A Virtual Reality Muscle–Computer Interface for Neurorehabilitation in Chronic Stroke: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
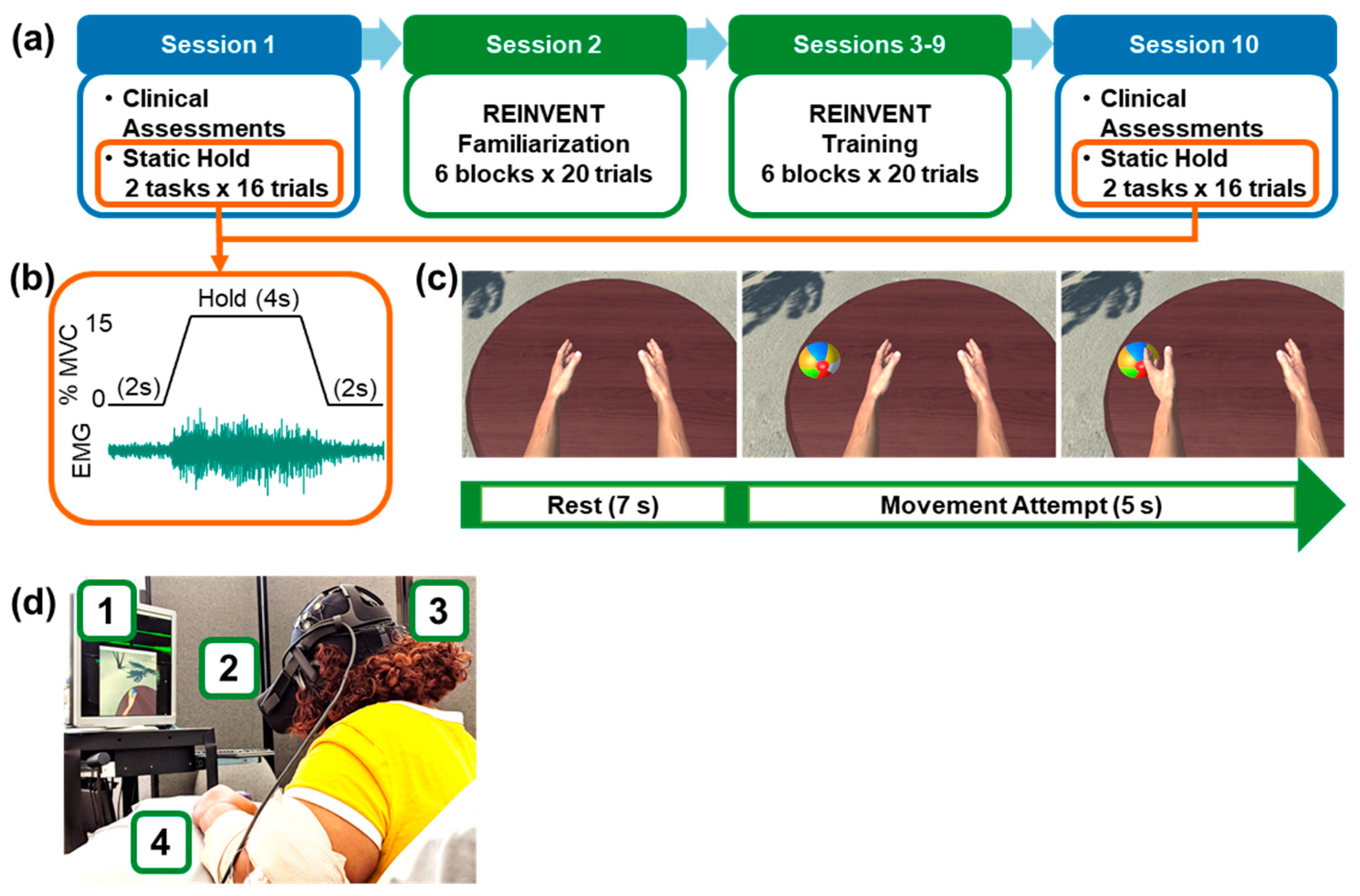
2.2. Study Timeline
2.3. Clinical Assessments (Sessions 1 and 10)
- Fugl–Meyer assessment for the upper extremity (FMA-UE). This scale measures sensorimotor impairment of the upper limb following a hemiplegic stroke, including movement, coordination, and reflexes, and provides a score that ranges from 0 (greatest impairment) to 66 (least impairment) [37].
- Action research arm test (ARAT). This scale measures functional performance of the upper limb in terms of the ability to functionally manipulate objects with different sizes, weights, and shapes, and provides a score that ranges from 0 (greatest impairment) to 57 (least impairment) [38].
- Montreal cognitive assessment (MOCA). This is an assessment of cognitive impairments evaluating visuospatial abilities, memory, attention, concentration, language, and orientation, and provides a score that ranges from 0 (greatest impairment) to 30 (least impairment) [39].
- Sixteen-question stroke impact scale (SIS-16). This assessment consists of a series of self-reported questions evaluating quality of life as related to strength, hand function, mobility, and activities of daily living, and provides a total score that ranges from 16 (greatest impairment) to 80 (least impairment) [40].
- Wrist range of motion (ROM). Using a goniometer, we recorded the maximum degrees of passive and active wrist extension, wrist flexion, ulnar deviation, and radial deviation. Activities of daily life usually require 40 degrees of wrist extension, 40 degrees of wrist flexion, and 40 degrees of combined ulnar and radial deviation [41].
2.4. Additional Data Acquired
- Grip strength (GS). In each session, we recorded maximal grip force from the more affected hand using an analog dynamometer, while recording the associated EMG.
- Simulator sickness questionnaire (SSQ). In sessions 2 and 9, we evaluated each participant’s comfort with the VR environment using this 16-question survey covering oculomotor discomfort, disorientation, and nausea. The total score ranges from 0 (no sickness induced) to 63 (highest values of sickness) [42].
- Finally, we qualitatively evaluated the participants’ overall experience and feedback in terms of enjoyment and ease of use with a free-form questionnaire at the end of the experiment.
2.5. Physiological Recordings and Analysis
2.6. Static Hold Task: Characterization of Muscle Control during EMG Amplitude Target Tracking (Sessions 1 and 10)
2.7. Wrist Extensor Training in Virtual Reality (Sessions 2–9)
2.8. Statistical Analyses
2.8.1. Behavioral and Neuromuscular Changes Following Training
2.8.2. Changes across Training Sessions
3. Results
3.1. Feasibility
3.2. Behavioral Changes Following Training
3.3. Changes of Muscle Control during EMG Amplitude Target Tracking
3.4. Neuromuscular Changes Following Training
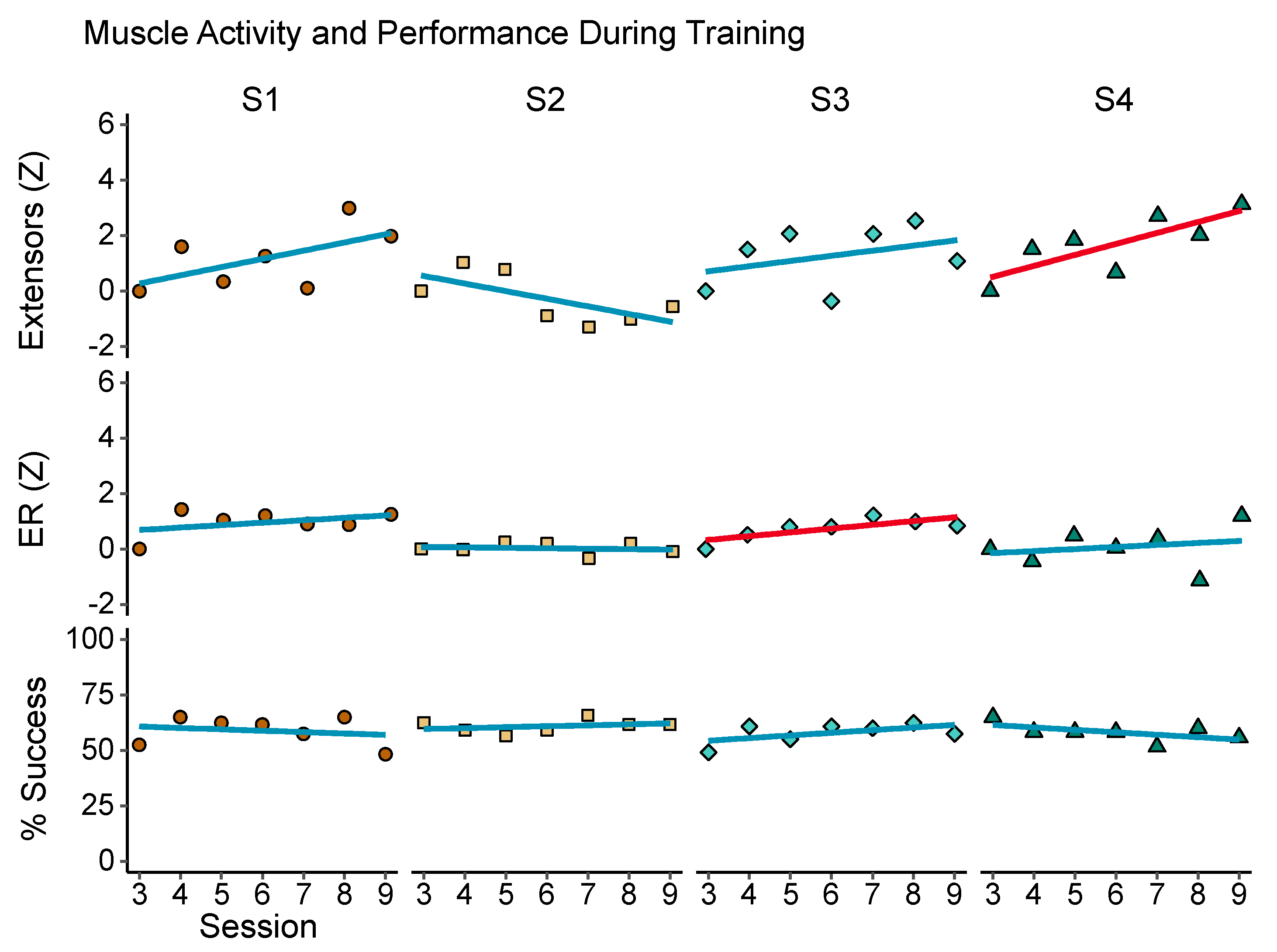
3.5. Changes across Training Sessions
4. Discussion
4.1. Summary
4.2. Feasibility and Acceptability
4.3. Clinical Assessments
4.4. Neuromuscular Control
4.5. Training Effects versus Task Performance
4.6. Limitations and Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Statements
Data Availability
References
- Ballester, B.R.; Maier, M.; Duff, A.; Cameirão, M.; Bermúdez, S.; Duarte, E.; Cuxart, A.; Rodríguez, S.; San Segundo Mozo, R.M.; Verschure, P.F.M.J. A critical time window for recovery extends beyond one-year post-stroke. J. Neurophysiol. 2019, 122, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Lohse, K.R.; Lang, C.E.; Boyd, L.A. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke 2014, 45, 2053–2058. [Google Scholar] [CrossRef]
- McCabe, J.; Monkiewicz, M.; Holcomb, J.; Pundik, S.; Daly, J.J. Comparison of Robotics, Functional Electrical Stimulation, and Motor Learning Methods for Treatment of Persistent Upper Extremity Dysfunction After Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2015, 96, 981–990. [Google Scholar] [CrossRef]
- Ward, N.S.; Brander, F.; Kelly, K. Intensive upper limb neurorehabilitation in chronic stroke: Outcomes from the Queen Square programme. J. Neurol. Neurosurg. Psychiatry 2019, 90, 498–506. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Fong, K.N.K.; Zhang, J.J.; Chan, J.; Ting, K.H. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Cervera, M.A.; Soekadar, S.R.; Ushiba, J.; Millán, J.D.R.; Liu, M.; Birbaumer, N.; Garipelli, G. Brain-computer interfaces for post-stroke motor rehabilitation: A meta-analysis. Ann. Clin. Transl. Neurol. 2018, 5, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Soekadar, S.R.; Birbaumer, N.; Slutzky, M.W.; Cohen, L.G. Brain–machine interfaces in neurorehabilitation of stroke. Neurobiol. Dis. 2015, 83, 172–179. [Google Scholar] [CrossRef]
- Remsik, A.; Young, B.; Vermilyea, R.; Kiekhoefer, L.; Abrams, J.; Evander Elmore, S.; Schultz, P.; Nair, V.; Edwards, D.; Williams, J.; et al. A review of the progression and future implications of brain-computer interface therapies for restoration of distal upper extremity motor function after stroke. Expert Rev. Med. Devices 2016, 13, 445–454. [Google Scholar] [CrossRef]
- Alkoby, O.; Abu-Rmileh, A.; Shriki, O.; Todder, D. Can We Predict Who Will Respond to Neurofeedback? A Review of the Inefficacy Problem and Existing Predictors for Successful EEG Neurofeedback Learning. Neuroscience 2018, 378, 155–164. [Google Scholar] [CrossRef]
- Peters, B.; Bieker, G.; Heckman, S.M.; Huggins, J.E.; Wolf, C.; Zeitlin, D.; Fried-Oken, M. Brain-Computer Interface Users Speak Up: The Virtual Users’ Forum at the 2013 International Brain-Computer Interface Meeting. Arch. Phys. Med. Rehabil. 2015, 96, S33–S37. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.J.; Vaughan, T.M. BCI in practice. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 228, pp. 389–404. ISBN 978-0-12-804216-8. [Google Scholar]
- Mulas, M.; Folgheraiter, M.; Gini, G. An EMG-Controlled Exoskeleton for Hand Rehabilitation. In Proceedings of the IEEE 9th International Conference on Rehabilitation Robotics, 2005. ICORR 2005, Chicago, IL, USA, 28 June–1 July 2005; pp. 371–374. [Google Scholar]
- Ngeo, J.; Tamei, T.; Shibata, T.; Orlando, M.F.F.; Behera, L.; Saxena, A.; Dutta, A. Control of an optimal finger exoskeleton based on continuous joint angle estimation from EMG signals. In Proceedings of the 2013 IEEE 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 338–341. [Google Scholar]
- Armagan, O.; Tascioglu, F.; Oner, C. Electromyographic Biofeedback in the Treatment of the Hemiplegic Hand: A Placebo-Controlled Study. Am. J. Phys. Med. Rehabil. 2003, 82, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Beer, R.F.; Dewald, J.P.A.; Rymer, W.Z. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: Evidence for disturbed control of limb dynamics. Exp. Brain Res. 2000, 131, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Dewald, J.P.A.; Pope, P.S.; Given, J.D.; Buchanan, T.S.; Rymer, W.Z. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 1995, 118, 495–510. [Google Scholar] [CrossRef]
- Zackowski, K.M.; Dromerick, A.W.; Sahrmann, S.A.; Thach, W.T.; Bastian, A.J. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain 2004, 127, 1035–1046. [Google Scholar] [CrossRef]
- Arpa, S. Does electromyographic biofeedback improve exercise effects in hemiplegic patients? A pilot randomized controlled trial. J. Rehabil. Med. 2019, 51, 109–112. [Google Scholar] [CrossRef]
- Glanz, M.; Klawansky, S.; Stason, W.; Berkey, C.; Shah, N.; Phan, H.; Chalmers, T.C. Biofeedback therapy in poststroke rehabilitation: A meta-analysis of the randomized controlled trials. Arch. Phys. Med. Rehabil. 1995, 76, 508–515. [Google Scholar] [CrossRef]
- Kim, J.-H. The effects of training using EMG biofeedback on stroke patients upper extremity functions. J. Phys. Ther. Sci. 2017, 29, 1085–1088. [Google Scholar] [CrossRef]
- Schleenbaker, R.E.; Mainous, A.G. Electromyographic biofeedback for neuromuscular reeducation in the hemiplegic stroke patient: A meta-analysis. Arch. Phys. Med. Rehabil. 1993, 74, 1301–1304. [Google Scholar] [CrossRef]
- Woodford, H.J.; Price, C.I. EMG biofeedback for the recovery of motor function after stroke. Cochrane Database Syst. Rev. 2007, 2007. [Google Scholar] [CrossRef]
- Mugler, E.M.; Tomic, G.; Singh, A.; Hameed, S.; Lindberg, E.W.; Gaide, J.; Alqadi, M.; Robinson, E.; Dalzotto, K.; Limoli, C.; et al. Myoelectric Computer Interface Training for Reducing Co-Activation and Enhancing Arm Movement in Chronic Stroke Survivors: A Randomized Trial. Neurorehabilit. Neural Repair 2019, 33, 284–295. [Google Scholar] [CrossRef]
- Wright, Z.A.; Zev Rymer, W.; Slutzky, M.W. Reducing abnormal muscle co-activation after stroke using a myoelectric-computer interface: A pilot study. Neurorehabilit. Neural Repair 2014, 28, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Pardo, O.M.; Lefebvre, S.; Neureither, M.; Saldana, D.; Jahng, E.; Liew, S.-L. Effects of a Brain-Computer Interface with Virtual Reality (VR) Neurofeedback: A Pilot Study in Chronic Stroke Patients. Front. Hum. Neurosci. 2019, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-W.; Lin, Y.-H.; Zhu, J.-D.; Wu, C.-Y.; Lin, Y.-P.; Chen, C.-C. Treatment Effects of Upper Limb Action Observation Therapy and Mirror Therapy on Rehabilitation Outcomes after Subacute Stroke: A Pilot Study. Behav. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sugg, K.; Müller, S.; Winstein, C.; Hathorn, D.; Dempsey, A. Does Action Observation Training with Immediate Physical Practice Improve Hemiparetic Upper-Limb Function in Chronic Stroke? Neurorehabilit. Neural Repair 2015, 29, 807–817. [Google Scholar] [CrossRef]
- Thieme, H.; Morkisch, N.; Mehrholz, J.; Pohl, M.; Behrens, J.; Borgetto, B.; Dohle, C. Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- von Carlowitz-Ghori, K.; Bayraktaroglu, Z.; Hohlefeld, F.U.; Losch, F.; Curio, G.; Nikulin, V.V. Corticomuscular coherence in acute and chronic stroke. Clin. Neurophysiol. 2014, 125, 1182–1191. [Google Scholar] [CrossRef]
- Krauth, R.; Schwertner, J.; Vogt, S.; Lindquist, S.; Sailer, M.; Sickert, A.; Lamprecht, J.; Perdikis, S.; Corbet, T.; del Millán, J.R.; et al. Cortico-Muscular Coherence Is Reduced Acutely Post-stroke and Increases Bilaterally During Motor Recovery: A Pilot Study. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, Y.; Liu, H. Corticomuscular Coherence and Its Applications: A Review. Front. Hum. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Rossiter, H.E.; Eaves, C.; Davis, E.; Boudrias, M.-H.; Park, C.; Farmer, S.; Barnes, G.; Litvak, V.; Ward, N.S. Changes in the location of cortico-muscular coherence following stroke. NeuroImage Clin. 2013, 2, 50–55. [Google Scholar] [CrossRef]
- Zheng, Y.; Peng, Y.; Xu, G.; Li, L.; Wang, J. Using Corticomuscular Coherence to Reflect Function Recovery of Paretic Upper Limb after Stroke: A Case Study. Front. Neurol. 2017, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.C.; Goldsmith, J.; Harran, M.D.; Xu, J.; Kim, N.; Schambra, H.M.; Luft, A.R.; Celnik, P.; Krakauer, J.W.; Kitago, T. A Short and Distinct Time Window for Recovery of Arm Motor Control Early After Stroke Revealed with a Global Measure of Trajectory Kinematics. Neurorehabilit. Neural Repair 2017, 31, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Lyle, R.C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Duncan, P.W.; Lai, S.M.; Bode, R.K.; Perera, S.; DeRosa, J. Stroke impact scale-16: A brief assessment of physical function. Neurology 2003, 60, 291–296. [Google Scholar] [CrossRef]
- Ryu, J.; Cooney, W.P.; Askew, L.J.; An, K.N.; Chao, E.Y.S. Functional ranges of motion of the wrist joint. J. Hand Surg. 1991, 16, 409–419. [Google Scholar] [CrossRef]
- Kennedy, R.S.; Lane, N.E.; Kevin, S.; Lilienthal, M.G. The International Journal of Aviation Psychology Simulator Sickness Questionnaire: An Enhanced Method for Quantifying Simulator Sickness. Int. J. Aerosp. Psychol. 1993, 3, 203–220. [Google Scholar] [CrossRef]
- Belardinelli, P.; Laer, L.; Ortiz, E.; Braun, C.; Gharabaghi, A. Plasticity of premotor cortico-muscular coherence in severely impaired stroke patients with hand paralysis. Neuroimage Clin. 2017, 14, 726–733. [Google Scholar] [CrossRef]
- Fang, Y.; Daly, J.J.; Sun, J.; Hvorat, K.; Fredrickson, E.; Pundik, S.; Sahgal, V.; Yue, G.H. Functional corticomuscular connection during reaching is weakened following stroke. Clin. Neurophysiol. 2009, 120, 994–1002. [Google Scholar] [CrossRef]
- Fisher, K.M.; Zaaimi, B.; Williams, T.L.; Baker, S.N.; Baker, M.R. Beta-band intermuscular coherence: A novel biomarker of upper motor neuron dysfunction in motor neuron disease. Brain 2012, 135, 2849–2864. [Google Scholar] [CrossRef]
- Mima, T.; Toma, K.; Koshy, B.; Hallett, M. Coherence Between Cortical and Muscular Activities After Subcortical Stroke. Stroke 2001, 32, 2597–2601. [Google Scholar] [CrossRef]
- Norton, J.A.; Gorassini, M.A. Changes in Cortically Related Intermuscular Coherence Accompanying Improvements in Locomotor Skills in Incomplete Spinal Cord Injury. J. Neurophysiol. 2006, 95, 2580–2589. [Google Scholar] [CrossRef]
- Pan, L.-L.H.; Yang, W.-W.; Kao, C.-L.; Tsai, M.-W.; Wei, S.-H.; Fregni, F.; Chen, V.C.-F.; Chou, L.-W. Effects of 8-week sensory electrical stimulation combined with motor training on EEG-EMG coherence and motor function in individuals with stroke. Sci. Rep. 2018, 8, 9217. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Andreas Edgar Kothe, C. Artifact Removal Techniques with Signal Reconstruction. U.S. Patent Application No. 14/895,440, 28 April 2016. [Google Scholar]
- Boonstra, T.W.; Breakspear, M. Neural mechanisms of intermuscular coherence: Implications for the rectification of surface electromyography. J. Neurophysiol. 2012, 107, 796–807. [Google Scholar] [CrossRef]
- Farina, D.; Negro, F.; Jiang, N. Identification of Common Synaptic Inputs to Motor Neurons from the Rectified Electromyogram. J. Physiol. (Lond.) 2013. [Google Scholar] [CrossRef]
- Mehrkanoon, S.; Breakspear, M.; Boonstra, T.W. The reorganization of corticomuscular coherence during a transition between sensorimotor states. NeuroImage 2014, 100, 692–702. [Google Scholar] [CrossRef]
- Baker, S.N. ‘Pooled coherence’ can overestimate the significance of coupling in the presence of inter-experiment variability. J. Neurosci. Methods 2000, 96, 171–172. [Google Scholar] [CrossRef]
- Halliday, D.M.; Rosenberg, J.R. On the application, estimation and interpretation of coherence and pooled coherence. J. Neurosci. Meth. 2000, 100, 173–174. [Google Scholar] [CrossRef]
- Amjad, A.M.; Halliday, D.M.; Rosenberg, J.R.; Conway, B.A. An extended difference of coherence test for comparing and combining several independent coherence estimates: Theory and application to the study of motor units and physiological tremor. J. Neurosci. Meth. 1997, 73, 69–79. [Google Scholar] [CrossRef]
- Carter, G.C. Coherence and time delay estimation. Proc. IEEE 1987, 75, 236–255. [Google Scholar] [CrossRef]
- Rosenberg, J.R.; Amjad, A.M.; Breeze, P.; Brillinger, D.R.; Halliday, D.M. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog. Biophys. Mol. Biol. 1989, 53, 1–31. [Google Scholar] [CrossRef]
- Kattla, S.; Lowery, M.M. Fatigue related changes in electromyographic coherence between synergistic hand muscles. Exp. Brain Res. 2010, 202, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Labstreaming Layer. Available online: https://github.com/sccn/labstreaminglayer (accessed on 22 June 2020).
- Vourvopoulos, A.; Bermúdezi Badia, S. Motor priming in virtual reality can augment motor-imagery training efficacy in restorative brain-computer interaction: A within-subject analysis. J. Neuroeng. Rehabil. 2016, 13, 1–14. [Google Scholar] [CrossRef]
- Ellis, M.D.; Schut, I.; Dewald, J.P.A. Flexion synergy overshadows flexor spasticity during reaching in chronic moderate to severe hemiparetic stroke. Clin. Neurophysiol. 2017, 128, 1308–1314. [Google Scholar] [CrossRef]
- Winstein, C.J.; Wolf, S.L.; Schweighofer, N. Task-Oriented Training to Promote Upper Extremity Recovery. In Stroke Recovery and Rehabilitation; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Kilner, J.M.; Baker, S.N.; Salenius, S.; Jousmäki, V.; Hari, R.; Lemon, R.N. Task-dependent modulation of 15-30 Hz coherence between rectified EMGs from human hand and forearm muscles. J. Physiol. 1999, 516, 559–570. [Google Scholar] [CrossRef]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically Important Differences for the Upper-Extremity Fugl-Meyer Scale in People with Minimal to Moderate Impairment Due to Chronic Stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef]
- Bundy, D.T.; Souders, L.; Baranyai, K.; Leonard, L.; Schalk, G.; Coker, R.; Moran, D.W.; Huskey, T.; Leuthardt, E.C. Contralesional Brain–Computer Interface Control of a Powered Exoskeleton for Motor Recovery in Chronic Stroke Survivors. Stroke 2017, 48, 1908–1915. [Google Scholar] [CrossRef]
- Kristeva, R.; Patino, L.; Omlor, W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. NeuroImage 2007, 36, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, T.W. The potential of corticomuscular and intermuscular coherence for research on human motor control. Front. Hum. Neurosci. 2013, 7, 855. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.F. Rhythmicity, synchronization and binding in human and primate motor systems. J. Physiol. 1998, 509, 3. [Google Scholar] [CrossRef]
- Mima, T.; Hallett, M. Corticomuscular coherence: A review. J. Clin. Neurophysiol. 1999, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Chwodhury, A.; Raza, H.; Dutta, A.; Nishad, S.S.; Saxena, A.; Prasad, G. A study on cortico-muscular coupling in finger motions for exoskeleton assisted neuro-rehabilitation. In Proceedings of the 2015 IEEE 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 4610–4614. [Google Scholar]
- Guo, Z.; Qian, Q.; Wong, K.; Zhu, H.; Huang, Y.; Hu, X.; Zheng, Y. Altered Corticomuscular Coherence (CMCoh) Pattern in the Upper Limb During Finger Movements After Stroke. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.H.; Zibrandtsen, I.C.; Wienecke, T.; Kjaer, T.W.; Christensen, M.S.; Nielsen, J.B.; Langberg, H. Corticomuscular coherence in the acute and subacute phase after stroke. Clin. Neurophysiol. 2017, 128, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.R.; Baker, S.N. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J. Physiol. 2003, 546, 931–942. [Google Scholar] [CrossRef]
- Matsuya, R.; Ushiyama, J.; Ushiba, J. Inhibitory interneuron circuits at cortical and spinal levels are associated with individual differences in corticomuscular coherence during isometric voluntary contraction. Sci. Rep. 2017, 7, 44417. [Google Scholar] [CrossRef]
- Power, H.A.; Norton, J.A.; Porter, C.L.; Doyle, Z.; Hui, I.; Chan, K.M. Transcranial direct current stimulation of the primary motor cortex affects cortical drive to human musculature as assessed by intermuscular coherence. J. Physiol. 2006, 577, 795–803. [Google Scholar] [CrossRef]
- Braun, C.; Staudt, M.; Schmitt, C.; Preissl, H.; Birbaumer, N.; Gerloff, C. Crossed cortico-spinal motor control after capsular stroke. Eur. J. Neurosci. 2007, 25, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- de Noordhout, A.M.; Rapisarda, G.; Bogacz, D.; Gérard, P.; De Pasqua, V.; Pennisi, G.; Delwaide, P.J. Corticomotoneuronal synaptic connections in normal man: An electrophysiological study. Brain 1999, 122 Pt 7, 1327–1340. [Google Scholar] [CrossRef]
- Palmer, E.; Ashby, P. Corticospinal projections to upper limb motoneurones in humans. J. Physiol. (Lond.) 1992, 448, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.-T.; Francisco, G.E.; Zhou, P.; Rymer, W.Z. A Unifying Pathophysiological Account for Post-stroke Spasticity and Disordered Motor Control. Front Neurol. 2019, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- McPherson, J.G.; Chen, A.; Ellis, M.D.; Yao, J.; Heckman, C.J.; Dewald, J.P.A. Progressive recruitment of contralesional cortico-reticulospinal pathways drives motor impairment post stroke. J. Physiol. 2018, 596, 1211–1225. [Google Scholar] [CrossRef]
- Owen, M.; Ingo, C.; Dewald, J.P.A. Upper Extremity Motor Impairments and Microstructural Changes in Bulbospinal Pathways in Chronic Hemiparetic Stroke. Front. Neurol. 2017, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Maslovat, D.; Drummond, N.M.; Hajj, J.; Leguerrier, A.; Carlsen, A.N. High-intensity transcranial magnetic stimulation reveals differential cortical contributions to prepared responses. J. Neurophysiol. 2019, 121, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.O.; Andrade, C.I. On the relationship between features extracted from EMG and force for constant and dynamic protocols. In Proceedings of the 2012 IEEE Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 3392–3395. [Google Scholar]
- Suresh, N.L.; Concepcion, N.S.; Madoff, J.; Rymer, W.Z. Anomalous EMG–force relations during low-force isometric tasks in hemiparetic stroke survivors. Exp. Brain Res. 2015, 233, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Sakurada, T.; Koike, Y.; Kansaku, K. A hybrid BMI-based exoskeleton for paresis: EMG control for assisting arm movements. J. Neural Eng. 2017, 14, 016015. [Google Scholar] [CrossRef] [PubMed]
- Leeb, R.; Sagha, H.; Chavarriaga, R.; del Millán, J.R. A hybrid brain–computer interface based on the fusion of electroencephalographic and electromyographic activities. J. Neural Eng. 2011, 8, 025011. [Google Scholar] [CrossRef]
- REINVENT Data. Available online: https://github.com/npnl/REINVENT_data (accessed on 22 June 2020).



| Participant | Sex | Age | Onset (Months) | Paresis | FMA-UE | MOCA |
|---|---|---|---|---|---|---|
| 1 | Male | 66 | 34 | Left | 19 | 23 |
| 2 | Male | 42 | 34 | Right | 22 | 17 |
| 3 | Male | 64 | 56 | Left | 14 | 22 |
| 4 | Female | 53 | 28 | Left | 20 | 22 |
| Assessment | t | p | Pre | Post |
|---|---|---|---|---|
| ARAT | 2.61 | 0.079 | 5.75 (8.85) | 7 (9.38) |
| Extension | 2.27 | 0.108 | 6.75 (7.81) | 10.5 (8.19) |
| FMA-UE | 2.43 | 0.093 | 18.75 (3.40) | 23.25 (4.19) |
| Grip More Imp. | 1.25 | 0.299 | 8.67 (6.99) | 9.44 (5.78) |
| SIS-16 | 5.67 | 0.011 * | 58.5 (10.08) | 62.75 (9.29) |
| Activity | t | p | Pre | Post |
|---|---|---|---|---|
| ER | 2.58 | 0.082 | 8.94 × 10−16 (1.65 × 10−15) | 0.80 (0.62) |
| Extensors | 1.81 | 0.168 | −1.99 × 10−16 (1.32 × 10−15) | 1.41 (1.56) |
| Grip | 1.53 | 0.224 | 7.67 (6.39) | 10.13 (4) |
| Flexors | 0.91 | 0.431 | −4.71 × 10−16 (7.37 × 10−16) | 0.51 (1.11) |
| Success | −0.40 | 0.719 | 57.29 (7.65) | 55.83 (5.57) |
| Threshold | −0.03 | 0.981 | 36.82 (19.35) | 36.44 (21.44) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin-Pardo, O.; Laine, C.M.; Rennie, M.; Ito, K.L.; Finley, J.; Liew, S.-L. A Virtual Reality Muscle–Computer Interface for Neurorehabilitation in Chronic Stroke: A Pilot Study. Sensors 2020, 20, 3754. https://doi.org/10.3390/s20133754
Marin-Pardo O, Laine CM, Rennie M, Ito KL, Finley J, Liew S-L. A Virtual Reality Muscle–Computer Interface for Neurorehabilitation in Chronic Stroke: A Pilot Study. Sensors. 2020; 20(13):3754. https://doi.org/10.3390/s20133754
Chicago/Turabian StyleMarin-Pardo, Octavio, Christopher M. Laine, Miranda Rennie, Kaori L. Ito, James Finley, and Sook-Lei Liew. 2020. "A Virtual Reality Muscle–Computer Interface for Neurorehabilitation in Chronic Stroke: A Pilot Study" Sensors 20, no. 13: 3754. https://doi.org/10.3390/s20133754
APA StyleMarin-Pardo, O., Laine, C. M., Rennie, M., Ito, K. L., Finley, J., & Liew, S.-L. (2020). A Virtual Reality Muscle–Computer Interface for Neurorehabilitation in Chronic Stroke: A Pilot Study. Sensors, 20(13), 3754. https://doi.org/10.3390/s20133754





