motor areas, may be better suited (e.g., prefrontal sites or nose tip). Artefacts are more difficult to detect at sites covering the motor areas because muscle force generation, movement kinematics, and movement-related artefacts have the same time course. Therefore, further technical improvements to reduce artefacts beforehand or to identify artefacts better [
44] and correct them are necessary to investigate motor behavior in movements including the whole body (as in sports: for example, motor learning or differentiating high from low performance in movement execution). One example to identify artefact sources was given in Study 3 when using the gyroscope to identify artefacts generated by rapid head movements.
Known artefact sources are the EMG activity and sweating artefacts. The latter cannot be filtered out when movement frequency is in the same range (as seen in one subject of Study 4). Other sources of artefacts may rely on small movements of the electrode cables relative to the cap which were still possible; and the translational head movement during rowing in an electromagnetically noisy environment (the room was not shielded) may have generated small currents in the cables, as in a generator (according to Faraday’s law). However, in both cases, the active electrodes should be less vulnerable to this artefact sources. Furthermore, for the second case, artefacts should be larger when movement speed increases, which was not observed.
To investigate further possible sources of motion artefacts, ref. [
45] used a phantom head to simulate motion artefacts in EEG data and found that artefacts increased with movement frequency as well as with movement amplitude, that is, in general with the acceleration of the phantom head. “We speculate that the major source of such artefacts is micro-movement of the recording electrodes in relation to the scalp surface“ [
45]. Their data showed that artefacts strongly increased when the head acceleration was larger than 1.5 g. Based on these results, additional measures of head acceleration using a triaxial acceleration sensor (Move II, Movisens GmbH, Karlsruhe/Germany,
www.movisens.com) attached to the Emotiv headset were analysed. These revealed values between 0.85 g in low-intensive rowing (75 W, 20 strokes/min) and 2.5 g in high-intensive rowing (360 W, 30 strokes/min). That is, the lower quality of VEPs in Study 4 at the rowing conditions with higher intensity may partly depend on such micro-movements of the electrodes independently of whether passive or active electrodes are used, because the main advantage of active electrodes is that these are less susceptible to cable sway artefacts.
Another pitfall with a physiological origin might be that different neuronal activities are superimposed, which hinders the detection of relevant activities. Ref. [
23] observed that the amplitude of the motor potential correlates very high (r = 0.93) with force output in an isometric elbow flexion task and reported values of up to 8 µV/150 N. In rowing, these values (assuming a physiological source) are considerably higher (about 100 µV and 1000 N in Study 4). In contrast to these efferent activities, probably generated from the pyramidal cells in the primary motor cortex [
46,
47], other motor-related activities (originating from the premotor cortex or the SMA) are very small. For example, in two of our own visuomotor tracking studies, the effects related to motor learning were below 1 µV [
12,
13]. That is, if generally the activities related to motor learning or motor programming (e.g., in rowing the perception and adaptation of within-crew differences of rowing technique [
27]) are in this amplitude range, these will be difficult to detect when the activity related to force execution is much higher. This hypothesis could be tested comparing rowing with other movement activities with a high force output, such as cycling on a stationary bike, which has the advantage that artefacts due to head movements and the related electrode movements could be minimised.
As a general recommendation for future ERP studies in an intensive-movement paradigm, cyclic movements where sub-movements (within one cycle) can be identified are advantageous. Movement kinematics and dynamics should be kept constant and can be controlled by providing feedback continuously about key performance factors (force, power, movement speed). Alternatively, after some training sessions to gain experience, participants should be able to control these factors intrinsically. For tasks (e.g., cognitive tests) not directly related to the movement itself, it has to be considered that movement intensity should be kept at the same level during the test. Furthermore, the test performance may decrease due to non-attention when the participant fatigues because intensity is too high.