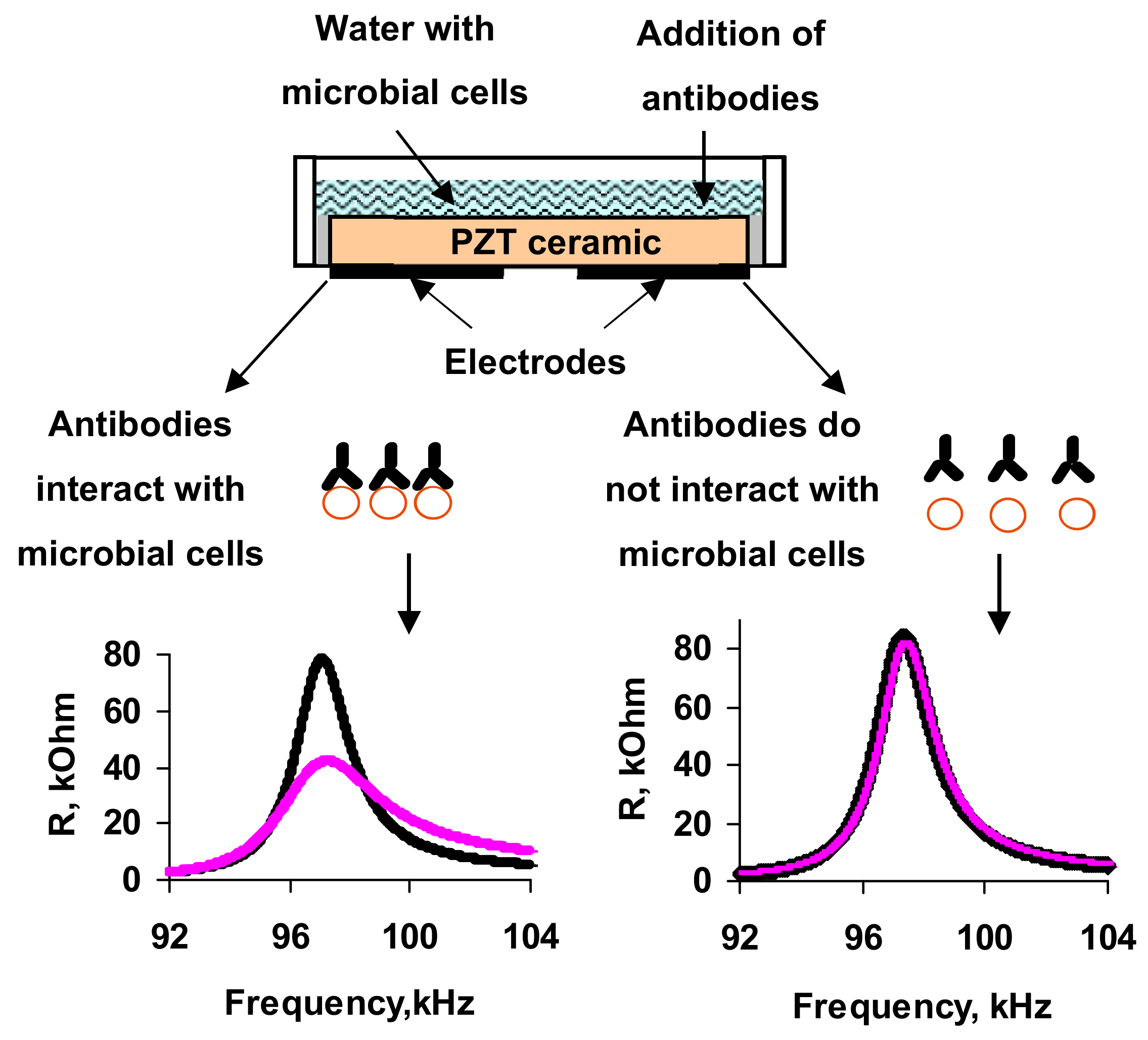
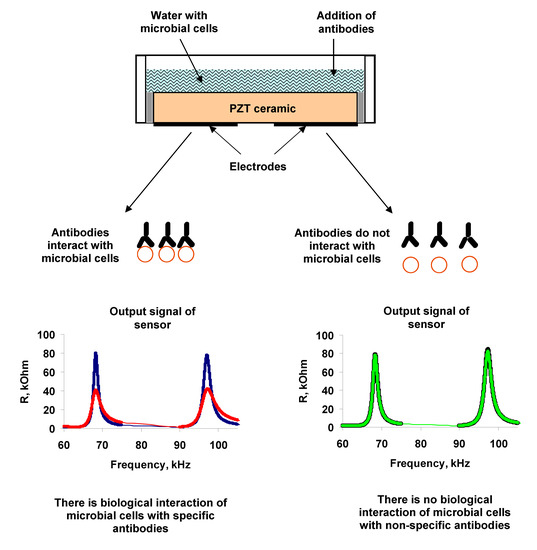
3.1. Measurements in Distilled Water
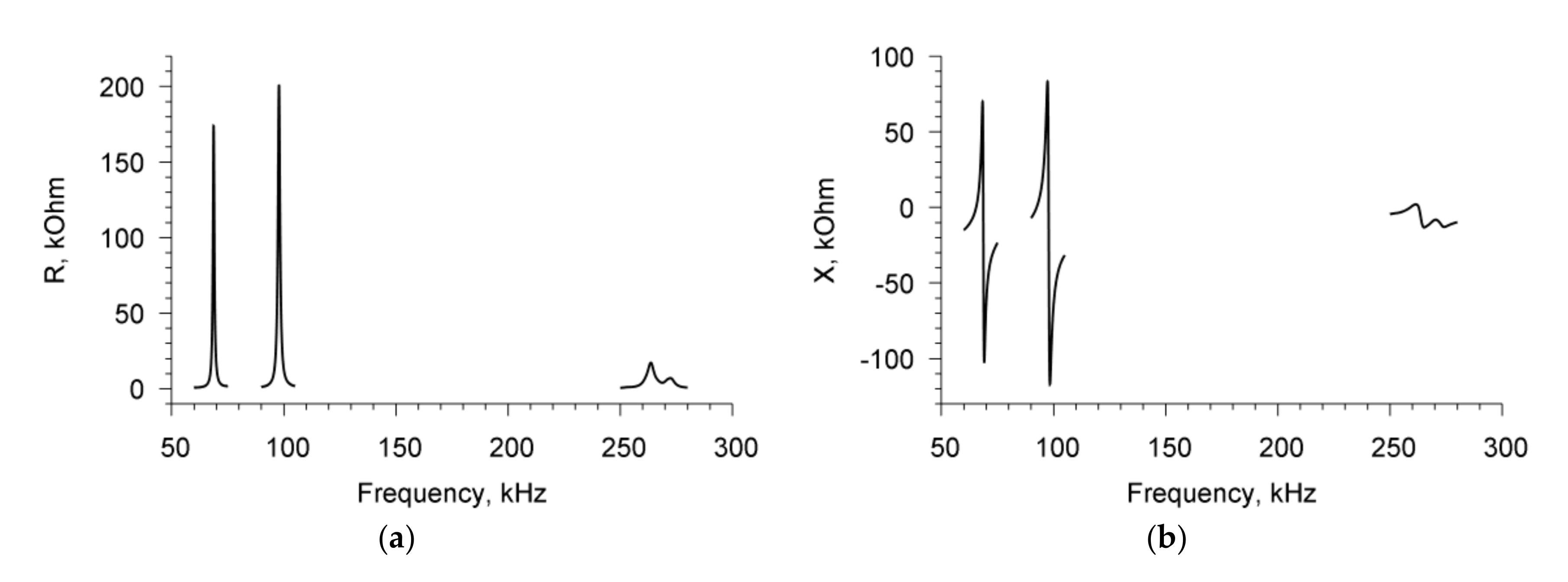
Measurements of the parameters of the sensor with an empty container showed the presence of three resonances in the frequency dependences of the real (R) (
Figure 2a) and imaginary (X) (
Figure 2b) parts of the electrical impedance of the resonator. These resonant peaks were observed near the frequencies of 68.7, 97.8, and 264 kHz, and had the values of electromechanical coupling coefficient of 15%, 14%, and 5%, respectively [
25].
In the next step, distilled water was added to the container. The addition of distilled water with a conductivity of 4.5 μS/cm led to the significant decrease in the maximum value of the real part of the electrical impedance (Rmax) for all resonant peaks in comparison with an empty container. This is apparently due to the fact that, firstly, the liquid layer exerted a massive load on the resonator. Secondly, the acoustic oscillations excited in this resonator were not purely shear, but had a small normal displacement component, which led to small radiation attenuation.
It was found that the quality factor of each resonance in contact with the liquid turned out to be significantly higher than one of a resonator with a longitudinal acoustic wave based on lithium niobate [
13,
14,
15]. This is due to the fact that in a piezoceramic resonator the shear component of the mechanical displacement is prevalent, which significantly reduces the radiation loss of the acoustic wave when the surface of the resonator contacts the liquid [
25].
Then, microbial cells, re-suspended in distilled water with a given concentration (102–108 cell/mL), were added to the container. It was shown that the addition of distilled water with microbial cells in container had practically no effect on the frequency dependences of the electrical impedance of resonator in comparison with adding the water without cells.
The surface of a microbe cell is a set of complicated antigen complexes. The distinctive feature of antibodies to form specific compounds with the appropriate antigens is used for determination of microorganisms. The same principle is employed to identify the microorganisms with the help of biosensor systems by using the Abs. Therefore, as a selective agent for determining bacteria, we used antibodies specific to them.
In the next measurement step the specific Abs were introduced into the cell suspension of A. brasilense Sp7. Different amounts of microbial cells (102, 103, 104, 106 and 108, cells/mL) and specific Abs (1, 2, 4, and 6 μg/mL) were used in the experiments. The addition of specific Abs to A. brasilense Sp7 led to a significant decrease in the real and imaginary parts of the electrical impedance of the resonator for almost all values of the studied cell concentrations excepting 102 cells/mL.
As already noted, as an informative parameter, we used the maximum value of the real part of the electric impedance of the resonator because it was shown earlier that this parameter is preferable for measuring the conductivity and viscosity of a liquid [
17].
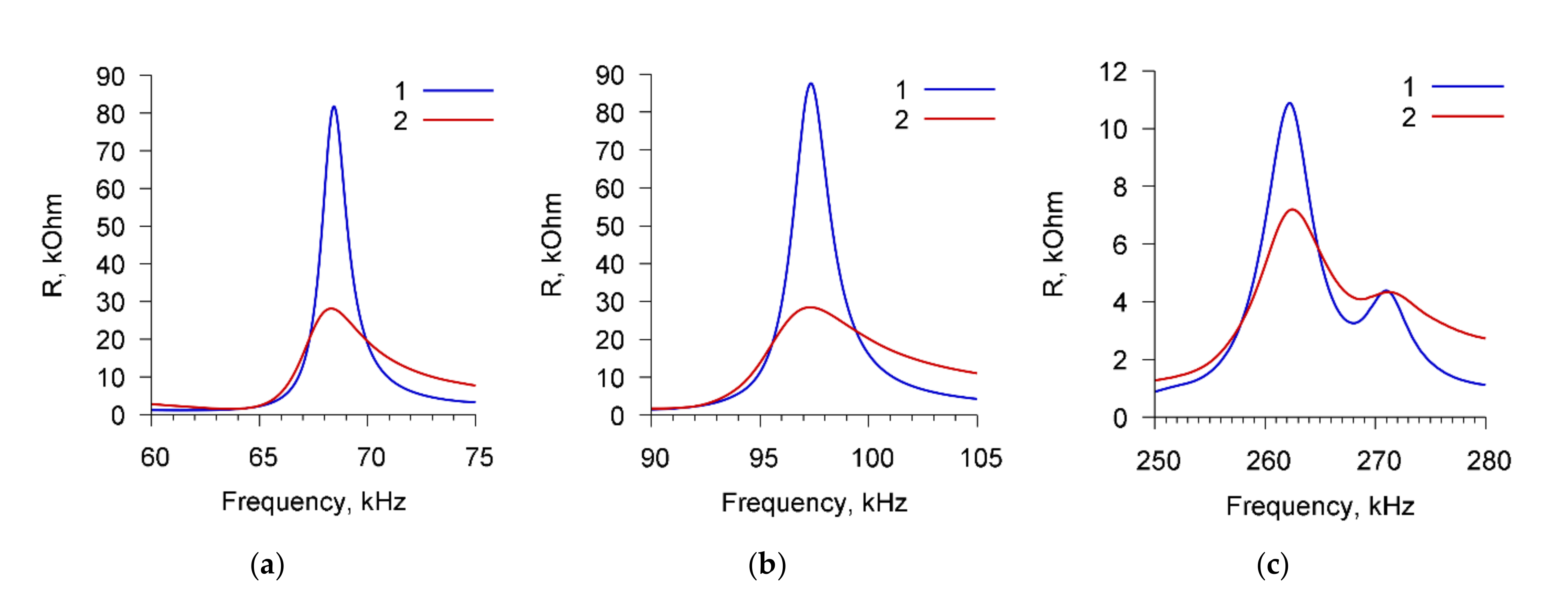
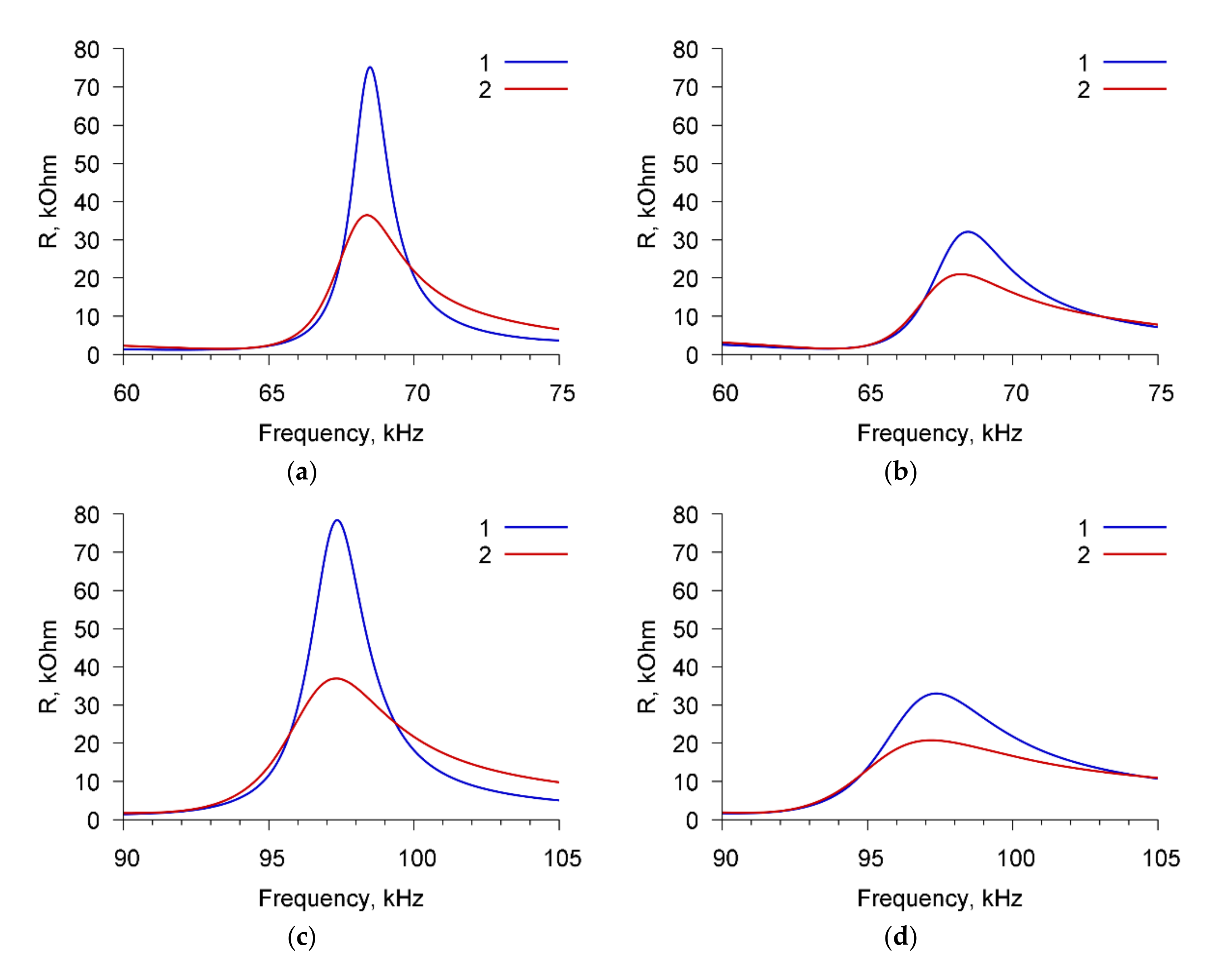
Figure 3 presents, as an example, the frequency dependencies of the real part of the electric impedance of the sensor for cell suspension of
A. brasilense Sp7 with concentration of 10
3 cells/mL before (curve 1) and after (curve 2) adding the specific Abs with the amount of 4 μg/mL. The dependencies are presented for three resonance peaks near the frequencies: (a) 68.7 kHz, (b) 97.8 kHz, and (c) 264 kHz.
One can see that the addition of specific Abs to the suspension of microbial cells leads to a decrease in the maximum value of the real part of the electrical impedance (Rmax) by 63.5, 65.6, and 4.1 kOhm for resonance peaks near frequencies of 68.7, 97.8, and 264 kHz, respectively.
The changes in the parameters of the sensor can be explained as follows. When specific antibodies are added to the cell suspension, the active center of the antibody (Ab) joins with the antigenic (Ag) determinant. This highly specific process proceeds in aqueous solutions at a high speed. O-antigen of the Gram-negative bacteria is associated with lipopolysaccharide LPS of the cell wall. The determinant groups of this complex antigen are the terminal repeating units of polysaccharide chains attached to its main part. At the moment of binding the antibody to the determinant group of the antigen, a conformational rearrangement of the active center of the antibody occurs and the active antibody adapts to the antigen. The binding of antibodies to antigenic determinants on the cell surface is recorded by changing the polarization properties of the Ag-Ab complex. The interaction of an antigen molecule with an antibody or its active Fab fragment is accompanied, in turn, by changes in the spatial structure of the antigen molecule. This affects the change in the integrity of bacterial membranes, and leads to the release of cell contents into suspension and to the increase in its electrical conductivity. This increase in conductivity is recorded by the acoustic sensor as a decrease in the real and imaginary parts of the electrical impedance of the resonator near the resonant frequencies.
To exclude the nonspecific interactions, we used
E. coli K-12 cells and Abs specific for
A. brasilense Sp7 cells.
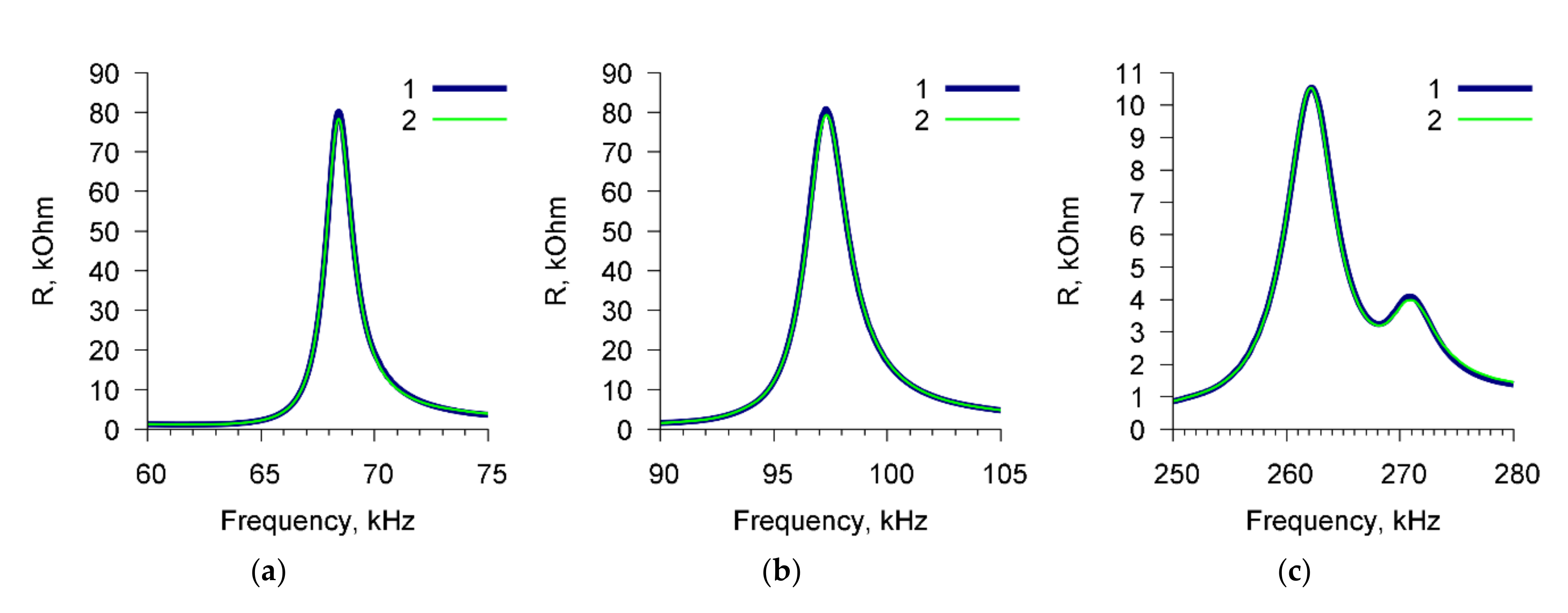
Figure 4 shows, as an example, the frequency dependencies of the real part of the electric impedance of the sensor for a suspension of
E. coli K-12 cells (10
3 cells/mL) before (curve 1) and after (curve 2) the addition of Abs specific for
A. brasilense Sp7 (4 μg/mL). These dependencies are presented for three resonance peaks near frequencies: (a) 68.7 kHz, (b) 97.8 kHz, and (c) 264 kHz. It can be seen that in this case no changes in the sensor parameters are observed.
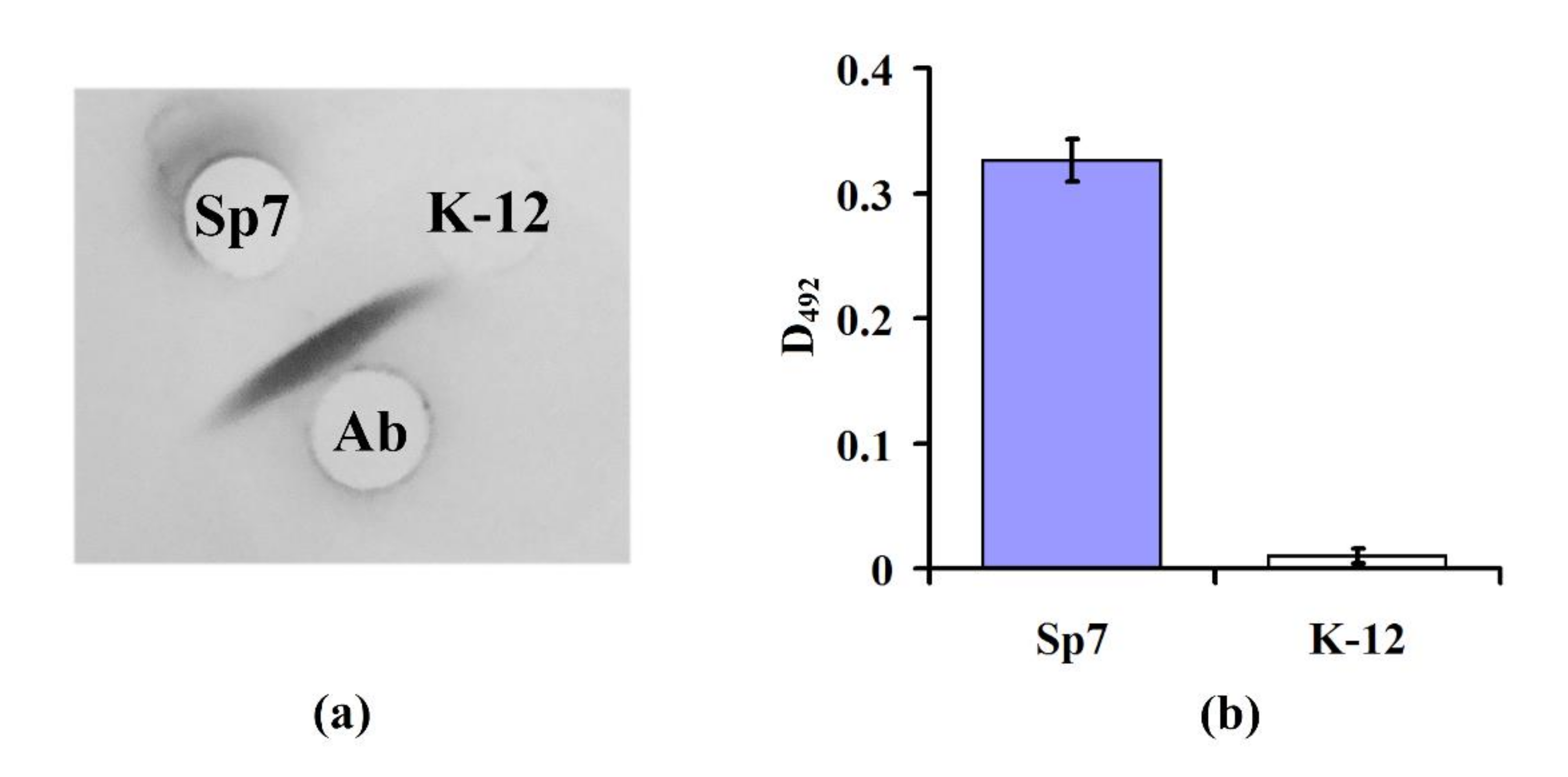
To determine the specificity of Abs to the O-antigen of
A. brasilense Sp7, an immunodiffusion analysis and enzyme-linked immunosorbent assay (ELISA) were performed. During double immunodiffusion, the specific antigen and antibody placed into wells of the agar gel diffuse towards each other and form precipitation in the form of a band at the zone of their meeting. According to the results of immunodiffusion, one can clearly see (
Figure 5a) that Abs interacted only with the extract of
A. brasilense Sp7 cells (formation of the antigen–antibody complex immunoprecipitation as the band in agarose gel) and did not interact with the extract of
E. coli K-12 (no immunoprecipitation band). Thus, the Abs against O-antigen of
A. brasilense Sp7 specifically formed the insoluble antigen–antibody complex only with the surface antigens of
A. brasilense Sp7 [
26] and did not form it with antigens of
E. coli K-12. ELISA is characterized by the unique specificity of the immunochemical reaction, i.e., antibodies bind exclusively to certain antigens and are highly sensitive to antigen detection. The direct specificity of the Abs used in the work was also determined by ELISA.
The data presented in
Figure 5b shows that Abs detected
A. brasilense Sp7 cells, but did not detect
E. coli K-12. These results indicate the absence of specific antigenic determinants on the cell surface of
E. coli K-12.
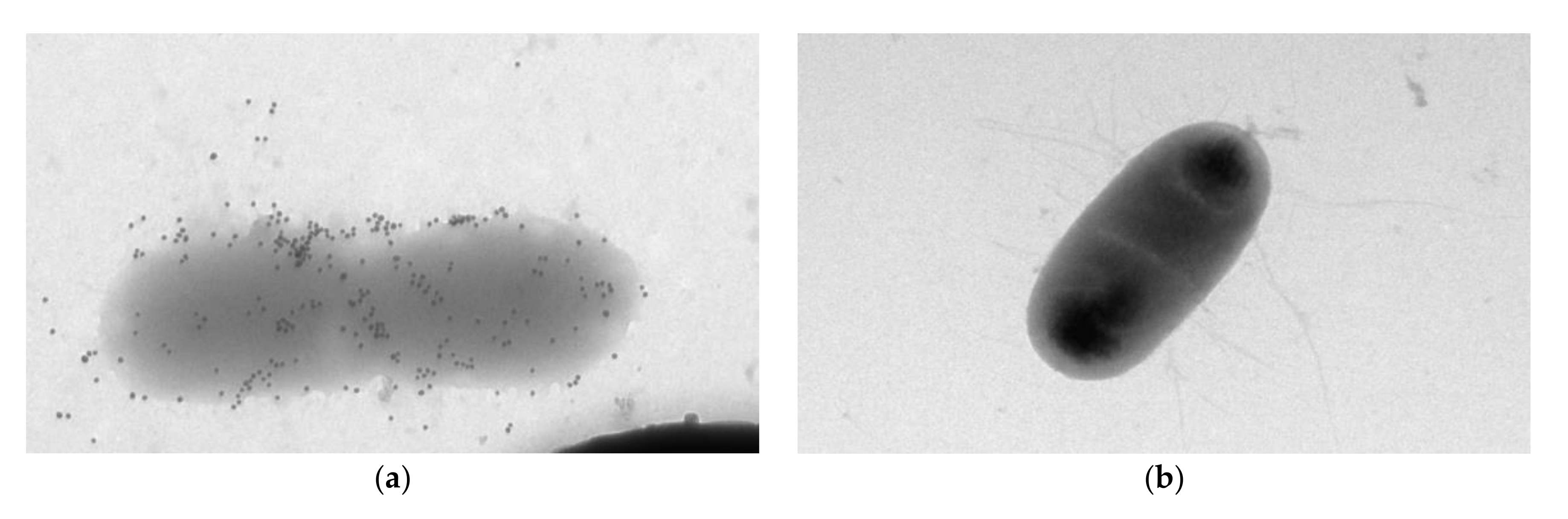
Additionally, the electron microscopy of microbial cells was performed during their interaction with specific and nonspecific antibodies.
Figure 6a presents the electron microscopic identification of
A. brasilense Sp7 with Abs specific to
A. brasilense Sp7 labeled with colloidal gold. Electron micrographs show that the markers of accumulation occur on the entire surface of the cells.
Figure 6b shows a photograph of
E. coli K-12 with Abs specific to
A. brasilense Sp7. One can see that in this case no accumulation of antibodies around the cell is observed.
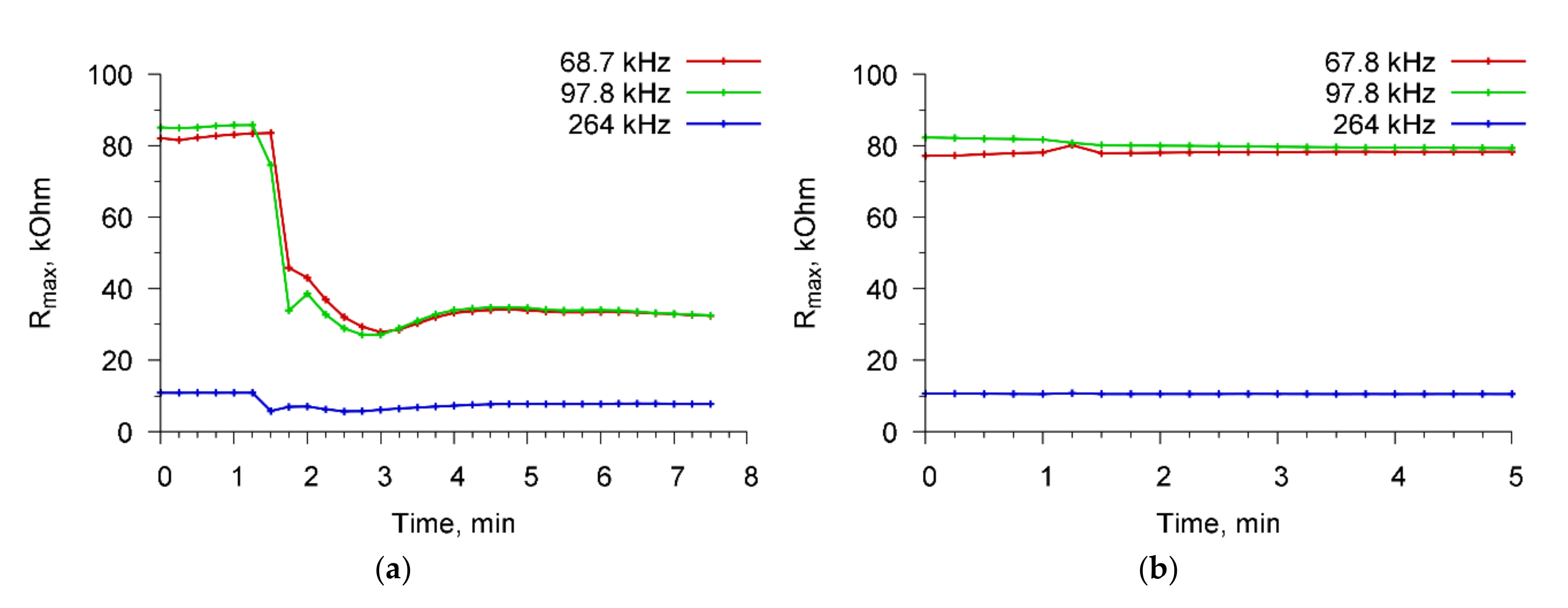
Figure 7 shows the time dependences of the maximum value of the real part of the resonator electrical impedance (R
max) for resonant peaks near 68.7, 97.8, and 264 kHz for a specific interaction “
A. brasilense Sp7—specific Abs”(a) and non-specific interaction “
E. coli K-12—Abs specific for
A. brasilense Sp7”(b).
At the initial time, a cell suspension was introduced into the container, and sensor readings were taken at intervals of 15 s. One could see no changes in the value of R
max. Then (at 100th second), a specific/nonspecific reagent was introduced into the container with the cell suspension and the sensor readings were again recorded. It is seen that in the case of a specific interaction (
Figure 7a), the value of R
max decreases sharply, and then the saturation process takes place. In the case of nonspecific interaction (
Figure 7b), no changes in the sensor parameters occurred. The analysis time in all cases did not exceed 4 min.
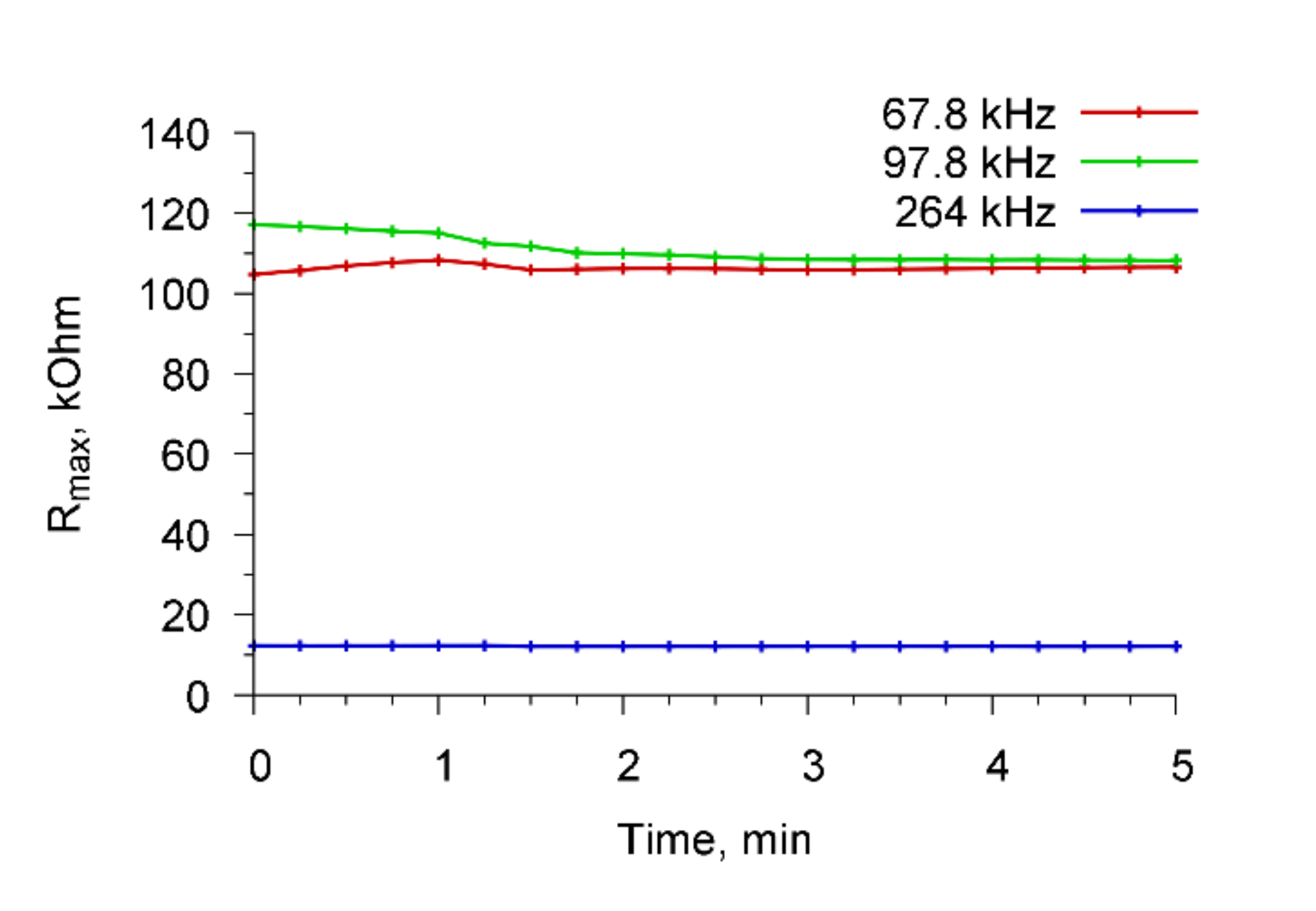
We also performed the control experiments when Abs, specific to
A. brasilense Sp7, were added to a container with water in the absence of microbial cells.
Figure 8 shows the time dependencies of the real part of the electrical impedance of the resonator for the resonance peaks near frequencies 68.7, 97.8, and 264 kHz when Abs (4 μg/mL) specific for
A. brasilense Sp7 cells were added to the container with water. It is seen that the addition of antibodies to water at the absence of cells did not lead to a change in the electrical impedance of the sensor.
Based on the measured frequency dependencies of the real part of the electrical impedance of the sensor, the dependencies of the change in the maximum value of the real part of the electrical impedance (ΔR
max) on the cell concentration was constructed for a fixed value of the number of specific antibodies added to the cell suspension.
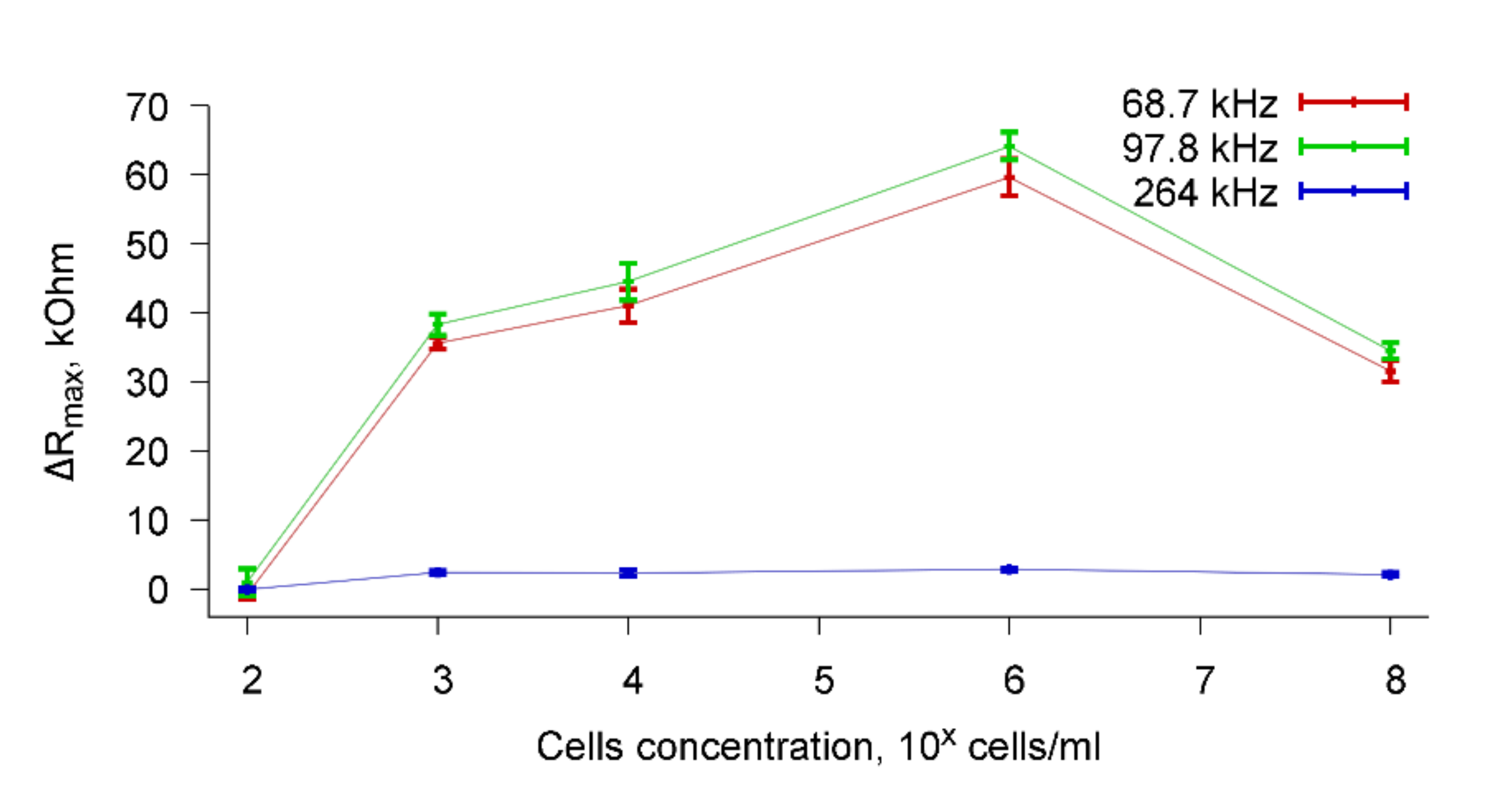
Figure 9 shows, as an example, the dependencies of ΔR
max on the concentration of
A. brasilense Sp7 cells when specific antibodies were added to the container with a cell suspension in an amount of 4 μg/mL. Dependences are presented for resonance peaks near frequencies 68.7, 97.8, and 264 kHz. It can be seen that the addition of specific Abs to a suspension of cells with a concentration of 10
2 cells/mL practically does not change the R
max value (ΔR
max = 0). This can be explained by the fact that in this case the concentration of cells in the water is very low and the sensor cannot detect a change in the conductivity of the cell suspension due to the specific biological interaction of microbial cells with antibodies.
Significant changes in ΔR
max are observed when specific antibodies are added to a cell suspension with concentrations of 10
3–10
8 cells/mL. The variation range ΔR
max in these cases is 30–60 kOhm for resonance peaks near frequencies of 68.7 and 97.8 kHz, and 4–6 kOhm for a peak near the frequency of 264 kHz.
Figure 9 shows that for cell suspensions with a concentration of 10
8 cells/mL, a decrease in ΔR
max is observed compared with a concentration of 10
3–10
6 cells/mL for resonance peaks near the frequencies of 68.7 and 97.8 kHz. This is because the addition of a cell suspension with a concentration of 10
8 cells/mL to a liquid container leads to some decrease in sensor signal even before the addition of specific Abs.
To explain this fact, we measured the viscosity and conductivity of cell suspensions with different concentrations and compared them with the viscosity and conductivity of distilled water before adding the cells. It was found that the viscosity of the cell suspension with any concentration of cells was equal to the viscosity of distilled water and amounted to 0.77–0.78 mPa s. With regard to the conductivity, the addition of cells with a concentration of 102–106 cells/mL to distilled water did not lead to a change in the conductivity of the cell suspension compared with the conductivity of distilled water. However, the addition of the cells with a concentration of 108 cells/mL to distilled water led to an increase in conductivity from 4.5 μS/cm to 6.0 μS/cm. This can be explained by the fact that a high-frequency current (68–264 kHz) can pass not only through the space between the cells, but also through the cells.
Thus, we can say that the lower limit of determination of microbial cells using the studied sensor based on PZT ceramics is 10
3 cells/mL. This value is an order of magnitude higher than the detection limit of the sensor based on lithium niobite [
14,
15].
The dependencies of a change in the maximum value of the real part of the electrical impedance (ΔR
max) on the number of specific Abs (from 1 to 6 μg/mL) added to the cell suspension with a given value of their concentration were also constructed.
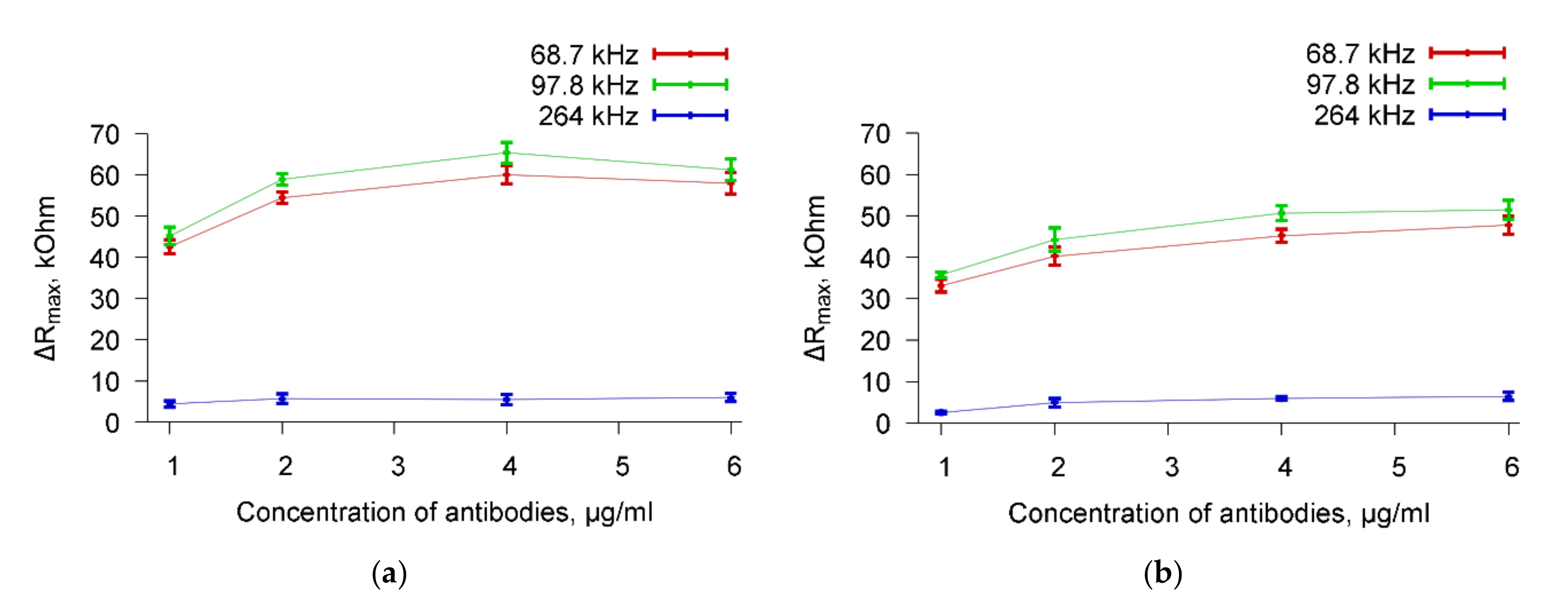
Figure 10 shows, as an example, the dependencies of ΔR
max on the concentration of specific Abs added to a suspension of
A. brasilense Sp7 cells with the concentrations 10
3 cells/mL (
Figure 10a) and 10
8 cells/mL (
Figure 10b) for three resonance peaks near frequencies 68.7, 97.8, and 264 kHz.
Figure 10a shows, that adding 1 μg/mL of antibodies to a cell suspension with a concentration of 10
3 cells/mL leads to a change in R
max (ΔR
max) of 42, 45, and 4.5 kOhm for resonances near the frequencies of 68.7, 97.8, and 264 kHz, respectively. The similar ΔR
max values are equal to 57, 65, and 6 kOhm for the quantity of antibodies of 5 μg/mL.
Figure 10b presents the same data for a cell suspension with a concentration of 10
8 cells/mL. In this case, the ΔR
max values were 32, 35, and 3 kOhm (the quantity of antibodies = 1 μg/mL) and 45, 50, and 5 kOhm (the quantity of antibodies = 5 μg/mL) for resonances near 68.7, 97.8, and 264 kHz, respectively. One can see a decrease in the sensor response for the concentration of 10
8 cells/mL as compared to the concentration of 10
3 cells/mL. An explanation of this fact is given after the commentary on
Figure 9.
Thus, it was shown that the addition of specific Abs to the cell suspension leads to a significant change in Rmax even with their minimal amount. Similar dependencies were constructed for other concentrations of microbial cells.
3.2. The Measurements in Buffers with Different Conductivity
As already noted, an important point is the ability to analyze cells in real liquids having high electrical conductivity (tap and industrial water, water in reservoirs, etc.). Therefore, in this section, we studied microbial cells in buffer solutions with different conductivities when interacting with antibodies using an acoustic sensor based on a resonator with a lateral electric field made of PZT ceramics. To carry out this measurement cycle, a measurement methodology was used similar to the procedure described in
Section 2. At the first stage, buffer solution with a given conductivity was added to the liquid container and the sensor readings were measured. Then, the container was cleaned and dried, and a microbial buffer solution was added. After the stabilization of the sensor signal, we added Abs specific or nonspecific to the cells and again recorded the sensor readings. In this measurement cycle,
A. brasilense Sp7 cells and
E. coli cells with a concentration of 10
3 cells/mL were used. The concentration of Abs was invariable and amounted to 6 μg/mL. The buffer solutions with a conductivity of 10, 20, 50, 100, 300, 500, and 1000 μS/cm were used.
As a result of the experiments, the frequency dependencies of the real part of the electrical impedance of the resonator were obtained for all values of the conductivity of the buffer solutions. It was shown that with an increase in the conductivity of the solution, the value of the real part of the electric impedance of the resonator decreased. These data are in good agreement with the data obtained earlier in [
25], where the influence of liquid conductivity on the characteristics of a resonator based on PZT ceramics was studied. Adding microbial cells to the buffer solution, as in experiments with distilled water, did not affect the performance of the sensor. Subsequent addition of specific antibodies to the cell suspension led to a significant decrease in the real part of the electrical impedance of the sensor.
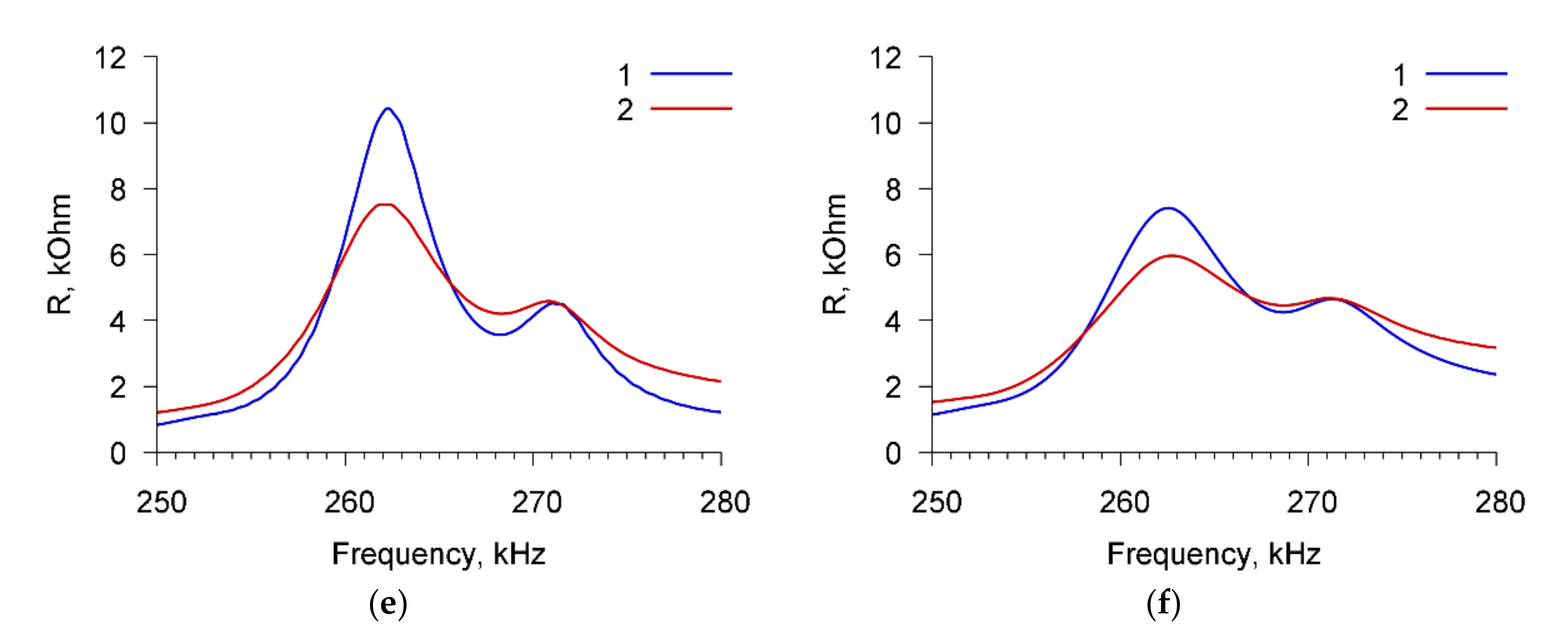
Figure 11 shows, as an example, the frequency dependencies of the real part of the electric impedance of the sensor filled by buffer solution with the conductivities of 10 μS/cm (
Figure 11a,c,e) and 50 μS/cm (
Figure 11b,d,f) with the cells
A. brasilense Sp7 before (curve 1) and after (curve 2) adding the specific Abs with concentration of 6 μg/mL. The cell concentration is 10
3 cells/mL.
One can see that the addition of specific Abs to a buffer solution with a conductivity of 10 μS/cm and microbial cells
A. brasilense Sp7 (
Figure 11a,c,e) leads to a decrease in the maximum value of the real part of the electrical impedance R
max by 32.1, 36.4, and 2.8 kOhm for resonant peaks near frequencies 67.8, 98.7, and 264 kHz, respectively. For a buffer solution with a conductivity of 50 μS/cm containing cells
A. brasilense Sp7, the maximum value of R
max decreases by 11.3, 12.4, and 1.5 kOhm due to the addition of specific Abs for the above-mentioned resonance peaks (
Figure 11b,d,f).
Based on the frequency dependencies of the real part of the electrical impedance of the sensor presented in
Figure 11, the change in ΔR
max due to the addition of the specific Abs as function of the conductivity of the buffer solution with cells
A. brasilense Sp7 were constructed.
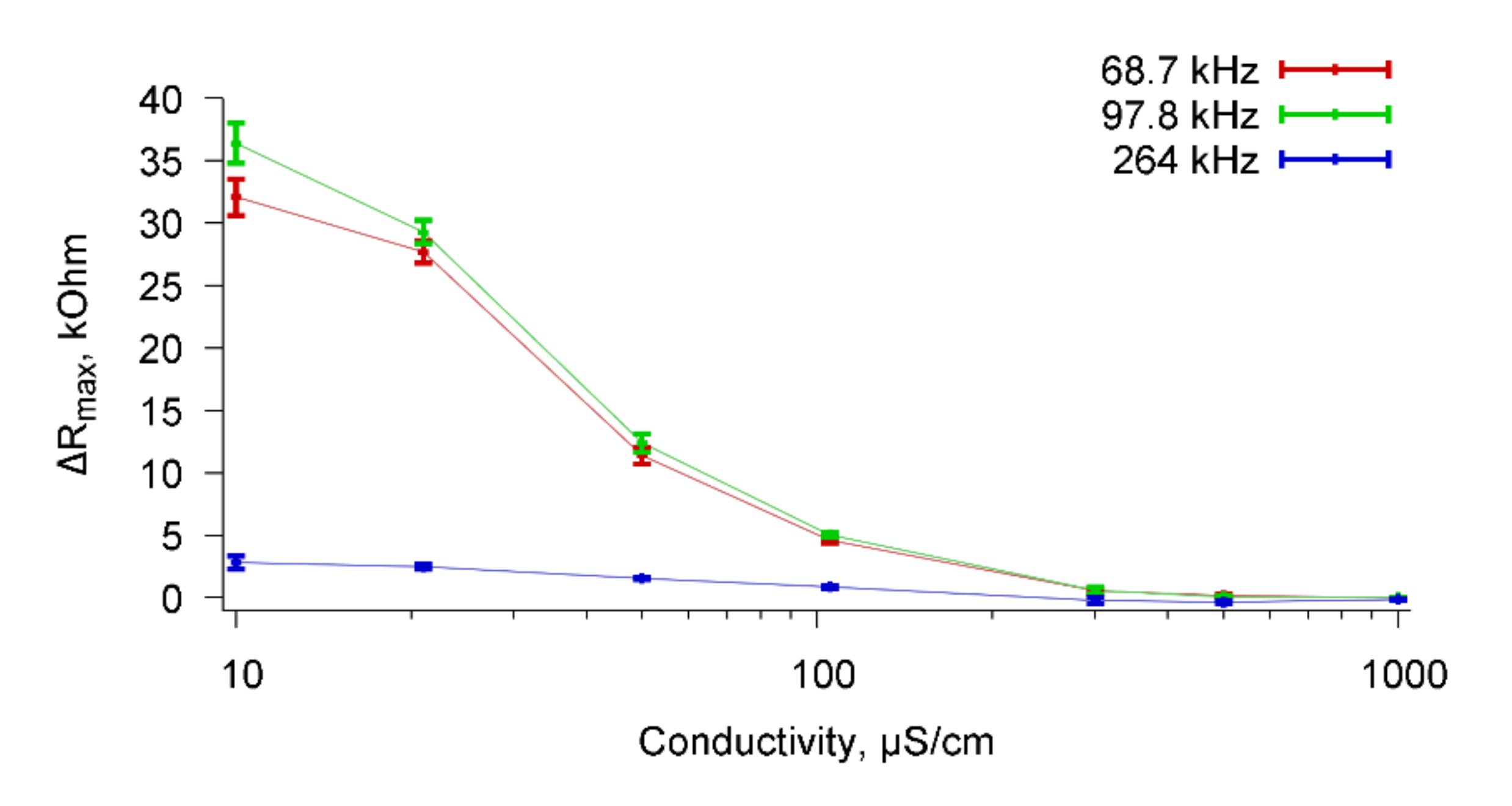
Figure 12 shows these dependencies for resonance peaks near frequencies 67.8, 98.7, and 264 kHz.
It can be seen that the value of ΔRmax decreases with increasing conductivity of the buffer solutions and reaches zero for the conductivity of 1000 μS/cm for all observed resonance peaks.
Table 1 shows the values of ΔR
max for suspensions of
A. brasilense Sp7 cells with a concentration of 10
3 cells/mL based on distilled water (4.5 μS/cm) and buffer solutions (10–1000 μS/cm) due to the addition of specific Abs in an amount of 6 μg/mL for resonant peaks near frequencies of 67.8, 98.7, and 264 kHz.
From the data given in the
Table 1 it can be seen that the sensor can successfully detect specific reactions of microbial cells with Abs in solutions with conductivity up to 300 μS/cm. For the solutions with the higher conductivity, the measurement data are comparable with the measurement error. Additionally, the measurements were carried out with the samples of tap water (conductivity = 1000 μS/cm), as well as with the samples of purified drinking water (conductivity = 500 μS/cm). The data obtained are presented in the lower two rows of
Table 1. They are well comparable with the data for buffer solutions.
As the control experiments, the interactions of a cell suspension with non-specific reagents were investigated. For this purpose, we used
E. coli K-12 cells and Abs specific for
A. brasilense Sp7 cells and non-specific for
E. coli K-12 cells.
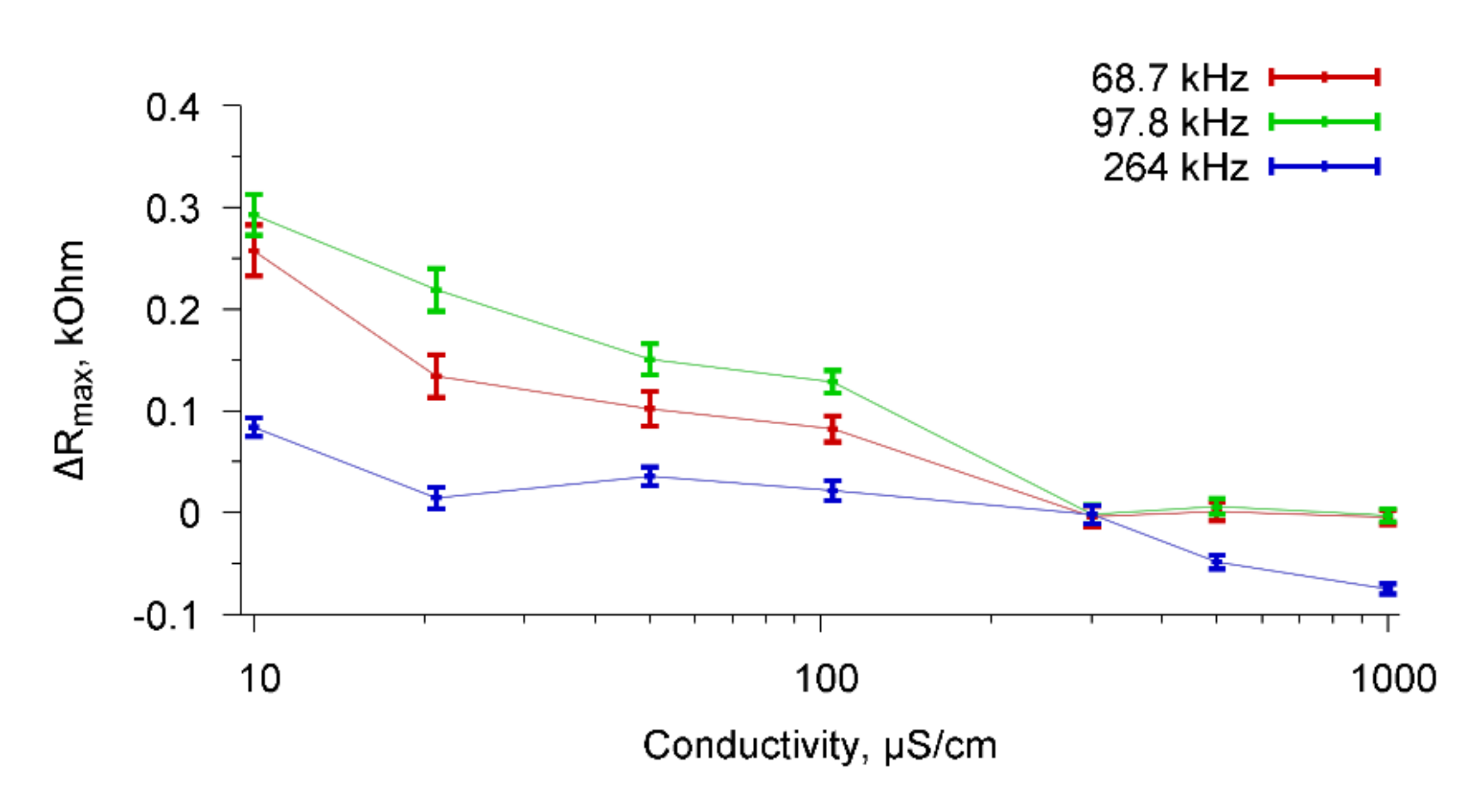
Figure 13 shows the dependencies of ΔR
max on the conductivity of the buffer solution when Abs specific for
A. brasilense Sp7 cells was added to the buffer with
E. coli K-12 cells for resonance peaks near frequencies 67.8, 98.7, and 264 kHz.
One can see that the nonspecific interactions practically did not lead to a change in the parameters of the sensor for each value of the conductivity of buffer solution. Thus, a sensor based on a resonator with a lateral electric field made of PZT ceramics confidently distinguishes specific biological interactions from non-specific interactions. This means that the sensor can be used not only for the detection of microbial cells in the aquatic environment, but also for their identification.
It should be noted that in most works devoted to acoustic biological sensors [
7,
10,
12], active layers with immobilized antibodies are used. Such an approach is distinguished, first, by the long analysis time (more than 6 h [
12]) and, second, by the need to remove the spent active film after each analysis. A biological sensor based on a piezoelectric resonator with a lateral electric field allows bacteria to be analyzed directly in the liquid phase without an active layer for several minutes. The team of authors of this paper has previously shown the possibility of using a resonator with a lateral electric field based on lithium niobate to record various biological interactions of bacterial cells with antibodies, bacteriophages, and mini-antibodies [
13,
14,
15] with the sensitivity threshold of 10
4 cells/mL. However, the quality factor of the pointed resonator on the plate of lithium niobate in contact with the liquid was found to be too low [
26], since the excited longitudinal wave in such a resonator led to radiation loss in the liquid. The sensor based on a resonator made of PZT ceramics presented in this paper allowed a reduction in the value of the threshold up to 10
3 cells/mL. This may be explained by the fact that for the dominant mode of the PZT resonator the shear component of the mechanical displacement is predominant and this greatly reduces radiation loss upon contact with the liquid. It has been also shown that the sensor confidently detects specific biological interactions of microbial cells in aqueous solutions with conductivity up to 300 μS/cm. Such conductivity is characteristic, for example, of well-treated tap water.
The results obtained showed that the magnitude of the change in the output parameters of the investigated sensor depends on the concentration of bacterial cells in the cell suspension. This opens up the prospects for further work in terms of quantitative analysis of microbial cells. Furthermore, we plan to continue research on this sensor in order to study its capabilities for rapid analysis of viral particles.