Advancements in Microfabricated Gas Sensors and Microanalytical Tools for the Sensitive and Selective Detection of Odors
Abstract
1. Introduction
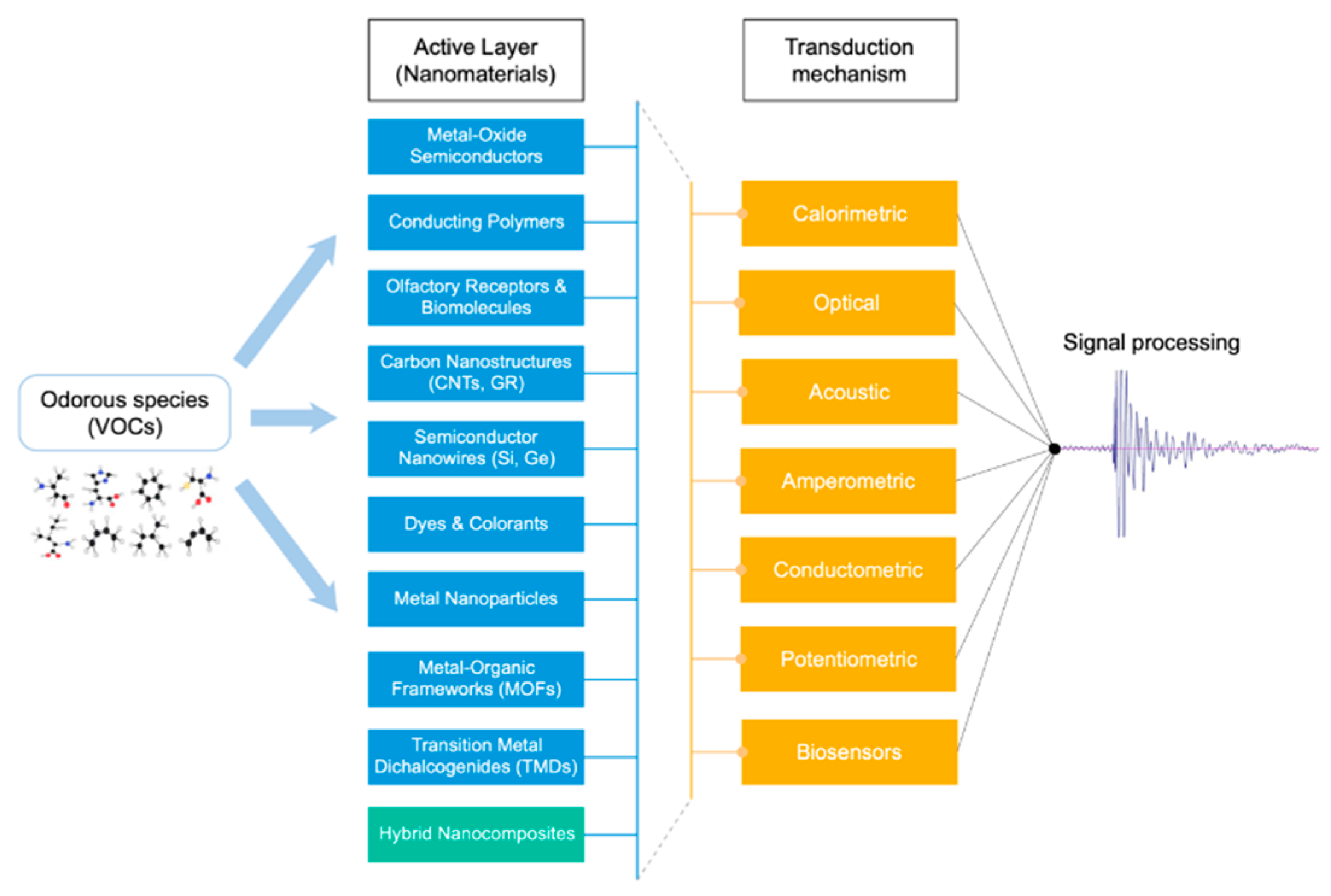
2. Gas Sensors for VOCs Detection
2.1. Transduction Mechanisms
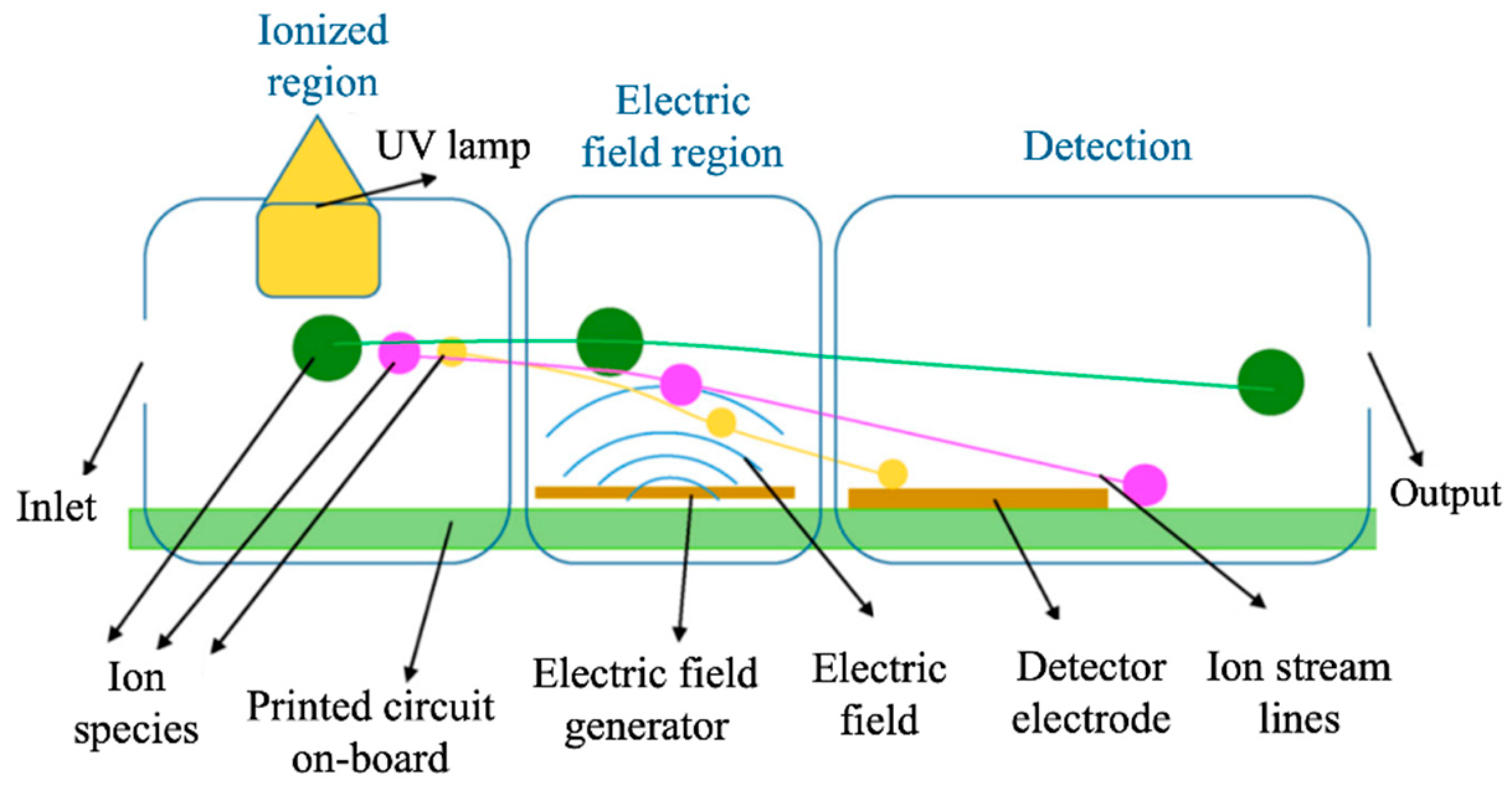
2.1.1. Optical Devices
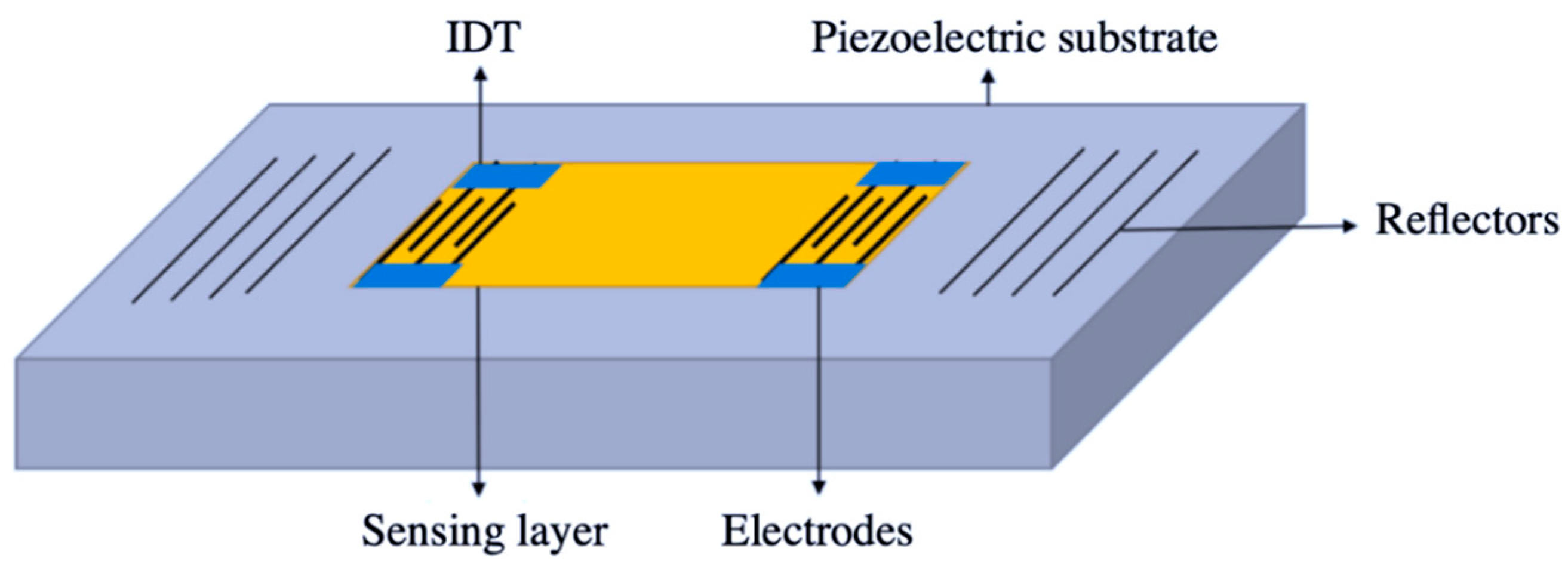
2.1.2. Gravimetric Devices
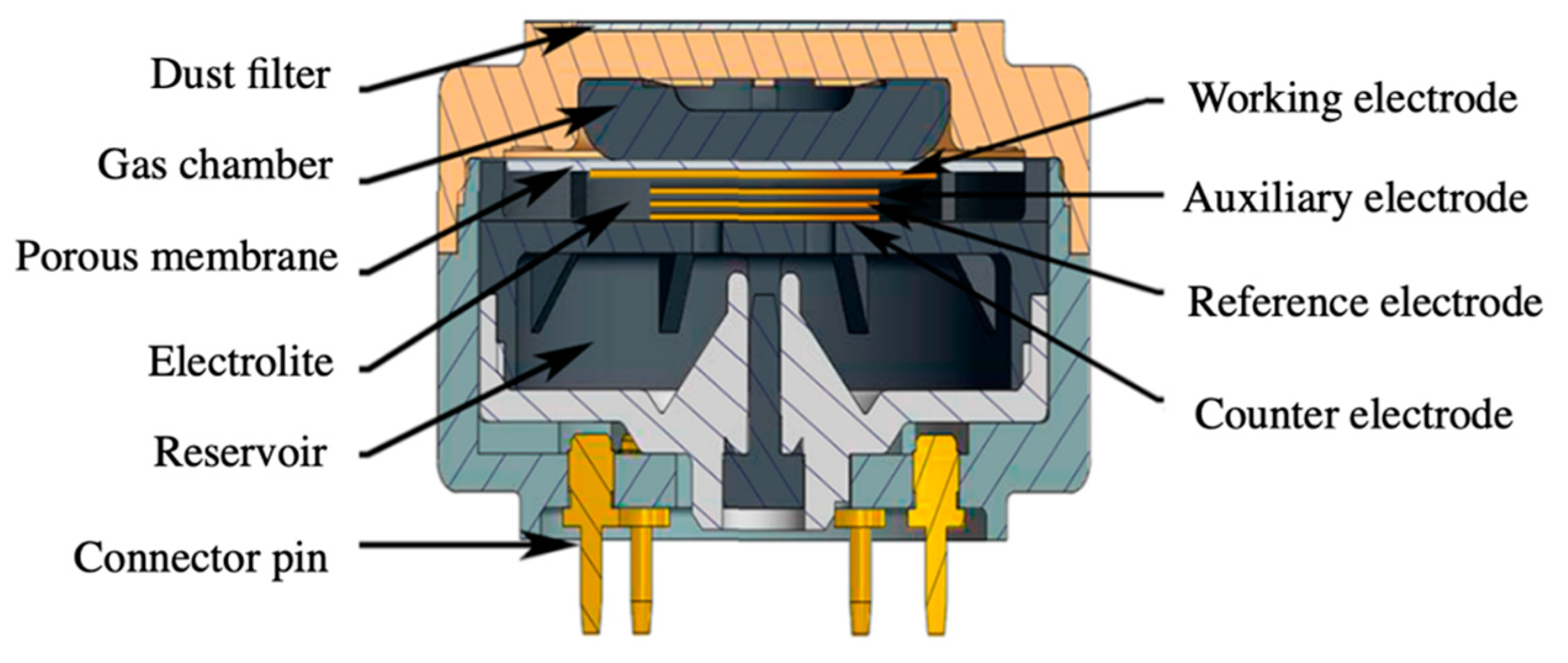
2.1.3. Electrochemical Devices
2.1.4. Calorimetric Devices
2.2. Functional Sensing Material
2.2.1. Metal Oxide Semiconductors (MOS)
2.2.2. Polymeric Materials
2.2.3. Carbon Nanostructures
2.2.4. Biological Composites
2.2.5. Other Nanomaterials
3. Microanalytical Tools for VOCs Discrimination
3.1. Microgas Chromatographs (µGC)
3.2. Microfluidic-Based Devices
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sichu, L. Overview of Odor Detection Instrumentation and the Potential for Human Odor Detection in Air Matrices. 2009, pp. 5–43. Available online: https://www.mitre.org/sites/default/files/pdf/09_4536.pdf (accessed on 7 January 2020).
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- De Luca, R.; Botelho, D. The unconscious perception of smells as a driver of consumer responses: A framework integrating the emotion-cognition approach to scent marketing. AMS Rev. 2019, 1–17. [Google Scholar] [CrossRef]
- Litman, T. Autonomous Vehicle Implementation Predictions: Implications for Transport Planning; Victoria Transport Policy Institute: Victoria, BC, Canada, 2014. [Google Scholar]
- Son, M.; Lee, J.Y.; Ko, H.J.; Park, T.H. Bioelectronic Nose: An Emerging Tool for Odor Standardization. Trends Biotechnol. 2017, 35, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Szulczyński, B.; Gębicki, J. Currently Commercially Available Chemical Sensors Employed for Detection of Volatile Organic Compounds in Outdoor and Indoor Air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro-and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Donarelli, M.; Ottaviano, L. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS2, WS2 and Phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef]
- Mohamed, E.F. Nanotechnology: Future of Environmental Air Pollution Control. Environ. Manag. Sustain. Dev. 2017, 6, 429. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic-Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef]
- Jalal, A.H.; Alam, F.; Roychoudhury, S.; Umasankar, Y.; Pala, N.; Bhansali, S. Prospects and Challenges of Volatile Organic Compound Sensors in Human Healthcare. ACS Sens. 2018, 3, 1246–1263. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Choi, K.J.; Jang, H.W. One-dimensional oxide nanostructures as gas-sensing materials: Review and issues. Sensors 2010, 10, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef] [PubMed]
- Pavelko, R.G.; Vasiliev, A.A.; Llobet, E.; Vilanova, X.; Sevastyanov, V.G.; Kuznetsov, N.T. Selectivity problem of metal oxide based sensors in the presence of water vapors. Procedia Eng. 2010, 5, 111–114. [Google Scholar] [CrossRef][Green Version]
- Bindra, P.; Hazra, A. Selective detection of organic vapors using TiO2 nanotubes based single sensor at room temperature. Sens. Actuators B Chem. 2019, 290, 684–690. [Google Scholar] [CrossRef]
- He, J.; Xu, L.; Wang, P.; Wang, Q. A high precise E-nose for daily indoor air quality monitoring in living environment. Integration 2017, 58, 286–294. [Google Scholar] [CrossRef]
- Fitzgerald, J.E.; Bui, E.T.H.; Simon, N.M.; Fenniri, H. Artificial Nose Technology: Status and Prospects in Diagnostics. Trends Biotechnol. 2017, 35, 33–42. [Google Scholar] [CrossRef]
- Schroeder, V.; Evans, E.D.; Wu, Y.C.M.; Voll, C.C.A.; McDonald, B.R.; Savagatrup, S.; Swager, T.M. Chemiresistive Sensor Array and Machine Learning Classification of Food. ACS Sens. 2019, 4, 2101–2108. [Google Scholar] [CrossRef]
- Biniecka, M.; Caroli, S. Analytical methods for the quantification of volatile aromatic compounds. TrAC Trends Anal. Chem. 2011, 30, 1756–1770. [Google Scholar] [CrossRef]
- Huikko, K.; Kostiainen, R.; Kotiaho, T. Introduction to micro-analytical systems: Bioanalytical and pharmaceutical applications. Eur. J. Pharm. Sci. 2003, 20, 149–171. [Google Scholar] [CrossRef]
- Giungato, P.; Di Gilio, A.; Palmisani, J.; Marzocca, A.; Mazzone, A.; Brattoli, M.; Giua, R.; de Gennaro, G. Synergistic approaches for odor active compounds monitoring and identification: State of the art, integration, limits and potentialities of analytical and sensorial techniques. TrAC Trends Anal. Chem. 2018, 107, 116–129. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Hu, W.; Wan, L.; Jian, Y.; Ren, C.; Jin, K.; Su, X.; Bai, X.; Haick, H.; Yao, M.; Wu, W. Electronic Noses: From Advanced Materials to Sensors Aided with Data Processing. Adv. Mater. Technol. 2019, 4, 1–38. [Google Scholar] [CrossRef]
- Lussac, E.; Barattin, R.; Cardinael, P.; Agasse, V. Review on Micro-Gas Analyzer Systems: Feasibility, Separations and Applications. Crit. Rev. Anal. Chem. 2016, 46, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Zampolli, S.; Elmi, I.; Mancarella, F.; Betti, P.; Dalcanale, E.; Cardinali, G.C.; Severi, M. Real-time monitoring of sub-ppb concentrations of aromatic volatiles with a MEMS-enabled miniaturized gas-chromatograph. Sens. Actuators B Chem. 2009, 141, 322–328. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, M.; Ahmadi, A.; Rousseau, J.; Nejad, H.R.; Hoorfar, M. On-Chip Electronic Nose for Wine Tasting: A Digital Microfluidic Approach. IEEE Sens. J. 2017, 17, 4322–4329. [Google Scholar] [CrossRef]
- Mehrabi, P.; Hui, J.; Montazeri, M.M.; Nguyen, K.T.; Logel, A.; O’Brian, A.; Hoorfar, M. Smelling through Microfluidic Olfaction Technology. 2018. Available online: https://yorkspace.library.yorku.ca/xmlui/handle/10315/35359 (accessed on 11 March 2020).
- Paknahad, M.; Ghafarinia, V.; Hossein-Babaei, F. A microfluidic gas analyzer for selective detection of biomarker gases. In Proceedings of the 2012 IEEE Sensors Applications Symposium (SAS), Brescia, Italy, 7–9 February 2012; pp. 10–14. [Google Scholar] [CrossRef]
- Paknahad, M. Development of Highly Selective Single Sensor Microfluidic-Based Gas Detector. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2017. [Google Scholar] [CrossRef]
- Schiavon, M.; Martini, L.M.; Corrà, C.; Scapinello, M.; Coller, G.; Tosi, P.; Ragazzi, M. Characterisation of volatile organic compounds (VOCs) released by the composting of different waste matrices. Environ. Pollut. 2017, 231, 845–853. [Google Scholar] [CrossRef]
- Jha, S.K. Characterization of human body odor and identification of aldehydes using chemical sensor. Rev. Anal. Chem. 2017, 36. [Google Scholar] [CrossRef]
- James, D.; Scott, S.M.; Ali, Z.; O’Hare, W.T. Chemical sensors for electronic nose systems. Microchim. Acta 2005, 149, 1–17. [Google Scholar] [CrossRef]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Application of electronic nose systems for assessing quality of medicinal and aromatic plant products: A review. J. Appl. Res. Med. Aromat. Plants 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Wu, J.; Yin, M.J.; Seefeldt, K.; Dani, A.; Guterman, R.; Yuan, J.; Zhang, A.P.; Tam, H.Y. In situ Μ-printed optical fiber-tip CO2 sensor using a photocrosslinkable poly(ionic liquid). Sens. Actuators B Chem. 2018, 259, 833–839. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, Y.H.; Park, K.S.; Eom, J.B.; Kim, M.J.; Rho, B.S.; Choi, H.Y. Interferometric fiber optic sensors. Sensors 2012, 12, 2467–2486. [Google Scholar] [CrossRef]
- Yin, M.J.; Gu, B.; An, Q.F.; Yang, C.; Guan, Y.L.; Yong, K.T. Recent development of fiber-optic chemical sensors and biosensors: Mechanisms, materials, micro/nano-fabrications and applications. Coord. Chem. Rev. 2018, 376, 348–392. [Google Scholar] [CrossRef]
- Fu, H.; Jiang, Y.; Ding, J.; Zhang, J.; Zhang, M.; Zhu, Y.; Li, H. Zinc oxide nanoparticle incorporated graphene oxide as sensing coating for interferometric optical microfiber for ammonia gas detection. Sens. Actuators B Chem. 2018, 254, 239–247. [Google Scholar] [CrossRef]
- Korposh, S.; Selyanchyn, R.; Yasukochi, W.; Lee, S.W.; James, S.W.; Tatam, R.P. Optical fibre long period grating with a nanoporous coating formed from silica nanoparticles for ammonia sensing in water. Mater. Chem. Phys. 2012, 133, 784–792. [Google Scholar] [CrossRef]
- Pacquiao, M.R.; de Luna, M.D.G.; Thongsai, N.; Kladsomboon, S.; Paoprasert, P. Highly fluorescent carbon dots from enokitake mushroom as multi-faceted optical nanomaterials for Cr 6+ and VOC detection and imaging applications. Appl. Surf. Sci. 2018, 453, 192–203. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, Y.; Feng, C.; Wang, L.; Ding, Y.; Hu, A. Conjugated Polymer Nanoparticles Based Fluorescent Electronic Nose for the Identification of Volatile Compounds. Anal. Chem. 2018, 90, 4815–4822. [Google Scholar] [CrossRef]
- Thongsai, N.; Tanawannapong, N.; Praneerad, J.; Kladsomboon, S.; Jaiyong, P.; Paoprasert, P. Real-time detection of alcohol vapors and volatile organic compounds via optical electronic nose using carbon dots prepared from rice husk and density functional theory calculation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 278–287. [Google Scholar] [CrossRef]
- Zhong, X.; Li, D.; Du, W.; Yan, M.; Wang, Y.; Huo, D.; Hou, C. Rapid recognition of volatile organic compounds with colorimetric sensor arrays for lung cancer screening. Anal. Bioanal. Chem. 2018, 410, 3671–3681. [Google Scholar] [CrossRef]
- Azzouz, A.; Kumar, V.; Kim, K.H.; Ballesteros, E.; Rhadfi, T.; Malik, A.K. Advances in colorimetric and optical sensing for gaseous volatile organic compounds. TrAC Trends Anal. Chem. 2019, 118, 502–516. [Google Scholar] [CrossRef]
- Yasuda, T.; Yonemura, S.; Tani, A. Comparison of the characteristics of small commercial NDIR CO2 sensor models and development of a portable CO2 measurement device. Sensors 2012, 12, 3641–3655. [Google Scholar] [CrossRef] [PubMed]
- Agbroko, S.O.; Covington, J. A novel, low-cost, portable PID sensor for the detection of volatile organic compounds. Sens. Actuators B Chem. 2018, 275, 10–15. [Google Scholar] [CrossRef]
- Zhu, H.; Nidetz, R.; Zhou, M.; Lee, J.; Buggaveeti, S.; Kurabayashi, K.; Fan, X. Flow-through microfluidic photoionization detectors for rapid and highly sensitive vapor detection. Lab Chip 2015, 15, 3021–3029. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.V.; Choi, I.Y.; Son, Y.S.; Kim, J.C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, F.; Liao, H.; Yin, X.; Liu, Y.; Yu, B. A novel spectrum analysis technique for odor sensing in optical electronic nose. Sens. Actuators B Chem. 2016, 222, 769–779. [Google Scholar] [CrossRef]
- Palzer, S. Photoacoustic-based gas sensing: A review. Sensors 2020, 20, 2745. [Google Scholar] [CrossRef]
- Dello Russo, S.; Zhou, S.; Zifarelli, A.; Patimisco, P.; Sampaolo, A.; Giglio, M.; Iannuzzi, D.; Spagnolo, V. Photoacoustic spectroscopy for gas sensing: A comparison between piezoelectric and interferometric readout in custom quartz tuning forks. Photoacoustics 2020, 17, 100155. [Google Scholar] [CrossRef]
- Joe, H.E.; Yun, H.; Jo, S.H.; Jun, M.B.G.; Min, B.K. A review on optical fiber sensors for environmental monitoring. Int. J. Precis. Eng. Manuf. Green Technol. 2018, 5, 173–191. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhao, Y.; Lv, R.Q. A review for optical sensors based on photonic crystal cavities. Sens. Actuators A Phys. 2015, 233, 374–389. [Google Scholar] [CrossRef]
- Lopez-Torres, D.; Elosua, C.; Villatoro, J.; Zubia, J.; Rothhardt, M.; Schuster, K.; Arregui, F.J. Enhancing sensitivity of photonic crystal fiber interferometric humidity sensor by the thickness of SnO2 thin films. Sens. Actuators B Chem. 2017, 251, 1059–1067. [Google Scholar] [CrossRef]
- Kou, D.; Zhang, Y.; Zhang, S.; Wu, S.; Ma, W. High-sensitive and stable photonic crystal sensors for visual detection and discrimination of volatile aromatic hydrocarbon vapors. Chem. Eng. J. 2019, 375, 121987. [Google Scholar] [CrossRef]
- Arnau, A. Piezoelectric Transducers and Applications (Google eBook); Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Mujahid, A.; Dickert, F.L. Surface acousticwave (SAW) for chemical sensing applications of recognition layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef] [PubMed]
- Benetti, M.; Cannatà, D.; Verona, E.; Palla Papavlu, A.; Dinca, V.C.; Lippert, T.; Dinescu, M.; Di Pietrantonio, F. Highly selective surface acoustic wave e-nose implemented by laser direct writing. Sens. Actuators B Chem. 2019, 283, 154–162. [Google Scholar] [CrossRef]
- Yang, Y.; Nagano, K.; Chaoluomen, C.; Iwasa, T.; Fukuda, H. Analysis of surface acoustic wave propagation velocity in biological function-oriented odor sensor. J. Sens. 2018, 2018, 8129484. [Google Scholar] [CrossRef]
- Panneerselvam, G.; Thirumal, V.; Pandya, H.M. Review of surface acoustic wave sensors for the detection and identification of toxic environmental gases/vapours. Arch. Acoust. 2018, 43, 357–367. [Google Scholar] [CrossRef]
- Hamidon, M.N.; Skarda, V.; White, N.M.; Krispel, F.; Krempl, P.; Binhack, M.; Buff, W. Fabrication of high temperature surface acoustic wave devices for sensor applications. Sens. Actuators A Phys. 2005, 123–124, 403–407. [Google Scholar] [CrossRef]
- Vashist, S.K.; Vashist, P. Recent advances in quartz crystal microbalance-based sensors. J. Sens. 2011, 2011, 571405. [Google Scholar] [CrossRef]
- Hung, V.N.; Abe, T.; Minh, P.N.; Esashi, M. High-frequency one-chip multichannel quartz crystal microbalance fabricated by deep RIE. Sens. Actuators A Phys. 2003, 108, 91–96. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Chang, Y.; Zhang, R.; Tao, J.; Qu, H.; Duan, X. Detection of volatile organic compunds by high-Q piezotransduced single-crystal silicon bulk acoustic resonator arrays. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar] [CrossRef]
- Li, D.; Zu, X.; Ao, D.; Tang, Q.; Fu, Y.Q.; Guo, Y.; Bilawal, K.; Faheem, M.B.; Li, L.; Li, S.; et al. High humidity enhanced surface acoustic wave (SAW) H2S sensors based on sol–gel CuO films. Sens. Actuators B Chem. 2019, 294, 55–61. [Google Scholar] [CrossRef]
- Khot, L.R.; Panigrahi, S.; Lin, D. Development and evaluation of piezoelectric-polymer thin film sensors for low concentration detection of volatile organic compounds related to food safety applications. Sens. Actuators B Chem. 2011, 153, 1–10. [Google Scholar] [CrossRef]
- Weinberg, M.S.; Cunningham, B.T.; Clapp, C.W. Modeling flexural plate wave devices. J. Microelectromech. Syst. 2000, 9, 370–379. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Park, J.; Heldsinger, D.; Hsieh, M.D.; Zellers, E.T. Vapor recognition with an integrated array of polymer-coated flexural plate wave sensors. Sens. Actuators B Chem. 2000, 62, 121–130. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, D.J. Fabrication and characterization of ZnO films for biological sensor application of FPW device. In Proceedings of the 2006 15th IEEE International Symposium on the Applications of Ferroelectrics, Sunset Beach, NV, USA, 30 July–3 August 2006; pp. 10–13. [Google Scholar] [CrossRef]
- Reusch, M.; Hole, K.; Zukauskaite, A.; Lebedev, V.; Kurz, N.; Ambacher, O. Flexural plate wave sensors with buried IDT for sensing in liquids. In Proceedings of the 2017 IEEE Sensors, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Zheng, Z.; Yao, Y.; Sun, Y.; Yeow, J.T.W. Development of a highly sensitive humidity sensor based on the capacitive micromachined ultrasonic transducer. Sens. Actuators B Chem. 2019, 286, 39–45. [Google Scholar] [CrossRef]
- Fraser, J.D. Capacitive micromachined ultrasonic transducers. J. Acoust. Soc. Am. 2003, 113, 1194. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, K.K.; Kupnik, M.; Oralkan, Ö.; Khuri-Yakub, B.T. Chemical vapor detection using a capacitive micromachined ultrasonic transducer. Anal. Chem. 2011, 83, 9314–9320. [Google Scholar] [CrossRef]
- Emadi, T.A.; Buchanan, D.A. Design and fabrication of a novel MEMS capacitive transducer with multiple moving membrane, M3-CMUT. IEEE Trans. Electron Devices 2014, 61, 890–896. [Google Scholar] [CrossRef]
- Stedman, Q.; Park, K.K.; Khuri-Yakub, B.T. Distinguishing chemicals using CMUT chemical sensor array and artificial neural networks. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 162–165. [Google Scholar] [CrossRef]
- Stedman, Q.; Park, K.K.; Khuri-Yakub, B.T. An 8-channel CMUT chemical sensor array on a single chip. In Proceedings of the 2017 IEEE International Ultrasonics Symposium, Washington, DC, USA, 6–9 September 2017; Volume 1, pp. 1–4. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Constantino, N.; Seok, C.; Yamaner, F.Y.; Dean, R.A.; Oralkan, O. A CMUT—Based Electronic Nose for Real-Time Monitoring of Volatiles Emitted by Plants: Preliminary Results. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Zhou, J.; Welling, C.M.; Kawadiya, S.; Deshusses, M.A.; Grego, S.; Chakrabarty, K. Sensor-Array optimization based on mutual information for sanitation-related malodor alerts. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable electronic nose based on electrochemical sensors for food quality assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. TrAC Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Knake, R.; Jacquinot, P.; Hodgson, A.W.E.; Hauser, P.C. Amperometric sensing in the gas-phase. Anal. Chim. Acta 2005, 549, 1–9. [Google Scholar] [CrossRef]
- Hoherčáková, Z.; Opekar, F. Au/PVC composite—A new material for solid-state gas sensors: Detection of nitrogen dioxide in the air. Sens. Actuators B Chem. 2004, 97, 379–386. [Google Scholar] [CrossRef]
- Baron, R.; Saffell, J. Amperometric Gas Sensors as a Low Cost Emerging Technology Platform for Air Quality Monitoring Applications: A Review. ACS Sens. 2017, 2, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wen, W.; Zhang, X.; Wang, S. Highly sensitive amperometric biosensor based on electrochemically-reduced graphene oxide-chitosan/hemoglobin nanocomposite for nitromethane determination. Biosens. Bioelectron. 2016, 79, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Aliramezani, M.; Koch, C.R.; Hayes, R.E.; Patrick, R. Amperometric solid electrolyte NOx sensors—The effect of temperature and diffusion mechanisms. Solid State Ionics 2017, 313, 7–13. [Google Scholar] [CrossRef]
- Wan, H.; Yin, H.; Mason, A.J. Rapid measurement of room temperature ionic liquid electrochemical gas sensor using transient double potential amperometry. Sens. Actuators B Chem. 2017, 242, 658–666. [Google Scholar] [CrossRef]
- Silvester, D.S. New innovations in ionic liquid–based miniaturised amperometric gas sensors. Curr. Opin. Electrochem. 2019, 15, 7–17. [Google Scholar] [CrossRef]
- Tang, Y.; He, J.; Gao, X.; Yang, T.; Zeng, X. Continuous amperometric hydrogen gas sensing in ionic liquids. Analyst 2018, 143, 4136–4146. [Google Scholar] [CrossRef]
- Hussain, G.; Silvester, D.S. Detection of sub-ppm Concentrations of Ammonia in an Ionic Liquid: Enhanced Current Density Using “Filled” Recessed Microarrays. Anal. Chem. 2016, 88, 12453–12460. [Google Scholar] [CrossRef]
- Nagata, S.; Kameshiro, N.; Terutsuki, D.; Mitsuno, H.; Sakurai, T.; Niitsu, K.; Nakazato, K.; Kanzaki, R.; Ando, M. A high-density integrated odorant sensor array system based on insect cells expressing insect odorant receptors. In Proceedings of the 2018 IEEE Micro Electro Mechanical Systems (MEMS), Belfast, Northern Ireland, 21–25 January 2018; pp. 282–285. [Google Scholar] [CrossRef]
- Shinmyo, N.; Iwata, T.; Hashizume, K.; Kuroki, K.; Sawada, K. Development of potentiometric miniature gas sensor arrays feasible for small olfactory chips and gas recognition from their response patterns. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Sadaoka, Y.; Mori, M. Detection of VOC in air with a planar-type potentiometric gas sensor based on YSZ with a Pt electrode modified with TiO2. Sens. Actuators B Chem. 2017, 248, 878–885. [Google Scholar] [CrossRef]
- Bur, C.; Bastuck, M.; Puglisi, D.; Schütze, A.; Lloyd Spetz, A.; Andersson, M. Discrimination and quantification of volatile organic compounds in the ppb-range with gas sensitive SiC-FETs using multivariate statistics. Sens. Actuators B Chem. 2015, 214, 225–233. [Google Scholar] [CrossRef]
- Mondal, S.P.; Dutta, P.K.; Hunter, G.W.; Ward, B.J.; Laskowski, D.; Dweik, R.A. Development of high sensitivity potentiometric NOx sensor and its application to breath analysis. Sens. Actuators B Chem. 2011, 158, 292–298. [Google Scholar] [CrossRef]
- Nketia-Yawson, B.; Noh, Y.Y. Organic thin film transistor with conjugated polymers for highly sensitive gas sensors. Macromol. Res. 2017, 25, 489–495. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, O.S.; Lee, S.H.; Song, H.S.; Park, T.H.; Jang, J. Ultrasensitive flexible graphene based field-effect transistor (FET)-type bioelectronic nose. Nano Lett. 2012, 12, 5082–5090. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.S.; Tang, W.X.; Wang, G.E.; Nath, B.; Xu, G. MOF Thin Film-Coated Metal Oxide Nanowire Array: Significantly Improved Chemiresistor Sensor Performance. Adv. Mater. 2016, 28, 5229–5234. [Google Scholar] [CrossRef] [PubMed]
- Lv, A.; Wang, M.; Wang, Y.; Bo, Z.; Chi, L. Investigation into the Sensing Process of High-Performance H2S Sensors Based on Polymer Transistors. Chemistry 2016, 22, 3654–3659. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, H.; Qi, L.; Xiao, L. Conductometric acetone vapor sensor based on the use of gold-doped three-dimensional hierarchical porous zinc oxide microspheres. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Hasani, A.; Dehsari, H.S.; Gavgani, J.N.; Shalamzari, E.K.; Salehi, A.; Afshar Taromi, F.; Mahyari, M. Sensor for volatile organic compounds using an interdigitated gold electrode modified with a nanocomposite made from poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) and ultra-large graphene oxide. Microchim. Acta 2015, 182, 1551–1559. [Google Scholar] [CrossRef]
- Xie, Z.; Raju, M.V.R.; Brown, B.S.; Stewart, A.C.; Nantz, M.H.; Fu, X.A. Electronic nose for detection of toxic volatile organic compounds in air. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 1425–1428. [Google Scholar]
- Krainer, J.; Deluca, M.; Lackner, E.; Wimmer-Teubenbacher, R.; Sosada, F.; Gspan, C.; Rohracher, K.; Wachmann, E.; Koeck, A. CMOS Integrated Tungsten Oxide Nanowire Networks for ppb-level H2S Sensing. Procedia Eng. 2016, 168, 272–275. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, J.S.; Lee, H.J. Pt-doped SnO2 thin film based micro gas sensors with high selectivity to toluene and HCHO. Sens. Actuators B Chem. 2017, 248, 1011–1016. [Google Scholar] [CrossRef]
- Hartwig, M.; Zichner, R.; Joseph, Y. Inkjet-printed wireless chemiresistive sensors—A review. Chemosensors 2018, 6, 66. [Google Scholar] [CrossRef]
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-cost and flexible printed graphene-PEDOT:PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Khan, S.; Lorenzelli, L. Recent advances of conductive nanocomposites in printed and flexible electronics. Smart Mater. Struct. 2017, 26, 083001. [Google Scholar] [CrossRef]
- Suematsu, K.; Ma, N.; Watanabe, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of humid aging on the oxygen adsorption in SnO2 gas sensors. Sensors 2018, 18, 254. [Google Scholar] [CrossRef]
- Serry, M.; Voiculcscu, I.; Kobtan, A. Catalytic Hafnium Oxide Calorimetric MEMS Gas and Chemical Sensor. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018; pp. 2–5. [Google Scholar] [CrossRef]
- Vahidpour, F.; Oberländer, J.; Schöning, M.J. Flexible Calorimetric Gas Sensors for Detection of a Broad Concentration Range of Gaseous Hydrogen Peroxide: A Step Forward to Online Monitoring of Food-Package Sterilization Processes. Phys. Status Solidi Appl. Mater. Sci. 2018, 215, 1–7. [Google Scholar] [CrossRef]
- Lee, E.B.; Hwang, I.S.; Cha, J.H.; Lee, H.J.; Lee, W.B.; Pak, J.J.; Lee, J.H.; Ju, B.K. Micromachined catalytic combustible hydrogen gas sensor. Sens. Actuators B Chem. 2011, 153, 392–397. [Google Scholar] [CrossRef]
- Jildeh, Z.B.; Kirchner, P.; Oberländer, J.; Kremers, A.; Wagner, T.; Wagner, P.H.; Schöning, M.J. FEM-based modeling of a calorimetric gas sensor for hydrogen peroxide monitoring. Phys. Status Solidi Appl. Mater. Sci. 2017, 214. [Google Scholar] [CrossRef]
- Del Orbe, D.V.; Cho, I.; Kang, K.; Park, J.; Park, I. Low Power Thermo-Catalytic Gas Sensor Based on Suspended Noble-Metal Nanotubes for H2 Sensing. In Proceedings of the 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1407–1410. [Google Scholar] [CrossRef]
- Bíró, F.; Dücső, C.; Radnóczi, G.Z.; Baji, Z.; Takács, M.; Bársony, I. ALD nano-catalyst for micro-calorimetric detection of hydrocarbons. Sens. Actuators B Chem. 2017, 247, 617–625. [Google Scholar] [CrossRef]
- Arreola, J.; Keusgen, M.; Wagner, T.; Schöning, M.J. Combined calorimetric gas- and spore-based biosensor array for online monitoring and sterility assurance of gaseous hydrogen peroxide in aseptic filling machines. Biosens. Bioelectron. 2019, 143, 111628. [Google Scholar] [CrossRef]
- Illyaskutty, N.; Kansizoglu, O.; Akdag, O.; Ojha, B.; Knoblauch, J.; Kohler, H. Miniaturized single chip arrangement of a wheatstone bridge based calorimetric gas sensor. Chemosensors 2018, 6, 22. [Google Scholar] [CrossRef]
- Nemirovsky, Y.; Stolyarova, S.; Blank, T.; Bar-Lev, S.; Svetlitza, A.; Zviagintsev, A.; Brouk, I. A New Pellistor-Like Gas Sensor Based on Micromachined CMOS Transistor. IEEE Trans. Electron Devices 2018, 65, 5494–5498. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Mohtasebi, S.S.; Siadat, M.; Balasubramanian, S. Meat quality assessment by electronic nose (Machine Olfaction Technology). Sensors 2009, 9, 6058–6083. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.S.; Sanfelice, R.C.; Pavinatto, A.; Mattoso, L.H.C.; Correa, D.S. Hybrid nanomaterials designed for volatile organic compounds sensors: A review. Mater. Des. 2018, 156, 154–166. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Vishinkin, R.; Barash, O.; Nakhleh, M.K.; Haick, H. Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem. Soc. Rev. 2018, 47, 4781–4859. [Google Scholar] [CrossRef]
- Sayago, I.; Aleixandre, M.; Santos, J.P. Development of Tin oxide-based nanosensors for electronic nose environmental applications. Biosensors 2019, 9, 21. [Google Scholar] [CrossRef]
- Han, D.; Zhai, L.; Gu, F.; Wang, Z. Highly sensitive NO2 gas sensor of ppb-level detection based on In2O3 nanobricks at low temperature. Sens. Actuators B Chem. 2018, 262, 655–663. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Rahmanian, R.; Mozaffari, S.A.; Abedi, M. Disposable urea biosensor based on nanoporous ZnO film fabricated from omissible polymeric substrate. Mater. Sci. Eng. C 2015, 57, 387–396. [Google Scholar] [CrossRef]
- Tiggemann, L.; Ballen, S.; Bocalon, C.; Graboski, A.M.; Manzoli, A.; De Paula Herrmann, P.S.; Steffens, J.; Valduga, E.; Steffens, C. Low-cost gas sensors with polyaniline film for aroma detection. J. Food Eng. 2016, 180, 16–21. [Google Scholar] [CrossRef]
- Pirsa, S. Chemiresistive gas sensors based on conducting polymers. In Handbook of Research on Nanoelectronic Sensor Modeling and Applications; IGI Global: Hershey, PA, USA, 2017; pp. 150–180. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-based sensors: A review. Polym. Test. 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, W.; Yan, K.; Pan, L.; Yu, G. Doping engineering of conductive polymer hydrogels and their application in advanced sensor technologies. Chem. Sci. 2019, 10, 6232–6244. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tang, N.; Zhou, C.; Han, Z.; Qu, H.; Duan, X. A chemiresistive sensor array from conductive polymer nanowires fabricated by nanoscale soft lithography. Nanoscale 2018, 10, 20578–20586. [Google Scholar] [CrossRef] [PubMed]
- Waghuley, S.A.; Yenorkar, S.M.; Yawale, S.S.; Yawale, S.P. Application of chemically synthesized conducting polymer-polypyrrole as a carbon dioxide gas sensor. Sens. Actuators B Chem. 2008, 128, 366–373. [Google Scholar] [CrossRef]
- Wang, J.; Feng, B.; Wu, W. Conductive-carbon-black filled PDMS chemiresistor sensor for the detection of volatile organic compounds. In Proceedings of the NEMS 2011—6th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Kaohsiung, Taiwan, 20–23 February 2011; pp. 449–452. [Google Scholar] [CrossRef]
- Ruhhammer, J.; Zens, M.; Goldschmidtboeing, F.; Seifert, A.; Woias, P. Highly elastic conductive polymeric MEMS. Sci. Technol. Adv. Mater. 2015, 16. [Google Scholar] [CrossRef]
- Silva, L.I.B.; M-Costa, A.; Freitas, A.C.; Rocha-Santos, T.A.P.; Duarte, A.C. Polymeric nanofilm-coated optical fibre sensor for speciation of aromatic compounds. Int. J. Environ. Anal. Chem. 2009, 89, 183–197. [Google Scholar] [CrossRef]
- Zhao, C.; Han, F.; Li, Y.; Mao, B.; Kang, J.; Shen, C.; Dong, X. Volatile Organic Compound Sensor Based on PDMS Coated Fabry-Perot Interferometer with Vernier Effect. IEEE Sens. J. 2019, 19, 4443–4450. [Google Scholar] [CrossRef]
- Sayago, I.; Fernández, M.J.; Fontecha, J.L.; Horrillo, M.C.; Vera, C.; Obieta, I.; Bustero, I. New sensitive layers for surface acoustic wave gas sensors based on polymer and carbon nanotube composites. Sens. Actuators B Chem. 2012, 175, 67–72. [Google Scholar] [CrossRef]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-electrical gas sensors based on conducting polymer hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B Chem. 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Organic semiconductors in potentiometric gas sensors. J. Solid State Electrochem. 2009, 13, 41–49. [Google Scholar] [CrossRef]
- Bertoni, C.; Naclerio, P.; Viviani, E.; Dal Zilio, S.; Carrato, S.; Fraleoni-Morgera, A. Nanostructured p3ht as a promising sensing element for real-time, dynamic detection of gaseous acetone. Sensors 2019, 19, 1296. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Sharma, D.; Cobb, B.; Dodabalapur, A. Charge transport and trapping in organic field effect transistors exposed to polar analytes. Appl. Phys. Lett. 2011, 98, 28–31. [Google Scholar] [CrossRef]
- Wei, S.; Tian, F.; Ge, F.; Wang, X.; Zhang, G.; Lu, H.; Yin, J.; Wu, Z.; Qiu, L. Helical Nanofibrils of Block Copolymer for High-Performance Ammonia Sensors. ACS Appl. Mater. Interfaces 2018, 10, 22504–22512. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Tinku, S.; Lorenzelli, L.; Dahiya, R.S. Flexible tactile sensors using screen-printed P(VDF-TrFE) and MWCNT/PDMS composites. IEEE Sens. J. 2015, 15, 3146–3155. [Google Scholar] [CrossRef]
- Boday, D.J.; Muriithi, B.; Stover, R.J.; Loy, D.A. Polyaniline nanofiber-silica composite aerogels. J. Non-Cryst. Solids 2012, 358, 1575–1580. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Iakoubovskii, K.; Minami, N.; Karthigeyan, A. Optical gas sensing with dip-coated carbon nanotubes through the modulation of photoluminescence and optical absorption. J. Mater. Chem. 2012, 22, 4716–4719. [Google Scholar] [CrossRef]
- Sivaramakrishnan, S.; Rajamani, R.; Smith, C.S.; McGee, K.A.; Mann, K.R.; Yamashita, N. Carbon nanotube-coated surface acoustic wave sensor for carbon dioxide sensing. Sens. Actuators B Chem. 2008, 132, 296–304. [Google Scholar] [CrossRef]
- Liu, S.F.; Lin, S.; Swager, T.M. An Organocobalt-Carbon Nanotube Chemiresistive Carbon Monoxide Detector. ACS Sens. 2016, 1, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Park, S.; Yu, K.; Ruoff, R.S.; Ocola, L.E.; Rosenmann, D.; Chen, J. Toward practical gas sensing with highly reduced graphene oxide: A new signal processing method to circumvent run-to-run and device-to-device variations. ACS Nano 2011, 5, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, C.; Kwon, O.S. Conducting polymer based nanobiosensors. Polymers 2016, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Duan, H. 2D and 3D graphene materials: Preparation and bioelectrochemical applications. Biosens. Bioelectron. 2015, 65, 404–419. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, D.H.; Seo, D.K.; Kim, T.; Im, H.U.; Lee, H.M.; Yoo, J.B.; Hong, S.H.; Kang, T.J.; Kim, Y.H. Flexible hydrogen sensors using graphene with palladium nanoparticle decoration. Sens. Actuators B Chem. 2012, 169, 387–392. [Google Scholar] [CrossRef]
- Zhang, T.; Nix, M.B.; Yoo, B.Y.; Deshusses, M.A.; Myung, N.V. Electrochemically functionalized single-walled carbon nanotube gas sensor. Electroanalysis 2006, 18, 1153–1158. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Howard Fairbrother, D. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, H.; Shen, W.; Jian, J. Wearable electronic nose for human skin odor identification: A preliminary study. Sens. Actuators A Phys. 2019, 285, 395–405. [Google Scholar] [CrossRef]
- Seo, S.M.; Kang, T.J.; Cheon, J.H.; Kim, Y.H.; Park, Y.J. Facile and scalable fabrication of chemiresistive sensor array for hydrogen detection based on gold-nanoparticle decorated SWCNT network. Sens. Actuators B Chem. 2014, 204, 716–722. [Google Scholar] [CrossRef]
- Bora, A.; Mohan, K.; Pegu, D.; Gohain, C.B.; Dolui, S.K. A room temperature methanol vapor sensor based on highly conducting carboxylated multi-walled carbon nanotube/polyaniline nanotube composite. Sens. Actuators B Chem. 2017, 253, 977–986. [Google Scholar] [CrossRef]
- Alshammari, A.S.; Alenezi, M.R.; Lai, K.T.; Silva, S.R.P. Inkjet printing of polymer functionalized CNT gas sensor with enhanced sensing properties. Mater. Lett. 2017, 189, 299–302. [Google Scholar] [CrossRef]
- Cittadini, M.; Bersani, M.; Perrozzi, F.; Ottaviano, L.; Wlodarski, W.; Martucci, A. Graphene oxide coupled with gold nanoparticles for localized surface plasmon resonance based gas sensor. Carbon 2014, 69, 452–459. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, I.D. Recent Developments in 2D Nanomaterials for Chemiresistive-Type Gas Sensors. Electron. Mater. Lett. 2018, 14, 221–260. [Google Scholar] [CrossRef]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible Graphene-Based Wearable Gas and Chemical Sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef]
- Toda, K.; Furue, R.; Hayami, S. Recent progress in applications of graphene oxide for gas sensing: A review. Anal. Chim. Acta 2015, 878, 43–53. [Google Scholar] [CrossRef]
- Latif, U.; Dickert, F.L. Graphene hybrid materials in gas sensing applications. Sensors 2015, 15, 30504–30524. [Google Scholar] [CrossRef]
- Basu, S.; Bhattacharyya, P. Recent developments on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Wang, Y.; Yeow, J.T.W. A Review of Carbon Nanotubes-Based Gas Sensors. J. Sens. 2009, 2009, 493904. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, J.Y.; Hwang, H.R.; Kim, H.S.; Lee, J.H.; Seo, J.W.; Shin, U.S.; Lee, S.H. Simple and cost-effective method of highly conductive and elastic carbon nanotube/polydimethylsiloxane composite for wearable electronics. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Wei, Y.; Chen, H.; Zhu, Z.; Fan, P.; Xu, X.; Xie, B. Printing Carbon Nanotube-Embedded Silicone Elastomers via Direct Writing. ACS Appl. Mater. Interfaces 2018, 10, 44796–44802. [Google Scholar] [CrossRef] [PubMed]
- Dung, T.T.; Oh, Y.; Choi, S.J.; Kim, I.D.; Oh, M.K.; Kim, M. Applications and advances in bioelectronic noses for odour sensing. Sensors 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Terziyska, A.; Waltschewa, L.; Venkov, P. A new sensitive test based on yeast cells for studying environmental pollution. Environ. Pollut. 2000, 109, 43–52. [Google Scholar] [CrossRef]
- Baronian, K.H.R. The use of yeast and moulds as sensing elements in biosensors. Biosens. Bioelectron. 2004, 19, 953–962. [Google Scholar] [CrossRef]
- Sung, J.H.; Ko, H.J.; Park, T.H. Piezoelectric biosensor using olfactory receptor protein expressed in Escherichia coli. Biosens. Bioelectron. 2006, 21, 1981–1986. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, Y.; Zhang, Q.; Zhang, D.; Zhuang, S.; Li, H.; Liu, Q. Olfactory biosensor for insect semiochemicals analysis by impedance sensing of odorant-binding proteins on interdigitated electrodes. Biosens. Bioelectron. 2015, 67, 662–669. [Google Scholar] [CrossRef]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Bioelectronic nose: Current status and perspectives. Biosens. Bioelectron. 2017, 87, 480–494. [Google Scholar] [CrossRef]
- Jin, H.J.; Lee, S.H.; Kim, T.H.; Park, J.; Song, H.S.; Park, T.H.; Hong, S. Nanovesicle-based bioelectronic nose platform mimicking human olfactory signal transduction. Biosens. Bioelectron. 2012, 35, 335–341. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, J.H.; Park, J.; Hong, S.; Park, T.H. Bioelectronic nose combined with a microfluidic system for the detection of gaseous trimethylamine. Biosens. Bioelectron. 2015, 71, 179–185. [Google Scholar] [CrossRef]
- Yoon, W.; Lee, S.H.; Kwon, O.S.; Song, H.S.; Oh, E.H.; Park, T.H.; Jang, J. Polypyrrole nanotubes conjugated with human olfactory receptors: High-Performance transducers for FET-Type bioelectronic noses. Angew. Chem. Int. Ed. 2009, 48, 2755–2758. [Google Scholar] [CrossRef] [PubMed]
- Liu, A. Towards development of chemosensors and biosensors with metal-oxide-based nanowires or nanotubes. Biosens. Bioelectron. 2008, 24, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, V.S.; Garnier, G. Cellulose Nano-Films as Bio-Interfaces. Front. Chem. 2019, 7, 535. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, B.Y.; Jaworski, J.; Yokoyama, K.; Chung, W.J.; Wang, E.; Hong, S.; Majumdar, A.; Lee, S.W. Selective and sensitive TNT sensors using biomimetic polydiacetylene-coated CNT-FETs. ACS Nano 2011, 5, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Viter, R.; Tereshchenko, A.; Smyntyna, V.; Ogorodniichuk, J.; Starodub, N.; Yakimova, R.; Khranovskyy, V.; Ramanavicius, A. Toward development of optical biosensors based on photoluminescence of TiO2 nanoparticles for the detection of Salmonella. Sens. Actuators B Chem. 2017, 252, 95–102. [Google Scholar] [CrossRef]
- Dong, Z.M.; Jin, X.; Zhao, G.C. Amplified QCM biosensor for type IV collagenase based on collagenase-cleavage of gold nanoparticles functionalized peptide. Biosens. Bioelectron. 2018, 106, 111–116. [Google Scholar] [CrossRef]
- Xiao-wei, H.; Xiao-bo, Z.; Ji-yong, S.; Zhi-hua, L.; Jie-wen, Z. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Zou, X.; Wang, J.; Liu, X.; Wang, C.; Jiang, Y.; Wang, Y.; Xiao, X.; Ho, J.C.; Li, J.; Jiang, C.; et al. Rational design of sub-parts per million specific gas sensors array based on metal nanoparticles decorated nanowire enhancement-mode transistors. Nano Lett. 2013, 13, 3287–3292. [Google Scholar] [CrossRef]
- Sil, D.; Hines, J.; Udeoyo, U.; Borguet, E. Palladium Nanoparticle-Based Surface Acoustic Wave Hydrogen Sensor. ACS Appl. Mater. Interfaces 2015, 7, 5709–5714. [Google Scholar] [CrossRef]
- Murray, B.J.; Walter, E.C.; Penner, R.M. Amine vapor sensing with silver mesowires. Nano Lett. 2004, 4, 665–670. [Google Scholar] [CrossRef]
- Liu, Z.; Searson, P.C. Single nanoporous gold nanowire sensors. J. Phys. Chem. B 2006, 110, 4318–4322. [Google Scholar] [CrossRef] [PubMed]
- García, M.; García-Carmona, L.; Escarpa, A. Microfluidic system for enzymeless electrochemical determination of inulin using catalytically active metal nanowires. Microchim. Acta 2014, 182, 745–752. [Google Scholar] [CrossRef]
- Lee, J.H.; Mirzaei, A.; Kim, J.Y.; Kim, J.H.; Kim, H.W.; Kim, S.S. Optimization of the surface coverage of metal nanoparticles on nanowires gas sensors to achieve the optimal sensing performance. Sens. Actuators B Chem. 2020, 302, 127196. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Yan, F. Polypyrrole/silver composite nanotubes for gas sensors. Sens. Actuators B Chem. 2010, 145, 495–500. [Google Scholar] [CrossRef]
- Fennell, J.F.; Liu, S.F.; Azzarelli, J.M.; Weis, J.G.; Rochat, S.; Mirica, K.A.; Ravnsbæk, J.B.; Swager, T.M. Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem. Int. Ed. 2016, 55, 1266–1281. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Faustini, M.; Garrido, J.J. Effects of the porous texture and surface chemistry of silica xerogels on the sensitivity of fiber-optic sensors toward VOCs. Sens. Actuators B Chem. 2016, 222, 1166–1174. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.H. Metal organic frameworks for sensing applications. TrAC Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Wang, X.F.; Song, X.Z.; Sun, K.M.; Cheng, L.; Ma, W. MOFs-derived porous nanomaterials for gas sensing. Polyhedron 2018, 152, 155–163. [Google Scholar] [CrossRef]
- Koo, W.-T.; Jang, J.-S.; Kim, I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Sarkar, D.; Xie, X.; Kang, J.; Zhang, H.; Liu, W.; Navarrete, J.; Moskovits, M.; Banerjee, K. Functionalization of transition metal dichalcogenides with metallic nanoparticles: Implications for doping and gas-sensing. Nano Lett. 2015, 15, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Goel, N.; Hojamberdiev, M.; Kumar, M. Transition metal dichalcogenides-based flexible gas sensors. Sens. Actuators A Phys. 2020, 303, 111875. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, Y.S.; Kim, D.J. Two-Dimensional Transition Metal Dichalcogenides and Metal Oxide Hybrids for Gas Sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef] [PubMed]
- Ion Science Ltd. MiniPID 2 PID. Available online: https://www.ionscience-usa.com/wp-content/uploads/2016/07/MiniPID-2-PID-sensor-UK-V1.1.pdf (accessed on 27 August 2020).
- Penza, M.; Antolini, F.; Vittori-Antisari, M. Carbon nanotubes-based surface acoustic waves oscillating sensor for vapour detection. Thin Solid Films 2005, 472, 246–252. [Google Scholar] [CrossRef]
- SGX Sensortech. MiCS-2714 Datasheet (1107 rev 6). 2017. Available online: https://www.sgxsensortech.com/content/uploads/2014/08/1107_Datasheet-MiCS-2714.pdf (accessed on 28 August 2020).
- Environmental Sensors Co. Z-900 Hydrogen Sulfide Monitor. Available online: http://www.environmentalsensors.com/hydrogen-sulfide-z-900.html (accessed on 28 August 2020).
- Jha, S.K.; Yadava, R.D.S.; Hayashi, K.; Patel, N. Recognition and sensing of organic compounds using analytical methods, chemical sensors, and pattern recognition approaches. Chemom. Intell. Lab. Syst. 2019, 185, 18–31. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; Xie, J.; Ai, L.; Tian, H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2019, 293, 8–14. [Google Scholar] [CrossRef]
- Dong, W.; Guo, R.; Liu, M.; Shen, C.; Sun, X.; Zhao, M.; Sun, J.; Li, H.; Zheng, F.; Huang, M.; et al. Characterization of key odorants causing the roasted and mud-like aromas in strong-aroma types of base Baijiu. Food Res. Int. 2019, 125, 108546. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, J.; Qiao, Z.; Cao, X.; Luo, Q.; Zhao, J.; Wang, F.; Zhang, W. Assessment of β-glucans, phenols, flavor and volatile profiles of hulless barley wine originating from highland areas of China. Food Chem. 2019, 293, 32–40. [Google Scholar] [CrossRef]
- Muñoz, R.; Sivret, E.C.; Parcsi, G.; Lebrero, R.; Wang, X.; Suffet, I.H.; Stuetz, R.M. Monitoring techniques for odour abatement assessment. Water Res. 2010, 44, 5129–5149. [Google Scholar] [CrossRef]
- Jha, S.K.; Hayashi, K. Body odor classification by selecting optimal peaks of chemical compounds in GC–MS spectra using filtering approaches. Int. J. Mass Spectrom. 2017, 415, 92–102. [Google Scholar] [CrossRef]
- Karasek, F.W.; Clement, R.E. Chromatography-Mass Spectrometry Principles and Techniques; Elsevier Science B.V: Amsterdam, The Netherlands, 1988; pp. 5–8. [Google Scholar]
- Grabowska-Polanowska, B.; Miarka, P.; Skowron, M.; Sułowicz, J.; Wojtyna, K.; Moskal, K.; Śliwka, I. Development of sampling method and chromatographic analysis of volatile organic compounds emitted from human skin. Bioanalysis 2017, 9, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Recent Developments in Human Odor Detection Technologies. J. Forensic Sci. Criminol. 2014, 1, 1–12. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Namieśnik, J.; Płotka-Wasylka, J. Direct solid phase microextraction combined with gas chromatography—Mass spectrometry for the determination of biogenic amines in wine. Talanta 2018, 183, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.K.W.; Austin, C.C. Determination of complex mixtures of volatile organic compounds in ambient air: An overview. Anal. Bioanal. Chem. 2006, 386, 1089–1098. [Google Scholar] [CrossRef]
- Dräger Ltd. X-PID 9000/9500. 2019. Ntegrated, L.T.E. Multi-Gas Detection. Available online: https://www.draeger.com/Products/Content/x-pid-9000-9500-pi-9104798-en-master.pdf (accessed on 14 September 2020).
- Micro GC. Go Mobile with a Complete, Portable Gc. Available online: https://www.agilent.com/cs/library/brochures/5991-6041EN.pdf (accessed on 14 September 2020).
- Defiant Technologies. FROG-5000. Available online: https://www.defiant-tech.com/frog-portable-gas-chromatograph-gc (accessed on 14 September 2020).
- Cheung, K.; Velásquez-García, L.F.; Akinwande, A.I. Chip-scale quadrupole mass filters for portable mass spectrometry. J. Microelectromech. Syst. 2010, 19, 469–483. [Google Scholar] [CrossRef]
- Agah, M.; Lambertus, G.R.; Sacks, R.; Wise, K. High-Speed MEMS-Based Gas Chromatography. J. Microelectromech. Syst. 2006, 15, 1371–1378. [Google Scholar] [CrossRef]
- Sun, J.; Guan, F.; Cui, D.; Chen, X.; Zhang, L.; Chen, J. An improved photoionization detector with a micro gas chromatography column for portable rapid gas chromatography system. Sens. Actuators B Chem. 2013, 188, 513–518. [Google Scholar] [CrossRef]
- Jian, R.S.; Huang, Y.S.; Lai, S.L.; Sung, L.Y.; Lu, C.J. Compact instrumentation of a μ-GC for real time analysis of sub-ppb VOC mixtures. Microchem. J. 2013, 108, 161–167. [Google Scholar] [CrossRef]
- Zhong, Q.; Steinecker, W.H.; Zellers, E.T. Characterization of a high-performance portable GC with a chemiresistor array detector. Analyst 2009, 134, 283–293. [Google Scholar] [CrossRef]
- Shakeel, H.; Agah, M. High density semipacked separation columns with optimized atomic layer deposited phases. Sens. Actuators B Chem. 2017, 242, 215–223. [Google Scholar] [CrossRef]
- Akbar, M.; Restaino, M.; Agah, M. Chip-scale gas chromatography: From injection through detection. Microsyst. Nanoeng. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Ghosh, A.; Vilorio, C.R.; Hawkins, A.R.; Lee, M.L. Microchip gas chromatography columns, interfacing and performance. Talanta 2018, 188, 463–492. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Takemori, Y.; Matsuoka, S.; Kanai, M.; Nishimoto, T.; Ueda, M.; Komori, K. Development of μGC (micro gas chromatography) with high performance micromachined chip column. IEEJ Trans. Electr. Electron. Eng. 2009, 4, 358–364. [Google Scholar] [CrossRef]
- Azzouz, I.; Marty, F.; Bourouina, T. Recent advances in micro-gas chromatography-The opportunities and the challenges. In Proceedings of the 2017 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP), Bordeaux, France, 29 May–1 June 2017. [Google Scholar] [CrossRef]
- Bhushan, A.; Yemane, D.; Trudell, D.; Overton, E.B.; Goettert, J. Fabrication of micro-gas chromatograph columns for fast chromatography. Microsyst. Technol. 2007, 13, 361–368. [Google Scholar] [CrossRef]
- Han, B.; Wu, G.; Huang, H.; Liu, T.; Wang, J.; Sun, J.; Wang, H. A semi-packed micro GC column for separation of the NAFLD exhaled breath VOCs. Surf. Coat. Technol. 2019, 363, 322–329. [Google Scholar] [CrossRef]
- Sun, J.; Cui, D.; Chen, X.; Zhang, L.; Cai, H.; Li, H. Fabrication and characterization of microelectromechanical systems-based gas chromatography column with embedded micro-posts for separation of environmental carcinogens. J. Chromatogr. A 2013, 1291, 122–128. [Google Scholar] [CrossRef]
- Radadia, A.D.; Salehi-Khojin, A.; Masel, R.I.; Shannon, M.A. The effect of microcolumn geometry on the performance of micro-gas chromatography columns for chip scale gas analyzers. Sens. Actuators B Chem. 2010, 150, 456–464. [Google Scholar] [CrossRef]
- Sun, J.H.; Guan, F.Y.; Zhu, X.F.; Ning, Z.W.; Ma, T.J.; Liu, J.H.; Deng, T. Micro-fabricated packed gas chromatography column based on laser etching technology. J. Chromatogr. A 2016, 1429, 311–316. [Google Scholar] [CrossRef]
- Reidy, S.; Lambertus, G.; Reece, J.; Sacks, R. High-performance, static-coated silicon microfabricated columns for gas chromatography. Anal. Chem. 2006, 78, 2623–2630. [Google Scholar] [CrossRef]
- Zareian-Jahromi, M.A.; Ashraf-Khorassani, M.; Taylor, L.T.; Agah, M. Design, modeling and fabrication of mems-based multicapillary gas chromatography columns. J. Microelectromech. Syst. 2008, 18, 28–37. [Google Scholar]
- Regmi, B.P.; Agah, M. Micro Gas Chromatography: An Overview of Critical Components and Their Integration. Anal. Chem. 2018, 90, 13133–13150. [Google Scholar] [CrossRef] [PubMed]
- Collin, W.R.; Nuñovero, N.; Paul, D.; Kurabayashi, K.; Zellers, E.T. Comprehensive two-dimensional gas chromatographic separations with a temperature programmed microfabricated thermal modulator. J. Chromatogr. A 2016, 1444, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Reidy, S.M.; Block, B.P.; Wise, K.D.; Zellers, E.T.; Kurabayashi, K. Microfabricated thermal modulator for comprehensive two-dimensional micro gas chromatography: Design, thermal modeling, and preliminary testing. Lab Chip 2010, 10, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Kurabayashi, K. Influence of Thermal Time Constant of a Micro-Fabricated Thermal Modulator (μTM) for Comprehensive Microscale 2-D Gas Chromatography (μGC × μGC). J. Microelectromech. Syst. 2017, 26, 743–745. [Google Scholar] [CrossRef]
- Lee, J.; Zhou, M.; Zhu, H.; Nidetz, R.; Kurabayashi, K.; Fan, X. In situ calibration of micro-photoionization detectors in a multi-dimensional micro-gas chromatography system. Analyst 2016, 141, 4100–4107. [Google Scholar] [CrossRef]
- Sanchez, J.B.; Berger, F.; Daniau, W.; Blind, P.; Fromm, M.; Nadal, M.H. Development of a gas detection micro-device for hydrogen fluoride vapours. Sens. Actuators B Chem. 2006, 113, 1017–1024. [Google Scholar] [CrossRef]
- Liu, J.; Seo, J.H.; Li, Y.; Chen, D.; Kurabayashi, K.; Fan, X. Smart multi-channel two-dimensional micro-gas chromatography for rapid workplace hazardous volatile organic compounds measurement. Lab Chip 2013, 13, 818–825. [Google Scholar] [CrossRef]
- Fan, X.; Lee, J.; Zhu, H.; Zhou, M.; Nidetz, R.; Kurabayashi, K.; Sayler, S.; Neitzel, R.; Richardson, R.J. Portable multi-dimensional gas chromatography device for rapid field analysis of chemical compounds. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 654–659. [Google Scholar]
- Garg, A.; Akbar, M.; Vejerano, E.; Narayanan, S.; Nazhandali, L.; Marr, L.C.; Agah, M. Zebra GC: A mini gas chromatography system for trace-level determination of hazardous air pollutants. Sens. Actuators B Chem. 2015, 212, 145–154. [Google Scholar] [CrossRef]
- Zampolli, S.; Elmi, I.; Stürmann, J.; Nicoletti, S.; Dori, L.; Cardinali, G.C. Selectivity enhancement of metal oxide gas sensors using a micromachined gas chromatographic column. Sens. Actuators B Chem. 2005, 105, 400–406. [Google Scholar] [CrossRef]
- Lee, J.; Zhou, M.; Zhu, H.; Nidetz, R.; Kurabayashi, K.; Fan, X. Fully Automated Portable Comprehensive 2-Dimensional Gas Chromatography Device. Anal. Chem. 2016, 88, 10266–10274. [Google Scholar] [CrossRef]
- Narayanan, S.; Alfeeli, B.; Agah, M. A micro gas chromatography chip with an embedded non-cascaded thermal conductivity detector. Procedia Eng. 2010, 5, 29–32. [Google Scholar] [CrossRef]
- Bryant-Genevier, J.; Zellers, E.T. Toward a microfabricated preconcentrator-focuser for a wearable micro-scale gas chromatograph. J. Chromatogr. A 2015, 1422, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Babaei, F.; Hooshyar Zare, A.; Gharesi, M. Quantitative Assessment of Vapor Molecules Adsorption to Solid Surfaces by Flow Rate Monitoring in Microfluidic Channels. Anal. Chem. 2019, 91, 12827–12834. [Google Scholar] [CrossRef] [PubMed]
- Janfaza, S.; Kim, E.; O’Brien, A.; Najjaran, H.; Nikkhah, M.; Alizadeh, T.; Hoorfar, M. A Nanostructured Microfluidic Artificial Olfaction for Organic Vapors Recognition. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, M.; Bachhal, J.S.; Ahmadi, A.; Hoorfar, M. Characterization of channel coating and dimensions of microfluidic-based gas detectors. Sens. Actuators B Chem. 2017, 241, 55–64. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Hooshyar Zare, A.; Ghafarinia, V.; Erfantalab, S. Identifying volatile organic compounds by determining their diffusion and surface adsorption parameters in microfluidic channels. Sens. Actuators B Chem. 2015, 220, 607–613. [Google Scholar] [CrossRef]
- Ghafarinia, V.; Amini, A.; Paknahad, M. Gas identification by a single gas sensor equipped with microfluidic channels. Sens. Lett. 2012, 10, 845–849. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Hooshyar Zare, A.; Ghafarinia, V. Transient molecular diffusion in microfluidic channels: Modeling and experimental verification of the results. Sens. Actuators B Chem. 2016, 233, 646–653. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Orvatinia, M. Transient regime of gas diffusion-physisorption through a microporous barrier. IEEE Sens. J. 2005, 5, 1004–1010. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Shakerpour, S. Diffusion-physisorption of a trace material in a capillary tube. J. Appl. Phys. 2006, 100, 124917. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Ghafarinia, V. Gas analysis by monitoring molecular diffusion in a microfluidic channel. Anal. Chem. 2010, 82, 8349–8355. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Babaei, F.; Hooshyar Zare, A. The selective flow of volatile organic compounds in conductive polymer-coated microchannels. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, M.; Mcintosh, C.; Hoorfar, M. Selective detection of volatile organic compounds in microfluidic gas detectors based on “like dissolves like”. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Syed, J.A.; Tang, S.; Meng, X. Super-hydrophobic multilayer coatings with layer number tuned swapping in surface wettability and redox catalytic anti-corrosion application. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Lim, H.; Moon, S.J. Stable nonpolar solvent droplet generation using a poly(dimethylsiloxane) microfluidic channel coated with poly-p-xylylene for a nanoparticle growth. Biomed. Microdevices 2015, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Petrou, P.S.; Papageorgiou, D.P.; Kakabakos, S.E.; Tserepi, A.; Gogolides, E. Controlled protein adsorption on microfluidic channels with engineered roughness and wettability. Sens. Actuators B Chem. 2012, 161, 216–222. [Google Scholar] [CrossRef]
- Paknahad, M.; Bachhal, J.S.; Hoorfar, M. Diffusion-based humidity control membrane for microfluidic-based gas detectors. Anal. Chim. Acta 2018, 1021, 103–112. [Google Scholar] [CrossRef]
- Jha, S.K.; Marina, N.; Liu, C.; Hayashi, K. Human body odor discrimination by GC-MS spectra data mining. Anal. Methods 2015, 7, 9549–9561. [Google Scholar] [CrossRef]
- Szymańska, E. Modern data science for analytical chemical data—A comprehensive review. Anal. Chim. Acta 2018, 1028, 1–10. [Google Scholar] [CrossRef]
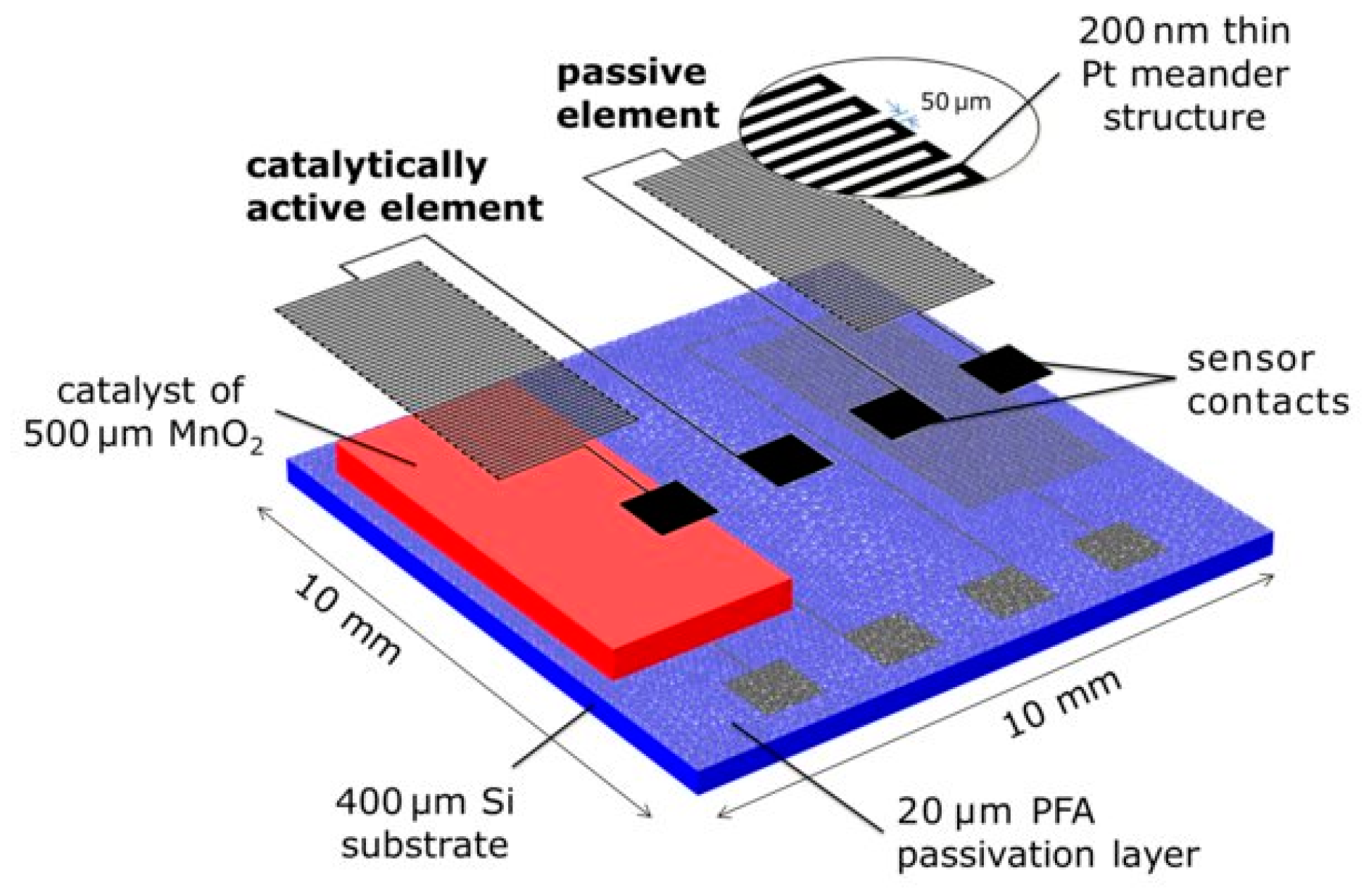

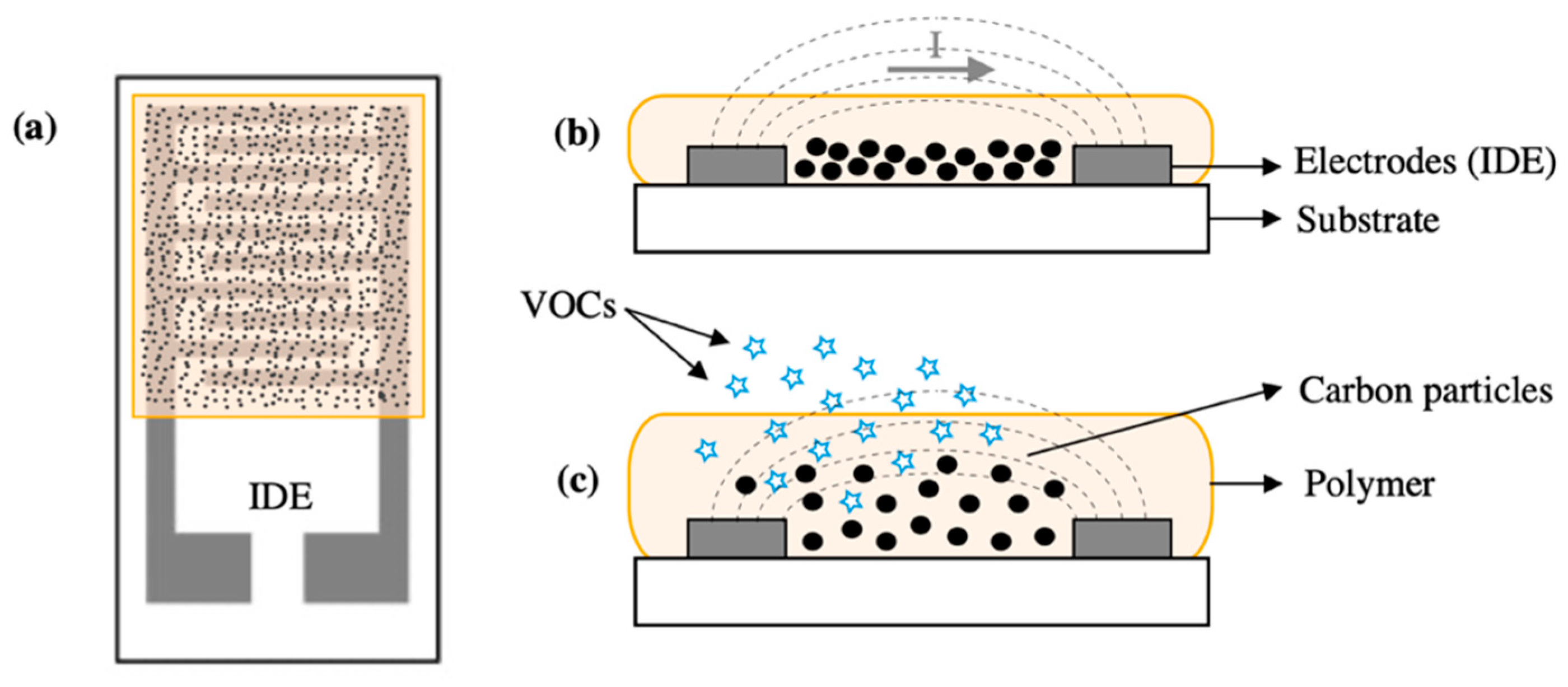
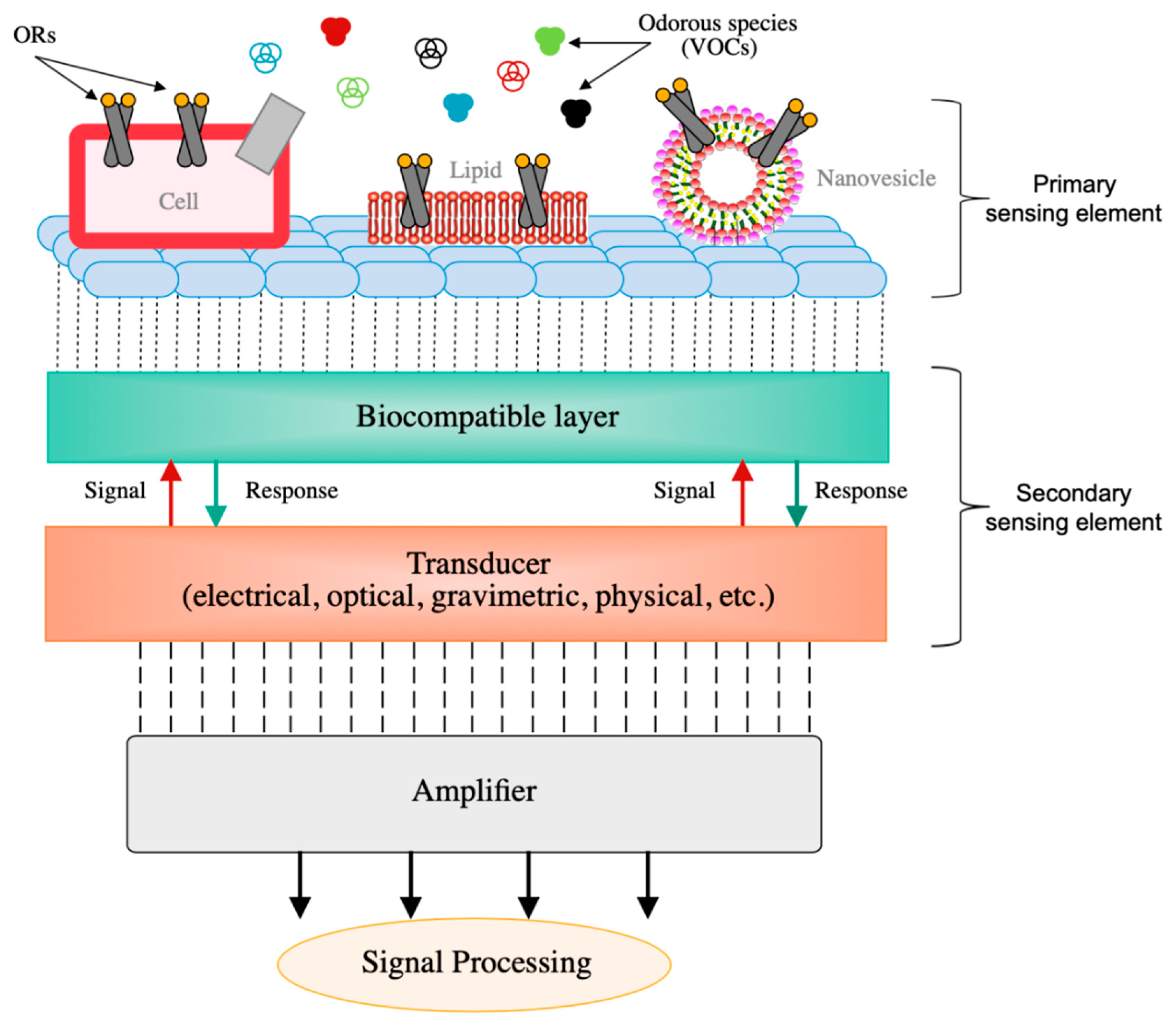
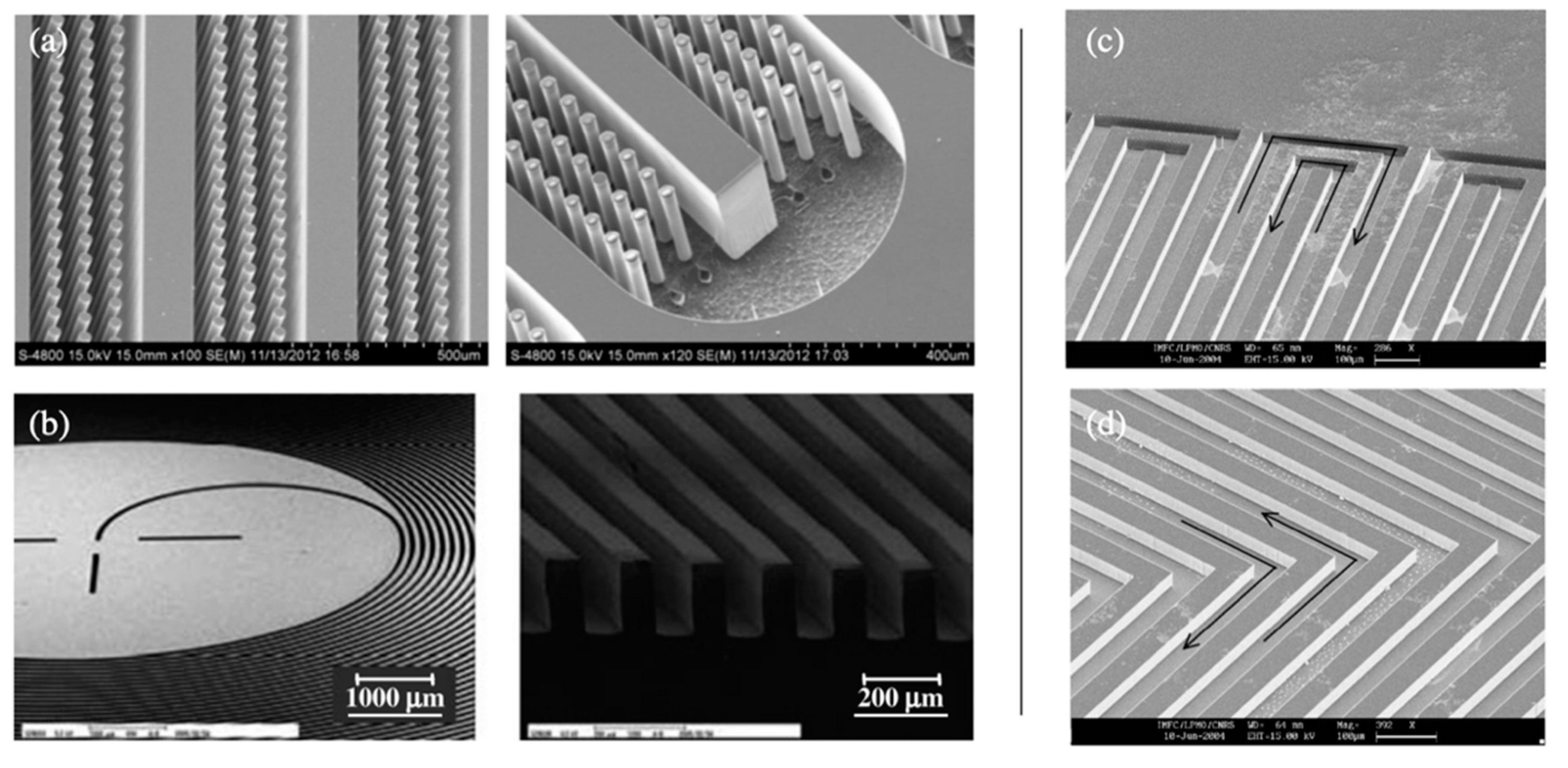
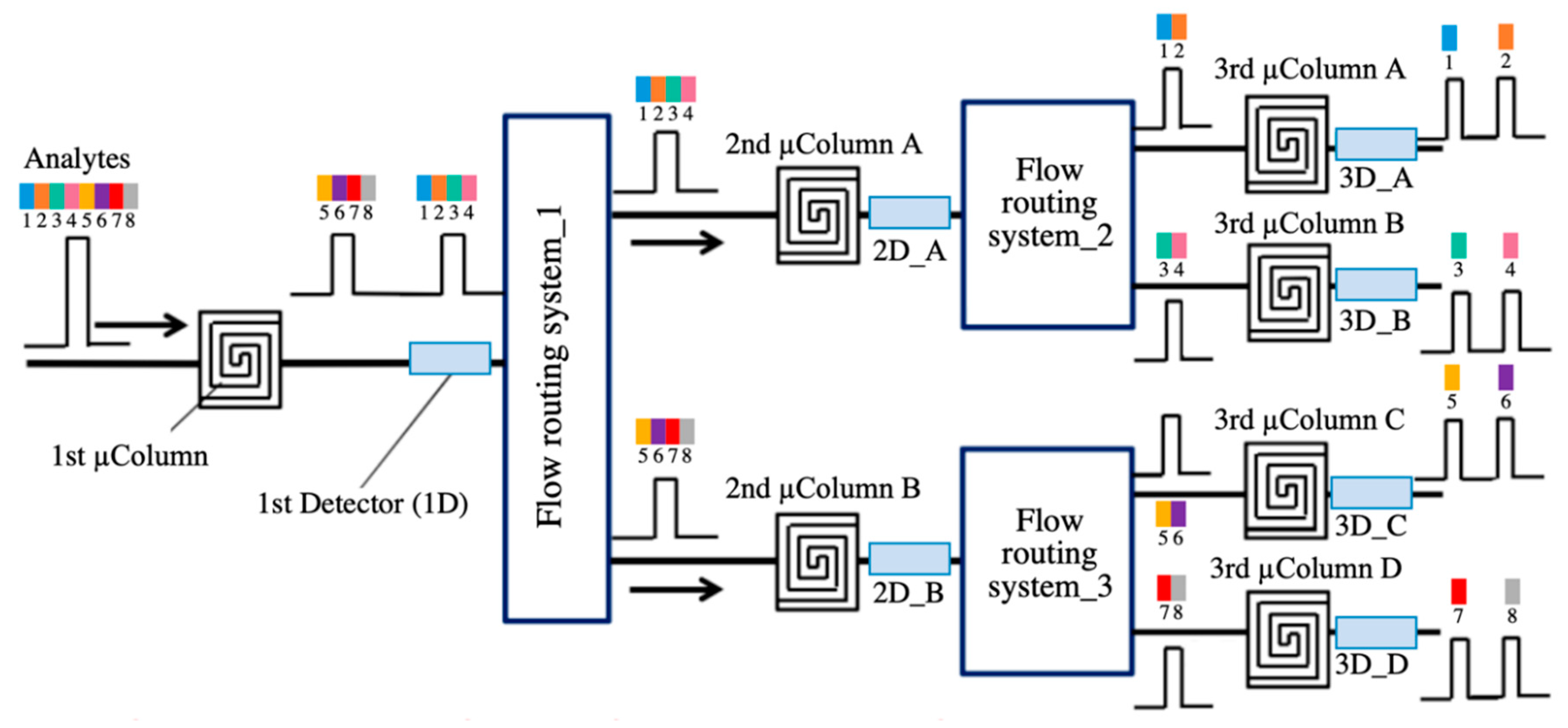
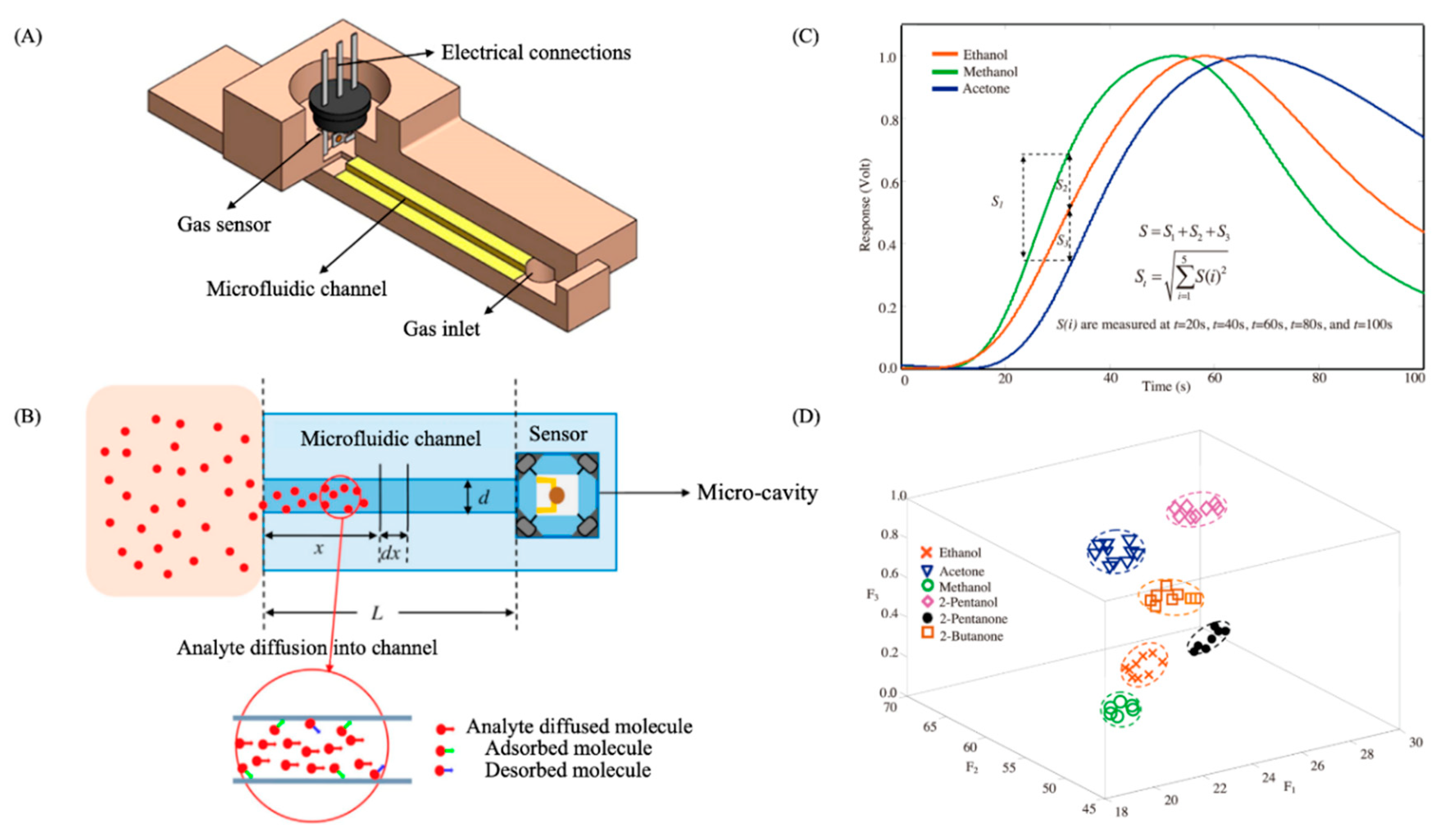
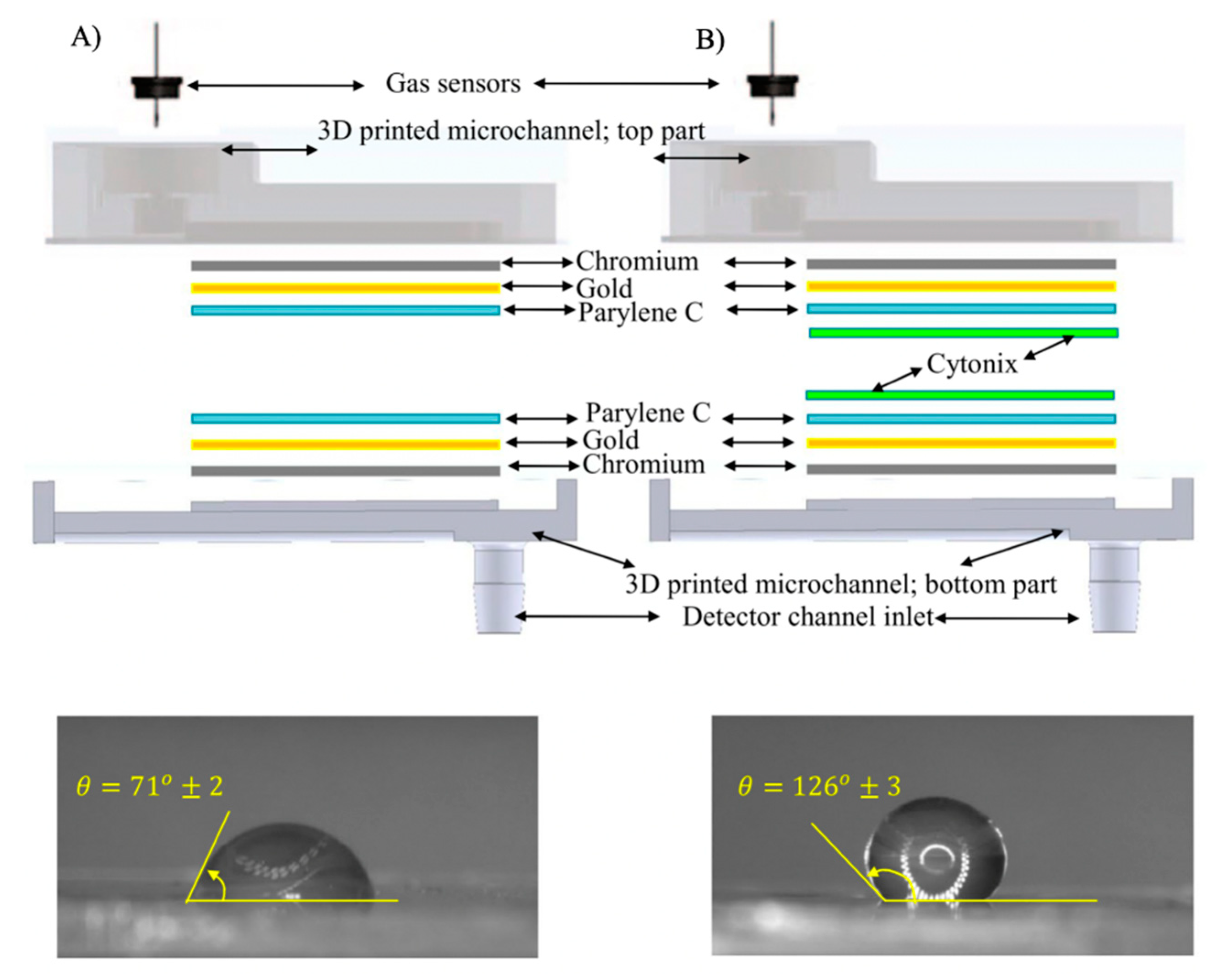
| Transduction Mechanism | Sensor Type | Dimensions | Active Layer | Sensitivity Range | LOD | Operating Conditions | Response Time | Manufacturing Techniques | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Traditional devices commercially available | |||||||||
| Optical | NDIR | L: 5–8.2 cm W: 3–5 cm H: 1.2–2 cm | – | 0–5.000 ppm | 2–20 ppm | 4.5–20 VDC | 20–120 s | – | [46] |
| Optical | PID (MiniPID 2) | Ø 20 mm C.V: 15 µL | – | 0–40 ppm | 1 ppb | 10.6 eV lamp 3–3.6 VDC | 8 s | – | [204] |
| Acoustic | SAW | L: 4.0 mm W: 1.0 mm H: 0.5 mm | 6–30 nm (CNTs) | 10–180 ppm | 1–10 ppm | Room temp. fr = 433.92 MHz noise: 3 kHz | 2–4 min | – | [205] |
| Conductometric | Chemiresistor (MiCS-2714) | D: 5 mm × 7 mm H: 2.25 mm | MOS | 0.1–10 ppm | 50 ppb | High temp. (220 °C/50 mW) | 12 s | – | [206] |
| Potentiometric | MOSFET (Z-900) | 4.75 cm × 2.5 cm × 1.5 cm | MOS | 0–50 ppm | 0.1 ppm | High temp. 9 V battery power | <30 s | – | [207] |
| Microfabricated devices | |||||||||
| Optical | µPID | 2.4 mm × 2.4 mm C.V: 1.3 µL | – | 0–1 ppb | 2–8 ppt | 10.6 eV lamp 5–6 VDC | 0.1 s | – | [48] |
| Gravimetric | SAW | 20 mm × 20 mm | 53.91 nm (CuO) | 0–50 ppm | 500 ppb | Room temp. fr = 198.98 MHz noise: <300 Hz | 10–90 s | Sol–gel | [66] |
| Gravimetric | CMUT | 4 mm × 1.5 mm Øe: 5.3 µm | 50 nm (Polymer) | 10–100 ppb | 51 ppt | Room temp. fr = 47.7 MHz noise: <2 Hz | <120 s | Direct wafer-bonding + local oxidation | [74] |
| Amperometric | RTILs | Øe: 1 mm VRTILs: 2–8 µL | 150 nm (Pt-TFEs) | 0.1–2 ppm | 20–110 ppb | Room temp. LSV (100 mV·s−1) | – | Electrodeposition | [90] |
| Conductometric | MOS-chemiresistor | 13.4 mm × 7 mm (Ag-Pd IDEs) | Nanobricks (In2O3) | 0.1–1 ppm | <100 ppb | Low temp. (50 °C) | 114 s | Electrochemical anodization | [125] |
| Potentiometric | Polymer-FET | Au-elec. (30 nm; 20 µm × 1 mm) | 20 nm (OSC-film) | 1–25 ppm | 1 ppb | Room temp. RH (45–70%) | 5 s | Dip coating | [99] |
| Potentiometric | Bioelectronic-FET | – | 12–15 nm (ORs + CNTs) | 10 ppt–1 ppb | 10 ppt | Room temp. | Real time (<5 s) | Photolithography | [180] |
| Feature | Conventional GC Columns [212,214] | µGC Columns [229,240] | Microfluidic Channels [253,261,262] |
|---|---|---|---|
| Typology | Capillary | Capillary Chip based | Chip based |
| Geometry 1 | 10–100 m (L) 0.18–0.53 mm () | 1–3 m (L) 50–500 µm (W) 50–800 µm (H) | 1–5 cm (L) 2–4 mm (W) 50–500 µm (H) |
| Selectivity 2 | L ; | L ; H/W | L ; W/H |
| Layout | Circular spiral | Serpentine Circular spiral Square spiral Wavy Zigzag Radiator | Straight |
| Cross section | Circular | Square Trapezoidal Semicircular Circular | Square Circular |
| Coating materials | Polymeric films (PDMS) Inorganic sorbents (silica, Al2O3) | Polymeric films (PDMS) Carbon–polymer hybrids Inorganic sorbents (silica, Al2O3) | Polymeric films (Parylene) Pure metals (Au) Metal oxides (ZnO) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ollé, E.P.; Farré-Lladós, J.; Casals-Terré, J. Advancements in Microfabricated Gas Sensors and Microanalytical Tools for the Sensitive and Selective Detection of Odors. Sensors 2020, 20, 5478. https://doi.org/10.3390/s20195478
Ollé EP, Farré-Lladós J, Casals-Terré J. Advancements in Microfabricated Gas Sensors and Microanalytical Tools for the Sensitive and Selective Detection of Odors. Sensors. 2020; 20(19):5478. https://doi.org/10.3390/s20195478
Chicago/Turabian StyleOllé, Enric Perarnau, Josep Farré-Lladós, and Jasmina Casals-Terré. 2020. "Advancements in Microfabricated Gas Sensors and Microanalytical Tools for the Sensitive and Selective Detection of Odors" Sensors 20, no. 19: 5478. https://doi.org/10.3390/s20195478
APA StyleOllé, E. P., Farré-Lladós, J., & Casals-Terré, J. (2020). Advancements in Microfabricated Gas Sensors and Microanalytical Tools for the Sensitive and Selective Detection of Odors. Sensors, 20(19), 5478. https://doi.org/10.3390/s20195478






