Instrumental Assessment of Stepping in Place Captures Clinically Relevant Motor Symptoms of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Clinical Testing
2.2. Stepping in Place
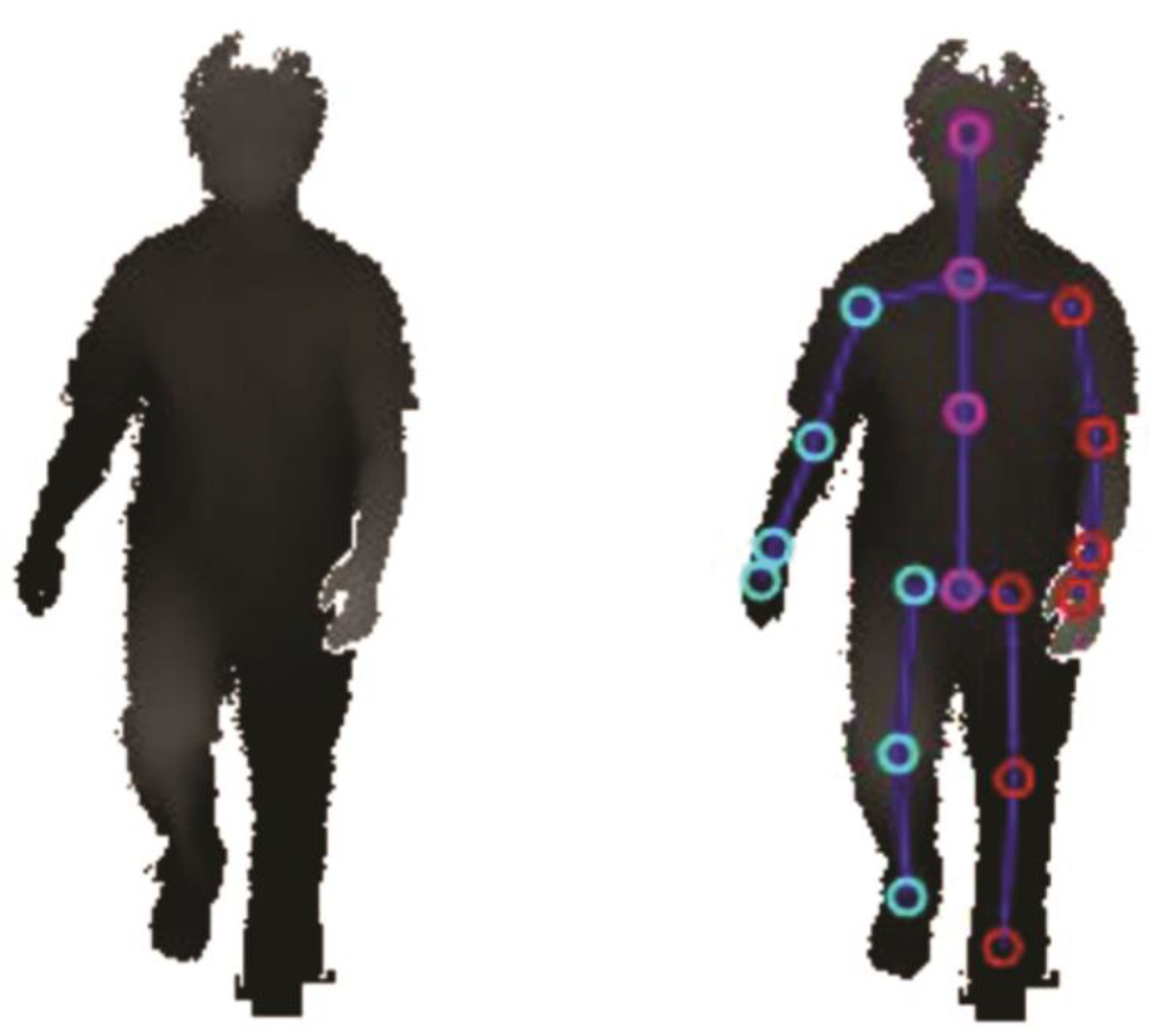
2.3. Technical Setup
2.4. Data Processing and Calculation of Kinematic Parameters
- To compensate for the subject’s position changes in the measurement area, we used the 3D positions of each knee as time series in relative position to the relating hip position. This eliminates possible errors due to the tendency to move towards the sensor.
- A median filter (window size 5 frames) was applied to smoothen the anterior–posterior knee movement signal and reduce noise.
- All minima of the filtered signal were detected and interpolated linearly, creating a minima-signal to provide a base level of minor landmark shifts over time caused by changes in the detected 3D user mask.
- The minima-signal was subtracted from the anterior–posterior knee movement signal to eliminate smaller measurement errors when the knees were straight.
- A threshold of 2.5 cm for anterior–posterior knee amplitude was defined as suitable to differentiate between step (>2.5 cm) and stance (<2.5 cm) phase. The threshold was identified by visual inspection of recordings.
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics and Analysis of Potential Confounding Effects
3.2. Relation of SIP Parameters to Disease Severity and Postural Instability
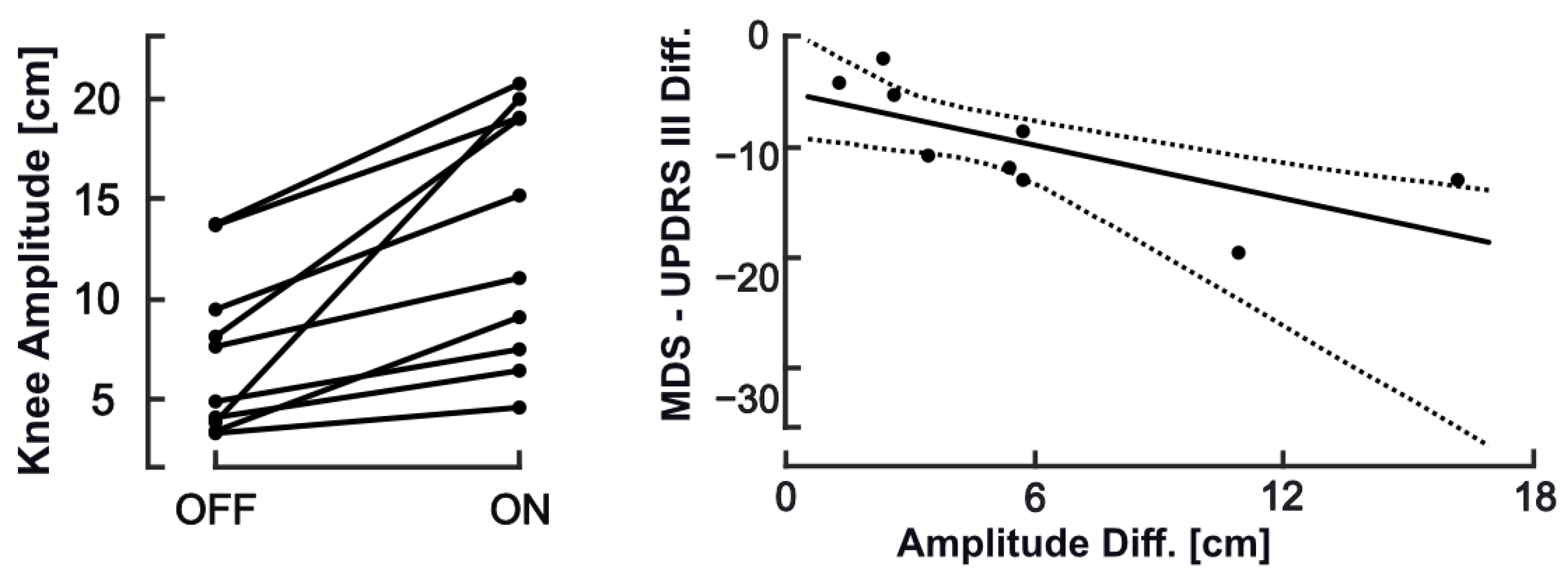
3.3. Comparison between Recordings Taken in ON vs. OFF States
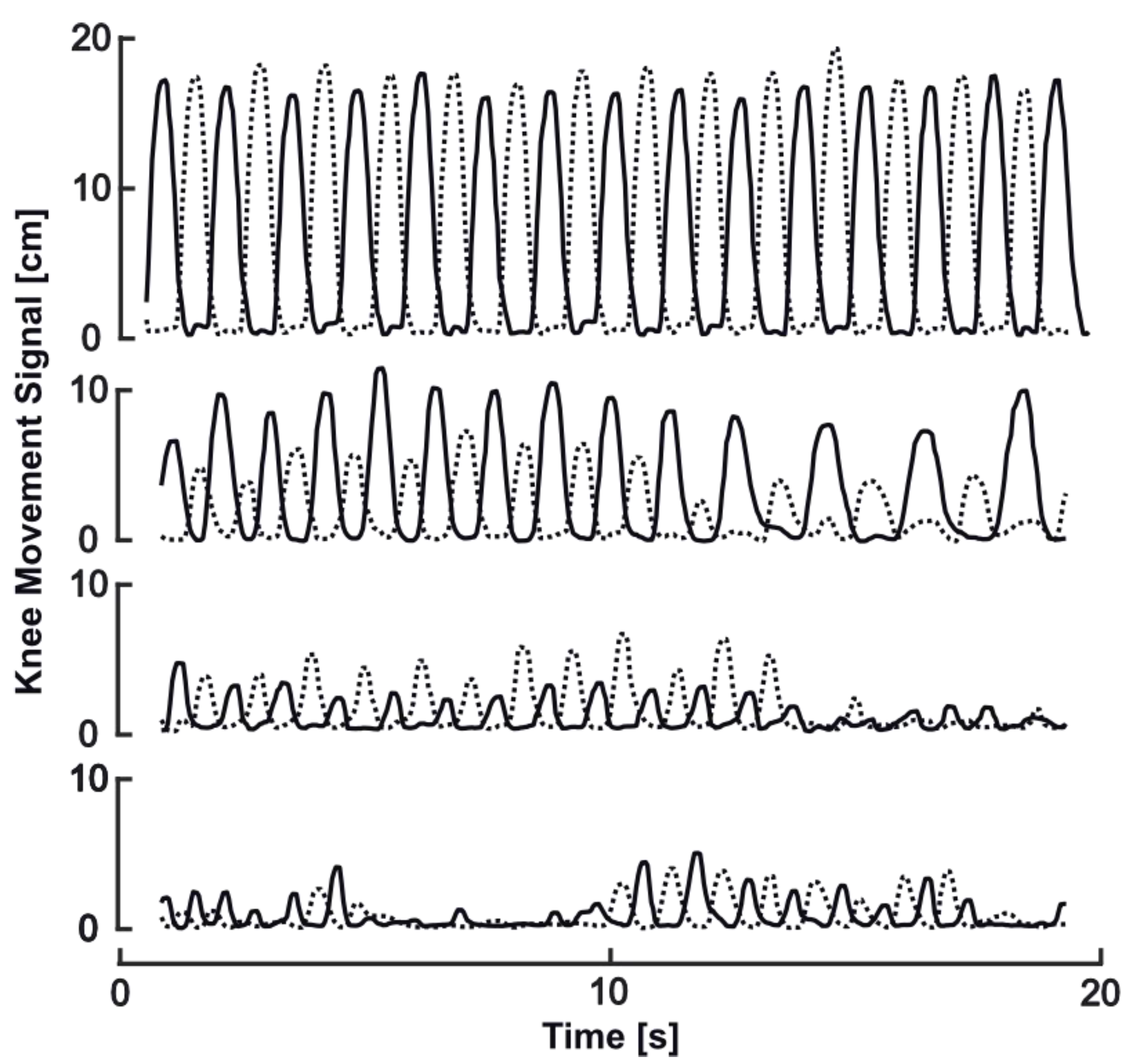
3.4. Implications of FOG and Other Motor Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Treves, T.A.; Simon, E.S.; Shabtai, H.; Orlov, Y.; Kandinov, B.; Paleacu, D.; Korczyn, A.D. Freezing of gait in patients with advanced Parkinson’s disease. J. Neural Transm. 2001, 108, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Martens, K.A.E.; Lukasik, E.L.; Georgiades, M.J.; Gilat, M.; Hall, J.; Walton, C.C.; Lewis, S.J. Predicting the onset of freezing of gait: A longitudinal study. Mov. Disord. 2018, 33, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Rochester, L.; Herman, T.; Vandenberghe, W.; Emil, G.E.; Thomaes, T.; Giladi, N. Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson’s disease and their carers. Gait Posture 2009, 30, 459–463. [Google Scholar] [CrossRef]
- Mancini, M.; Bloem, B.R.; Horak, F.B.; Lewis, S.J.G.; Nieuwboer, A.; Nonnekes, J. Clinical and methodological challenges for assessing freezing of gait: Future perspectives. Mov. Disord. 2019, 34, 783–790. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Ravina, B.; Seppi, K.; Coelho, M.; Poewe, W.; Rascol, O.; Goetz, C.G.; Sampaio, C. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2011, 26. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D. The New Diagnostic Criteria for Parkinson’s Disease, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Jeukens-Visser, M.; Lyons, K.E.; Rodriguez-Blazquez, C.; Selai, C.; Siderowf, A.; Welsh, M.; Poewe, W.; Rascol, O.; Sampaio, C.; et al. Health-related quality-of-life scales in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2011, 26, 2371–2380. [Google Scholar] [CrossRef]
- Jankovic, J. Motor fluctuations and dyskinesias in Parkinson’s disease: Clinical manifestations. Mov. Disord. 2005, 20 (Suppl. S11), S11–S16. [Google Scholar] [CrossRef]
- Buckley, C.; Alcock, L.; McArdle, R.; Rehman, R.Z.U.; Del Din, S.; Mazzà, C.; Yarnall, A.J.; Rochester, L. The role of movement analysis in diagnosing and monitoring neurodegenerative conditions: Insights from gait and postural control. Brain Sci. 2019, 9, 34. [Google Scholar] [CrossRef]
- Sánchez-Ferro, Á.; Elshehabi, M.; Godinho, C.; Salkovic, D.; Hobert, M.A.; Domingos, J.; van Uem, J.M.; Ferreira, J.J.; Maetzler, W. New methods for the assessment of Parkinson’s disease (2005 to 2015): A systematic review. Mov. Disord. 2016, 31, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Bonato, P.; Nahab, F.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson disease: Challenges and Opportunities on behalf of the MDS Taskforce on Technology HHS Public Access Author manuscript. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Maetzler, W.; Klucken, J.; Horne, M. A clinical view on the development of technology-based tools in managing Parkinson’s disease. Mov. Disord. 2016, 31, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Godinho, C.; Domingos, J.; Cunha, G.V.; Santos, A.T.; Fernandes, R.M.; Abreu, D.; Gonçalves, N.; Matthews, H.; Isaacs, T.; Duffen, J.; et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J. Neuroeng. Rehabil. 2016, 13, 24. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Mazzà, C.; Lord, S.; Rochester, L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov. Disord. 2016, 31, 1293–1313. [Google Scholar] [CrossRef]
- Maetzler, W.; Domingos, J.; Srulijes, K.; Ferreira, J.J.; Bloem, B.R. Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov. Disord. 2013, 28, 1628–1637. [Google Scholar] [CrossRef]
- Galna, B.; Jackson, D.; Schofield, G.; McNaney, R.; Webster, M.; Barry, G.; Mhiripiri, D.; Balaam, M.; Olivier, P.; Rochester, L. Retraining function in people with Parkinson’s disease using the Microsoft kinect: Game design and pilot testing. J. Neuroeng. Rehabil. 2014, 11, 60. [Google Scholar] [CrossRef]
- Behrens, J.; Pfüller, C.; Mansow-Model, S.; Otte, K.; Paul, F.; Brandt, A.U. Using perceptive computing in multiple sclerosis—The Short Maximum Speed Walk test. J. Neuroeng. Rehabil. 2014, 11, 89. [Google Scholar] [CrossRef]
- Ilg, W.; Schatton, C.; Schicks, J.; Giese, M.A.; Schöls, L.; Synofzik, M. Video game-based coordinative training improves ataxia in children with degenerative ataxia. Neurology 2012, 79, 2056–2060. [Google Scholar] [CrossRef]
- Otte, K.; Kayser, B.; Mansow-Model, S.; Verrel, J.; Paul, F.; Brandt, A.U.; Schmitz-Hübsch, T. Accuracy and reliability of the kinect version 2 for clinical measurement of motor function. PLoS ONE 2016, 11, e0166532. [Google Scholar] [CrossRef]
- Fukuda, T. The stepping test. Acta Oto-Laryngol. 1959, 50, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.K.; Nelson, A.J.; Ling, W.; Van Olden, C. Comparing stepping-in-place and gait ability in adults with and without hemiplegia. Arch. Phys. Med. Rehabil. 2001, 82, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nantel, J.; de Solages, C.; Bronte-Stewart, H. Repetitive stepping in place identifies and measures freezing episodes in subjects with Parkinson’s disease. Gait Posture 2011, 34, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Van DIjsseldonk, K.; Wang, Y.; Van Wezel, R.; Bloem, B.R.; Nonnekes, J. Provoking freezing of gait in clinical practice: Turning in place is more effective than stepping in place. J. Parkinson’s Dis. 2018, 8, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Giladi, N.; Balash, Y.; Peretz, C.; Hausdorff, J.M. Is freezing of gait in Parkinson’s disease related to asymmetric motor function? Ann. Neurol. 2005, 57, 656–663. [Google Scholar] [CrossRef]
- Dalton, C.; Sciadas, R.; Nantel, J. Executive function is necessary for the regulation of the stepping activity when stepping in place in older adults. Aging Clin. Exp. Res. 2016, 28, 909–915. [Google Scholar] [CrossRef][Green Version]
- Mentiplay, B.; Perraton, L.G.; Bower, K.J.; Pua, Y.-H.; McGaw, R.; Heywood, S.; Clark, R.A. Gait assessment using the Microsoft Xbox One Kinect: Concurrent validity and inter-day reliability of spatiotemporal and kinematic variables. J. Biomech. 2015, 48, 2166–2170. [Google Scholar] [CrossRef]
- Steinert, A.; Sattler, I.; Otte, K.; Röhling, H.; Mansow-Model, S.; Müller-Werdan, U. Using new camera-based technologies for gait analysis in older adults in comparison to the established GAITrite system. Sensors 2020, 20, 125. [Google Scholar] [CrossRef]
- Morris, R.; Lord, S.; Lawson, R.A.; Coleman, S.; Galna, B.; Duncan, G.W.; Khoo, T.K.; Yarnall, A.J.; Burn, D.J.; Rochester, L. Gait Rather Than Cognition Predicts Decline in Specific Cognitive Domains in Early Parkinson’s Disease. J. Gerontol. A Biol. Sci. Med Sci. 2017, 72, 1656–1662. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent domains of gait in older adults and associated motor and nonmotor attributes: Validation of a factor analysis approach. J. Gerontol. A Biol. Sci. Med Sci. 2013, 68, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Hausdorff, J.M. The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease. Mov. Disord. 2008, 23 (Suppl. S2), 444–450. [Google Scholar] [CrossRef] [PubMed]
- Santanna, A.; Salarian, A.; Wickstrom, N. A new measure of movement symmetry in early parkinsons disease patients using symbolic processing of inertial sensor data. IEEE Trans. Biomed. Eng. 2011, 58, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Galna, B.; Rochester, L. Moving forward on gait measurement: Toward a more refined approach. Mov. Disord. 2013, 28, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Galna, B.; Coleman, S.; Yarnall, A.; Burn, D.; Rochester, L. Cognition and gait show a selective pattern of association dominated by phenotype in incident Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 6. [Google Scholar] [CrossRef]
- Lord, S.; Baker, K.; Nieuwboer, A.; Burn, D.; Rochester, L. Gait variability in Parkinson’s disease: An indicator of non-dopaminergic contributors to gait dysfunction? J. Neurol. 2011, 258, 566–572. [Google Scholar] [CrossRef]
- Kroneberg, D.; Elshehabi, M.; Meyer, A.-C.; Otte, K.; Doss, S.; Paul, F.; Nussbaum, S.; Berg, D.; Kühn, A.A.; Maetzler, W.; et al. Less is more—Estimation of the number of strides required to assess gait variability in spatially confined settings. Front. Aging Neurosci. 2019, 11, 435. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Earhart, G.M.; McNeely, M.E. Can postural instability tests improve the prediction of future falls in people with Parkinson’s disease beyond knowing existing fall history? J. Neurol. 2016, 263, 133–139. [Google Scholar] [CrossRef]
- Ziegler, K.; Schroeteler, F.; Ceballos-Baumann, A.O.; Fietzek, U.M. A new rating instrument to assess festination and freezing gait in Parkinsonian patients. Mov. Disord. 2010, 25, 1012–1018. [Google Scholar] [CrossRef]
- Nieuwboer, A.; Giladi, N. Characterizing freezing of gait in Parkinson’s disease: Models of an episodic phenomenon. Mov. Disord. 2013, 28, 1509–1519. [Google Scholar] [CrossRef]
- Pintér, D.; Martinez-Martin, P.; Janszky, J.; Kovács, N. The Parkinson’s Disease Composite Scale Is Adequately Responsive to Acute Levodopa Challenge. Parkinsons Dis. 2019, 2019, 1412984. [Google Scholar] [CrossRef] [PubMed]
- Horváth, K.; Aschermann, Z.; Acs, P.; Deli, G.; Janszky, J.; Komoly, S.; Balázs, É.; Takacs, K.; Karádi, K.; Kovács, N. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism Relat. Disord. 2015, 21, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Yogev, G.; Plotnik, M.; Peretz, C.; Giladi, N.; Hausdorff, J.M. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: When does the bilateral coordination of gait require attention? Exp. Brain Res. 2007, 177, 336–346. [Google Scholar] [CrossRef] [PubMed]


| ALL | ON | OFF | ON-OFF | |
|---|---|---|---|---|
| N subjects | 25 | 20 | 13 | 10 |
| male | 18 | 15 | 8 | 6 |
| female | 7 | 5 | 5 | 4 |
| Age (years) | 65.3 (±9.4) | 65.5 (±11.05) | 66.2 (±8.0) | 65.3 (±8.7) |
| Weight (kg) | 75.0 (±13.5) | 74.1 (±13.5) | 76.3 (±12.5) | 76.2 (±13.8) |
| Height (cm) | 168.4 (±6.8) | 167.7 (±6.1) | 170.4 (±7.8) | 168.4 (±5.3) |
| Disease Duration (years) | 12.8 (±8.1) | 12.1 (±8.0) | 11.6 (±6.6) | 10.1 (±7.2) |
| MDS-UPDRS-III | 28.3 (±14.7) | 25.3 (±13.7) | 34.9 (±15.1) | ON: 28.8 (±13.4) |
| OFF: 37.2 (±14.5) | ||||
| N-item 11 (FOG) | 23/4/6/0/0 | 14/3/3/0/0 | 9/1/3/0/0 | ON: 8/2/0/0/0 |
| 0/1/2/3/4 | OFF: 7/3/0/0/0 | |||
| N-item 12 (Pull test) | 12/10/6/1/1 | 8/4/5/2/0 | 4/6/1/1/1 | ON: 4/3/2/1/0 |
| 0/1/2/3/4 | OFF: 1/6/1/1/1 |
| Parameter Name | Unit | Description |
|---|---|---|
| Cadence | Steps/min | Steps per minute |
| Knee Amplitude | cm | Anterior–posterior range of motion of knees |
| Asymmetry | % | Logarithmic ratio between knee amplitudes of larger side to smaller side |
| Average Step Time | s | Average time required for a step during the measurement |
| Longest Step Time | s | Maximal time required for a step during the measurement |
| Arrhythmicity | % | Ratio between standard deviation and average of the step time |
| Average Stance Time | s | Average time between step movements |
| Longest Stance Time | s | Maximal time between step movements |
| Descriptive Statistics | Confounder Analysis in ON (n = 20) | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | Pearson’s Correlation Coefficient r (p-Value) | |||||
| ALL (n = 33) | ON (n = 20) | OFF (n = 13) | Age | Height | Weight | |
| Cadence (steps/min) | 97.6 (27.3) | 96.6 (27.1) | 99.2 (28.6) | −0.271 (0.247) | 0.132 (0.682) | −0.358 (0.253) |
| Knee Amplitude (cm) | 12.5 (7.4) | 13.9 (5.5) | 10.2 (9.3) | 0.228 (0.334) | 0.151 (0.640) | 0.280 (0.378) |
| Asymmetry (%) | 18.2 (19.9) | 15.6 (14.1) | 22.1 (26.8) | −0.133 (0.577) | 0.066 (0.839) | −0.173 (0.591) |
| Average Step Time (s) | 0.72 (0.21) | 0.77 (0.21) | 0.64 (0.19) | −0.114 (0.632) | −0.108 (0.739) | −0.215 (0.503) |
| Longest Step Time (s) | 0.88 (0.24) | 0.93 (0.23) | 0.80 (0.26) | 0.209 (0.376) | −0.038 (0.906) | 0.259 (0.417) |
| Arrhythmicity (%) | 11.6 (5.58) | 11.3 (6.5) | 12.2 (4.0) | 0.264 (0.261) | 0.118 (0.715) | 0.350 (0.265) |
| Average Stance Time (s) | 0.65 (0.60) | 0.61 (0.60) | 0.72 (0.61) | 0.258 (0.272) | 0.108 (0.739) | 0.348 (0.267) |
| Longest Stance Time (s) | 1.69 (2.39) | 1.63 (2.75) | 1.77 (1.77) | −0.271 (0.247) | 0.132 (0.682) | −0.358 (0.253) |
| Spear. Corr. MDS-UPDRS III | Spear. Corr. Pull Test | |
|---|---|---|
| Rho (p-Value) | Rho (p-Value) | |
| Cadence (steps/min) | −0.234 (0.189) | −0.328 (0.072) |
| Knee Amplitude (cm) | −0.507 (0.003) | −0.436 (0.014) * |
| Asymmetry (%) | 0.202 (0.260) | 0.170 (0.361) |
| Average Step Time (s) | −0.287 (0.105) | −0.274 (0.136) |
| Longest Step Time (s) | −0.291 (0.101) | −0.242 (0.191) |
| Arrhythmicity (%) | 0.352 (0.045) * | 0.452 (0.011) * |
| Average Stance Time (s) | 0.374 (0.032) * | 0.374 (0.038) * |
| Longest Stance Time (s) | 0.523 (0.002) | 0.468 (0.008) |
| Mean (SD) OFF | Mean (SD) ON | Diff Abs. | Diff [%] | SRM | Paired t-Test p-Value | |
|---|---|---|---|---|---|---|
| MDS-UPDRS III | 37.2 (14.53) | 28.8 (13.37) | 10.64 | −28.6 | 1.69 | <0.001 |
| Cadence (steps/min) | 96.6 (29.0) | 96.9 (20.7) | 0.36 | 0.4 | −0.02 | 0.954 |
| Knee Amplitude (cm) | 7.08 (4.0) | 13.1 (6.2) | 6.05 | 85.4 | −1.34 | 0.002 |
| Asymmetry (%) | 21.9 (27.6) | 17.7 (18.3) | −4.30 | −19.6 | 0.14 | 0.007 |
| Average Step Time (s) | 0.61 (0.17) | 0.71 (0.14) | 0.09 | 14.5 | −1.09 | 0.007 |
| Longest Step Time (s) | 0.76 (0.21) | 0.81 (0.14) | 0.05 | 7.2 | −0.35 | 0.298 |
| Arrhythmicity (%) | 11.9 (3.50) | 8.46 (3.93) | −3.49 | −29.3 | 0.84 | 0.025 |
| Average Stance Time (s) | 0.80 (0.67) | 0.62 (0.51) | −0.18 | −23.0 | 0.55 | 0.114 |
| Longest Stance Time (s) | 1.88 (1.87) | 3.67 (8.04) | 1.79 | 94.6 | −0.27 | 0.423 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otte, K.; Ellermeyer, T.; Vater, T.-S.; Voigt, M.; Kroneberg, D.; Rasche, L.; Krüger, T.; Röhling, H.M.; Kayser, B.; Mansow-Model, S.; et al. Instrumental Assessment of Stepping in Place Captures Clinically Relevant Motor Symptoms of Parkinson’s Disease. Sensors 2020, 20, 5465. https://doi.org/10.3390/s20195465
Otte K, Ellermeyer T, Vater T-S, Voigt M, Kroneberg D, Rasche L, Krüger T, Röhling HM, Kayser B, Mansow-Model S, et al. Instrumental Assessment of Stepping in Place Captures Clinically Relevant Motor Symptoms of Parkinson’s Disease. Sensors. 2020; 20(19):5465. https://doi.org/10.3390/s20195465
Chicago/Turabian StyleOtte, Karen, Tobias Ellermeyer, Tim-Sebastian Vater, Marlen Voigt, Daniel Kroneberg, Ludwig Rasche, Theresa Krüger, Hanna Maria Röhling, Bastian Kayser, Sebastian Mansow-Model, and et al. 2020. "Instrumental Assessment of Stepping in Place Captures Clinically Relevant Motor Symptoms of Parkinson’s Disease" Sensors 20, no. 19: 5465. https://doi.org/10.3390/s20195465
APA StyleOtte, K., Ellermeyer, T., Vater, T.-S., Voigt, M., Kroneberg, D., Rasche, L., Krüger, T., Röhling, H. M., Kayser, B., Mansow-Model, S., Klostermann, F., Brandt, A. U., Paul, F., Lipp, A., & Schmitz-Hübsch, T. (2020). Instrumental Assessment of Stepping in Place Captures Clinically Relevant Motor Symptoms of Parkinson’s Disease. Sensors, 20(19), 5465. https://doi.org/10.3390/s20195465






