Age-Related Distinctions in EEG Signals during Execution of Motor Tasks Characterized in Terms of Long-Range Correlations
Abstract
1. Introduction
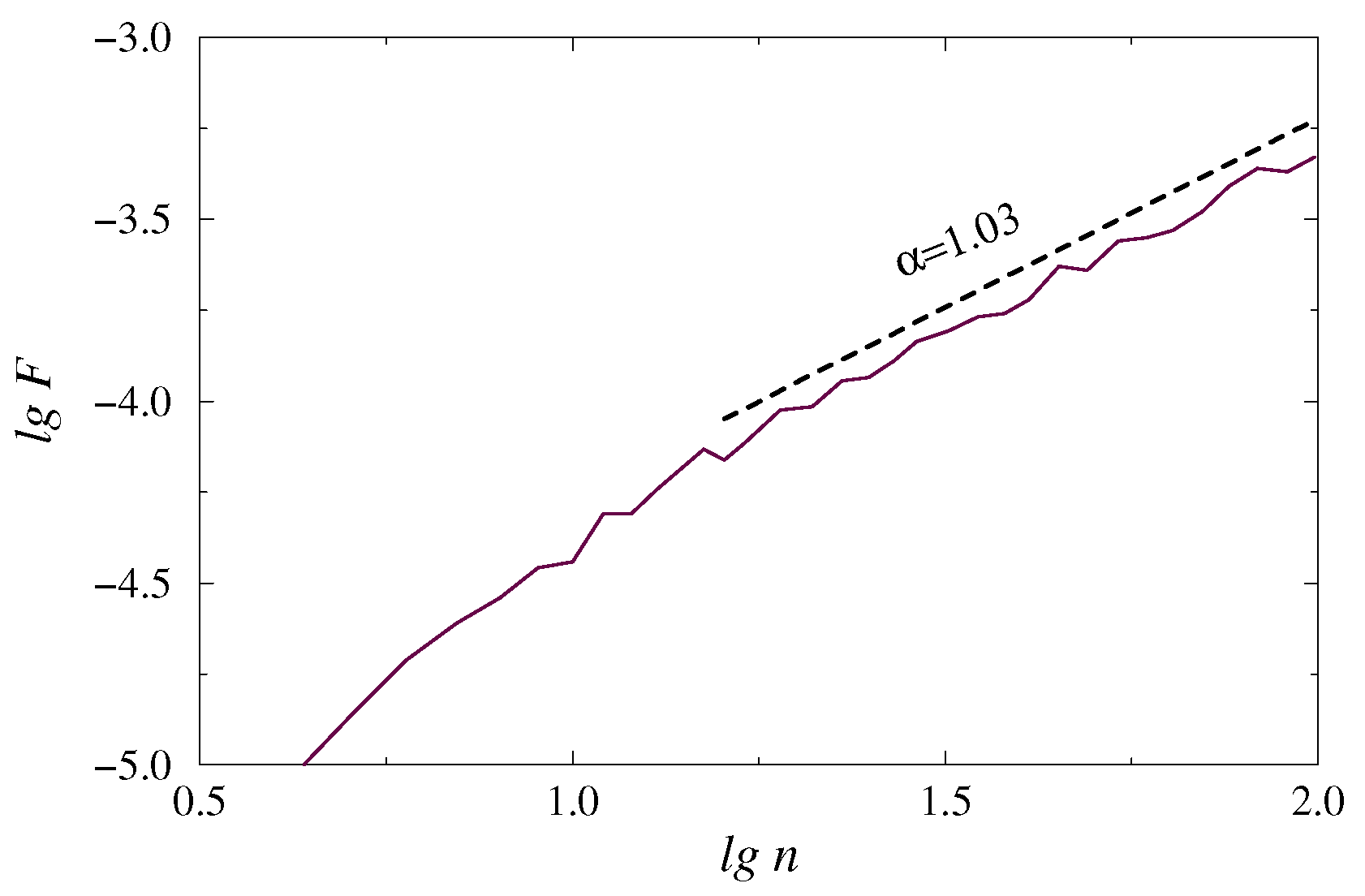
2. DFA Method Used for EEG Data Processing
- (1)
- Construction a profile of a signal , , (or random walk in terms of the theory of random processes).
- (2)
- Dividing the profile into non-overlapping segments of length n (n < N).
- (3)
- Fitting the trend within each segment with the least-squares method.
- (4)
- Computing RMS deviation of from .
- (5)
- Repeating steps 2–4 for different values of n to obtain an increasing dependence .
- (6)
- Estimation of the scaling exponentby representing Equation (3) in the log-log plot and linear fitting in the selected range of n. Such power-law behavior is typical for many stochastic processes, although can vary with n for inhomogeneous data sets.
3. Experiment
3.1. Subjects
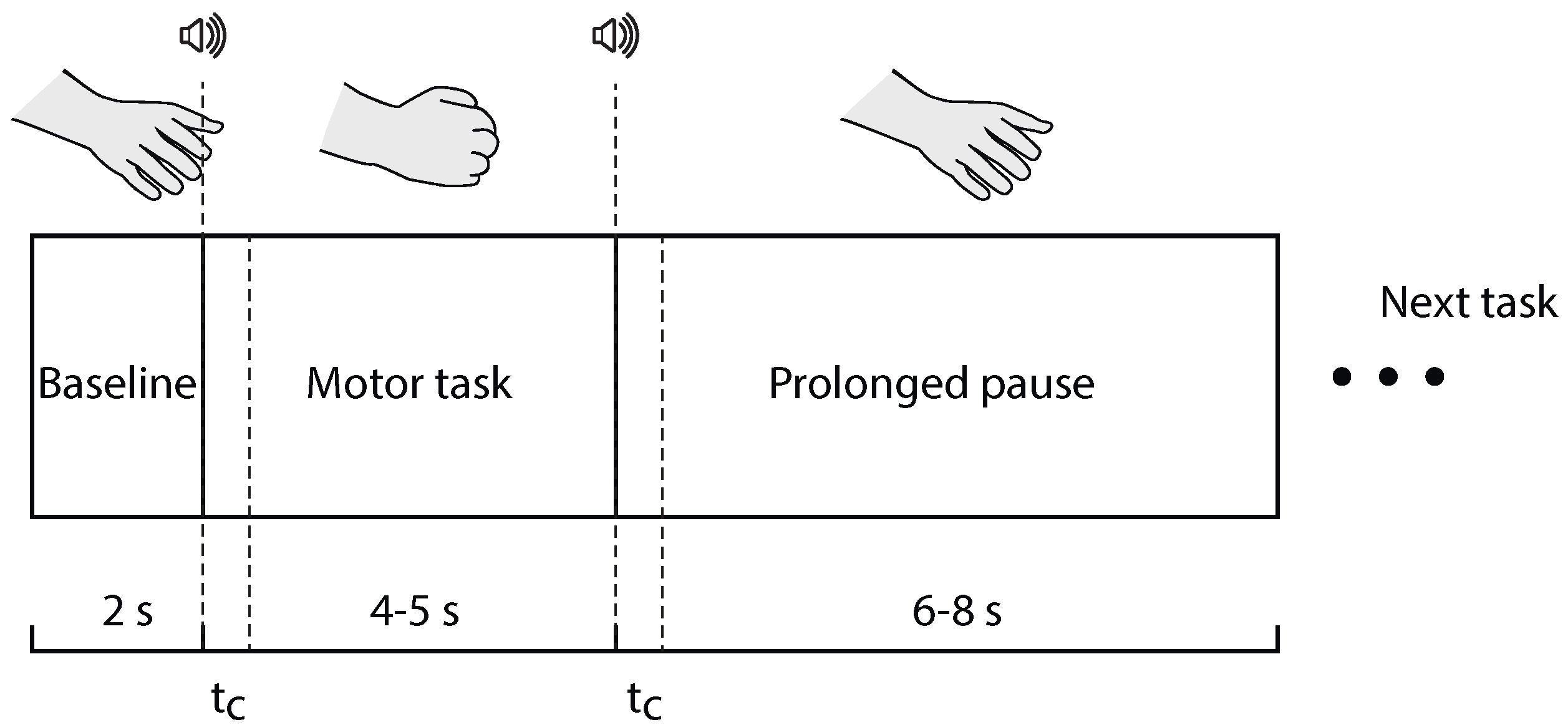
3.2. Experimental Procedures
3.3. EEG Data Acquisition and Preprocessing
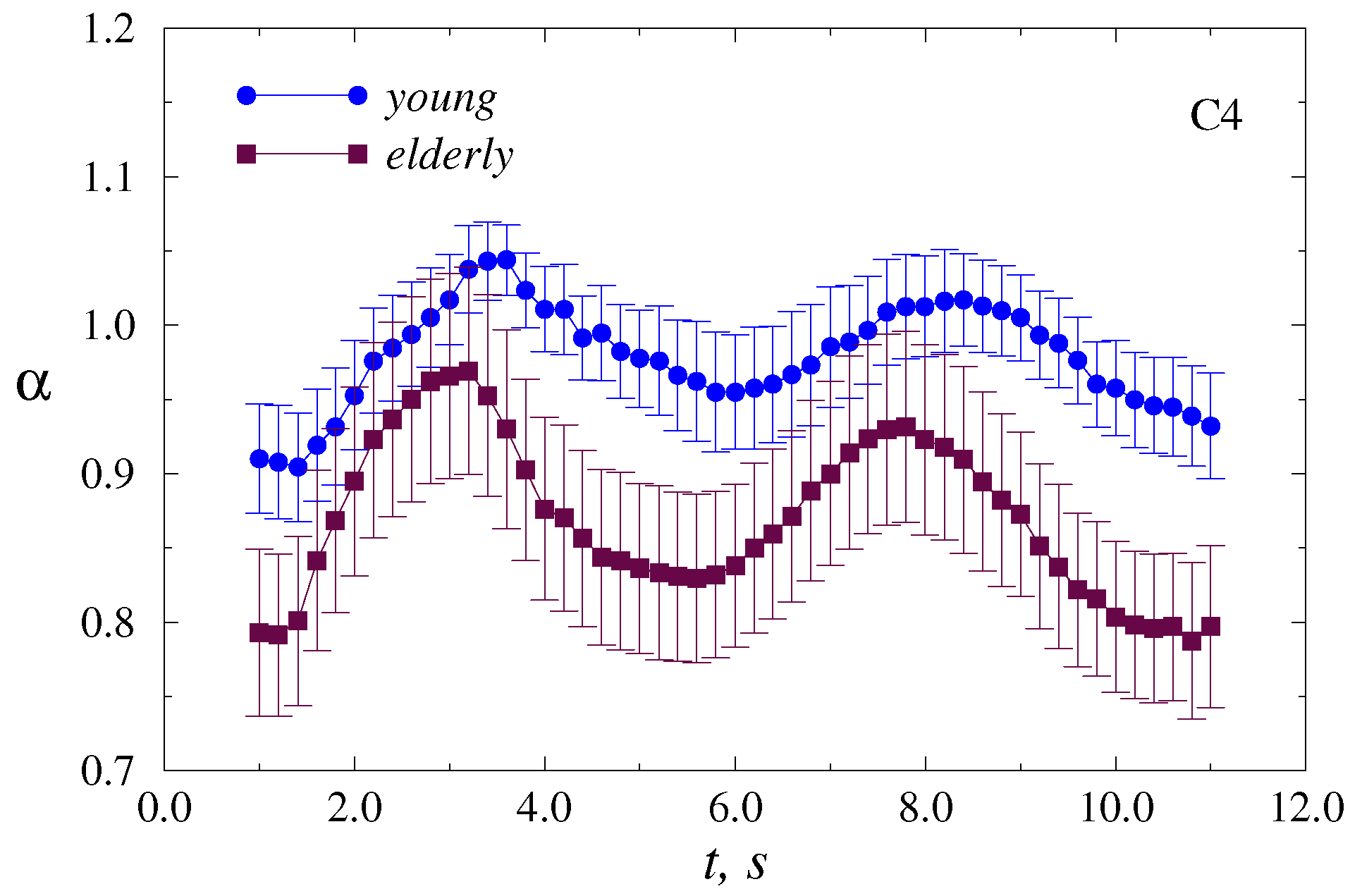
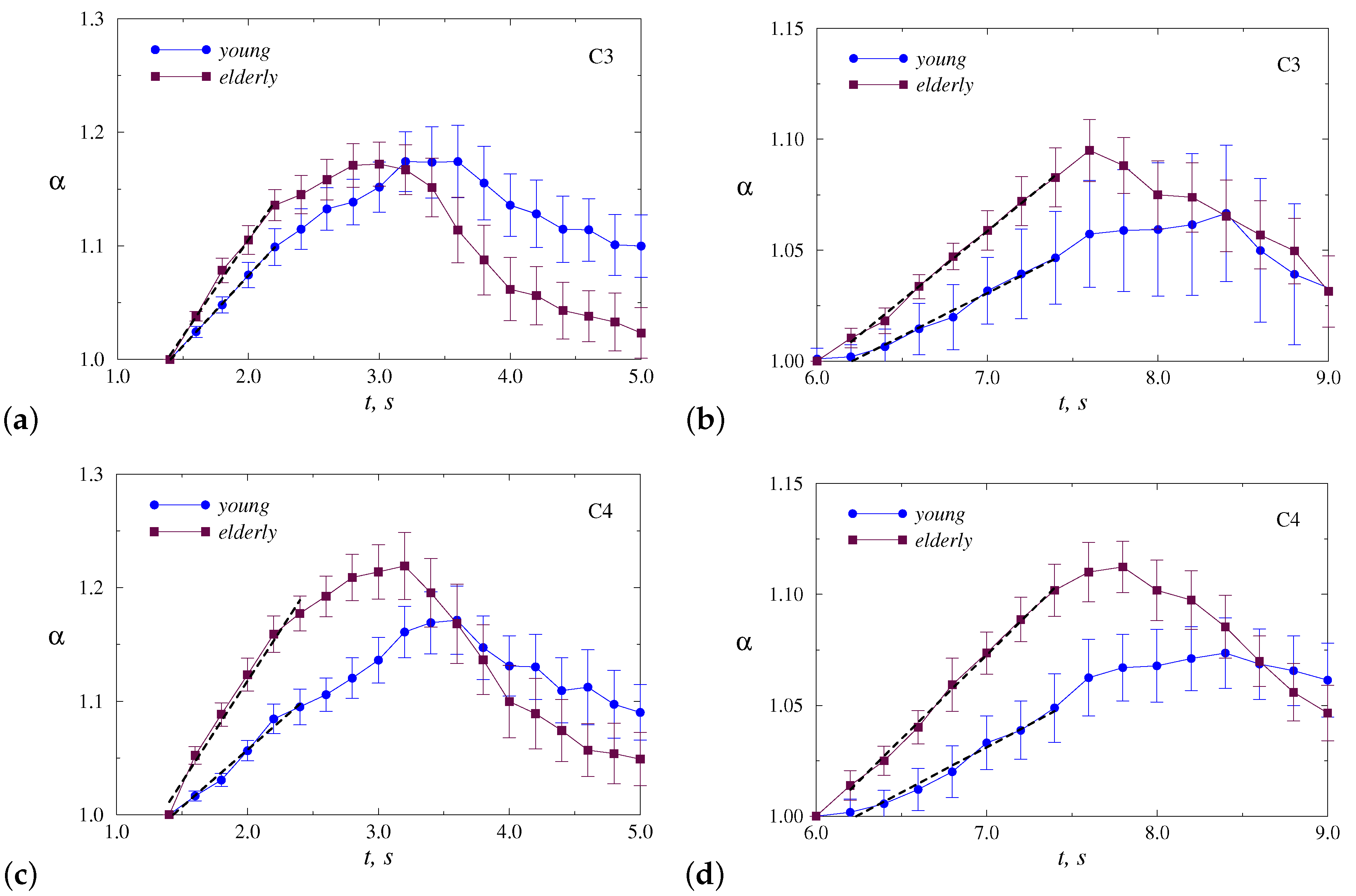
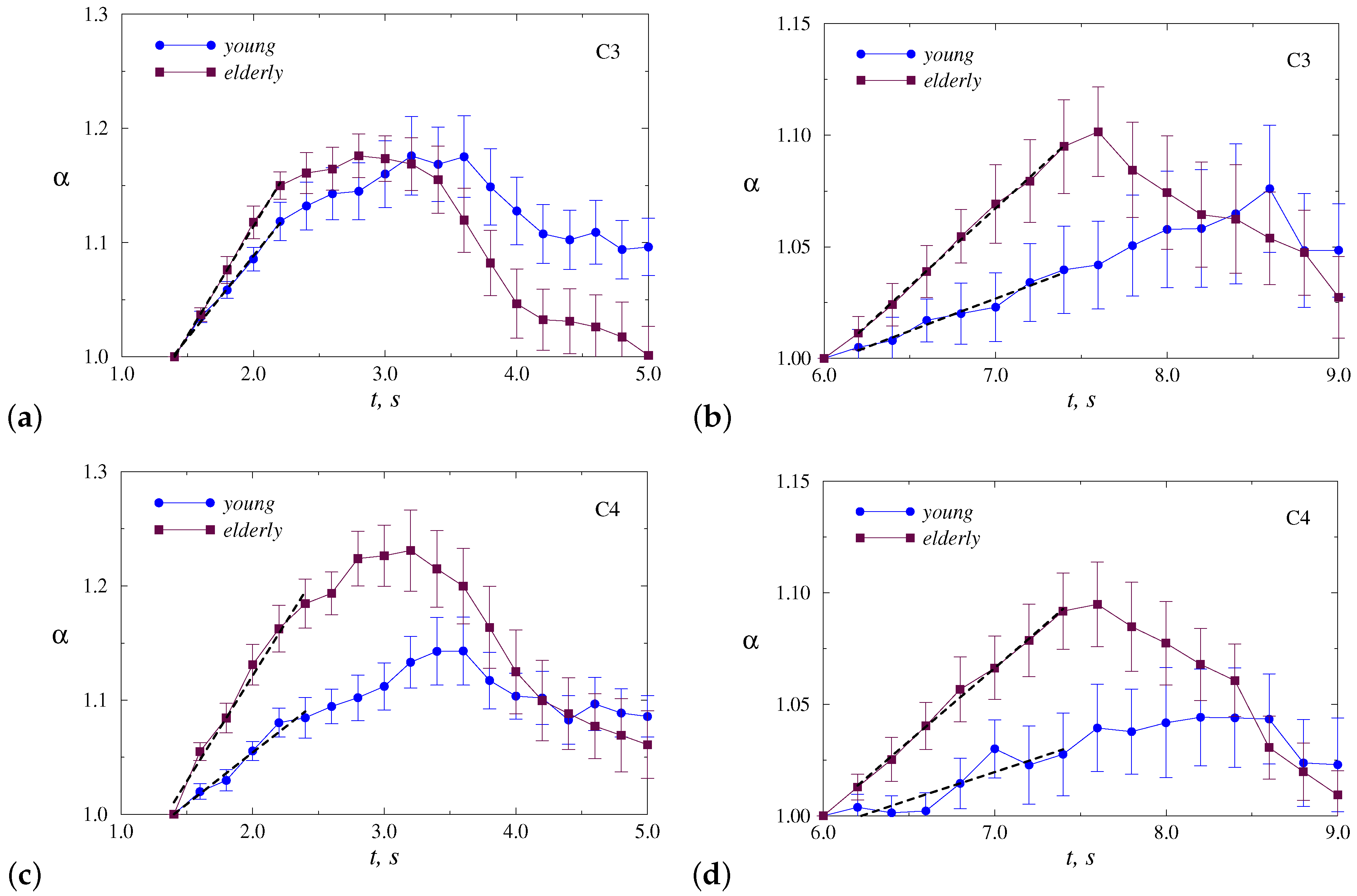
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DFA | Detrended fluctuation analysis |
| EEG | Electroencephalogram |
| RMS | Root mean square |
| SD | Standard deviation |
| SE | Standard error |
| LH | Left hand |
| RH | Right hand |
References
- Agosta, F.; Pievani, M.; Sala, S.; Geroldi, C.; Galluzzi, S.; Frisoni, G.B.; Filippi, M. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology 2011, 258, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gouw, A.A.; Hillebrand, A.; Tijms, B.M.; Stam, C.J.; van Straaten, E.C.; Pijnenburg, Y.A. Different functional connectivity and network topology in behavioral variant of frontotemporal dementia and Alzheimer’s disease: An EEG study. Neurobiol. Aging 2016, 42, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Steketee, R.M.; Meijboom, R.; de Groot, M.; Bron, E.E.; Niessen, W.J.; van der Lugt, A.; van Swieten, J.C.; Smits, M. Concurrent white and gray matter degeneration of disease-specific networks in early-stage Alzheimer’s disease and behavioral variant frontotemporal dementia. Neurobiol. Aging 2016, 43, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lindemer, E.R.; Greve, D.N.; Fischl, B.R.; Augustinack, J.C.; Salat, D.H. Regional staging of white matter signal abnormalities in aging and Alzheimer’s disease. Neuroimage Clin. 2017, 14, 156–165. [Google Scholar] [CrossRef]
- Yu, E.; Liao, Z.; Mao, D.; Zhang, Q.; Ji, G.; Li, Y.; Ding, Z. Directed functional connectivity of posterior cingulate cortex and whole brain in Alzheimer’s disease and mild cognitive impairment. Curr. Alzheimer Res. 2017, 14, 628–635. [Google Scholar] [CrossRef]
- Lin, Q.; Rosenberg, M.D.; Yoo, K.; Hsu, T.W.; O’Connell, T.P.; Chun, M.M. Resting-state functional connectivity predicts cognitive impairment related to Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 94. [Google Scholar] [CrossRef]
- Sorond, F.A.; Cruz-Almeida, Y.; Clark, D.J.; Viswanathan, A.; Scherzer, C.R.; De Jager, P.; Csiszar, A.; Laurienti, P.J.; Hausdorff, J.M.; Chen, W.G.; et al. Aging, the central nervous system, and mobility in older adults: Neural mechanisms of mobility impairment. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Gooijers, J.; de Xivry, J.J.O.; Swinnen, S.P.; Boisgontier, M.P. Two hands, one brain, and aging. Neurosci. Biobehav. Rev. 2017, 75, 234–256. [Google Scholar] [CrossRef]
- Smith, C.D.; Umberger, G.; Manning, E.; Slevin, J.; Wekstein, D.; Schmitt, F.; Markesbery, W.R.; Zhang, Z.; Gerhardt, G.A.; Kryscio, R.J.; et al. Critical decline in fine motor hand movements in human aging. Neurology 1999, 53, 1458. [Google Scholar] [CrossRef]
- Kalisch, T.; Wilimzig, C.; Kleibel, N.; Tegenthoff, M.; Dinse, H.R. Age-related attenuation of dominant hand superiority. PLoS ONE 2006, 1, e90. [Google Scholar] [CrossRef]
- Xifra-Porxas, A.; Niso, G.; Lariviére, S.; Kassinopoulos, M.; Baillet, S.; Mitsis, G.D.; Boudrias, M.-H. Older adults exhibit a more pronounced modulation of beta oscillations when performing sustained and dynamic handgrips. Neuroimage 2019, 201, 116037. [Google Scholar] [CrossRef] [PubMed]
- Heuninckx, S.; Wenderoth, N.; Swinnen, S.P. Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. J. Neurosci. 2008, 28, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Langan, J.; Peltier, S.; Bo, J.; Fling, B.W.; Welsh, R.C.; Seidler, R.D. Functional implications of age differences in motor system connectivity. Front. Syst. Neurosci. 2010, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Berchicci, M.; Lucci, G.; Pesce, C.; Spinelli, D.; Di Russo, F. Prefrontal hyperactivity in older people during motor planning. Neuroimage 2012, 62, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, J.; Peltsch, A.; Alahyane, N.; Brien, D.C.; Coe, B.C.; Garcia, A.; Munoz, D.P. Age related prefrontal compensatory mechanisms for inhibitory control in the antisaccade task. Neuroimage 2018, 165, 92–101. [Google Scholar] [CrossRef]
- Ward, N.S. Compensatory mechanisms in the aging motor system. Ageing Res. Rev. 2006, 5, 239–254. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Park, D.C. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol. Rev. 2014, 24, 55–370. [Google Scholar] [CrossRef]
- Anokhin, A.P.; Birbaumer, N.; Lutzenberger, W.; Nikolaev, A.; Vogel, F. Age increases brain complexity. Electroencephalogr. Clin. Neurophysiol. 1996, 99, 63–68. [Google Scholar] [CrossRef]
- Scheel, N.; Franke, E.; Münte, T.F.; Madany Mamlouk, A. Dimensional complexity of the resting brain in healthy aging, using a normalized MPSE. Front. Hum. Neurosci. 2018, 12, 451. [Google Scholar] [CrossRef]
- Nobukawa, S.; Yamanishi, T.; Nishimura, H.; Wada, Y.; Kikuchi, M.; Takahashi, T. Atypical temporal-scale-specific fractal changes in Alzheimer’s disease EEG and their relevance to cognitive decline. Cogn. Neurodyn. 2019, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Labate, D.; La Foresta, F.; Palamara, I.; Morabito, G.; Bramanti, A.; Zhang, Z.; Morabito, F.C. EEG complexity modifications and altered compressibility in mild cognitive impairment and Alzheimer’s disease. In Recent Advances of Neural Network Models and Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 163–173. [Google Scholar]
- Muzy, J.-F.; Bacry, E.; Arneodo, A. Wavelets and multifractal formalism for singular signals: Application to turbulence data. Phys. Rev. Lett. 1991, 67, 3515–3518. [Google Scholar] [CrossRef] [PubMed]
- Meyer, Y. Wavelets: Algorithms & Applications; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1993. [Google Scholar]
- Daubechies, I. Ten Lectures on Wavelets; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1992. [Google Scholar]
- Hramov, A.E.; Koronovskii, A.A.; Makarov, V.A.; Pavlov, A.N.; Sitnikova, E. Wavelets in Neuroscience; Springer: Berlin/Heidelberg, Germnay, 2015. [Google Scholar]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.-C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. A 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Huang, N.E.; Shen, Z.; Long, S.R. A new view of nonlinear water waves—The Hilbert spectrum. Annu. Rev. Fluid Mech. 1999, 31, 417–457. [Google Scholar] [CrossRef]
- Maksimenko, V.A.; Kurkin, S.A.; Pitsik, E.N.; Musatov, V.Y.; Runnova, A.E.; Efremova, T.Y.; Hramov, A.E.; Pisarchik, A.N. Artificial neural network classification of motor-related EEG: An increase in classification accuracy by reducing signal complexity. Complexity 2018, 2018, 9385947. [Google Scholar] [CrossRef]
- Chholak, P.; Niso, G.; Maksimenko, V.A.; Kurkin, S.A.; Frolov, N.S.; Pitsik, E.N.; Hramov, A.E.; Pisarchik, A.N. Visual and kinesthetic modes affect motor imagery classification in untrained subjects. Sci. Rep. 2019, 9, 9838. [Google Scholar] [CrossRef]
- Peng, C.-K.; Buldyrev, S.V.; Havlin, S.; Simons, M.; Stanley, H.E.; Goldberger, A.L. Mosaic organization of DNA nucleotides. Phys. Rev. E 1994, 49, 1685–1689. [Google Scholar] [CrossRef]
- Peng, C.-K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef]
- Stanley, H.E.; Amaral, L.A.N.; Goldberger, A.L.; Havlin, S.; Ivanov, P.C.; Peng, C.-K. Statistical physics and physiology: Monofractal and multifractal approaches. Physica A 1999, 270, 309–324. [Google Scholar] [CrossRef]
- Hu, K.; Ivanov, P.C.; Chen, Z.; Carpena, P.; Stanley, H.E. Effect of trends on detrended fluctuation analysis. Phys. Rev. E 2001, 64, 011114. [Google Scholar] [CrossRef]
- Chen, Z.; Ivanov, P.C.; Hu, K.; Stanley, H.E. Effect of nonstationarities on detrended fluctuation analysis. Phys. Rev. E 2002, 65, 041107. [Google Scholar] [CrossRef]
- Bryce, R.M.; Sprague, K.B. Revisiting detrended fluctuation analysis. Sci. Rep. 2012, 2, 315. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.H.; Gu, G.F.; Jiang, Z.Q.; Zhou, W.X.; Sornette, D. Comparing the performance of FA, DFA and DMA using different synthetic long-range correlated time series. Sci. Rep. 2012, 2, 835. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, V.A.; Pavlov, A.; Runnova, A.E.; Nedaivozov, V.; Grubov, V.; Koronovskii, A.; Pchelintseva, S.V.; Pitsik, E.; Pisarchik, A.N.; Hramov, A.E. Nonlinear analysis of brain activity, associated with motor action and motor imaginary in untrained subjects. Nonlinear Dyn. 2018, 91, 2803–2817. [Google Scholar] [CrossRef]
- Pavlov, A.N.; Runnova, A.E.; Maksimenko, V.A.; Pavlova, O.N.; Grishina, D.S.; Hramov, A.E. Detrended fluctuation analysis of EEG patterns associated with real and imaginary arm movements. Physica A 2018, 509, 777–782. [Google Scholar] [CrossRef]
- Pavlov, A.N.; Dubrovsky, A.I.; Koronovskii, A.A., Jr.; Pavlova, O.N.; Semyachkina-Glushkovskaya, O.V.; Kurths, J. Extended detrended fluctuation analysis of electroencephalograms signals during sleep and the opening of the blood-brain barrier. Chaos 2020, 30, 073138. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Ausloos, M. Application of the detrended fluctuation analysis (DFA) method for describing cloud breaking. Physica A 1999, 274, 349–354. [Google Scholar] [CrossRef]
- Heneghan, C.; McDarby, G. Establishing the relation between detrended fluctuation analysis and power spectral density analysis for stochastic processes. Phys. Rev. E 2000, 62, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Talkner, P.; Weber, R.O. Power spectrum and detrended fluctuation analysis: Application to daily temperatures. Phys. Rev. E 2000, 62, 150–160. [Google Scholar] [CrossRef]
- Kantelhardt, W.; Koscielny-Bunde, E.; Rego, H.H.A.; Havlin, S.; Bunde, A. Detecting long-range correlations with detrended fluctuation analysis. Physica A 2001, 295, 441–454. [Google Scholar] [CrossRef]
- Frolov, N.S.; Grubov, V.V.; Maksimenko, V.A.; Lüttjohann, A.; Makarov, V.V.; Pavlov, A.N.; Sitnikova, E.; Pisarchik, A.N.; Kurths, J.; Hramov, A.E. Statistical properties and predictability of extreme epileptic events. Sci. Rep. 2019, 9, 7243. [Google Scholar] [CrossRef]
- Pavlov, A.N.; Abdurashitov, A.S.; Koronovskii, A.A., Jr.; Pavlova, O.N.; Semyachkina-Glushkovskaya, O.V.; Kurths, J. Detrended fluctuation analysis of cerebrovascular responses to abrupt changes in peripheral arterial pressure in rats. Commun. Nonlinear Sci. Numer. Simulat. 2020, 85, 105232. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C. Motor imagery activates primary sensorimotor area in humans. Neurosci. Lett. 1997, 239, 65–68. [Google Scholar] [CrossRef]
- Frolov, N.S.; Pitsik, E.N.; Maksimenko, V.A.; Grubov, V.V.; Kiselev, A.R.; Wang, Z.; Hramov, A.E. Age-related slowing down in the motor initiation in elderly adults. PLoS ONE 2020, 15, e0233942. [Google Scholar] [CrossRef] [PubMed]
- Dushanova, J.; Christov, M. The effect of aging on EEG brain oscillations related to sensory and sensorimotor functions. Adv. Med. Sci. 2014, 59, 61–67. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Oja, E. Independent component analysis: Algorithms and applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef]
- Frolov, N.; Pitsik, E.; Grubov, V.; Kiselev, A.; Maksimenko, V.; Hramov, A. EEG Dataset for the Analysis of Age-Related Changes in Motor-Related Cortical Activity during a Series of Fine Motor Tasks Performance. Available online: https://figshare.com/articles/EEG_dataset_for_the_analysis_of_age-related_changes_in_motor-related_cortical_activity_during_a_series_of_fine_motor_tasks_performance/12301181/1 (accessed on 14 May 2020).
- Pitsik, E.; Frolov, N.; Badarin, A.; Hramov, A. Recurrence quantification analysis reveals the link between EEG signal’s complexity and reduced motor brain response under healthy aging. Chaos 2020, in press. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Velichkovsky, B.; Nedoluzhko, A.; Goldberg, E.; Efimova, O.; Sharko, F.; Rastorguev, S.; Krasivskaya, A.; Sharaev, M.; Korosteleva, A.; Ushakov, V. New insights into the human brain’s cognitive organization: Views from the top, from the bottom, from the left and, particularly, from the right. Procedia Comput. Sci. 2020, 169, 547–557. [Google Scholar] [CrossRef]
- Ferreri, F.; Guerra, A.; Vollero, L.; Ponzo, D.; Maatta, S.; Mervaala, E.; Iannello, G.; Di Lazzaro, V. Age-related changes of cortical excitability and connectivity in healthy humans: Non-invasive evaluation of sensorimotor network by means of TMS-EEG. Neuroscience 2017, 357, 255–263. [Google Scholar] [CrossRef][Green Version]
- Bardouille, T.; Bailey, L.; CamCAN Group. Evidence for age-related changes in sensorimotor neuromagnetic responses during cued button pressing in a large open-access dataset. NeuroImage 2019, 193, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Cassady, K.; Gagnon, H.; Lalwani, P.; Simmonite, M.; Foerster, B.; Park, D.; Peltier, S.J.; Petrou, M.; Taylor, S.F.; Weissman, D.H.; et al. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. NeuroImage 2018, 186, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Lin, M.-Y.; Yang, S.-H. Age effect on automatic inhibitory function of the somatosensory and motor cortex: An MEG study. Front. Aging Neurosci. 2018, 10, 53. [Google Scholar] [CrossRef]
- Hramov, A.E.; Grubov, V.; Badarin, A.; Maksimenko, V.A.; Pisarchik, A.N. Functional near-infrared spectroscopy for the classification of motor-related brain activity on the sensor-level. Sensors 2020, 20, 2362. [Google Scholar] [CrossRef] [PubMed]


| All Motor Tasks | Dominant Hand (RH) | Non-Dominant Hand (LH) | |
|---|---|---|---|
| clenching a fist (C3) | |||
| young | 0.095 ± 0.012 | 0.102 ± 0.011 | 0.089 ± 0.012 |
| elderly | 0.113 ± 0.013 | 0.107 ± 0.014 | 0.124 ± 0.011 |
| clenching a fist (C4) | |||
| young | 0.087 ± 0.013 | 0.101 ± 0.013 | 0.072 ± 0.014 |
| elderly | 0.121 ± 0.015 | 0.116 ± 0.016 | 0.127 ± 0.015 |
| unclenching a fist (C3) | |||
| young | 0.038 ± 0.008 | 0.049 ± 0.008 | 0.026 ± 0.009 |
| elderly | 0.054 ± 0.007 | 0.054 ± 0.006 | 0.053 ± 0.008 |
| unclenching a fist (C4) | |||
| young | 0.040 ± 0.009 | 0.057 ± 0.009 | 0.024 ± 0.010 |
| elderly | 0.068 ± 0.008 | 0.081 ± 0.008 | 0.054 ± 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlov, A.N.; Pitsik, E.N.; Frolov, N.S.; Badarin, A.; Pavlova, O.N.; Hramov, A.E. Age-Related Distinctions in EEG Signals during Execution of Motor Tasks Characterized in Terms of Long-Range Correlations. Sensors 2020, 20, 5843. https://doi.org/10.3390/s20205843
Pavlov AN, Pitsik EN, Frolov NS, Badarin A, Pavlova ON, Hramov AE. Age-Related Distinctions in EEG Signals during Execution of Motor Tasks Characterized in Terms of Long-Range Correlations. Sensors. 2020; 20(20):5843. https://doi.org/10.3390/s20205843
Chicago/Turabian StylePavlov, Alexey N., Elena N. Pitsik, Nikita S. Frolov, Artem Badarin, Olga N. Pavlova, and Alexander E. Hramov. 2020. "Age-Related Distinctions in EEG Signals during Execution of Motor Tasks Characterized in Terms of Long-Range Correlations" Sensors 20, no. 20: 5843. https://doi.org/10.3390/s20205843
APA StylePavlov, A. N., Pitsik, E. N., Frolov, N. S., Badarin, A., Pavlova, O. N., & Hramov, A. E. (2020). Age-Related Distinctions in EEG Signals during Execution of Motor Tasks Characterized in Terms of Long-Range Correlations. Sensors, 20(20), 5843. https://doi.org/10.3390/s20205843






