Reconstruction of 12-Lead Electrocardiogram from a Three-Lead Patch-Type Device Using a LSTM Network
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Setup
2.2.1. Hardware
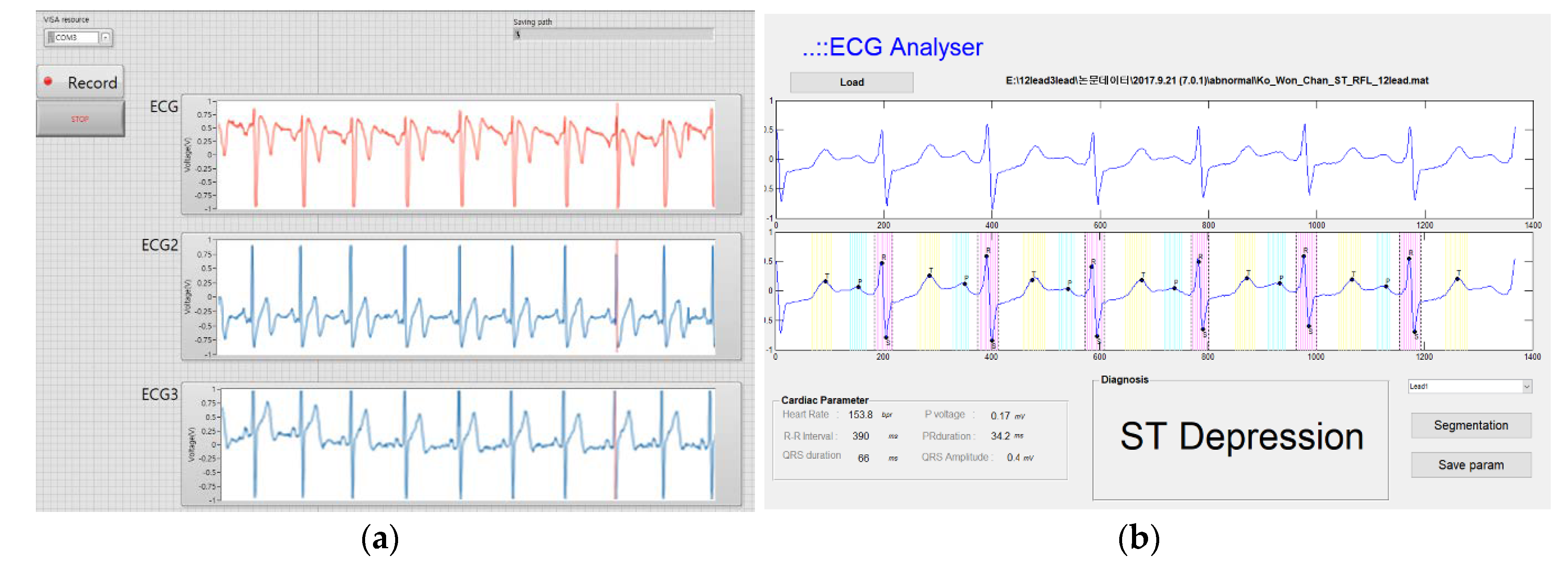
2.2.2. Software
2.3. Measurement Protocols (Data Acquisition Program)
2.4. Reconstruction Model
2.4.1. Linear Regression
2.4.2. Long Short-Term (LSTM) Network
2.5. Statistics
3. Results
3.1. Identicalness
3.2. Measurement of Normal Parameters
3.3. Detection of Pathologic Findings
3.3.1. Left Ventricular Hypertrophy (LVH)
3.3.2. ST Change
3.3.3. Wide QRS
3.4. Training and Test Performed with a Time Interval
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Plonsey, J.M. 12-lead ECG system. Bioelectromagnetism 1995, 15, 23–34. [Google Scholar]
- Finlay, D.D.; Nugent, C.D.; Kellett, J.G.; Donnelly, M.P.; McCullagh, P.J.; Black, N.D. Synthesising the 12-lead electrocardiogram: Trends and challenges. Eur. J. Intern. Med. 2007, 18, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Dower, G.E.; Yakush, A.; Nazzal, S.B.; Jutzy, R.V.; Ruiz, C.E. Deriving the 12-lead electrocardiogram from four (EASI) electrodes. J. Electrocardiol 1988, 21, S182–S187. [Google Scholar] [CrossRef]
- Tomasic, I.; Frljak, S.; Trobec, R. Estimating the Universal Positions of Wireless Body Electrodes for Measuring Cardiac Electrical Activity. IEEE. Trans. Biomed. Eng. 2013, 60, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, D.S.; Kwon, H.B.; Kim, D.Y.; Park, K.S. Reconstruction of 12-lead ECG Using a Single-patch Device. Methods Inf. Med. 2017, 56, 319–327. [Google Scholar] [CrossRef]
- Hadzievski, L.; Bojovic, B.; Vukcevic, V.; Belicev, P.; Pavlovic, S.; Vasilijevic-Pokrajcic, Z. A novel mobile transtelephonic system with synthesized 12-lead ECG. IEEE Trans. Inf. Technol. Biomed. 2004, 8, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Gulrajani, R.M. The forward and inverse problems of electrocardiography. IEEE Eng. Med. Biol. Mag. 1998, 17, 84–101. [Google Scholar] [CrossRef]
- Atoui, H.; Fayn, J.; Rubel, P. A neural network approach for patient-specific 12-lead ECG synthesis in patient monitoring environments. Comput. Cardiol. 2004, 31, 161–164. [Google Scholar]
- Atoui, H.; Fayn, J.; Rubel, P. A novel neural-network model for deriving standard 12-lead ECGs from serial three-lead ECGs: Application to self-care. IEEE Trans. Inf. Technol. Biomed 2010, 14, 883–890. [Google Scholar] [CrossRef]
- Hou, B.; Yang, J.; Wang, P.; Yan, R. LSTM Based Auto-Encoder Model for ECG Arrhythmias Classification. IEEE Trans. Instrum. Meas. 2019, 69, 1232–1240. [Google Scholar] [CrossRef]
- Puurtinen, M.; Hyttinen, J.; Malmivuo, J. Optimizing bipolar electrode location for wireless ECG measurement-analysis of ECG signal strength and deviation between individuals. Int. J. Bioelectro. magn. 2005, 7, 236–239. [Google Scholar]
- Puurtinen, M.; Viik, J.; Hyttinen, J. Best electrode locations for a small bipolar ECG device: Signal strength analysis of clinical data. Ann. Biomed. Eng. 2009, 37, 331–336. [Google Scholar] [CrossRef]
- Bailey, J.J.; Berson, A.S.; GarsonJr, A.; Horan, L.G.; Macfarlane, P.W.; Mortara, D.W.; Zywietz, C.Z. Recommendations for standardization and specifications in automated electrocardiography: Bandwidth and digital signal processing. A report for health professionals by an ad hoc writing group of the Committee on Electrocardiography and Cardiac Electrophysiology of the Council on Clinical Cardiology, American Heart Association. Circulation 1990, 81, 730–739. [Google Scholar] [PubMed]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Laguna, P.; Jane, R.; Caminal, P. Automatic detection of wave boundaries in multilead ECG signals: Validation with the CSE database. Comput. Biomed. Res. 1994, 27, 45–60. [Google Scholar] [CrossRef]
- Trobec, R.; Tomasic, I. Synthesis of the 12-lead electrocardiogram from differential leads. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 615–621. [Google Scholar] [CrossRef]
- Tomasic, I.; Trobec, R. Electrocardiographic systems with reduced numbers of leads-synthesis of the 12-lead ECG. IEEE Rev. Biomed. Eng. 2014, 7, 126–142. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Scherer, J.A.; Jenkins, J.M.; Nicklas, J.M. Synthesis of the 12-lead electrocardiogram from a 3-lead subset using patient-specific transformation vectors. An algorithmic approach to computerized signal synthesis. J. Electrocardiol 1989, 22, 128. [Google Scholar] [CrossRef]
- Cady, L.D. Computed relationship of standard electrocardiographic leads. Med. Res. Eng. 1969, 8, 37–42. [Google Scholar]
- Nelwan, S.P.; Kors, J.A.; Meij, S.H.; Van Bemmel, J.H.; Simoons, M.L. Reconstruction of the 12-lead electrocardiogram from reduced lead sets. J. Electrocardiol 2004, 37, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Soman, V.V.; Lombardi, J.P.; Rajbhandari, P.P.; Dhakal, T.P.; Wilson, W.; Poliks, M.; Ghose, K.; Turner, J.N.; Jin, Z. Heart Monitor Using Flexible Capacitive ECG Electrodes. IEEE Trans. Instrum. Meas. 2019, 10, 1–8. [Google Scholar]
- Lobodzinski, S.S. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog. Cardiovasc. Dis. 2013, 56, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Torfs, T.; Smeets, C.P.; Geng, D.; Berset, T.; Auwera, J.V.; Vandervoort, P.; Grieten, L. Clinical validation of a low-power and wearable ECG patch for long term full-disclosure monitoring. J. Electrocardiol 2014, 47, 881–889. [Google Scholar] [CrossRef]
- Lee, D.; Kwon, H.; Lee, H.; Seo, C.; Park, K. Optimal Lead Position in Patch-Type Monitoring Sensors for Reconstructing 12-Lead ECG Signals with Universal Transformation Coefficient. Sensors 2020, 20, 963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Frick, K. All-ECG: A Least-number of Leads ECG Monitor for Standard 12-lead ECG Tracking during Motion. In Proceedings of the 2019 IEEE Healthcare Innovations and Point of Care Technologies, Bethesda, MD, USA, 20–22 November 2019; pp. 103–106. [Google Scholar] [CrossRef]
- Tomasic, I.; Trobec, R.; Lindén, M. Can the regression trees be used to model relation between ECG leads. In International Internet of Things Summit; Springer: Rome, Italy, 2015; pp. 467–472. [Google Scholar]
- Zhu, H.; Pan, Y.; Cheng, K.T.; Huan, R. A lightweight piecewise linear synthesis method for standard 12-lead ECG signals based on adaptive region segmentation. PLoS ONE 2018, 13, e0206170. [Google Scholar] [CrossRef]










| Outcome | I | II | III | aVR | aVL | aVF | V1 | V2 | V3 | V4 | V5 | V6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient (mean) | Linear Regression | 0.76 | 0.75 | 0.70 | 0.77 | 0.68 | 0.71 | 0.75 | 0.77 | 0.78 | 0.75 | 0.74 | 0.75 |
| LSTM Network | 0.92 | 0.96 | 0.95 | 0.96 | 0.93 | 0.95 | 0.93 | 0.96 | 0.96 | 0.96 | 0.96 | 0.95 | |
| Root Mean Square Error (μV) (mean) | Linear Regression | 23.40 | 74.46 | 64.09 | 45.16 | 68.26 | 32.05 | 34.69 | 48.58 | 58.90 | 59.49 | 66.53 | 62.27 |
| LSTM Network | 20.81 | 17.42 | 18.01 | 47.76 | 20.85 | 18.02 | 20.68 | 15.56 | 16.12 | 16.06 | 16.09 | 17.58 | |
| ECG Parameter | Standard ECG | Synthesized ECG by Linear Regression | Synthesized ECG by LSTM Network | p-value | |
|---|---|---|---|---|---|
| Axis (degree) | 58.6 ± 35.2 | 60.2 ± 35.0 | 61.0 ± 35.0 | n.s | |
| P wave: Amplitude in lead II (mV) | 1.73 ± 0.63 * | 1.17 ± 0.93 * | 1.57 ± 0.72 | 0.004 | |
| PR interval (ms) | 152.7 ± 22.6 | 143.4 ± 35.1 | 148.5 ± 27.8 | n.s | |
| QRS | Duration (ms) | 57.4 ± 11.0 | 56.9 ± 9.2 | 56.0 ± 10.3 | n.s |
| Total Voltage (mV) | 140.7 ± 31.2 * | 110.2 ± 30.6 * | 139.9 ± 32.4 | 0.004 | |
| QT duration (ms) | 57.4 ± 11.0 | 56.9 ± 9.2 | 56.0 ± 10.3 | n.s | |
| T wave amplitude (mV) | V4 | 2.5 ± 2.1 | 2.6 ± 1.7 | 2.6 ± 2.3 | n.s |
| V5 | 2.5 ± 2.0 | 2.6 ± 1.9 | 2.5 ± 2.2 | n.s | |
| V6 | 2.1 ± 1.6 | 2.1 ± 1.5 | 2.2 ± 1.7 | n.s | |
| Synthesized ECG by Linear Regression | Synthesized ECG by LSTM Network | |||
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| LVH (n = 11) | 64 | 100 | 100 | 100 |
| ST elevation ant. and Inf. combined (n = 6) | 63 | 95 | 100 | 100 |
| ST depression in inf. and lat. combined (n = 13) | 46 | 88 | 100 | 100 |
| Wide QRS (n = 6) | 100 | 83 | 100 | 100 |
| Pathologic Q (n = 8) | 86 | 96 | 100 | 100 |
| T wave inversion in V4-6 (n = 12) | 83 | 100 | 92 | 100 |
| Study | Source ECG | Synthesis Method | Subjects | Performance (Average CC) |
|---|---|---|---|---|
| Atoui, H et al. [8] | Subset of standard ECG (I, II, V2) | Neural Network and Linear Regression | 120 patients | 0.948 |
| Trobec, R et al. [16] | Three bipolar lead | Linear Regression | 30 normal, 35 patients | 0.959 (median) |
| Lee, D et al. [25] | Three bipolar lead | Artificial Neural Network | 14 normal | 0.920 |
| Zhang, Q et al. [26] | Subset of standard ECG (I, II, V2) | Linear Regression, LSTM network | 20 patients | 0.820 |
| Tomašić, I et al. [27] | Three bipolar lead | Regression Trees | 20 normal, 22 patients | 0.985 |
| Zhy et al. [28] | Subset of standard ECG (I, II, V2) | Linear Regression | 39 patients | 0.947 |
| Our system | Three bipolar lead | LSTM network | 30 normal, 30 patients | 0.950 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, J.; Yang, S.; Lee, J.; Ku, Y.; Kim, H.C. Reconstruction of 12-Lead Electrocardiogram from a Three-Lead Patch-Type Device Using a LSTM Network. Sensors 2020, 20, 3278. https://doi.org/10.3390/s20113278
Sohn J, Yang S, Lee J, Ku Y, Kim HC. Reconstruction of 12-Lead Electrocardiogram from a Three-Lead Patch-Type Device Using a LSTM Network. Sensors. 2020; 20(11):3278. https://doi.org/10.3390/s20113278
Chicago/Turabian StyleSohn, Jangjay, Seungman Yang, Joonnyong Lee, Yunseo Ku, and Hee Chan Kim. 2020. "Reconstruction of 12-Lead Electrocardiogram from a Three-Lead Patch-Type Device Using a LSTM Network" Sensors 20, no. 11: 3278. https://doi.org/10.3390/s20113278
APA StyleSohn, J., Yang, S., Lee, J., Ku, Y., & Kim, H. C. (2020). Reconstruction of 12-Lead Electrocardiogram from a Three-Lead Patch-Type Device Using a LSTM Network. Sensors, 20(11), 3278. https://doi.org/10.3390/s20113278





