Characterization of Arabica and Robusta Coffees by Ion Mobility Sum Spectrum
Abstract
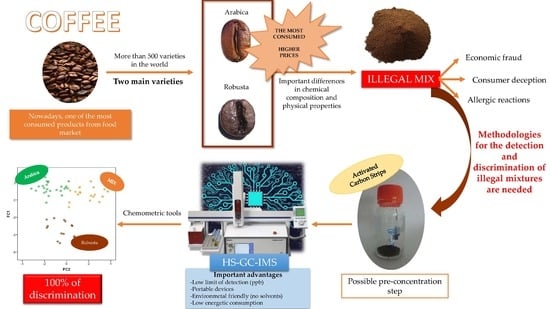
1. Introduction
2. Materials and Methods
2.1. Coffee Samples
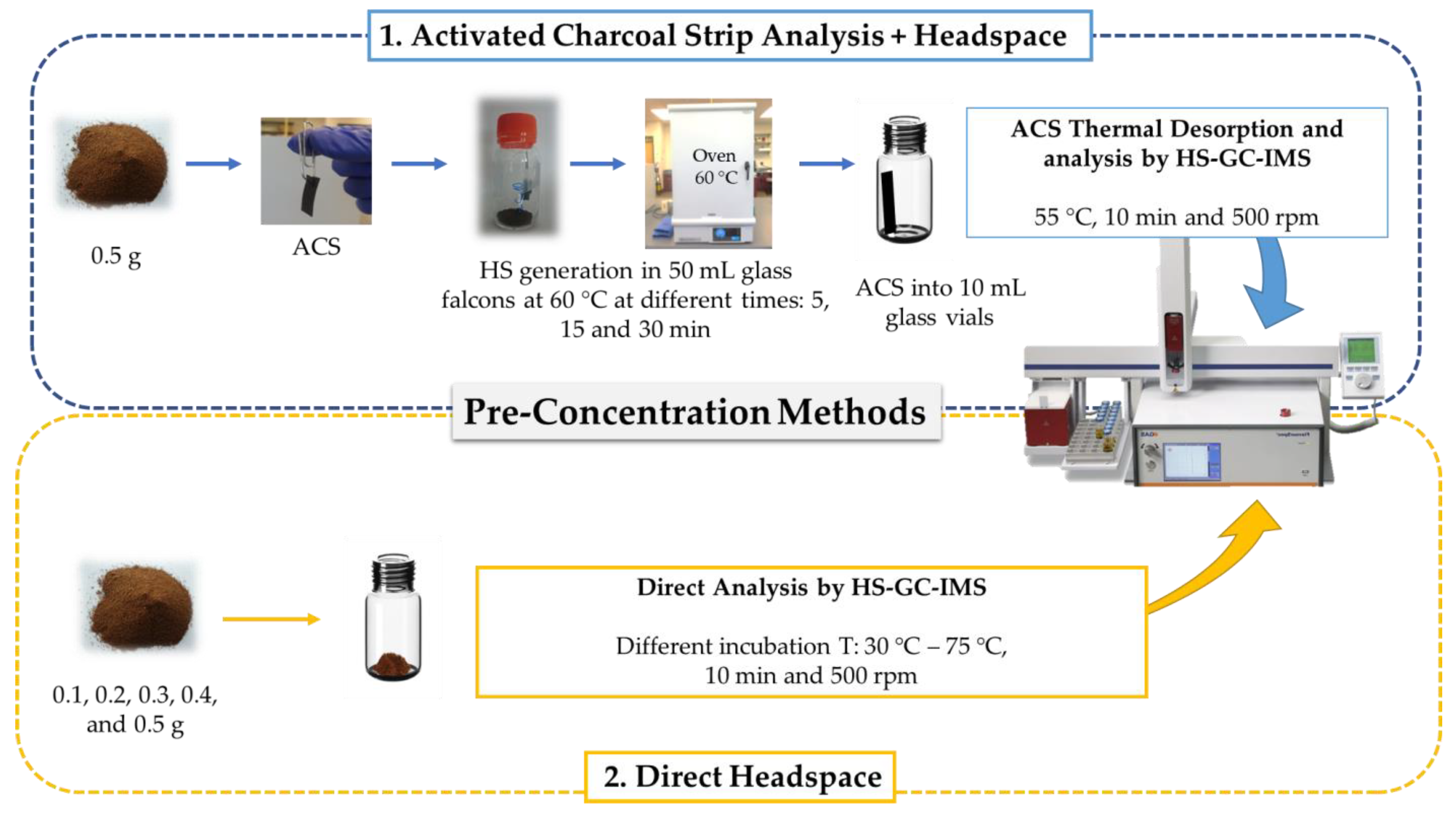
2.2. Pre-Concentration Methods
2.2.1. Activated Charcoal Strip Analysis + Headspace (ACS + HS)
2.2.2. Direct Headspace (HS)
2.3. GC-IMS Analysis
2.4. Data Analysis
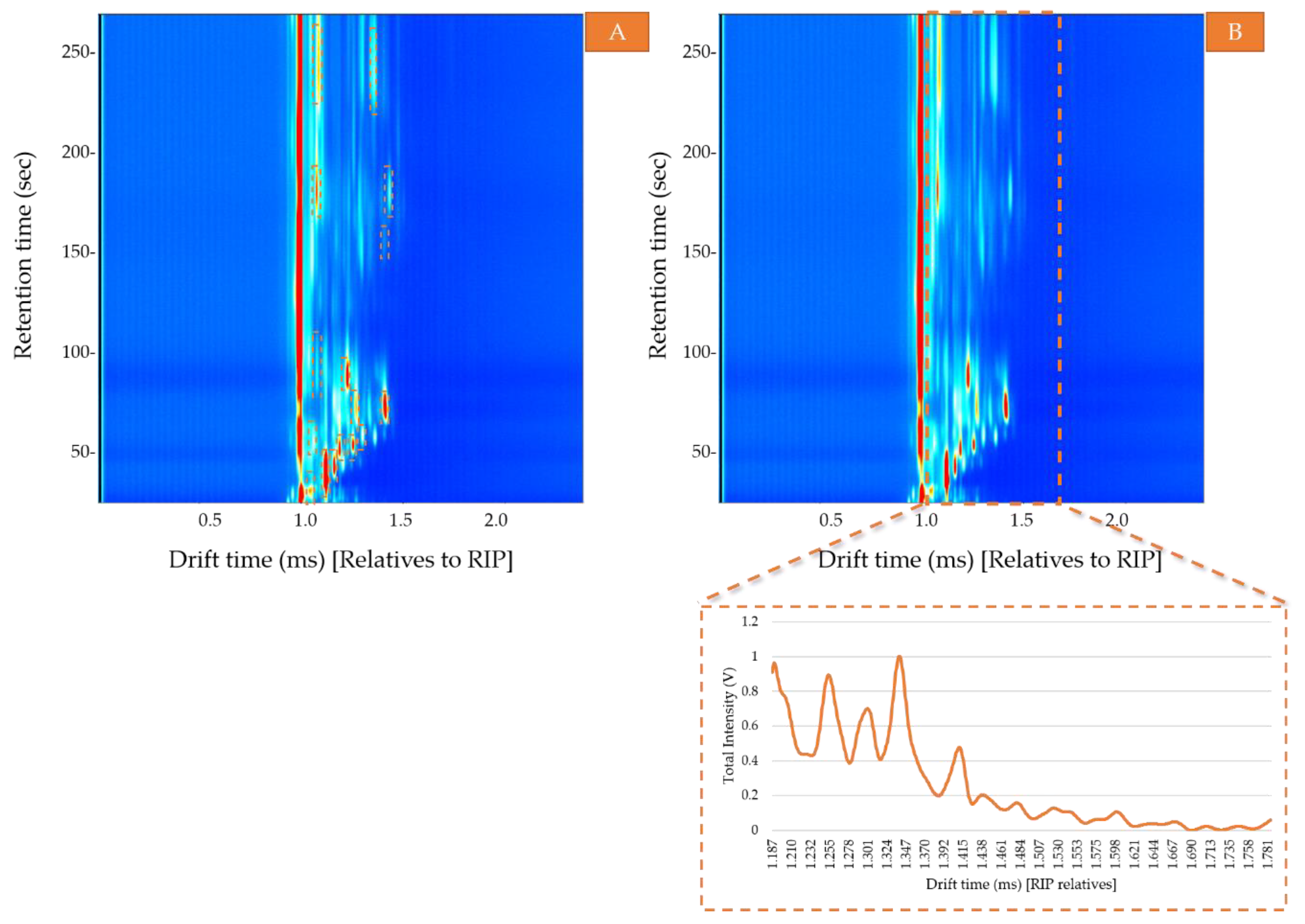
- Area. The total area calculated as the sum of the VOCs areas obtained by the two pre-concentration methods—ACS and non-preconcentrated samples. For that purpose, the area of each compound was selected and calculated using LAV software (Figure 2A). Then, the sum of all the areas was used to determine the optimal conditions to obtain the maximum signal (area) corresponding to the total VOC content and to evaluate the headspace differences between the pre-concentrated samples and non-pretreated samples.
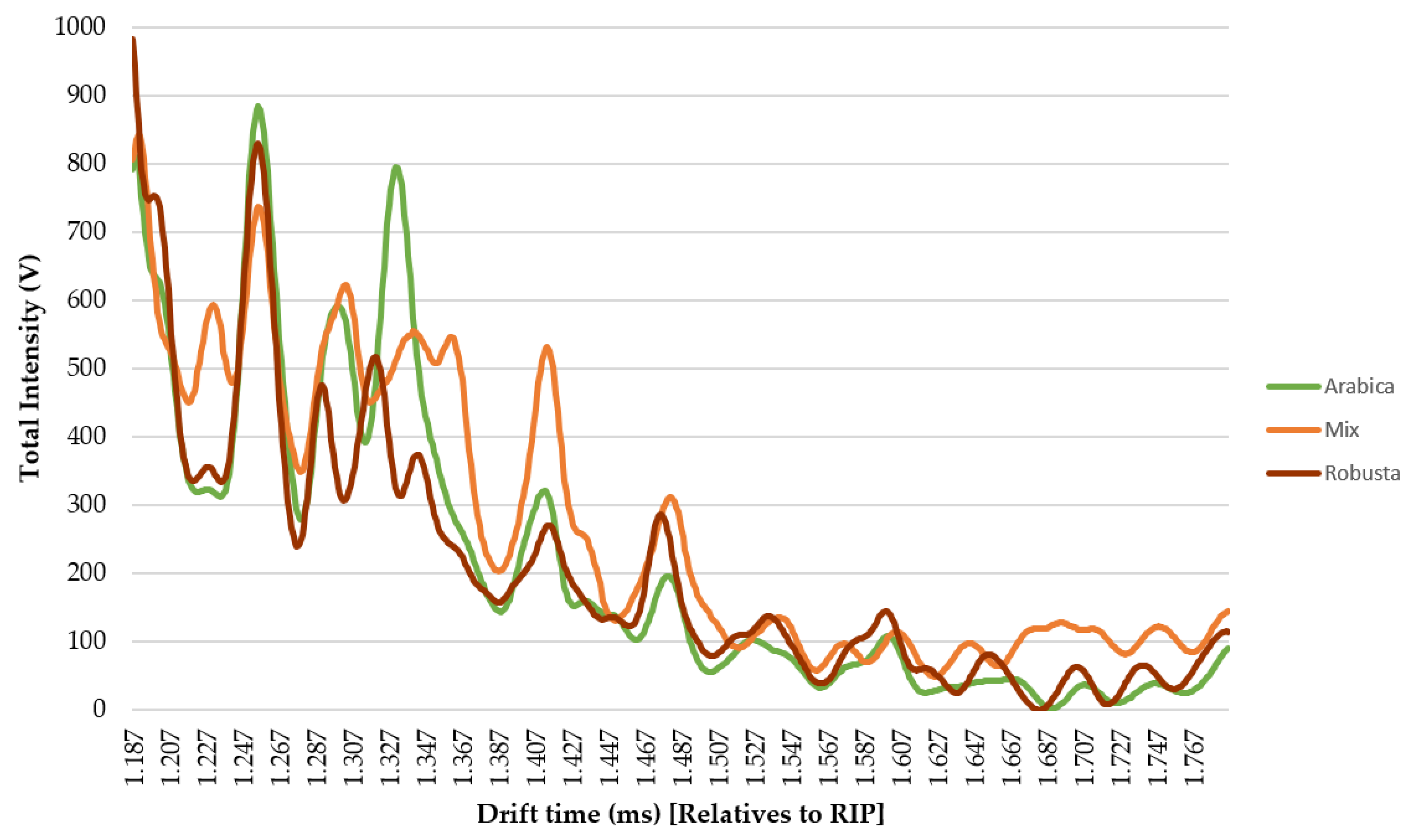
- Ion mobility sum spectrum (IMSS). Once the optimal sample preparation method had been established, a total of 30 samples were analyzed under those conditions. IMSS was used to evaluate the capacity of the HS-GC-IMS to discriminate between the Arabica, Robusta, and mixture samples. IMSS is defined as the sum of intensities across the chromatographic profile, this results in a spectrum in which each drift time acts as a “sensor,” and the total volatile compounds intensity collected at each drift time is equivalent to a multiple sensor signal. It supposes that no chromatographic information has been used (Figure 2B). i.e., data on the total intensities at 4500 drift times, from 0.000 ms to 4.500 ms (relatives to RIP). The reaction ion peak (RIP) represents the total available ions generated by the ion source and this signal is used as the reference to determine each compound drift time. A specific zone was selected, as it was the range where volatiles compounds were detected, resulting in a spectrum with a total of 599 drift times from 1.187 ms to 1.786 ms. Each spectrum was normalized by assigning one unit value to its maximum intensity. The data obtained from the IMSS was arranged in matrixes named Dm×n where m is the number of samples and n is the number of drift times.
2.5. Standardization Procedure
3. Results
3.1. Comparison between the Two Pre-Concentration Methods
3.1.1. Activated Charcoal Strip Analysis + Headspace (ACS + HS)
3.1.2. Headspace (HS)
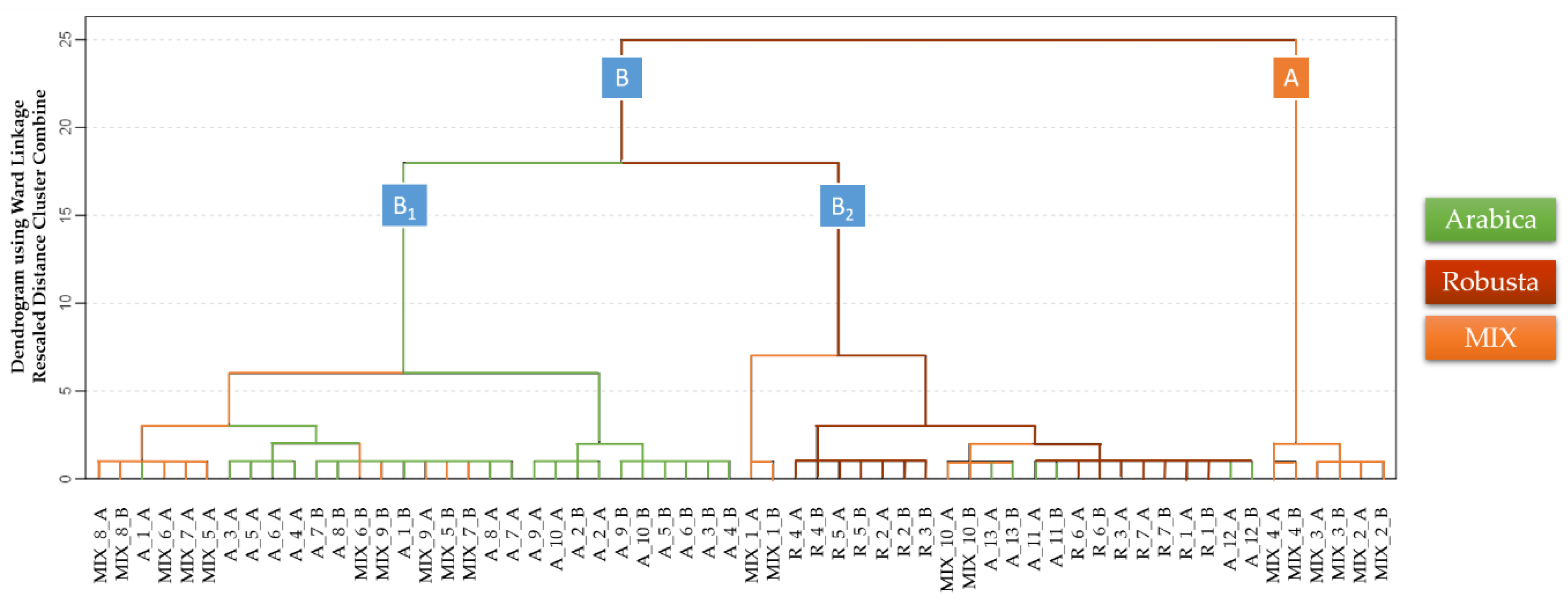
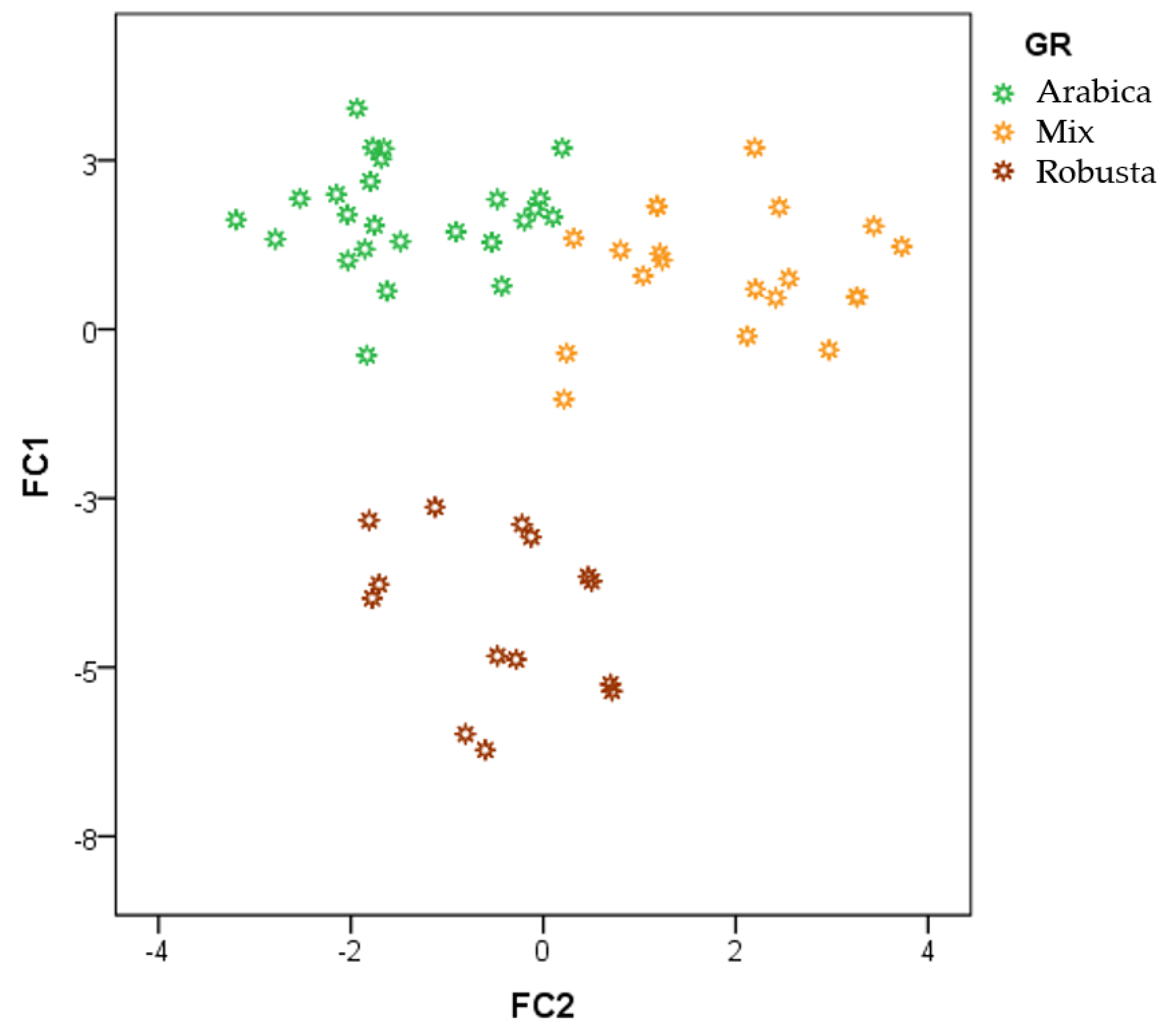
3.2. Discrimination of Arabica, Robusta, and Mixed Coffee Samples by HS-GCIMS
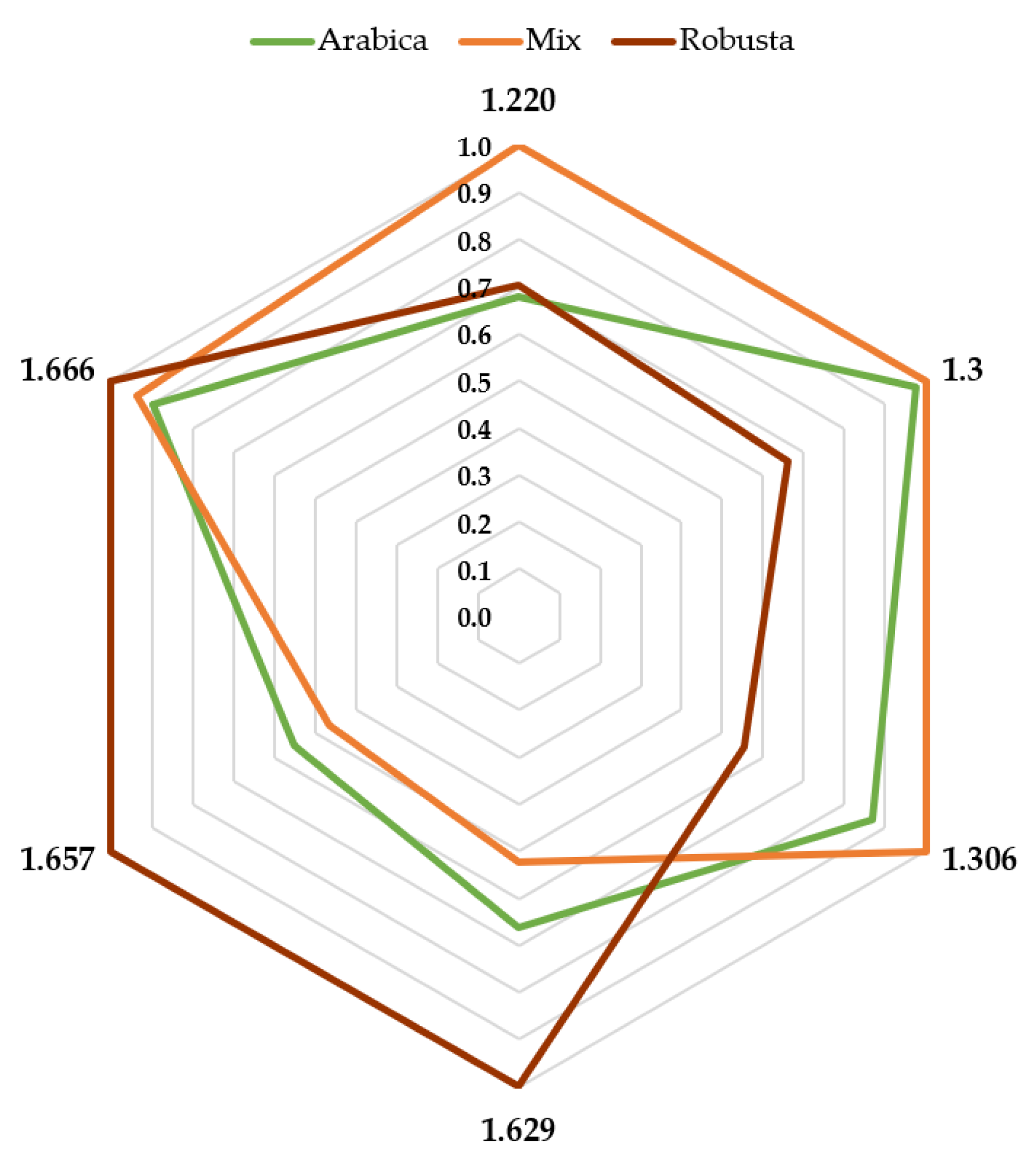
3.3. Greenness Assessment of the Developed Analytical Procedures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samoggia, A.; Riedel, B. Coffee consumption and purchasing behavior review: Insights for further research. Appetite 2018, 129, 70–81. [Google Scholar] [CrossRef]
- Mejia, E.G.; Ramirez-Mares, M.V. Impact of caffeine and coffee on our health. Trends Endocrinol. Metab. 2014, 25, 489–492. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Silva, L.T.; de Souza, C.O.; da Silva, J.R.; Albuquerque, E.C.C.; Druzian, J.I. Coffee-cocoa additives for bio-based antioxidant packaging. Food Packag. Shelf Life 2018, 18, 37–41. [Google Scholar] [CrossRef]
- Palmieri, M.G.S.; Cruz, L.T.; Bertges, F.S.; Húngaro, H.M.; Batista, L.R.; da Silva, S.S.; Fonseca, M.J.V.; Rodarte, M.P.; Vilela, F.M.P.; Amaral, M. Enhancement of antioxidant properties from green coffee as promising ingredient for food and cosmetic industries. Biocatal. Agric. Biotechnol. 2018, 16, 43–48. [Google Scholar] [CrossRef]
- Çelik, E.E.; Gökmen, V. A study on interactions between the insoluble fractions of different coffee infusions and major cocoa free antioxidants and different coffee infusions and dark chocolate. Food Chem. 2018, 255, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Gouveia, L.F.; Chiari, B.G.; Paiva, A.; Isaac, V.; Pinto, P.; Simões, P.; Almeida, A.J.; Ribeiro, H.M. The green generation of sunscreens: Using coffee industrial sub-products. Ind. Crops Prod. 2016, 80, 93–100. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; DiNicolantonio, J.J. Coffee for Cardioprotection and Longevity. Prog. Cardiovasc. Dis. 2018, 61, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Carlström, M.; Bahadoran, Z.; Azizi, F. Long-term effects of coffee and caffeine intake on the risk of pre-diabetes and type 2 diabetes: Findings from a population with low coffee consumption. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, J.A. Coffee Consumption and Prevention of Cirrhosis: In Support of the Caffeine Hypothesis. Gene Expr. 2018, 18, 1–3. [Google Scholar] [CrossRef]
- Munoz, D.G.; Fujioka, S. Caffeine and Parkinson disease. Neurology 2018, 90, 205–206. [Google Scholar] [CrossRef]
- Schmit, S.L.; Rennert, H.S.; Rennert, G.; Gruber, S.B. Coffee Consumption and the Risk of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Cid, M.C.; de Peña, M.-P. Coffee: Analysis and Composition. Encycl. Food Health 2016, 225–231. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Simões, C.; Maciel, E.; Domingues, P.; Domingues, M.R.M.; Coimbra, M.A. Data on coffee composition and mass spectrometry analysis of mixtures of coffee related carbohydrates, phenolic compounds and peptides. Data Br. 2017, 13, 145–161. [Google Scholar] [CrossRef]
- Clarke, R.J. COFFEE: Roast and Ground. Encycl. Food Sci. Nutr. 2003, 1487–1493. [Google Scholar] [CrossRef]
- De Oliveira, A.L.; Cruz, P.M.; Eberlin, M.N.; Cabral, F.A. Brazilian roasted coffee oil obtained by mechanical expelling: Compositional analysis by GC-MS. Food Sci. Technol. 2005, 25, 677–682. [Google Scholar] [CrossRef]
- Chu, B.; Yu, K.; Zhao, Y.; He, Y. Development of Noninvasive Classification Methods for Different Roasting Degrees of Coffee Beans Using Hyperspectral Imaging. Sensors 2018, 18, 1259. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.K.; Lee, K.-G. Effect of reversed coffee grinding and roasting process on physicochemical properties including volatile compound profiles. Innov. Food Sci. Emerg. Technol. 2017, 44, 97–102. [Google Scholar] [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Beverly, D.; Fryer, P.J.; Bakalis, S.; Lopez-Quiroga, E.; Farr, R. Mathematical modelling of the steam stripping of aroma from roast and ground coffee. In Proceedings of the Energy Procedia, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 161, pp. 157–164. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the impacts of postharvest processing on the aroma formation of coffee beans—A review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef]
- Anthony, F.; Bertrand, B.; Quiros, O.; Wilches, A.; Lashermes, P.; Berthaud, J.; Charrier, A. Genetic diversity of wild coffee (Coffea arabica L.) using molecular markers. Euphytica 2001, 118, 53–65. [Google Scholar] [CrossRef]
- Wintgens, J.N. Coffee: Growing, Processing, Sustainable Production, 2nd ed.; Wintgens, J.N., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2004; pp. 1–1000. ISBN 9783527619627. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Q.; Linforth, R.; Fisk, I.D.; Yang, N. Modifying Robusta coffee aroma by green bean chemical pre-treatment. Food Chem. 2019, 272, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; de Toledo Benassi, M. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Flament, I. Coffee Flavor Chemistry, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; pp. 1–424. ISBN 978-0-471-72038-6. [Google Scholar]
- Coffee Market Reports. The Current State of the Global Coffee Trade. Available online: www.ico.org (accessed on 1 March 2020).
- Folmer, B. The Craft and Science of Coffee, 1st ed.; Academic Press: London, UK, 2017; pp. 1–556. ISBN 978-0-12-803520-7. [Google Scholar]
- Dias, R.C.E.; Valderrama, P.; Março, P.H.; dos Santos Scholz, M.B.; Edelmann, M.; Yeretzian, C. Quantitative assessment of specific defects in roasted ground coffee via infrared-photoacoustic spectroscopy. Food Chem. 2018, 255, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Frega, N.G.; Pacetti, D.; Mozzon, M.; Balzano, M. Authentication of Coffee Blends. In Coffee in Health and Disease Prevention, 1st ed.; Press, A., Ed.; Elsevier: London, UK, 2015; pp. 107–115. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, T.; Liu, F.; He, Y. Identification of Coffee Varieties Using Laser-Induced Breakdown Spectroscopy and Chemometrics. Sensors 2017, 18, 95. [Google Scholar] [CrossRef]
- Combes, M.-C.; Joët, T.; Lashermes, P. Development of a rapid and efficient DNA-based method to detect and quantify adulterations in coffee (Arabica versus Robusta). Food Control 2018, 88, 198–206. [Google Scholar] [CrossRef]
- Couto, C.C.; Santos, T.F.; Mamede, A.M.G.N.; Oliveira, T.C.; Souza, A.M.; Freitas-Silva, O.; Oliveira, E.M.M. Coffea arabica and C. canephora discrimination in roasted and ground coffee from reference material candidates by real-time PCR. Food Res. Int. 2019, 115, 227–233. [Google Scholar] [CrossRef]
- Pauli, E.D.; Barbieri, F.; Garcia, P.S.; Madeira, T.B.; Acquaro, V.R.; Scarminio, I.S.; da Camara, C.A.P.; Nixdorf, S.L. Detection of ground roasted coffee adulteration with roasted soybean and wheat. Food Res. Int. 2014, 61, 112–119. [Google Scholar] [CrossRef]
- Marek, G.; Dobrzański, B., Jr.; Oniszczuk, T.; Combrzyński, M.; Ćwikła, D.; Rusinek, R. Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries Using an Electronic Nose and GC-MS. Sensors 2020, 20, 2124. [Google Scholar] [CrossRef]
- Borsdorf, H.; Eiceman, G.A. Ion Mobility Spectrometry: Principles and Applications. Appl. Spectrosc. Rev. 2006, 41, 323–375. [Google Scholar] [CrossRef]
- Fernández-Maestre, R. Ion mobility spectrometry: History, characteristics and applications. Rev. U.D.C.A Act. Div. Cient. 2012, 15, 467–479. [Google Scholar]
- Garrido-Delgado, R.; Arce, L.; Pérez-Marín, C.C.; Valcárcel, M. Use of ion mobility spectroscopy with an ultraviolet ionization source as a vanguard screening system for the detection and determination of acetone in urine as a biomarker for cow and human diseases. Talanta 2009, 78, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Sabo, M.; Matejčík, Š. Corona Discharge Ion Mobility Spectrometry with Orthogonal Acceleration Time of Flight Mass Spectrometry for Monitoring of Volatile Organic Compounds. Anal. Chem. 2012, 84, 5327–5334. [Google Scholar] [CrossRef] [PubMed]
- Vautz, W.; Franzke, J.; Zampolli, S.; Elmi, I.; Liedtke, S. On the potential of ion mobility spectrometry coupled to GC pre-separation—A tutorial. Anal. Chim. Acta 2018, 1024, 52–64. [Google Scholar] [CrossRef]
- Li, F.; Xie, Z.; Schmidt, H.; Sielemann, S.; Baumbach, J. Ion mobility spectrometer for online monitoring of trace compounds. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 1563–1574. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Mercader-Trejo, F.; Sielemann, S.; de Bruyn, W.; Arce, L.; Valcárcel, M. Direct classification of olive oils by using two types of ion mobility spectrometers. Anal. Chim. Acta 2011, 696, 108–115. [Google Scholar] [CrossRef]
- Camara, M.; Gharbi, N.; Cocco, E.; Guignard, C.; Behr, M.; Evers, D.; Orlewski, P. Fast screening for presence of muddy/earthy odorants in wine and in wine must using a hyphenated gas chromatography-differential ion mobility spectrometry (GC/DMS). Int. J. Ion Mobil. Spectrom. 2011, 14, 39–47. [Google Scholar] [CrossRef]
- Márquez-Sillero, I.; Cárdenas, S.; Valcárcel, M. Direct determination of 2,4,6-tricholoroanisole in wines by single-drop ionic liquid microextraction coupled with multicapillary column separation and ion mobility spectrometry detection. J. Chromatogr. A 2011, 1218, 7574–7580. [Google Scholar] [CrossRef]
- Vautz, W.; Baumbach, J.I.; Jung, J. Beer Fermentation Control Using Ion Mobility Spectrometry—Results of a Pilot Study. J. Inst. Brew. 2006, 112, 157–164. [Google Scholar] [CrossRef]
- Vautz, W.; Baumbach, J.I. Analysis of Bio-Processes using Ion Mobility Spectrometry. Eng. Life Sci. 2008, 8, 19–25. [Google Scholar] [CrossRef]
- Ruzsanyi, V.; Baumbach, J.I.; Eiceman, G.A. Detection of the mold markers using ion mobility spectrometry. Int. J. Ion Mobil. Spectrom. 2003, 6, 53–57. [Google Scholar]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Barbero, G.F.; Palma, M. Novel method based on ion mobility spectroscopy for the quantification of adulterants in honeys. Food Control 2020, 107236. [Google Scholar] [CrossRef]
- Nongonierma, A.; Voilley, A.; Cayot, P.; Le Quéré, J.-L.; Springett, M. Mechanisms of Extraction of Aroma Compounds from Foods, Using Adsorbents. Effect of Various Parameters. Food Rev. Int. 2006, 22, 51–94. [Google Scholar] [CrossRef]
- Feist, B.; Mikula, B. Preconcentration of heavy metals on activated carbon and their determination in fruits by inductively coupled plasma optical emission spectrometry. Food Chem. 2014, 147, 302–306. [Google Scholar] [CrossRef]
- Moon, S.Y.; Baek, S.Y.; Kim, M.R. Determination of aroma profiles of coffee cultivated in Goheung, Korea by gas chromatography–ion mobility spectrometry (2019). Korean J. Food Preserv. 2019, 26, 576–585. [Google Scholar] [CrossRef]
- Gloess, A.N.; Yeretzian, C.; Knochenmuss, R.; Groessl, M. On-line analysis of coffee roasting with ion mobility spectrometry–mass spectrometry (IMS–MS). Int. J. Mass Spectrom. 2018, 424, 49–57. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Palma, M.; Barbero, G.F. A screening method based on headspace-ion mobility spectrometry to identify adulterated honey. Sensors 2019, 19, 1621. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Novel method based on ion mobility spectrometry sum spectrum for the characterization of ignitable liquids in fire debris. Talanta 2019, 199, 189–194. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
| Time (min) | Average Sum Area ± Standard Deviation |
|---|---|
| 5 a | 1839 ± 24 |
| 15 b | 2309 ± 42 |
| 30 b | 2431 ± 12 |
| Sample Weight (g) | Average Sum Area ± Standard Deviation |
|---|---|
| 0.5 a | 9209 ± 2 |
| 0.4 b | 9141 ± 23 |
| 0.3 c | 9062 ± 78 |
| 0.2 d | 8920 ± 62 |
| 0.1 d | 8412 ± 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotr Konieczka, P.; Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Characterization of Arabica and Robusta Coffees by Ion Mobility Sum Spectrum. Sensors 2020, 20, 3123. https://doi.org/10.3390/s20113123
Piotr Konieczka P, Aliaño-González MJ, Ferreiro-González M, Barbero GF, Palma M. Characterization of Arabica and Robusta Coffees by Ion Mobility Sum Spectrum. Sensors. 2020; 20(11):3123. https://doi.org/10.3390/s20113123
Chicago/Turabian StylePiotr Konieczka, Paweł, María José Aliaño-González, Marta Ferreiro-González, Gerardo F. Barbero, and Miguel Palma. 2020. "Characterization of Arabica and Robusta Coffees by Ion Mobility Sum Spectrum" Sensors 20, no. 11: 3123. https://doi.org/10.3390/s20113123
APA StylePiotr Konieczka, P., Aliaño-González, M. J., Ferreiro-González, M., Barbero, G. F., & Palma, M. (2020). Characterization of Arabica and Robusta Coffees by Ion Mobility Sum Spectrum. Sensors, 20(11), 3123. https://doi.org/10.3390/s20113123