Analysis of Control Characteristics between Dominant and Non-Dominant Hands by Transient Responses of Circular Tracking Movements in 3D Virtual Reality Space
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Experimental Setup
2.2. Experimental Procedures
2.3. Movement Tasks
2.4. Data Analysis
3. Results
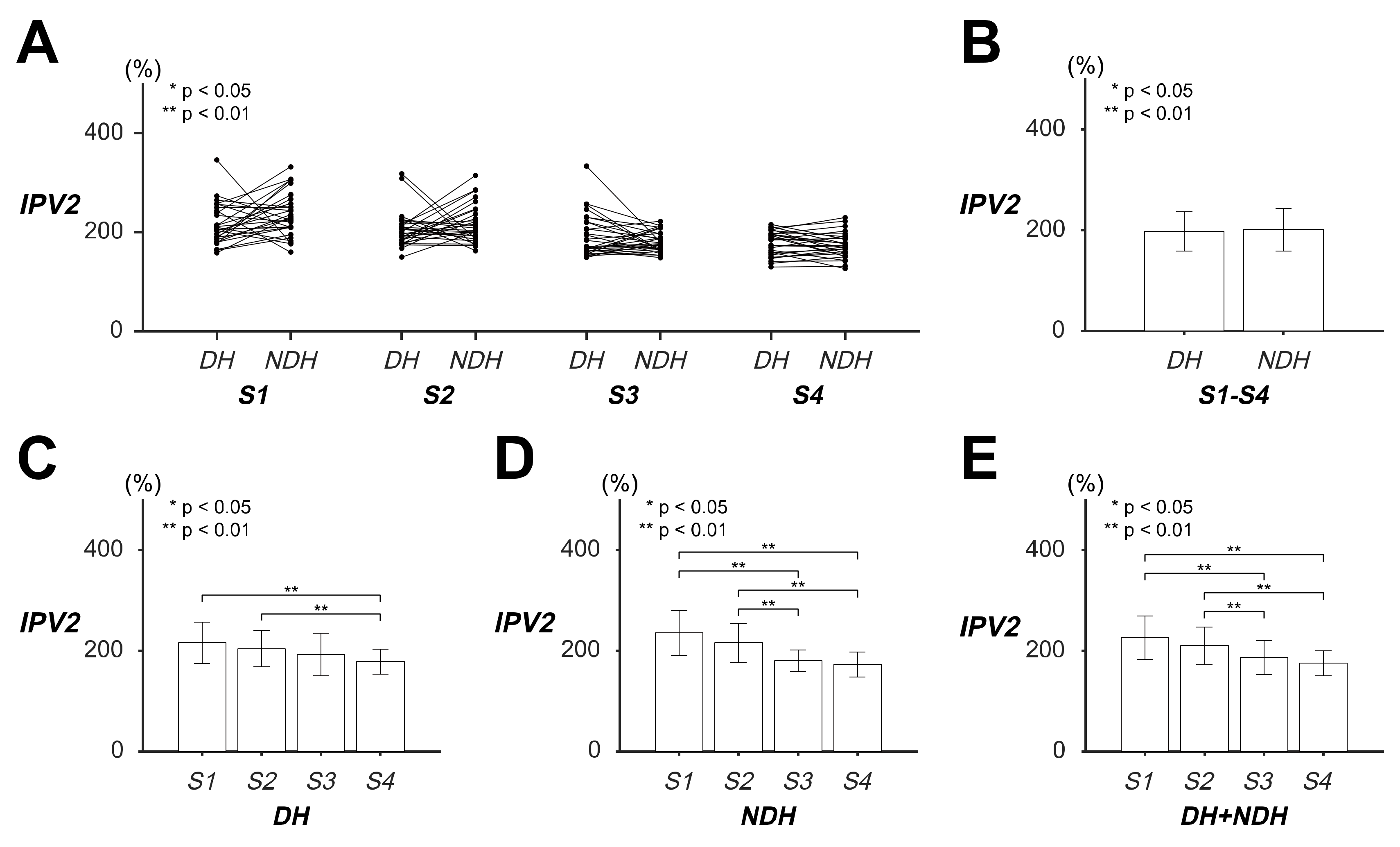
3.1. Differences in the Transient Response of Tracking Movement Based on IPV2
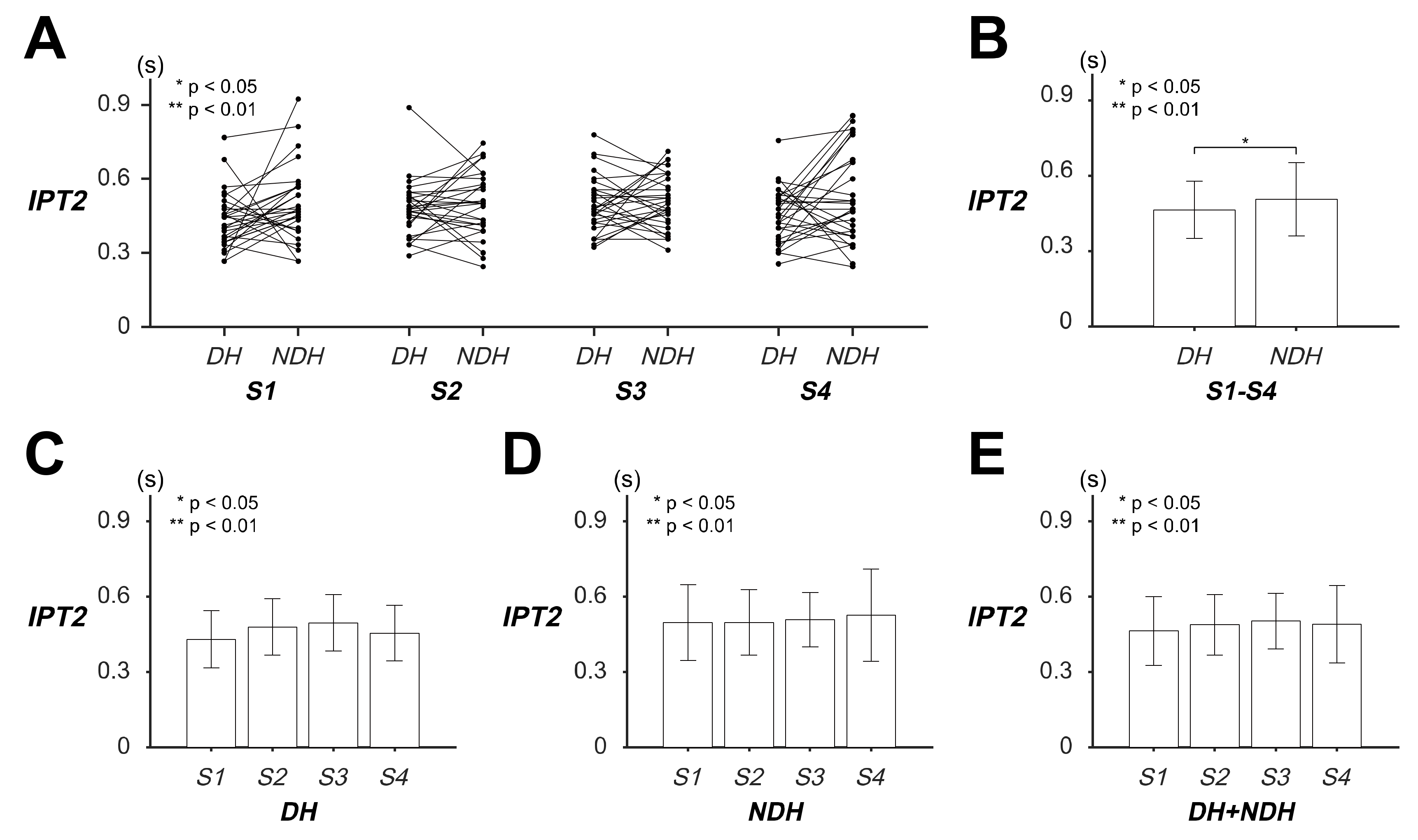
3.2. Differences in Transient Response of Tracking Movement Based on IPT2
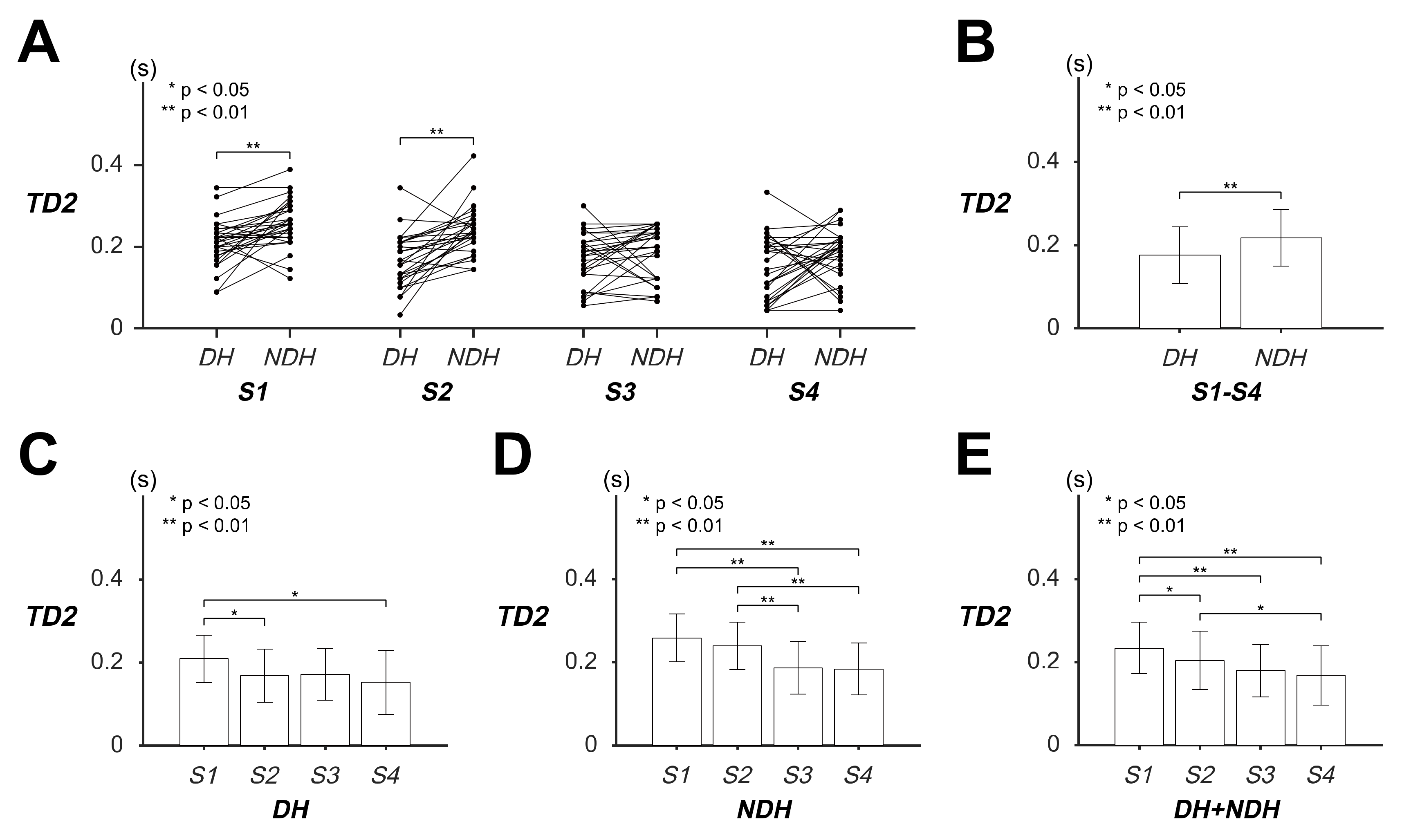
3.3. Differences in Transient Response of Tracking Movement Based on TD2
4. Discussion
4.1. Speed-Dependent Features of IPV2
4.2. Temporal Analysis of IPT2 and TD2
4.3. Clinical Application and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wolpert, D.M.; Ghahramani, Z.; Jordan, M.I. An internal model for sensorimotor integration. Science 1995, 269, 1880. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Miall, R.C.; Kawato, M. Internal models in the cerebellum. Trends Cogn. Sci. 1998, 2, 338–347. [Google Scholar] [CrossRef]
- Flash, T.; Hogan, N. The coordination of arm movements: An experimentally confirmed mathematical model. J. Neurosci. 1985, 5, 1688–1703. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Kawato, M.; Suzuki, R. Formation and control of optimal trajectory in human multijoint arm movement. Biol. Cybern. 1989, 61, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.D.; Scheidt, R.A.; Reinkensmeyer, D.J. Impedance control and internal model formation when reaching in a randomly varying dynamical environment. J. Neurophysiol. 2001, 86, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Kakei, S.; Hoffman, D.S.; Strick, P.L. Muscle and movement representations in the primary motor cortex. Science 1999, 285, 2136–2139. [Google Scholar] [CrossRef]
- Bastian, A.J. Learning to predict the future: The cerebellum adapts feedforward movement control. Curr. Opin. Neurol. 2006, 16, 645–649. [Google Scholar] [CrossRef]
- Todorov, E. Optimality principles in sensorimotor control. Nat. Neurosci. 2004, 7, 907–915. [Google Scholar] [CrossRef]
- Kakei, S.; Hoffman, D.S.; Strick, P.L. Sensorimotor transformations in cortical motor areas. Neurosci. Res. 2003, 46, 1–10. [Google Scholar] [CrossRef]
- Kambara, H.; Shin, D.; Koike, Y. A computational model for optimal muscle activity considering muscle viscoelasticity in wrist movements. J. Neurophysiol. 2013, 109, 2145–2160. [Google Scholar] [CrossRef]
- Yokota, H.; Naito, M.; Mizuno, N.; Ohshima, S. Framework for visual-feedback training based on a modified self-organizing map to imitate complex motion. Proc. Inst. Mech. Eng. Part P 2020, 234, 49–58. [Google Scholar] [CrossRef]
- Giannousi, M.; Kioumourtzoglou, E. The effects of verbal and visual feedback on performance and learning freestyle swimming in novice swimmers. Kinesiology 2017, 49, 65–73. [Google Scholar] [CrossRef]
- Ewerton, M.; Rother, D.; Weimar, J.; Kollegger, G.; Wiemeyer, J.; Peters, J.; Maeda, G. Assisting movement training and execution with visual and haptic feedback. Front. Neurorobot. 2018, 12, 1–18. [Google Scholar] [CrossRef]
- Steinberg, F.; Pixa, N.H.; Doppelmayr, M. Mirror visual feedback training improves intermanual transfer in a sport-specific task: A comparison between different skill levels. Neural Plast. 2016, 1–11. [Google Scholar] [CrossRef]
- Knudsen, E.W.; Holledig, M.; Knudsen, E.W.; Holledig, M.; Bach-Nielsen, S.S.; Peterson, R.K.; Zanescu, B.-C.; Nielsen, M.J.; Helweg, K.; Overholt, D.; et al. Audio-visual feedback for self-monitoring posture in ballet training. In Proceedings of the NIME 2017, Copenhagen, Denmark, 15–18 May 2017; 2017; pp. 71–76. [Google Scholar]
- Zajac, F.E. Muscle coordination of movement: A perspective. J. Biomech. 1993, 26, 109–124. [Google Scholar] [CrossRef]
- Scott, S.H.; Cluff, T.; Lowrey, C.R.; Takei, T. Feedback control during voluntary motor actions. Curr. Opin. Neurobiol. 2015, 33, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Wakeling, J.M.; Blake, O.M.; Chan, H.K. Muscle coordination is key to the power output and mechanical efficiency of limb movements. J. Exp. Biol. 2010, 213, 487–492. [Google Scholar] [CrossRef]
- Sabes, P.N. The planning and control of reaching movements. Curr. Opin. Neurol. 2000, 10, 740–746. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Ghilardi, M.-F.; Ghez, C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat. Neurosci. 1999, 2, 1026–1031. [Google Scholar] [CrossRef]
- Georgopoulos, A.P.; Grillner, S. Visuomotor coordination in reaching and locomotion. Science 1989, 245, 1209–1210. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M.; Arbib, M.A.; Rizzolatti, G.; Sakata, H. Grasping objects: The cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995, 18, 314–320. [Google Scholar] [CrossRef]
- Lacquaniti, F.; Caminiti, R. Visuo-motor transformations for arm reaching. Eur. J. Neurosci. 1998, 10, 195–203. [Google Scholar] [CrossRef]
- Zelaznik, H.N.; Hawkins, B.; Kisselburgh, L. Rapid visual feedback processing in single aiming movements. J. Mot. Behav. 1983, 15, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.C.; Soechting, J.F. Manual tracking in two dimensions. J. Neurophysiol. 2000, 83, 3483–3496. [Google Scholar] [CrossRef] [PubMed]
- Ishida, F.; Sawada, Y.E. Human hand moves proactively to the external stimulus: An evolutional strategy for minimizing transient error. Phys. Rev. Lett. 2004, 93, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Miall, R.; Weir, D.; Stein, J. Planning of movement parameters in a visuo-motor tracking task. Behav. Brain Res. 1988, 27, 1–8. [Google Scholar] [CrossRef]
- Russell, D.M.; Sternad, D. Sinusoidal visuomotor tracking: Intermittent servo-control or coupled oscillations? J. Mot. Behav. 2001, 33, 329–349. [Google Scholar] [CrossRef]
- Miall, R.; Weir, D.; Stein, J. Manual tracking of visual targets by trained monkeys. Behav. Brain Res. 1986, 20, 185–201. [Google Scholar] [CrossRef]
- Gollee, H.; Mamma, A.; Loram, I.D.; Gawthrop, P.J. Frequency-domain identification of the human controller. Biol. Cybern. 2012, 106, 359–372. [Google Scholar] [CrossRef]
- Miall, R.; Weir, D.; Stein, J. Intermittency in human manual tracking tasks. J. Mot. Behav. 1993, 27, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Sakaguchi, Y. Periodic change in phase relationship between target and hand motion during visuo-manual tracking task: Behavioral evidence for intermittent control. Hum. Mov. Sci. 2014, 33, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Roitman, A.V.; Massaquoi, S.G.; Takahashi, K.; Ebner, T.J. Kinematic analysis of manual tracking in monkeys: Characterization of movement intermittencies during a circular tracking task. J. Neurophysiol. 2004, 91, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Roitman, A.V.; Pasalar, S.; Johnson, M.T.V.; Ebner, T.J. Position, direction of movement, and speed tuning of cerebellar purkinje cells during circular manual tracking in monkey. J. Neurosci. 2005, 25, 9244–9257. [Google Scholar] [CrossRef] [PubMed]
- Roitman, A.V.; Pasalar, S.; Ebner, T.J. Single trial coupling of purkinje cell activity to speed and error signals during circular manual tracking. Exp. Brain Res. 2008, 192, 241–251. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kakei, S.; Kim, J. Motor control characteristics for circular tracking movements of human wrist. Adv. Robot. 2017, 31, 29–39. [Google Scholar] [CrossRef]
- Choi, W.; Lee, J.; Yanagihara, N.; Li, L.; Kim, J. Development of a quantitative evaluation system for visuo-motor control in three-dimensional virtual reality space. Sci. Rep. 2018, 8, 134–139. [Google Scholar] [CrossRef]
- Choi, W.; Lee, J.; Li, L. Analysis of three-dimensional circular tracking movements based on temporo-spatial parameters in polar coordintates. Appl. Sci. 2020, 10, 621. [Google Scholar] [CrossRef]
- Yoshikatsu, H.; Yurie, T.; Kazuya, S.; Ken, S.; Yasuji, S. Intermittently-visual tracking experiments reveal the roles of error-correction and predictive mechanisms in the human visual-motor control system. Proc. Soc. Instrum. Control Eng. 2010, 46, 391–400. [Google Scholar]
- Fine, J.M.; Ward, K.L.; Amazeen, E.L. Manual coordination with intermittent targets: Velocity information for prospective control. Acta Psychol. 2014, 149, 24–31. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sawada, Y. Transition from an antiphase error-correction mode to a synchronization mode in interaction hand tracking. Phys. Rev. E 2013, 88, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Keele, S.W.; Posner, M.I. Processing of visual feedback in rapid movements. J. Exp. Psychol. 1968, 77, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kagamihara, Y.; Tomatsu, S.; Kakei, S. The functional role of the cerebellum in visually guided tracking movement. Cerebellum 2012, 11, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Tanaka, R. Contribution of kinesthesia on human visuomotor elbow tracking movements. Neurosci. Lett. 1981, 26, 245–249. [Google Scholar] [CrossRef]
- Beppu, H.; Nagaoka, M.; Tanaka, R. Analysis of cerebellar motor disorders by visually-guided elbow tracking movement. Brain 1984, 107, 787–809. [Google Scholar] [CrossRef]
- Beppu, H.; Nagaoka, M.; Tanaka, R. Analysis of cerebellar motor disorders by visually-guided elbow tracking movement. 2. contribution of the visual cues on slow ramp pursuit. Brain 1987, 110, 1–18. [Google Scholar] [CrossRef]
- Mathew, J.; Sarlegna, F.R.; Bernier, P.-M.; Danion, F.R. Handedness matters for motor control but not for prediction. eNeuro 2019, 93, 1–4. [Google Scholar] [CrossRef]
- Hoffmann, E.R. Movement time of right- and left-handers using their preferred and non-preferred hands. Int. J. Ind. Ergon. 1997, 19, 49–57. [Google Scholar] [CrossRef]
- Simon, J.R.; De Crow, T.W.; Lincoln, R.S.; Smith, K.U. Effects of handedness on tracking accuracy. Mot. Skills Res. Exchange 1952, 4, 53–57. [Google Scholar] [CrossRef]
- Flowers, K. Handedness and controlled movement. Br. J. Psychol. 1975, 66, 39–52. [Google Scholar] [CrossRef]
- Todor, J.I.; Kyprie, P.M.; Price, H.L. Lateral asymmetries in arm, wrist and finger movements. Cortex 1982, 18, 515–523. [Google Scholar] [CrossRef]
- Wiberg, A.; Ng, M.; Omran, Y.A.; Alfaro-Almagro, F.; McCarthy, P.; Marchini, J.; Bennett, D.L.; Smith, S.; Douaud, G.; Furniss, D. Handedness, language areas and neuropsychiatric diseases: Insights from brain imaging and genetics. Brain 2019, 142, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Parma, V.; Brasselet, R.; Zoia, S.; Bulgheroni, M.; Castiello, U. The origin of human handedness and its role in pre-birth motor control. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Golnarafhi, F.; Kuo, B.C. Automatic Control Systems, 9th ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 283–293. [Google Scholar]
- Wing, A.M. Motor control: Mechanisms of motor equivalence in handwriting. Curr. Biol. 2000, 10, 245–248. [Google Scholar] [CrossRef]
- Plamondon, R.; Alimi, A.M. Speed/accuracy trade-offs in target-directed movements. Behav. Brain Sci. 1997, 20, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Parziale, A.; Senatore, R.; Marcelli, A. Exploring speed–accuracy tradeoff in reaching movements: A neurocomputational model. Neural Comput. Appl. 2020, 1–27. [Google Scholar] [CrossRef]
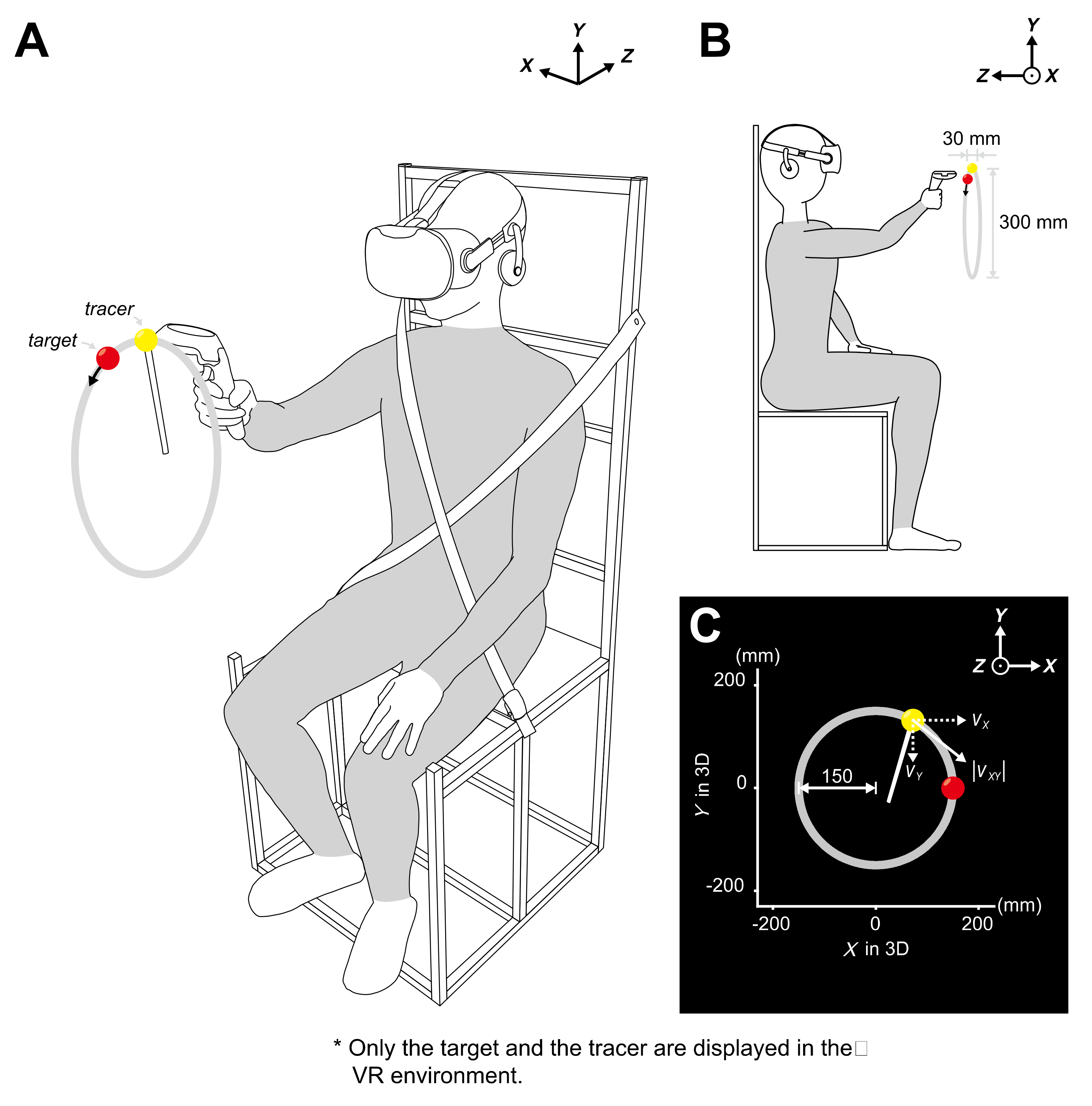

| Subject No. | Age (Years) | Sex | Dominant Hand |
|---|---|---|---|
| 1 | 25 | M | R |
| 2 | 24 | M | R |
| 3 | 24 | F | L |
| 4 | 24 | M | R |
| 5 | 25 | M | R |
| 6 | 22 | F | R |
| 7 | 22 | F | R |
| 8 | 22 | F | R |
| 9 | 22 | F | R |
| 10 | 24 | M | L |
| 11 | 26 | M | R |
| 12 | 22 | M | R |
| 13 | 22 | F | R |
| 14 | 23 | F | R |
| 15 | 23 | F | R |
| 16 | 22 | F | R |
| 17 | 35 | M | R |
| 18 | 24 | F | R |
| 19 | 26 | M | R |
| 20 | 25 | M | R |
| 21 | 23 | M | R |
| 22 | 26 | M | R |
| 23 | 26 | M | R |
| 24 | 24 | M | R |
| 25 | 23 | M | R |
| 26 | 23 | M | L |
| 27 | 25 | M | R |
| 28 | 33 | M | R |
| 29 | 30 | M | R |
| Variable | Hand Factor | Speed Factor | Total | |||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |||
| IPV2 (%) | DH | 216.01 (±40.992) | 204.53 (±36.119) | 192.56 (±42.834) | 178.31 (±24.868) | 197.85 (±39.011) |
| NDH | 235.65 (±44.350) | 215.90 (±38.679) | 180.60 (±20.895) | 172.86 (±25.152) | 201.25 (±42.034) | |
| DH + NDH | 225.83 (±43.471) | 210.21 (±37.532) | 186.58 (±33.944) | 175.58 (±24.942) | ||
| IPT2 (s) | DH | 0.4299 (±0.1142) | 0.4789 (±0.1126) | 0.4954 (±0.1126) | 0.4540 (±0.1105) | 0.4646 (±0.1138) |
| NDH | 0.4966 (±0.1513) | 0.4966 (±0.1306) | 0.5084 (±0.1087) | 0.5261 (±0.1837) | 0.5069 (±0.1448) | |
| DH + NDH | 0.4632 (±0.1371) | 0.4877 (±0.1212) | 0.5019 (±0.1099) | 0.4900 (±0.1546) | ||
| TD2 (s) | DH | 0.2092 (±0.0571) | 0.1686 (±0.0645) | 0.1720 (±0.0626) | 0.1525 (±0.0768) | 0.1756 (±0.0681) |
| NDH | 0.2586 (±0.0579) | 0.2395 (±0.0571) | 0.1870 (±0.0637) | 0.1843 (±0.0626) | 0.2173 (±0.0679) | |
| DH + NDH | 0.2339 (±0.0622) | 0.2040 (±0.0701) | 0.1795 (±0.0631) | 0.1684 (±0.0713) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.; Choi, W.; Jo, H.; Lee, G.; Kim, J. Analysis of Control Characteristics between Dominant and Non-Dominant Hands by Transient Responses of Circular Tracking Movements in 3D Virtual Reality Space. Sensors 2020, 20, 3477. https://doi.org/10.3390/s20123477
Park W, Choi W, Jo H, Lee G, Kim J. Analysis of Control Characteristics between Dominant and Non-Dominant Hands by Transient Responses of Circular Tracking Movements in 3D Virtual Reality Space. Sensors. 2020; 20(12):3477. https://doi.org/10.3390/s20123477
Chicago/Turabian StylePark, Wookhyun, Woong Choi, Hanjin Jo, Geonhui Lee, and Jaehyo Kim. 2020. "Analysis of Control Characteristics between Dominant and Non-Dominant Hands by Transient Responses of Circular Tracking Movements in 3D Virtual Reality Space" Sensors 20, no. 12: 3477. https://doi.org/10.3390/s20123477
APA StylePark, W., Choi, W., Jo, H., Lee, G., & Kim, J. (2020). Analysis of Control Characteristics between Dominant and Non-Dominant Hands by Transient Responses of Circular Tracking Movements in 3D Virtual Reality Space. Sensors, 20(12), 3477. https://doi.org/10.3390/s20123477







