3D Microfluidic Devices in a Single Piece of Paper for the Simultaneous Determination of Nitrite and Thiocyanate
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Instruments
2.2. Solution Preparation
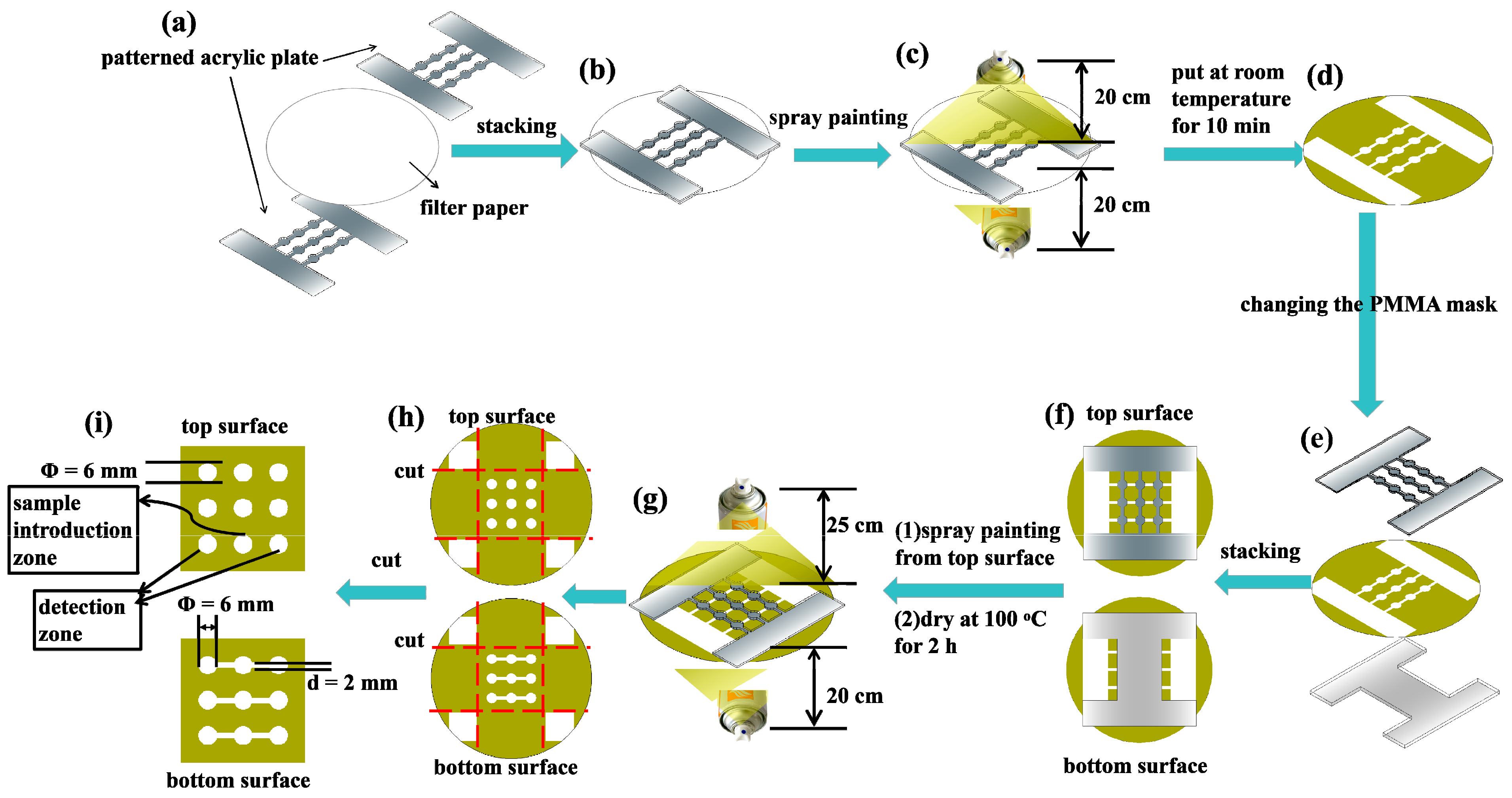
2.3. 3D sl-μPADs Fabricated by a Two-Step Spraying Technique
2.4. Simultaneous Colorimetric Detection of Nitrite and Thiocyanate
2.5. Recovery Test
3. Results and Discussion
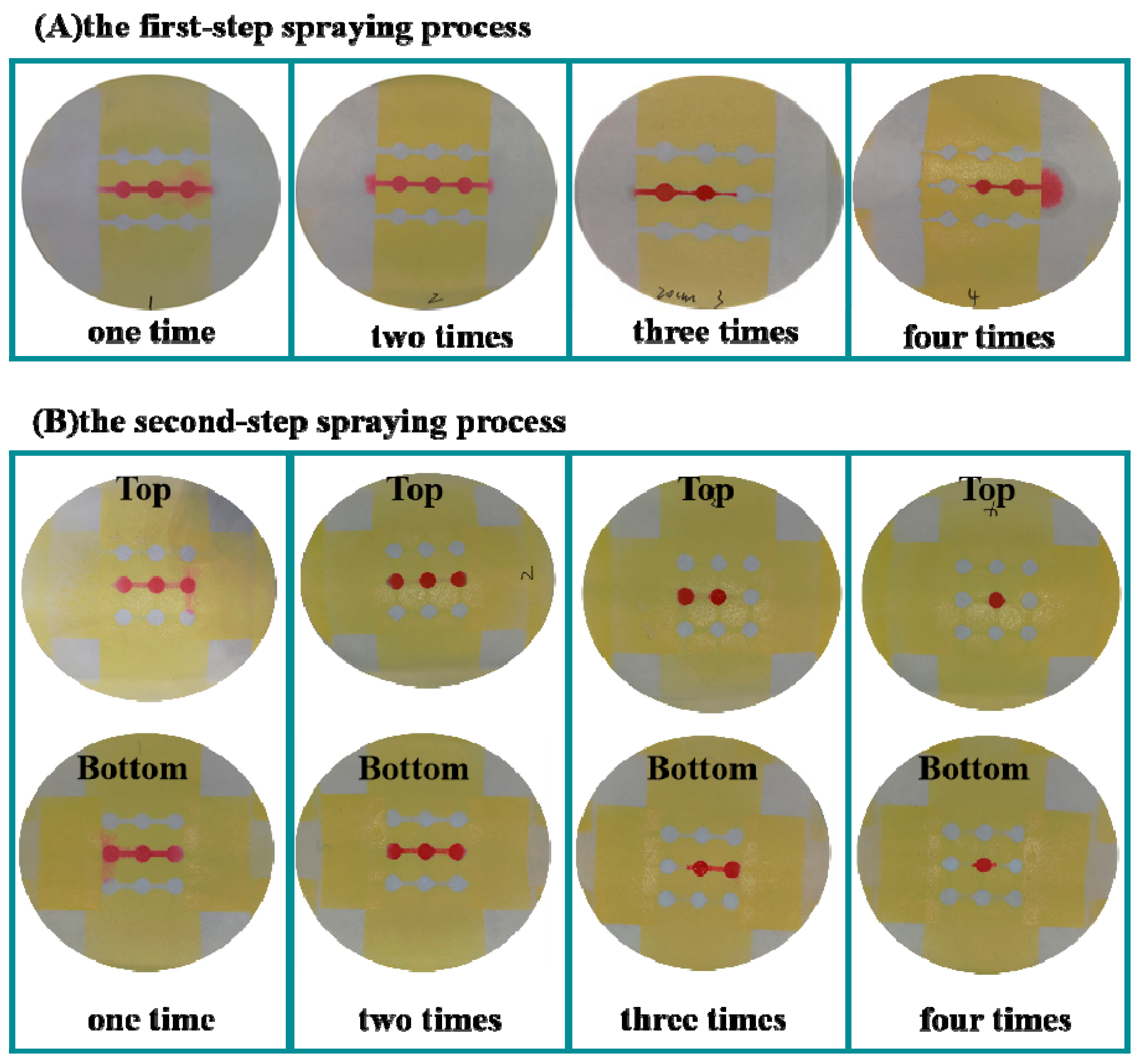
3.1. Conditions Optimization for Spraying Prototyping Process
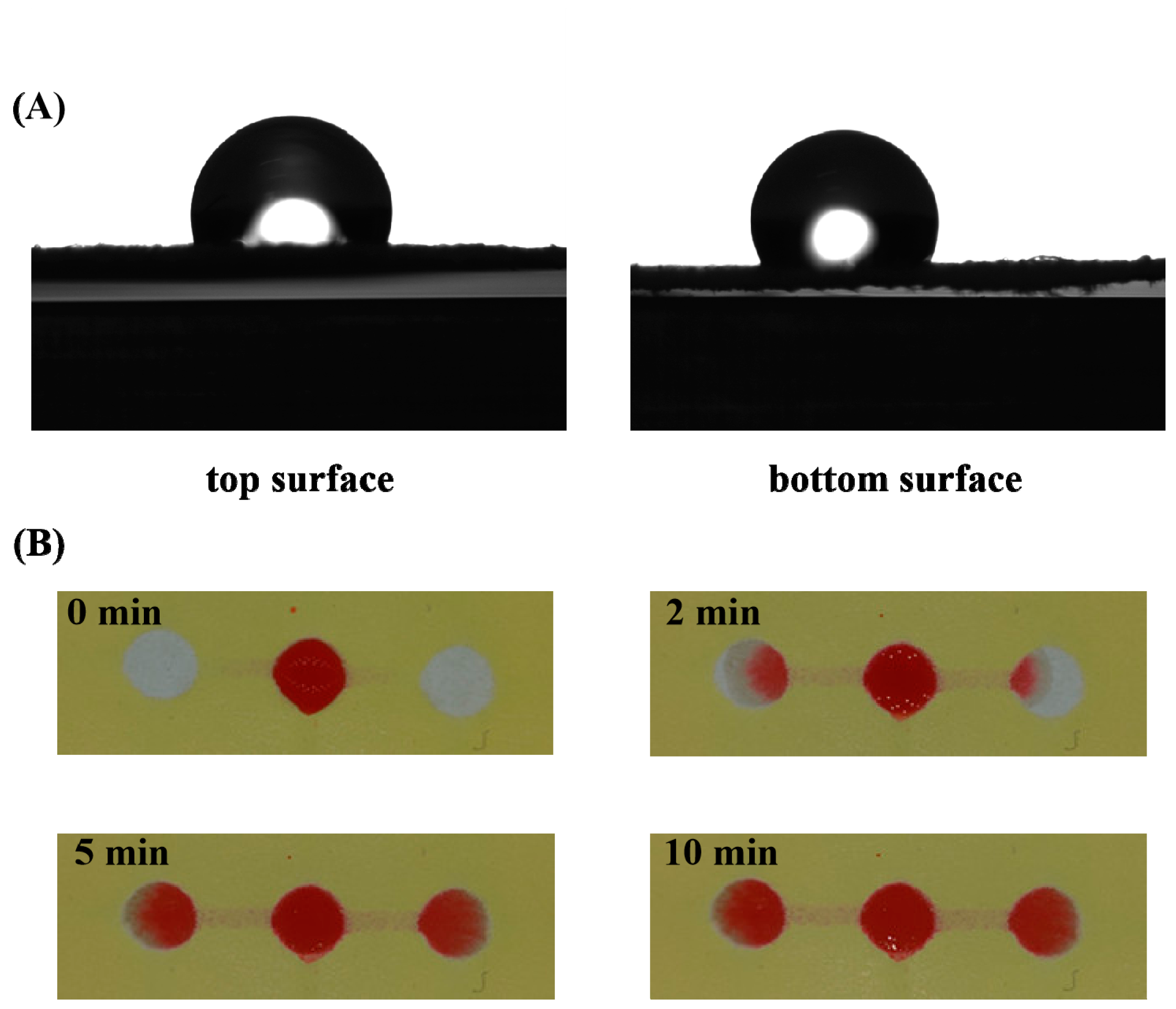
3.2. Characterization of the 3D sl-μPADs
3.3. The Feasibility Demonstration
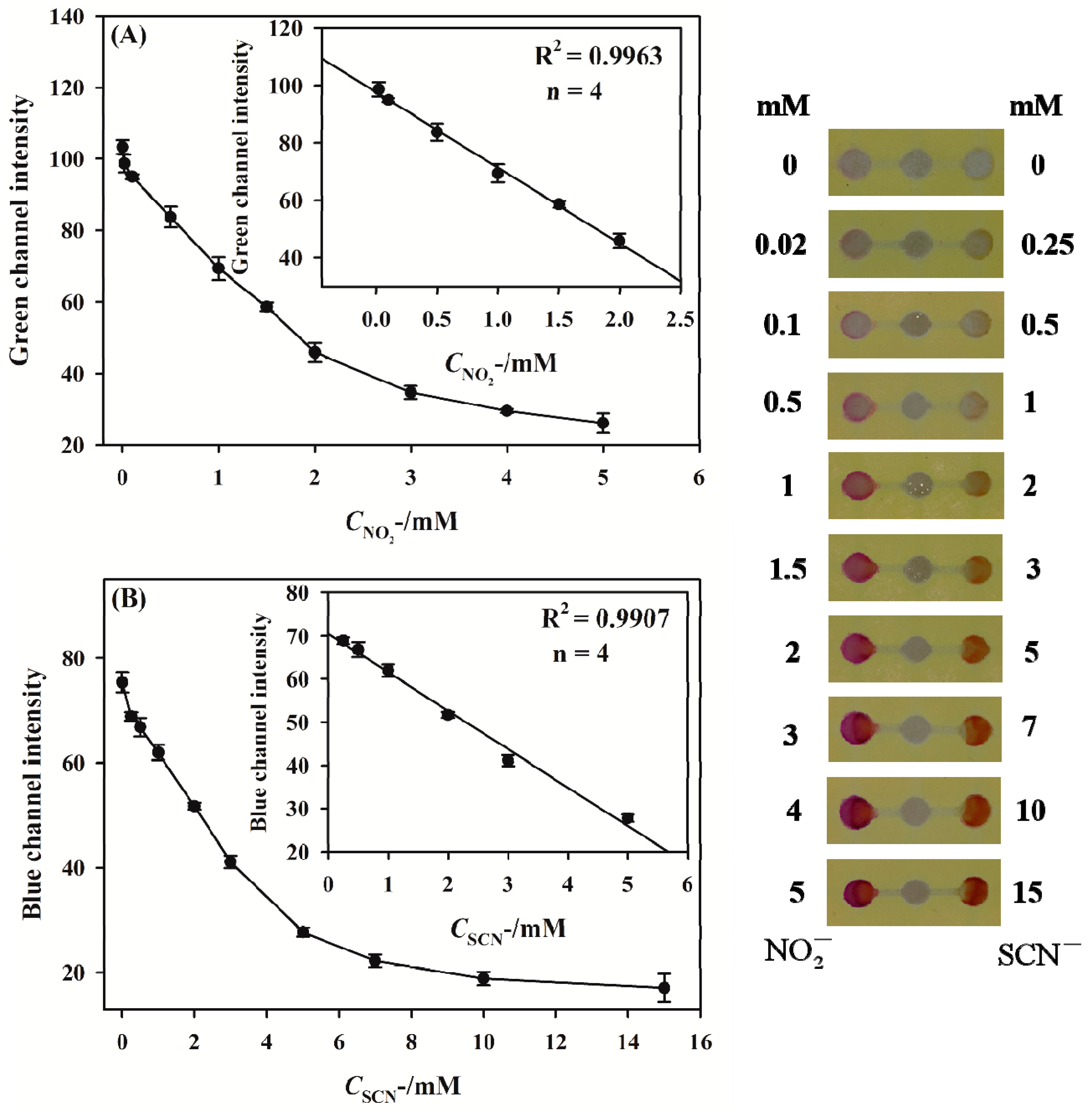
3.4. Simultaneous Detection of Nitrite and Thiocyanate
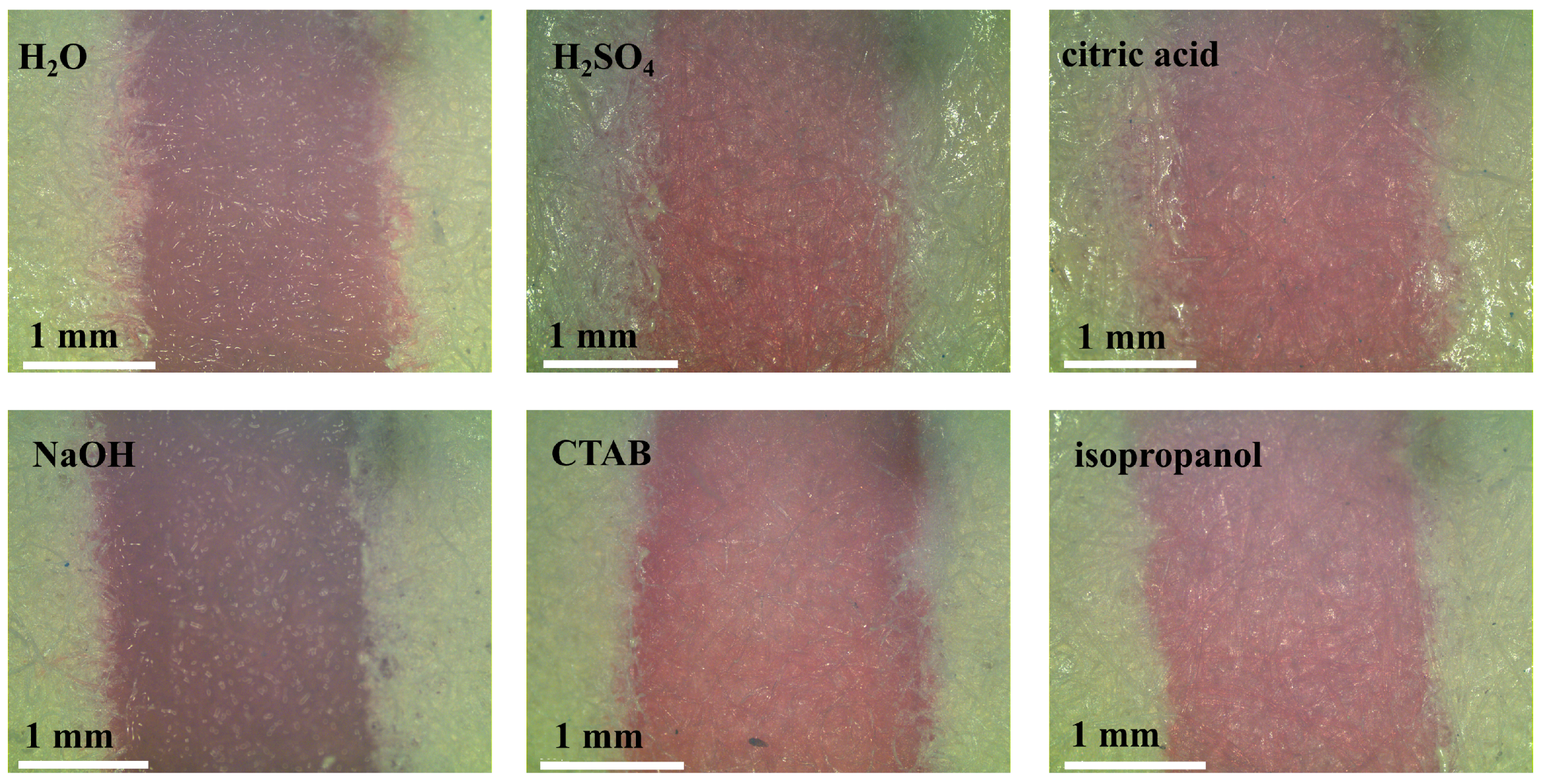
3.5. Specificity and Precision
3.6. Recovery Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, K.J.; Xie, W.Z.; Zhang, H.S.; Wang, H. Ultra-trace level determination of nitrite in human saliva by spectrofluorimetry using 1, 3, 5, 7-tetramethyl-8-(3, 4-diaminophenyl)-difluoroboradiaza-s-indacene. Microchim. Acta 2008, 161, 201–207. [Google Scholar] [CrossRef]
- Kuralay, F.; Dumangöz, M.; Tunç, S. Polymer/carbon nanotubes coated graphite surfaces for highly sensitive nitrite detection. Talanta 2015, 144, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.L.; Khin, C.; Pereira, J.C.M.; Suárez, S.A.; Iretskii, A.V.; Doctorovich, F.; Ford, P.C. Nitrite reduction mediated by heme models. Routes to NO and HNO? J. Am. Chem. Soc. 2013, 135, 4007–4017. [Google Scholar] [CrossRef] [PubMed]
- Kodamatani, H.; Yamazaki, S.; Saito, K.; Tomiyasu, T.; Komatsu, Y. Selective determination method for measurement of nitrite and nitrate in water samples using high-performance liquid chromatography with post-column photochemical reaction and chemiluminescence detection. J. Chromatogr. A 2009, 1216, 3163–3316. [Google Scholar] [CrossRef]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakian, T.; Ghaedi, H. Surface decoration of multi-walled carbon nanotubes modified carbon paste electrode with gold nanoparticles for electro-oxidation and sensitive determination of nitrite. Biosens. Bioelectron. 2014, 51, 379–385. [Google Scholar] [CrossRef]
- Tannenbaum, S.R.; Sinskey, A.J.; Weissman, M.; Bishop, W. Nitrite in human saliva: Its possible relationship to nitrosamine formation. J. Natl. Cancer Inst. 1974, 53, 75–784. [Google Scholar] [CrossRef]
- Hou, T.; Liu, Y.; Xu, L.; Wu, Y.; Ying, Y.; Wen, Y.; Guo, X.Y.; Yang, H. Au dotted magnetic graphene sheets for sensitive detection of thiocyanate. Sens. Actuators B Chem. 2017, 241, 376–382. [Google Scholar] [CrossRef]
- Song, J.; Wu, F.Y.; Wan, Y.Q.; Ma, L.H. Ultrasensitive turn-on fluorescent detection of trace thiocyanate based on fluorescence resonance energy transfer. Talanta 2015, 132, 619–624. [Google Scholar] [CrossRef]
- Glatz, Z.; Nováková, S.; Štěrbová, H. Analysis of thiocyanate in biological fluids by capillary zone electrophoresis. J. Chromatogr. A 2001, 916, 273–277. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Zong, S.; Cui, Y. Rapid and reproducible analysis of thiocyanate in real human serum and saliva using a droplet SERS-microfluidic chip. Biosens. Bioelectron. 2014, 62, 13–18. [Google Scholar] [CrossRef]
- Bhakta, S.A.; Borba, R.; Taba, J.M.; Garcia, C.D.; Carrilho, E. Determination of nitrite in saliva using microfluidic paper-based analytical devices. Anal. Chim. Acta 2014, 809, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Valdés, M.; Díaz-García, M. Determination of thiocyanate within physiological fluids and environmental samples: Current practice and future trends. Crit. Rev. Anal. Chem. 2004, 34, 9–23. [Google Scholar] [CrossRef]
- Ryder, M.I.; Fujitaki, R.; Johnson, G.; Hyun, W. Alterations of neutrophil oxidative burst by in vitro smoke exposure: Implications for oral and systemic diseases. Ann. Periodontol. 1998, 3, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Zheng, Y.; Huang, Z.; Cheng, Y.; Ye, J.; Chu, Q.; Huang, D. Study on the potential application of salivary inorganic anions in clinical diagnosis by capillary electrophoresis coupled with contactless conductivity detection. J. Chromatogr. B 2016, 1014, 70–74. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.; Sako, A.V.F.; Micke, G.A.; Vitali, L. A rapid method for simultaneous determination of nitrate, nitrite and thiocyanate in milk by CZE-UV using quaternary ammonium chitosan as electroosmotic flow inverter. J. Food Compos. Anal 2020, 88, 103455. [Google Scholar] [CrossRef]
- Tanaka, Y.; Naruishi, N.; Fukuya, H.; Sakata, J.; Saito, K.; Wakida, S.I. Simultaneous determination of nitrite, nitrate, thiocyanate and uric acid in human saliva by capillary zone electrophoresis and its application to the study of daily variations. J. Chromatogr. A 2004, 1051, 193–197. [Google Scholar] [CrossRef]
- Connolly, D.; Barron, L.; Paull, B. Determination of urinary thiocyanate and nitrate using fast ion-interaction chromatography. J. Chromatogr. B 2002, 767, 175–180. [Google Scholar] [CrossRef]
- Hu, W.; Haraguchi, H. Determination of Monovalent Inorganic Anions in Human Saliva by Ion Chromatography Using Microcolumn Coated with Micellar Zwitterionic Bile Acid Derivative. Bull. Chem. Soc. Jpn. 1993, 66, 1420–1423. [Google Scholar] [CrossRef]
- Prest, J.E.; Baldock, S.J.; Beardah, M.S.; Doyle, S.P.; Fielden, P.R.; Goddard, N.J.; Brown, B.J.T. Thiocyanate and nitrite analysis using miniaturised isotachophoresis on a planar polymer chip. Analyst 2011, 136, 3170–3176. [Google Scholar] [CrossRef]
- Bjergegaard, C.; Møller, P.; Sørensen, H. Determination of thiocyanate, iodide, nitrate and nitrite in biological samples by micellar electrokinetic capillary chromatography. J. Chromatogr. A 1995, 717, 409–414. [Google Scholar] [CrossRef]
- Chen, S.H.; Wu, H.L.; Tanaka, M.; Shono, T.; Funazo, K. Simultaneous gas chromatographic determination of iodide, nitrite, sulphide and thiocyanate anions by derivatization with pentafluorobenzyl bromide. J. Chromatogr. A 1987, 396, 129–137. [Google Scholar] [CrossRef]
- Chen, S.H.; Wu, H.L.; Tanaka, M.; Shono, T.; Funazo, K. Simultaneous gas chromatographic determination of cyanide, iodide, nitrite, sulphide and thiocyanate anions by derivatization with pentafluorobenzyl bromide and using a kryptand as phase-transfer catalyst. J. Chromatogr. A 1990, 502, 257–264. [Google Scholar] [CrossRef]
- Tanabe, S.; Kitahara, M.; Nawata, M.; Kawanabe, K. Determination of oxidizable inorganic anions by high-performance liquid chromatography with fluorescence detection and application to the determination of salivary nitrite and thiocyanate and serum thiocyanate. J. Chromatogr. B 1988, 424, 29–37. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Tian, T.; Jia, S.; Zhu, Z.; Ma, Y.; Sun, J.; Lin, Z.; Yang, C.J. Target-responsive DNA hydrogel mediated “stop-flow” microfluidic paper-based analytic device for rapid, portable and visual detection of multiple targets. Anal. Chem. 2015, 87, 4275–4282. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Lavilla, I.; Bendicho, C. Paper-based analytical device for instrumental-free detection of thiocyanate in saliva as a biomarker of tobacco smoke exposure. Talanta 2016, 147, 390–396. [Google Scholar] [CrossRef]
- Li, X.; Liu, X. Fabrication of three-dimensional microfluidic channels in a single layer of cellulose paper. Microfluid. Nanofluid 2014, 16, 819–827. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. P. Natl. A. Sci. 2008, 105, 19606–19611. [Google Scholar] [CrossRef]
- Lu, J.; Ge, S.; Ge, L.; Yan, M.; Yu, J. Electrochemical DNA sensor based on three-dimensional folding paper device for specific and sensitive point-of-care testing. Electrochim. Acta 2012, 80, 334–341. [Google Scholar] [CrossRef]
- Jeong, S.G.; Lee, S.H.; Choi, C.H.; Kim, J.; Lee, C.S. Toward instrument-free digital measurements: A three-dimensional microfluidic device fabricated in a single sheet of paper by double-sided printing and lamination. Lab Chip 2015, 15, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.M.; de Souza, F.R.; Garcia, P.T.; Rabelo, D.; Henry, C.S.; Coltro, W.K. Versatile fabrication of paper-based microfluidic devices with high chemical resistance using scholar glue and magnetic masks. Anal. Chim. Acta 2017, 974, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Nurak, T.; Praphairaksit, N.; Chailapakul, O. Fabrication of paper-based devices by lacquer spraying method for the determination of nickel (II) ion in waste water. Talanta 2013, 114, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Deng, M.H.; Yang, Y. New Single-Layered Paper-Based Microfluidic Devices for the Analysis of Nitrite and Glucose Built via Deposition of Adhesive Tape. Sensors 2019, 19, 4082. [Google Scholar] [CrossRef]
- Lewis, G.G.; DiTucci, M.J.; Baker, M.S.; Phillips, S.T. High throughput method for prototyping three-dimensional, paper-based microfluidic devices. Lab Chip 2012, 12, 2630–2633. [Google Scholar] [CrossRef]
- Yıldırmaz, G.; Akgöl, S.; Arıca, M.Y.; Sönmez, H.; Denizli, A. Polyhydroxyethylmethacrylate/polyhydroxybutyrate composite membranes for fluoride release. J. Appl. Polym. Sci. 2003, 87, 976–981. [Google Scholar] [CrossRef]
- Klasner, S.A.; Price, A.K.; Hoeman, K.W.; Wilson, R.S.; Bell, K.J.; Culbertson, C.T. Paper-based microfluidic devices for analysis of clinically relevant analytes present in urine and saliva. Anal. Bioanal. Chem. 2010, 397, 1821–1829. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2008, 43, 645–657. [Google Scholar] [CrossRef]
- Jones, E.A.; Hemmings, M.J. Spectrophotometric Determination by Flow Injection Analysis of Thiocyanate in Metallurgical Process Solutions Containing Free Cyanide and Metal Cyanide Complexes. S. Afr. J. Chem. 1989, 42, 6–8. [Google Scholar]
- Van Staden, J.F.; Botha, A. Spectrophotometric determination of thiocyanate by sequential injection analysis. Anal. Chim. Acta 2000, 403, 279–286. [Google Scholar] [CrossRef]
- Noiphung, J.; Nguyen, M.P.; Punyadeera, C.; Wan, Y.; Laiwattanapaisal, W.; Henry, C.S. Development of paper-based analytical devices for minimizing the viscosity effect in human saliva. Theranostics 2018, 8, 3797. [Google Scholar] [CrossRef] [PubMed]
- Shallan, A.I.; Smejkal, P.; Corban, M.; Guijt, R.M.; Breadmore, M.C. Cost-effective three-dimensional printing of visibly transparent microchips within minutes. Anal. Chem. 2014, 86, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Wei, J.; Xu, J.; Wang, Y.; Zheng, G. Paper-based three-dimensional microfluidic device for monitoring of heavy metals with a camera cell phone. Anal. Bioanal. Chem. 2014, 406, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Cook, B.S.; Fang, Y.; Tentzeris, M.M. Fully inkjet-printed microfluidics: A solution to low-cost rapid three-dimensional microfluidics fabrication with numerous electrical and sensing applications. Sci. Rep. 2016, 6, 35111. [Google Scholar] [CrossRef]
- Shende, V.; Biviji, A.T.; Akarte, N. Estimation and correlative study of salivary nitrate and nitrite in tobacco related oral squamous carcinoma and submucous fbrosis. J. Oral Maxillofac. Pathol. 2013, 17, 381–385. [Google Scholar] [CrossRef]
- Phonchai, A.; Srisukpan, T.; Riengrojpitak, S.; Wilairat, P.; Chantiwas, R. Simple and Rapid screening of the thiocyanate level in saliva for the identifcation of smokers and non- smokers by capillary electrophoresis with contactless conductivity detection. Anal. Methods. 2016, 8, 4983–4990. [Google Scholar] [CrossRef]
- Júnior, J.J.S.; Farias, M.A.; Silva, V.L.; Montenegro, M.C.; Araújo, A.N.; Lavorante, A.F.; Paim, A.P.S. Spectrophotometric determination of thiocyanate in human saliva employing micropumping multicommutation flow system. Spectrosc. Lett. 2013, 43, 213–219. [Google Scholar] [CrossRef]
- Daniel, W.L.; Han, M.S.; Lee, J.S.; Mirkin, C.A. Colorimetric nitrite and nitrate detection with gold nanoparticle probes and kinetic end points. J. Am. Chem. Soc. 2009, 131, 6362–6363. [Google Scholar] [CrossRef]
- Nilghaz, A.; Bagherbaigi, S.; Lam, C.L.; Mousavi, S.M.; Cόrcoles, E.P.; Wicaksono, D.H. Multiple semi-quantitative colorimetric assays in compact embeddable microfluidic cloth-based analytical device (μCAD) for effective point-of-care diagnostic. Microfluid. Nanofluid 2015, 19, 317–333. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Z.; Wang, M. Fabrication of a paper-based microfluidic device to readily determine nitrite ion concentration by simple colorimetric assay. J. Chem. Educ. 2015, 92, 733–736. [Google Scholar] [CrossRef]
- Chiang, C.K.; Kurniawan, A.; Kao, C.Y.; Wang, M.J. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2019, 194, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Sitanurak, J.; Fukana, N.; Wongpakdee, T.; Thepchuay, Y.; Ratanawimarnwong, N.; Amornsakchai, T.; Nacapricha, D. T-shirt ink for one-step screen-printing of hydrophobic barriers for 2D-and 3D-microfluidic paper-based analytical devices. Talanta 2019, 205, 120113. [Google Scholar] [CrossRef] [PubMed]
- Gavrilenko, N.A.; Volgina, T.N.; Urazov, E.V.; Gavrilenko, M.A. Transparent polymer sensor for visual and photometrical detection of thiocyanate in oilfield water. J. Petrol. Sci. Eng. 2019, 172, 960–963. [Google Scholar] [CrossRef]
- Mohammad, A.; Chahar, J.P.S. Thin-layer chromatographic separation, colorimetric determination and recovery of thiocyanate from photogenic waste, river and sea waters. J. Chromatogr. A 1997, 774, 373–377. [Google Scholar] [CrossRef]
- Acebal, C.C.; Sklenářová, H.; Škrlíková, J.; Šrámková, I.; Andruch, V.; Balogh, I.S.; Solich, P. Application of DV-SIA manifold for determination of thiocyanate ions in human saliva samples. Talanta 2012, 96, 107–112. [Google Scholar] [CrossRef]
- Davis, S.S. The rheological properties of saliva. Rheol. Acta 1971, 10, 28–35. [Google Scholar] [CrossRef]
- Stokes, J.R.; Davies, G.A. Viscoelasticity of human whole saliva collected after acid and mechanical stimulation. Biorheology 2007, 44, 141–160. [Google Scholar]





| Nitrite Assay | ||||||
| Sensing Reagents | Method | Response Time (min) | Volume (μL) | Linear Range (mM) | LOD (mM) | Refs. |
| Griess reagent | Colorimetric | 2 | 15 | 0.16 | [48] | |
| Griess reagent | Colorimetric | 0.087 | [49] | |||
| Griess reagent | Colorimetric | 2 | 1.40 | 0.156–1.25 | [50] | |
| Griess reagent | Colorimetric | 10 | 5 | 0.0156–1 | 0.0148 | [51] |
| Griess reagent | Colorimetric | 10 | 12 | 0.02–0.9 | 0.015 | [36] |
| Griess reagent | Colorimetric | 15 | 18 | 0.02–2 | 0.0096 | This work |
| Thiocyanate Assay | ||||||
| Sensing Reagents | Method | Response Time (min) | Volume (μL) | Linear Range (mM) | LOD (mM) | Refs. |
| Fe3+ | Colorimetric | 4 | 15 | 1–8 | 1.19 | [52] |
| Fe3+ | Colorimetric | 10 | 2 | 0.025–20 | 0.06 | [27] |
| Fe3+ | Colorimetric | 0.10–0.34 | 0.10 | [53] | ||
| Fe3+ | Colorimetric | 100 | 2.55–25.48 | 0.20 | [54] | |
| Astra phloxine | Spectrophotometic | 50 | 0.05–0.50 | 0.02 | [55] | |
| Fe3+ | Colorimetric | 15 | 18 | 0.25–5 | 0.074 | This work |
| Nitrite | Sample No. | Added (mM) | Found (mM) | RSD (%) | Recovery (%) |
| 1 | 0.1 | 0.11 | 5.63 | 110 | |
| 2 | 0.5 | 0.50 | 1.98 | 100 | |
| 3 | 1.5 | 1.43 | 1.88 | 95 | |
| Thiocyanate | Sample No. | Added (mM) | Found (mM) | RSD (%) | Recovery (%) |
| 1 | 0.5 | 0.54 | 2.71 | 108 | |
| 2 | 2 | 1.83 | 2.30 | 92 | |
| 3 | 3 | 3.13 | 2.84 | 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Deng, M.; Yang, Y.; Nie, B.; Zhao, S. 3D Microfluidic Devices in a Single Piece of Paper for the Simultaneous Determination of Nitrite and Thiocyanate. Sensors 2020, 20, 4118. https://doi.org/10.3390/s20154118
Yu P, Deng M, Yang Y, Nie B, Zhao S. 3D Microfluidic Devices in a Single Piece of Paper for the Simultaneous Determination of Nitrite and Thiocyanate. Sensors. 2020; 20(15):4118. https://doi.org/10.3390/s20154118
Chicago/Turabian StyleYu, Peng, Muhan Deng, Yi Yang, Beixi Nie, and Shaoyu Zhao. 2020. "3D Microfluidic Devices in a Single Piece of Paper for the Simultaneous Determination of Nitrite and Thiocyanate" Sensors 20, no. 15: 4118. https://doi.org/10.3390/s20154118
APA StyleYu, P., Deng, M., Yang, Y., Nie, B., & Zhao, S. (2020). 3D Microfluidic Devices in a Single Piece of Paper for the Simultaneous Determination of Nitrite and Thiocyanate. Sensors, 20(15), 4118. https://doi.org/10.3390/s20154118






