Preparation of Hydrophobic Film by Electrospinning for Rapid SERS Detection of Trace Triazophos
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterizations
2.3. Fabrication of Poly(styrene-co-butadiene) Nanofiber
2.4. Synthesis of Silver Nanoparticles
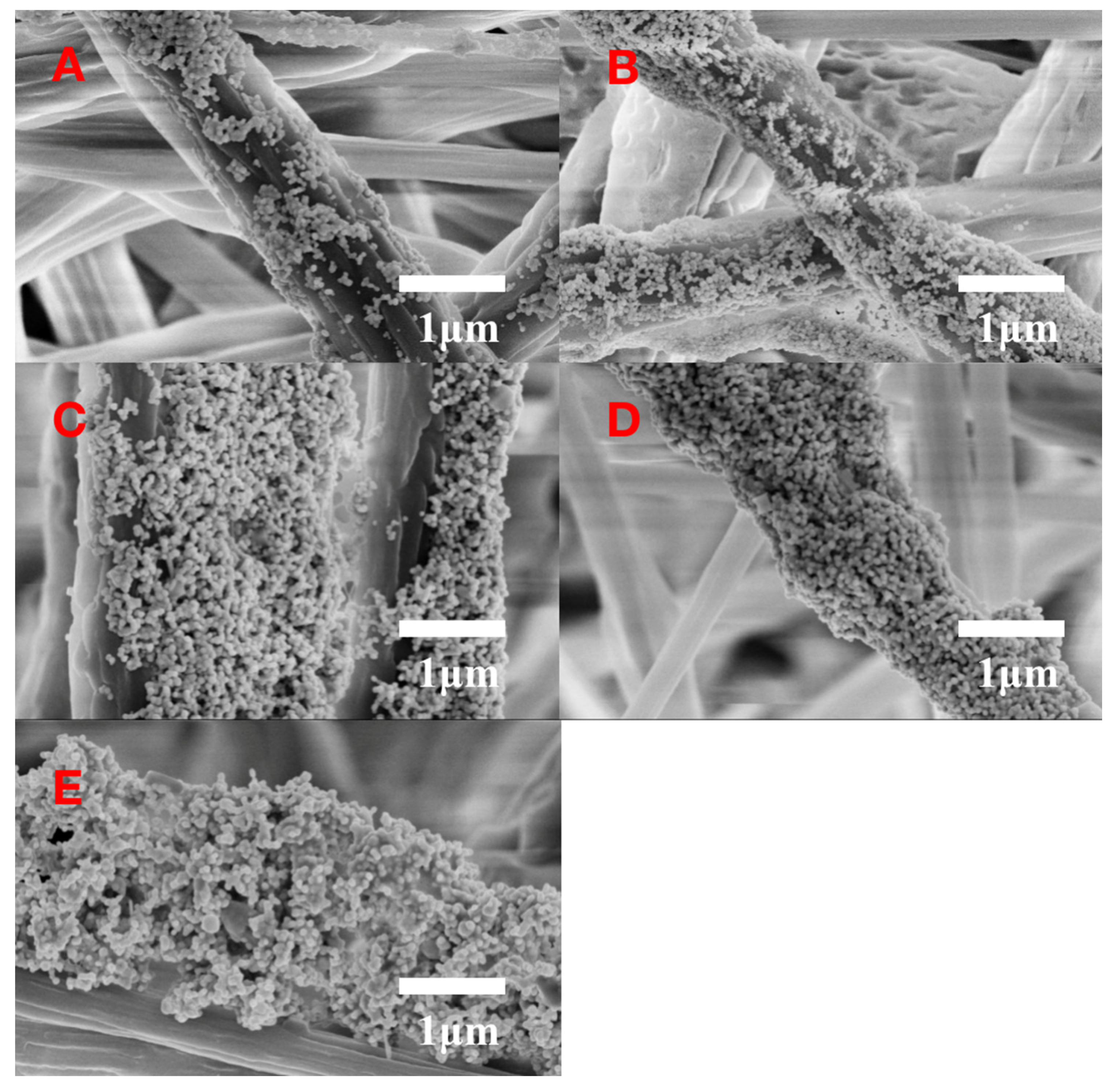
2.5. Fabrication of Ag NPs on the SB Substrate
2.6. SERS Analysis for Real Samples
3. Results and Discussion
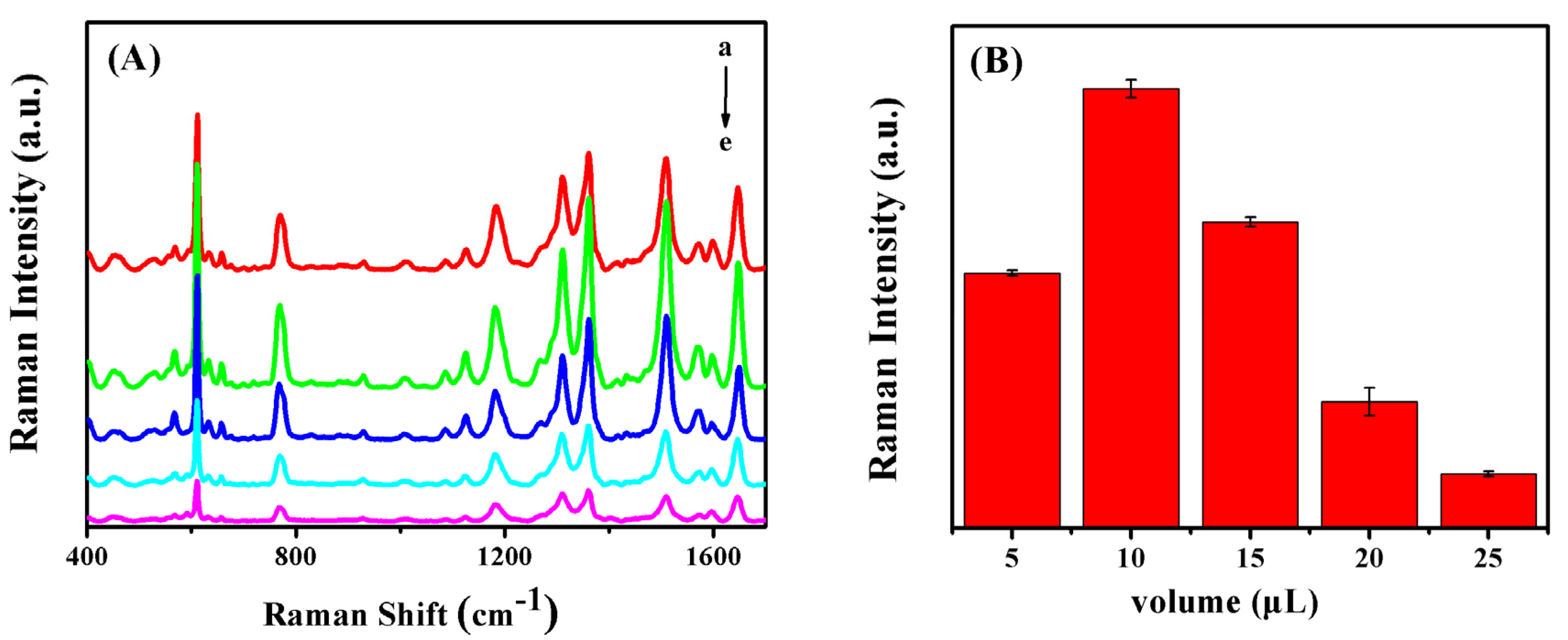
3.1. Optimization of Ag NPs Usage
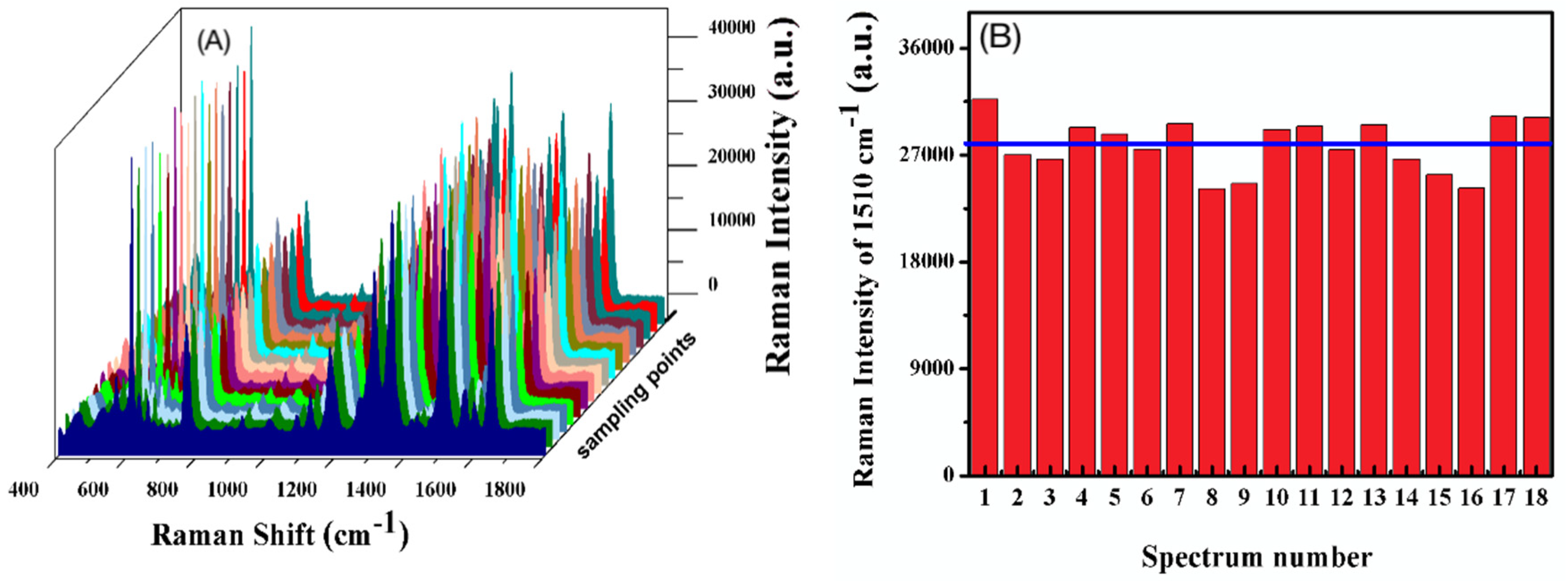
3.2. SERS Performance of Ag/SB Film
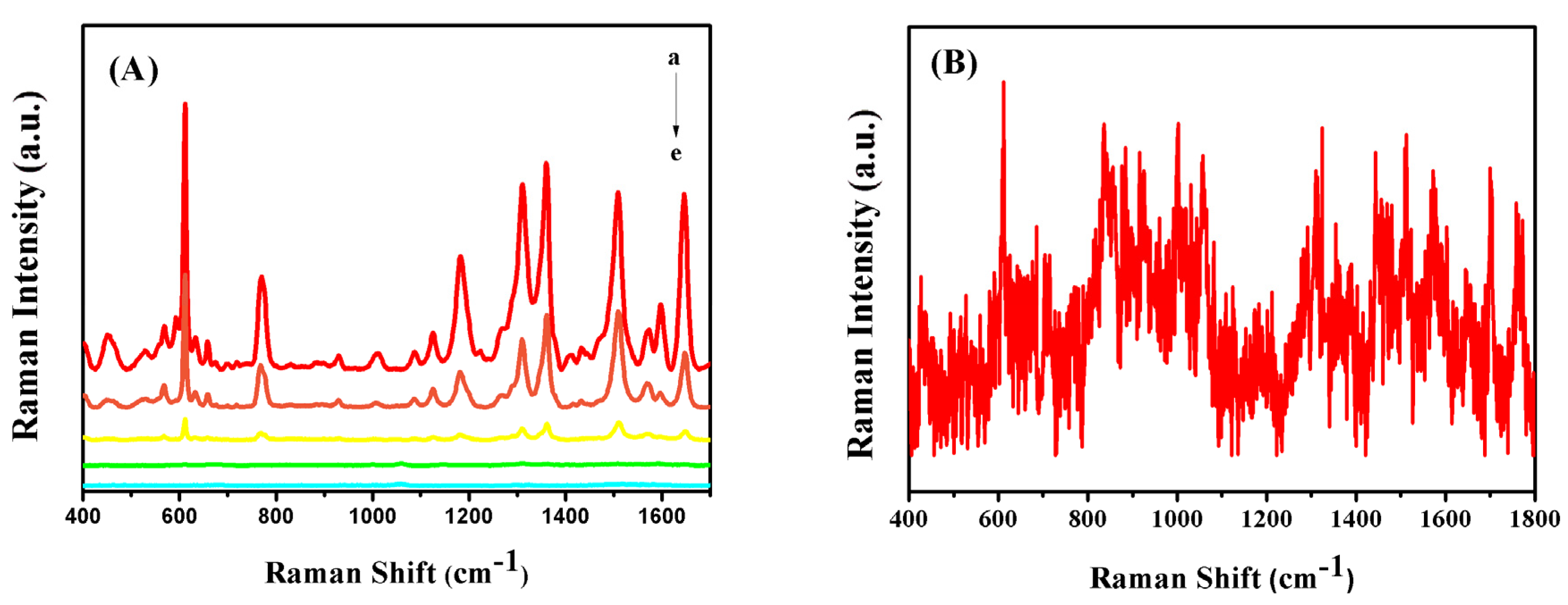
3.3. SERS Measurement of Triazophos
3.4. SERS Detection of Triazophos in/on Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halvorson, R.A.; Vikesland, P.J. Surface-Enhanced Raman Spectroscopy (SERS) for Environmental Analyses Environmental. Sci. Technol. 2010, 44, 7749–7755. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Premasiri, W.R.; Ziegler, L.D. Surface enhanced Raman spectroscopy of Chlamydia trachomatis and Neisseria gonorrhoeae for diagnostics, and extra-cellular metabolomics and biochemical monitoring. Sci. Rep. 2018, 8, 5163. [Google Scholar] [CrossRef]
- Liu, H.B.; Du, X.J.; Zang, Y.X.; Li, P.; Wang, S. SERS-Based Lateral Flow Strip Biosensor for Simultaneous Detection of Listeria monocytogenes and Salmonella enterica Serotype Enteritidis. Agric Food Chem. 2017, 65, 10290–10299. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lin, X.M.; Divan, R.; Jaeger, H.M. In-situ partial sintering of gold-nanoparticle sheets for SERS applications. Small 2011, 7, 3487–3492. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.W.; Pu, H.; Wei, Q. Ultrasensitive analysis of kanamycin residue in milk by SERS-based aptasensor. Talanta 2019, 197, 151–158. [Google Scholar] [CrossRef]
- Kahraman, M.; Balz, B.N.; Wachsmann-Hogiu, S. Hydrophobicity-driven self-assembly of proteinand silver nanoparticles for protein detection using surface-enhanced Raman scattering. Analyst 2013, 138, 2906–2913. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Pastoriza-Santos, I.; Rodriguez-Gonzalez, B.; Javier Garcia de Abajo, F.; Liz-Marzan, L.M. High-yield synthesis and optical response of gold nanostars. Nanotechnology 2008, 19, 15606. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhang, C.; Yuwen, L.; Chao, J.; Zuo, X.; Liu, X.; Song, C.; Fan, C.; Wang, L. Creating SERS hot spots on MoS2 nanosheets with in situ grown gold nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 18735–18741. [Google Scholar] [CrossRef]
- Rajapandiyan, P.; Yang, J. Sensitive cylindrical SERS substrate array for rapid microanalysis of nucleobases. Anal. Chem. 2012, 84, 10277–10282. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, F.; Guang, S.; Cai, Z. Low-cost and large-scale flexible SERS-cotton fabric as a wipe substrate for surface trace analysis. Appl. Surf. Sci. 2018, 436, 111–116. [Google Scholar] [CrossRef]
- Eshkeiti, A.; Narakathu, B.B.; Reddy, A.S.G.; Moorthi, A.; Atashbar, M.Z.; Rebrosova, E.; Rebros, M.; Joyce, M. Detection of heavy metal compounds using a novel inkjet printed surface enhanced Raman spectroscopy (SERS) substrate. Sens. Actuators B Chem. 2012, 171, 705–711. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, W.; Xu, L.; Wu, X.; Kuang, H.; Wang, L.; Xu, C. Ultrasensitive SERS detection of VEGF based on a self-assembled Ag ornamented-AU pyramid superstructure. Biosens. Bioelectron. 2015, 68, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Huang, Y.J.; Kannan, P.; Zhang, L.; Lin, Z.Y.; Zhang, J.W.; Chen, T.; Guo, L.H. Flexible and adhesive surface enhance Raman scattering active tape for rapid detection of pesticide residues in fruits and vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Politano, G.G.; Cazzanelli, E.; Versace, C.; Castriota, M.; Desiderio, G.; Davoli, M.; Vena, C.; Bartolino, R. Micro-Raman investigation of Ag/graphene oxide/Au sandwich structure. Mater. Res. Express 2019, 6, 75605. [Google Scholar] [CrossRef]
- He, R.X.; Liang, R.; Peng, P.; Zhou, Y.N. Effect of the size of silver nanoparticles on SERS signal enhancement. J. Nanoparticle Res. 2017, 19, 267. [Google Scholar] [CrossRef]
- Zhong, L.B.; Yin, J.; Zheng, Y.M.; Liu, Q.; Cheng, X.X.; Luo, F.H. Self-assembly of Au nanoparticles on PMMA template as flexible, transparent, and highly active SERS substrates. Anal. Chem. 2014, 86, 6262–6267. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Hou, C.; Huo, D.; Zhou, Y.; Zou, S.; Zhao, J.; Lei, Y. Flexible bipyramid-Au NPs based SERS tape sensing strategy for detecting methyl parathion on vegetable and fruit surface. Sens. Actuators B Chem. 2019, 28, 123–128. [Google Scholar] [CrossRef]
- Hoppmann, E.P.; Yu, W.W.; White, I.M. Highly sensitive and flexible inkjet printed SERS sensors on paper. Methods 2013, 63, 219–224. [Google Scholar] [CrossRef]
- Kong, X.M.; Reza, M.; Ma, Y.B.; Hinestroza, J.P.; Ahvenniemi, E.; Vuorinen, T. Assembly of metal nanoparticles on regenerated fibers from wood sawdust and de-inked pulp: Flexible substrates for surface enhanced Raman scattering (SERS) applications. Cellulose 2015, 22, 3645–3655. [Google Scholar] [CrossRef]
- Polavarapu, L.; Liz-Marzan, L.M. Towards low-cost flexible substrates for nanoplasmonic sensing. Phys. Chem. Chem. Phys. 2013, 15, 5288–5300. [Google Scholar] [CrossRef]
- Wu, W.; Liu, L.; Dai, Z.; Liu, J.; Yang, S.; Zhou, L.; Xiao, X.; Jiang, C.; Roy, V.A. Low-Cost, Disposable, Flexible and Highly Reproducible Screen Printed SERS Substrates for the Detection of Various Chemicals. Sci. Rep. 2015, 5, 10208. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Yang, S.R.; Hur, Y.H.; Kim, S.W.; Baek, K.M.; Yim, S.; Jang, H.I.; Park, J.H.; Lee, S.Y.; Park, C.O.; et al. High-resolution nanotransfer printing applicable to diverse surfaces via interface-targeted adhesion switching. Nat. Commun. 2014, 5, 5387. [Google Scholar] [CrossRef]
- Xu, L.L.; Zhang, H.; Tian, Y.; Jiao, A.X.; Li, S.; Tan, Y.; Chen, M.; Chen, F. Modified photochemical strategy to support highly-purity, dense and monodisperse Au nanospheres on graphene oxide for optimizing SERS detection. Talanta 2020, 209, 120535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Lv, K.P.; Cong, H.P.; Yu, S.H. Controlled assemblies of gold nanorods in PVA nanofiber matrix as flexible free-standing SERS substrates by electrospinning. Small 2012, 8, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Chamuah, N.; Bhuyan, N.; Das, P.P.; Ojah, N.; Choudhary, A.J.; Medhi, T.; Nath, P. Gold-coated electrospun PVA nanofibers as SERS substrate for detection of pesticides. Sens. Actuators B Chem. 2018, 273, 710–717. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, L.; Yan, Z.; Bai, L. Plasma-treated electrospun nanofibers as a template for the electrostatic assembly of silver nanoparticles. New J. Chem. 2018, 42, 11185–11191. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Fabrication of silver nanoparticles embedded into polyvinyl alcohol (Ag/PVA) composite nanofibrous films through electrospinning for antibacterial and surface-enhanced Raman scattering (SERS) activities. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 69, 462–469. [Google Scholar] [CrossRef]
- Li, X.; Cao, M.; Zhang, H.; Zhou, L.; Cheng, S.; Yao, J.L.; Fan, L.J. Surface-enhanced Raman scattering-active substrates of electrospun polyvinyl alcohol/gold-silver nanofibers. J. Colloid Interface Sci. 2012, 382, 28–35. [Google Scholar] [CrossRef]
- Zhang, C.L.; Lv, K.P.; Huang, H.T.; Cong, H.P.; Yu, S.H. Co-assembly of Au nanorods with Ag nanowires within polymer nanofiber matrix for enhanced SERS property by electrospinning. Nanoscale 2012, 4, 5348–5355. [Google Scholar] [CrossRef]
- Wu, H.; Lin, D.; Pan, W. High performance surface-enhanced Raman scattering substrate combining low dimensional and hierarchical nanostructures. Langmuir 2010, 26, 6865–6868. [Google Scholar] [CrossRef]
- Xu, S.; Sabino, F.P.; Janotti, A.; Chase, D.B.; Sparks, D.L.; Rabolt, J.F. Unique Surface Enhanced Raman Scattering Substrate for the Study of Arsenic Speciation and Detection. J. Phys. Chem. A 2018, 122, 9474–9482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gong, X.; Bao, Y.; Zhao, Y.; Xi, M.; Jiang, C.; Fong, H. Electrospun nanofibrous membranes surface-decorated with silver nanoparticles as flexible and active/sensitive substrates for surface-enhanced Raman scattering. Langmuir 2012, 28, 14433–14440. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Oh, K.; Choi, H.K.; Lee, S.G.; Youn, H.J.; Lee, H.L.; Jeong, D.H. Sub-nanomolar Sensitivity of Filter Paper-Based SERS Sensor for Pesticide Detection by Hydrophobicity Change of Paper Surface. ACS Sens. 2018, 3, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Prikhozhdenko, E.S.; Bratashov, D.N.; Gorin, D.A.; Yashchenok, A.M. Flexible surface-enhanced Raman scattering-active substrates based on nanofibrous membranes. Nanoparticle Res. 2018, 11, 4468–4488. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and Surface-Enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Holden, A.J.; Chen, L.; Shaw, I.C. Thermal Stability of Organophosphorus Pesticide Triazophos and Its Relevance in the Assessment of Risk to the Consumer of Triazophos Residues in Food. J. Agric. Food Chem. 2001, 49, 103–106. [Google Scholar] [CrossRef]
- Nouanthavong, S.; Nacapricha, D.; Henry, C.S.; Sameenoi, Y. Pesticide analysis using nanoceria-coated paper-based devices as a detection platform. Analyst 2016, 141, 1837–1846. [Google Scholar] [CrossRef]
- Fahimi-Kashani, N.; Hormozi-Nezhad, M.R. Gold-Nanoparticle-Based Colorimetric Sensor Array for Discrimination of Organophosphate Pesticides. Anal. Chem. 2016, 88, 8099–8106. [Google Scholar] [CrossRef]
- Galán-Cano, F.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Dispersive micro-solid phase extraction with ionic liquid-modified silica for the determination of organophosphate pesticides in water by ultra performance liquid chromatography. Microchem. J. 2013, 106, 311–317. [Google Scholar] [CrossRef]
- Du, D.; Huang, X.; Cai, J.; Zhang, A. Amperometric detection of triazophos pesticide using acetylcholinesterase biosensor based on multiwall carbon nanotube–chitosan matrix. Sens. Actuators B Chem. 2007, 127, 531–535. [Google Scholar] [CrossRef]
- Li, W.; Qiu, S.P.; Wu, Y.J. Triazophos residues and dissipation rates in wheat crops and soil. Ecotoxicol. Environ. Saf. 2008, 69, 312–316. [Google Scholar] [CrossRef]
- Yan, M.; Chen, G.; She, Y.; Ma, J.; Hong, S.H.; Shao, Y.; El-Aty, A.M.A.; Wang, M.; Wang, S.S.; Wang, J. Sensitive and Simple Competitive Biomimetic Nanozyme-Linked Immunosorbent Assay for Colorimetric and Surface-Enhanced Raman Scattering Sensing of Triazophos. J. Agric. Food Chem. 2019, 67, 9658–9666. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wu, T.; Chen, W.C.; Li, L.; Du, Y.P. A novel metastable state nanoparticle-enhanced Raman spectroscopy coupled with thin layer chromatography for determination of multiple pesticides. Food Chem. 2019, 270, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Y.; Tang, M.; Gong, Z.J.; Qiu, Z.P.; Wang, D.M.; Fan, M.K. Screening pesticide residues on fruit peels using portable Raman spectrometer combined with adhesive tape sampling. Food Chem. 2019, 295, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wong, K.W.; Wang, Q.; Zhou, W.F.; Tang, C.Y.; Fan, M.K.; Mei, J.; Lau, W.M. Silver-nanoparticles-loaded chitosan foam as a flflexible SERS substrate for active collecting analytes from both solid surface and solution. Talanta 2019, 191, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Bhamore, J.R.; Ganguly, P.; Kailasa, S.K. Molecular assembly of 3-mercaptopropinonic acid and guanidine acetic acid on silver nanoparticles for selective colorimetric detection of triazophos in water and food samples. Sens. Actuators B Chem. 2016, 233, 486–495. [Google Scholar] [CrossRef]
- Boroduleva, A.Y.; Wu, J.; Yang, Q.; Li, H.; Zhang, Q.; Li, P.W. Development of fluorescence polarization immunoassays for parallel detection of pesticides carbaryl and triazophos in wheat grains. Anal. Methods 2017, 9, 6814–6822. [Google Scholar] [CrossRef]
- Sun, J.; Gong, L.; Lu, Y.; Wang, D.; Gong, Z.; Fan, M. Dual functional PDMS sponge SERS substrate for the on-site detection of pesticides both on fruit surfaces and in juice. Analyst 2018, 143, 2689–2695. [Google Scholar] [CrossRef]
- Fu, L.Y.; Liu, X.J.; Hu, J.; Zhao, X.N.; Wang, H.L.; Wang, H.D. Application of dispersive liquid-liquid microextraction for the analysis of triazophos and carbaryl pesticides in water and fruit juice samples. Anal. Chim. Acta 2009, 632, 289–295. [Google Scholar] [CrossRef]


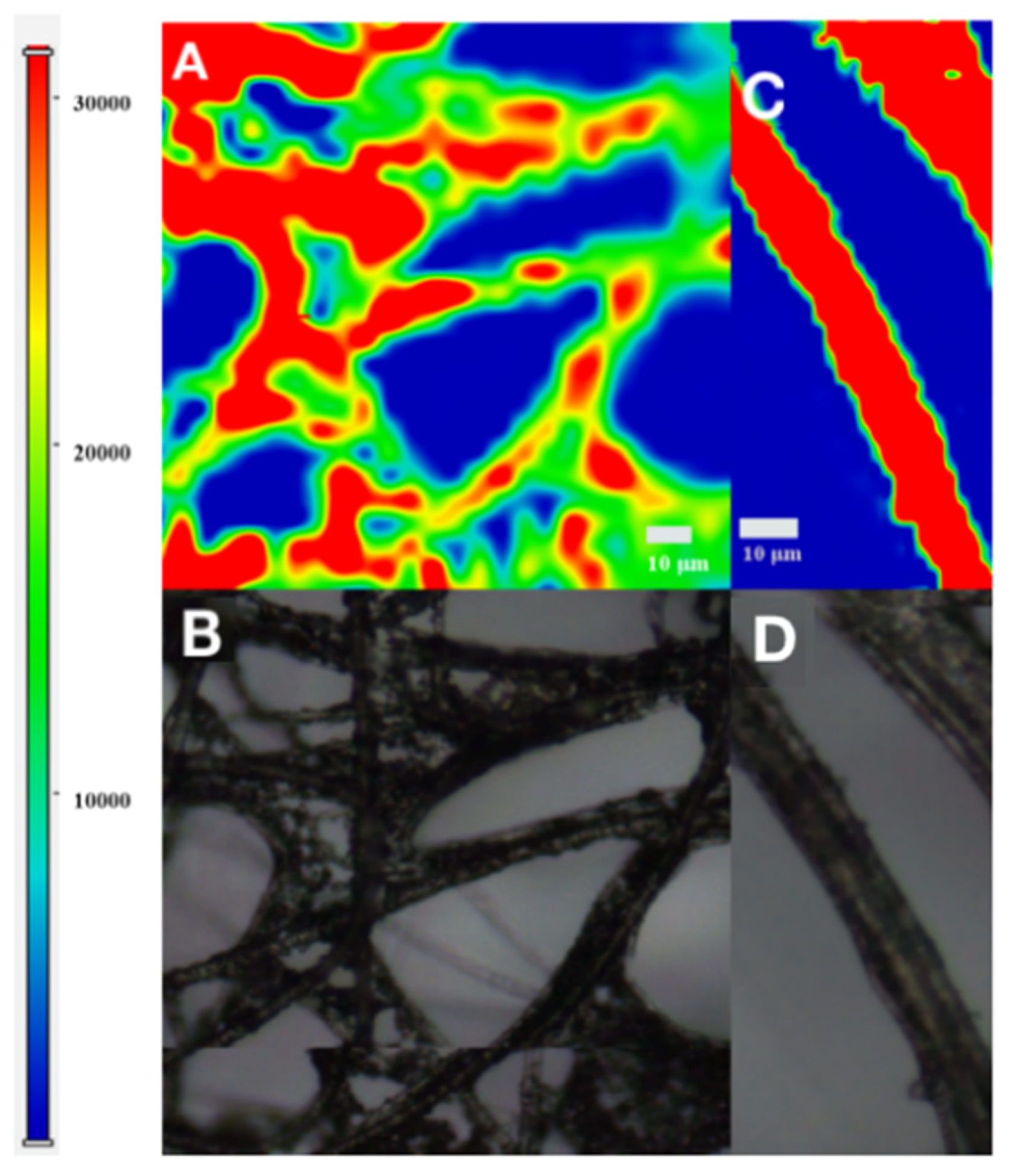
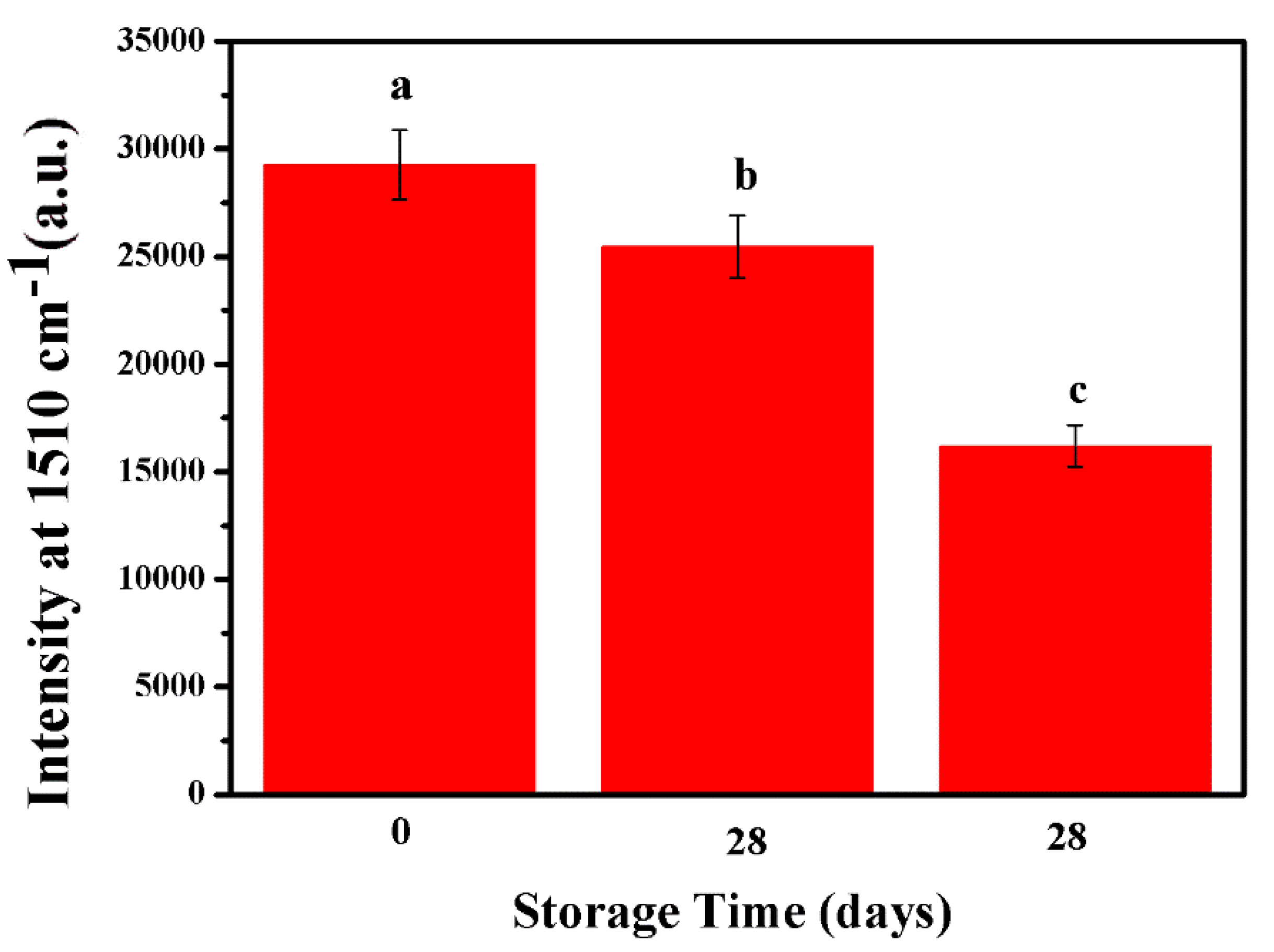
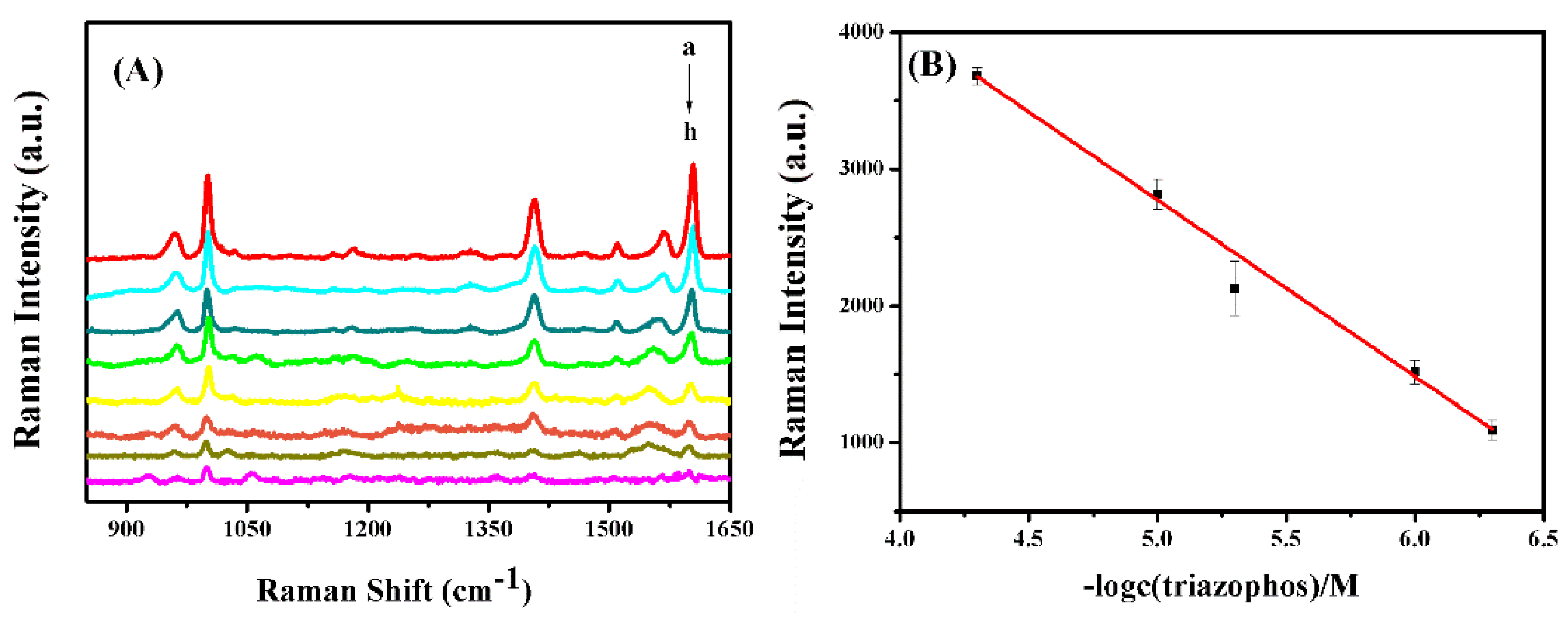
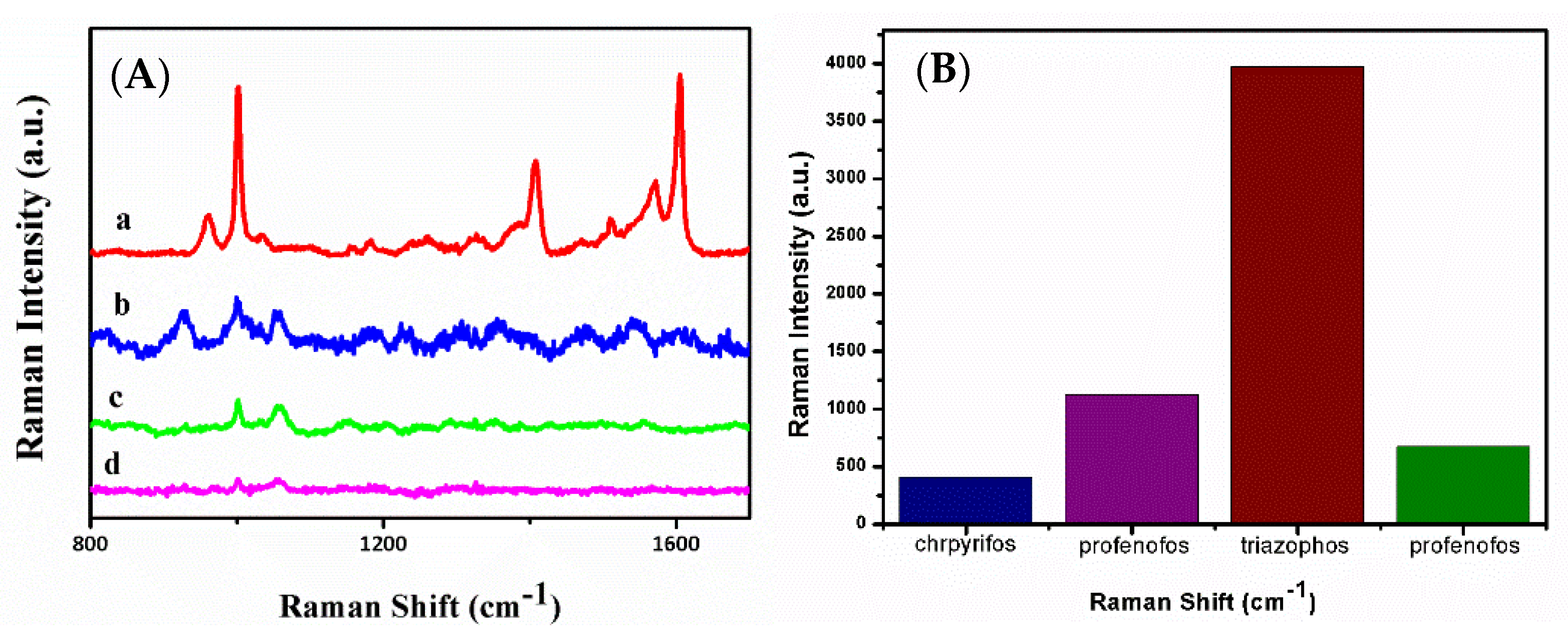
| Samples | Added (μM) | Detected (μM) | Recovery Rate (%) | RSD (%) |
|---|---|---|---|---|
| Apple peel | 50 | 51 | 102 | 7.6 |
| 10 | 8.7 | 87 | 7.7 | |
| 5 | 4.8 | 96 | 7.0 |
| Samples | Added (μM) | SERS (μM) | SERS Recovery (%) | HPLC (μM) | HPLC Recovery (%) |
|---|---|---|---|---|---|
| Apple juice | 50 | 49 | 98 | 49 | 98 |
| 10 | 8.5 | 85 | 9.1 | 91 | |
| 5 | 5.3 | 106 | 4 | 80 |
| Method | Nanomaterial | Detection Limit (M) | Recovery (%) | References |
|---|---|---|---|---|
| Colorimetry | MPA-GAA-Ag NPs | 5 × 10−7 | 92.18–99.64 | [46] |
| FPIA | - | 10−7 | 76–110 | [47] |
| SERS | PDMS-Ag NPs | 10−7 | - | [48] |
| HPLC-FLD | - | 6 × 10−8 | 86.3–104.2 | [49] |
| SERS | Ag/SB-film | 2.5 × 10−8 | 85–106 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, F.; Cao, J.; Ying, Y.; Liu, Y.; Wang, D.; Guo, X.; Wu, Y.; Wen, Y.; Yang, H. Preparation of Hydrophobic Film by Electrospinning for Rapid SERS Detection of Trace Triazophos. Sensors 2020, 20, 4120. https://doi.org/10.3390/s20154120
Shao F, Cao J, Ying Y, Liu Y, Wang D, Guo X, Wu Y, Wen Y, Yang H. Preparation of Hydrophobic Film by Electrospinning for Rapid SERS Detection of Trace Triazophos. Sensors. 2020; 20(15):4120. https://doi.org/10.3390/s20154120
Chicago/Turabian StyleShao, Fei, Jiaying Cao, Ye Ying, Ying Liu, Dan Wang, Xiaoyu Guo, Yiping Wu, Ying Wen, and Haifeng Yang. 2020. "Preparation of Hydrophobic Film by Electrospinning for Rapid SERS Detection of Trace Triazophos" Sensors 20, no. 15: 4120. https://doi.org/10.3390/s20154120
APA StyleShao, F., Cao, J., Ying, Y., Liu, Y., Wang, D., Guo, X., Wu, Y., Wen, Y., & Yang, H. (2020). Preparation of Hydrophobic Film by Electrospinning for Rapid SERS Detection of Trace Triazophos. Sensors, 20(15), 4120. https://doi.org/10.3390/s20154120






