A Fetal ECG Monitoring System Based on the Android Smartphone
Abstract
:1. Introduction
2. Materials and Methods
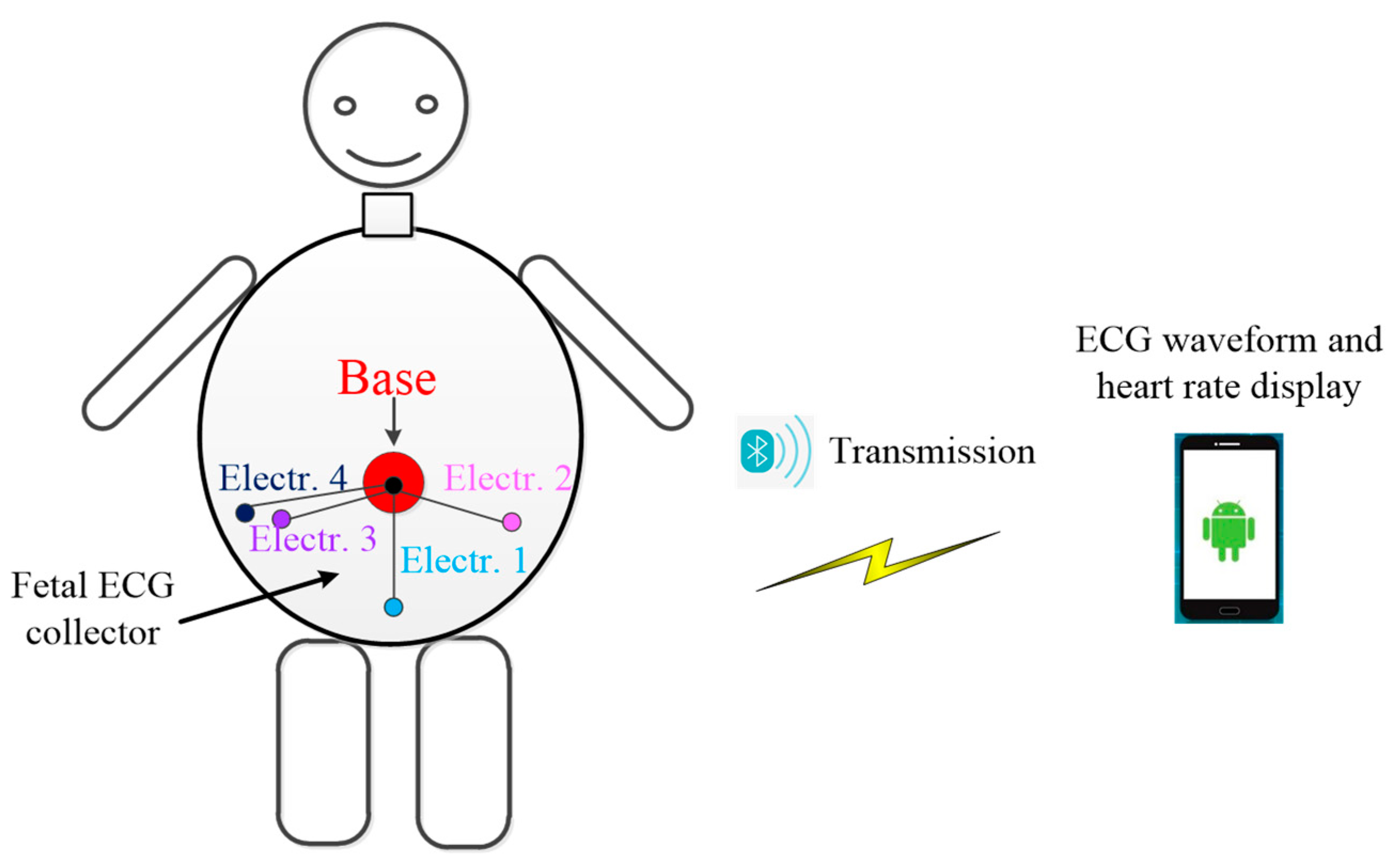
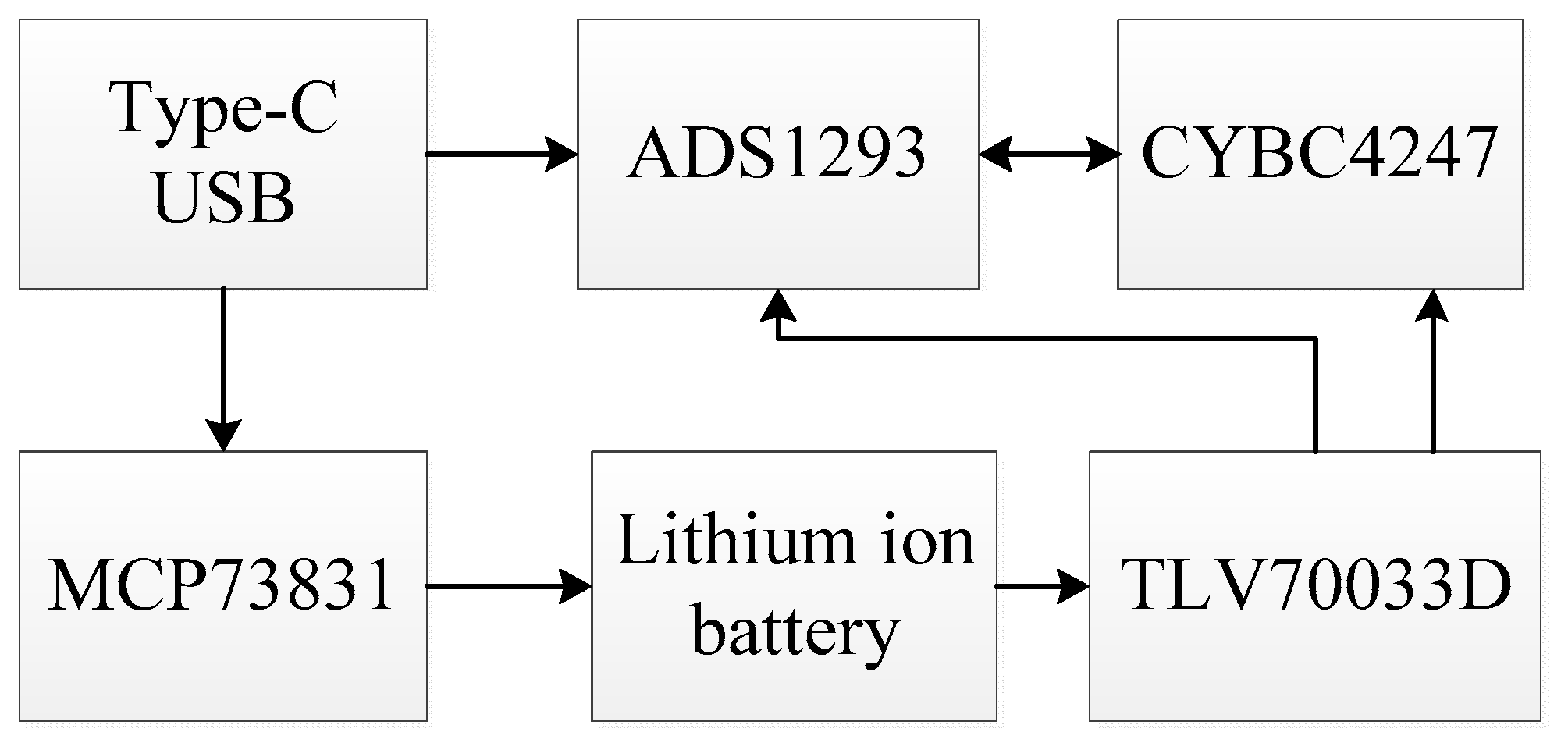
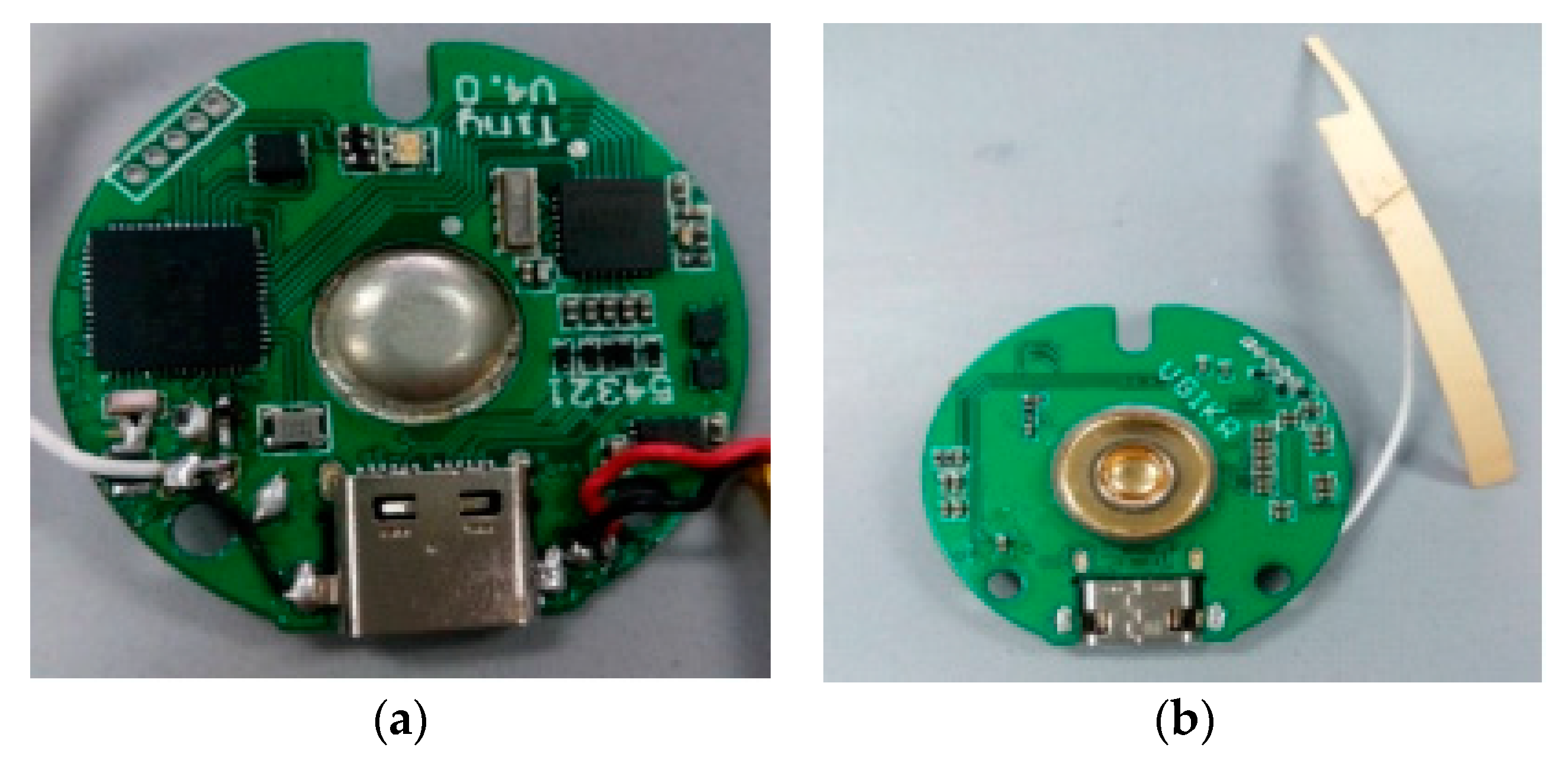
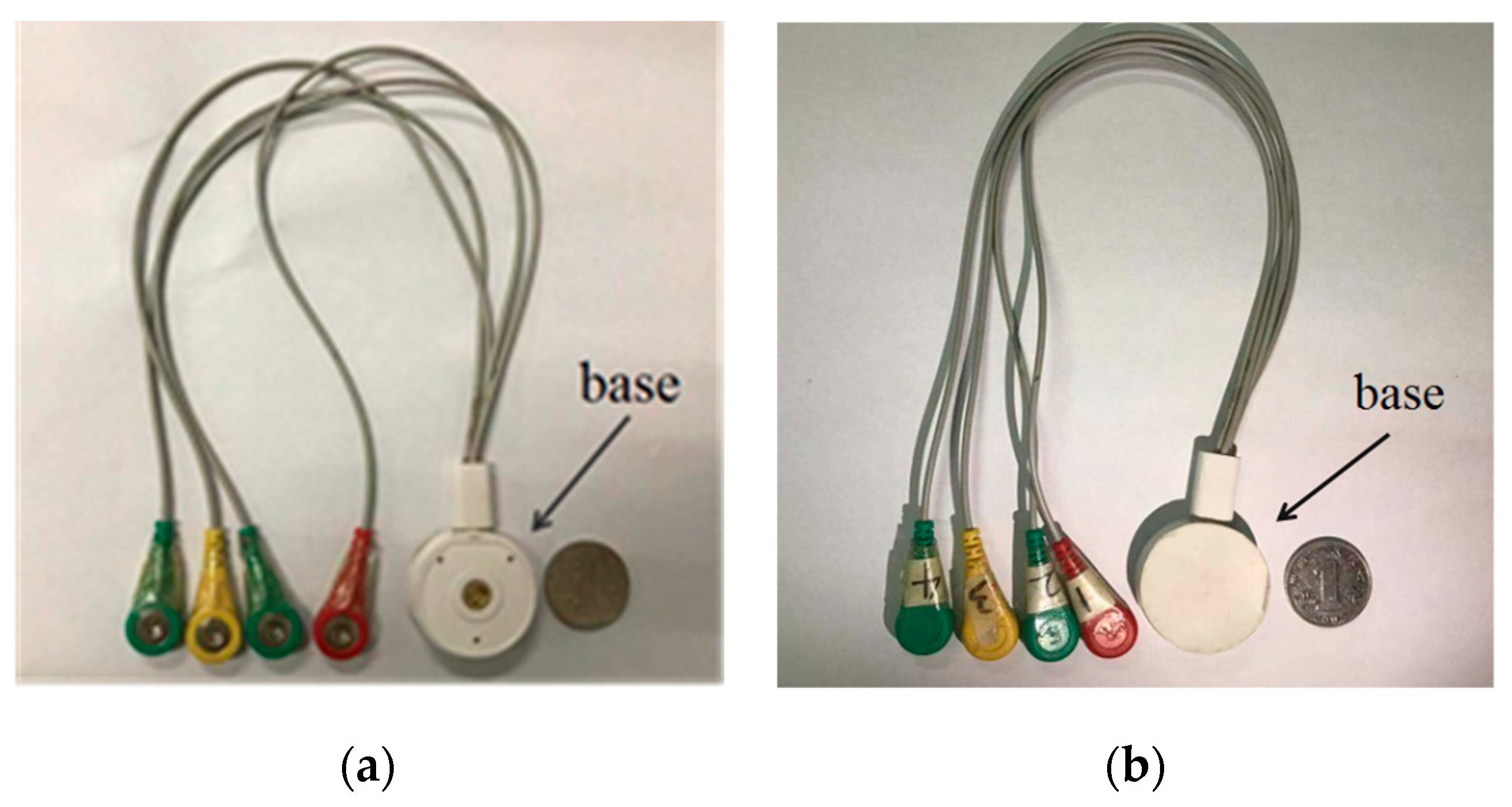
2.1. Hardware Design of Fetal ECG Collector
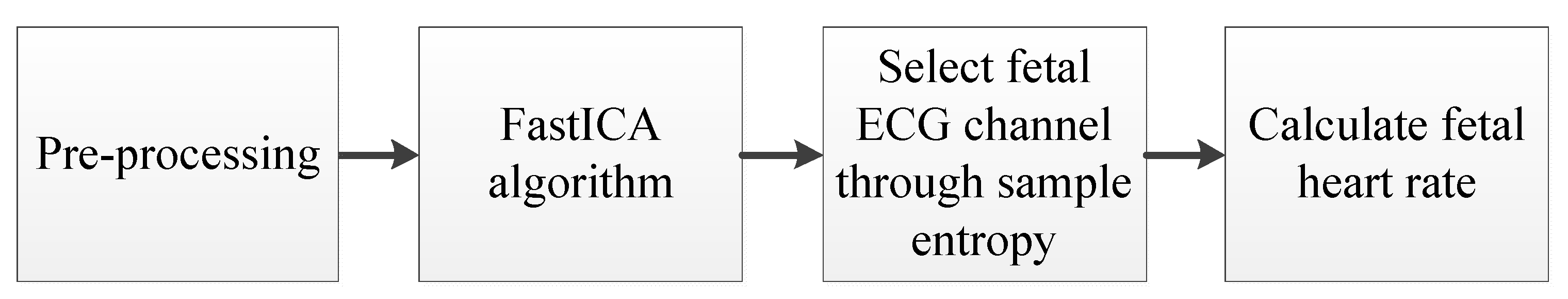
2.2. Fetal ECG Extraction Algorithm
2.2.1. Abdominal Signal Pre-Processing
2.2.2. FastICA Algorithm
2.2.3. Sample Entropy
2.3. Android Smartphone App Software Design
3. Results
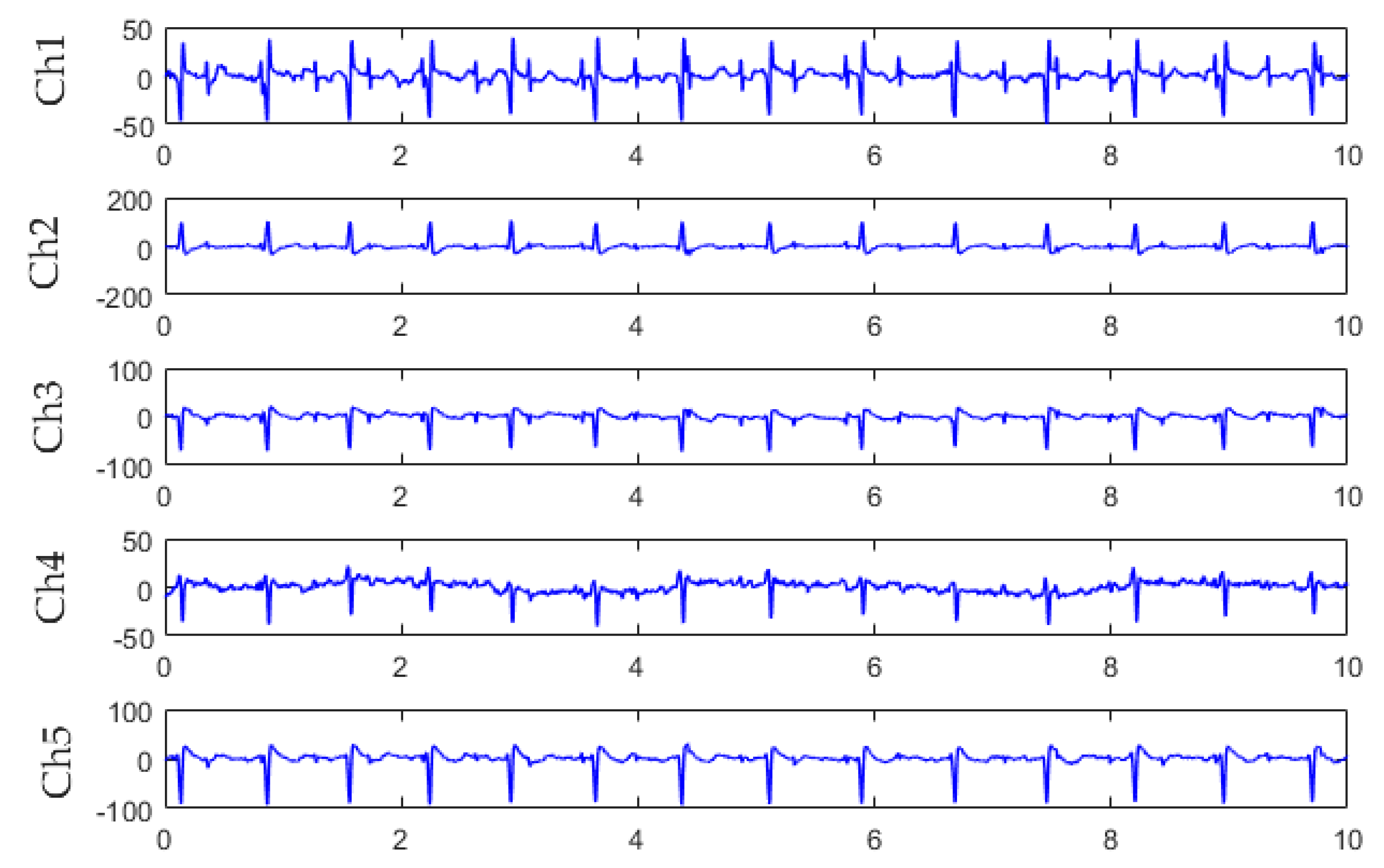
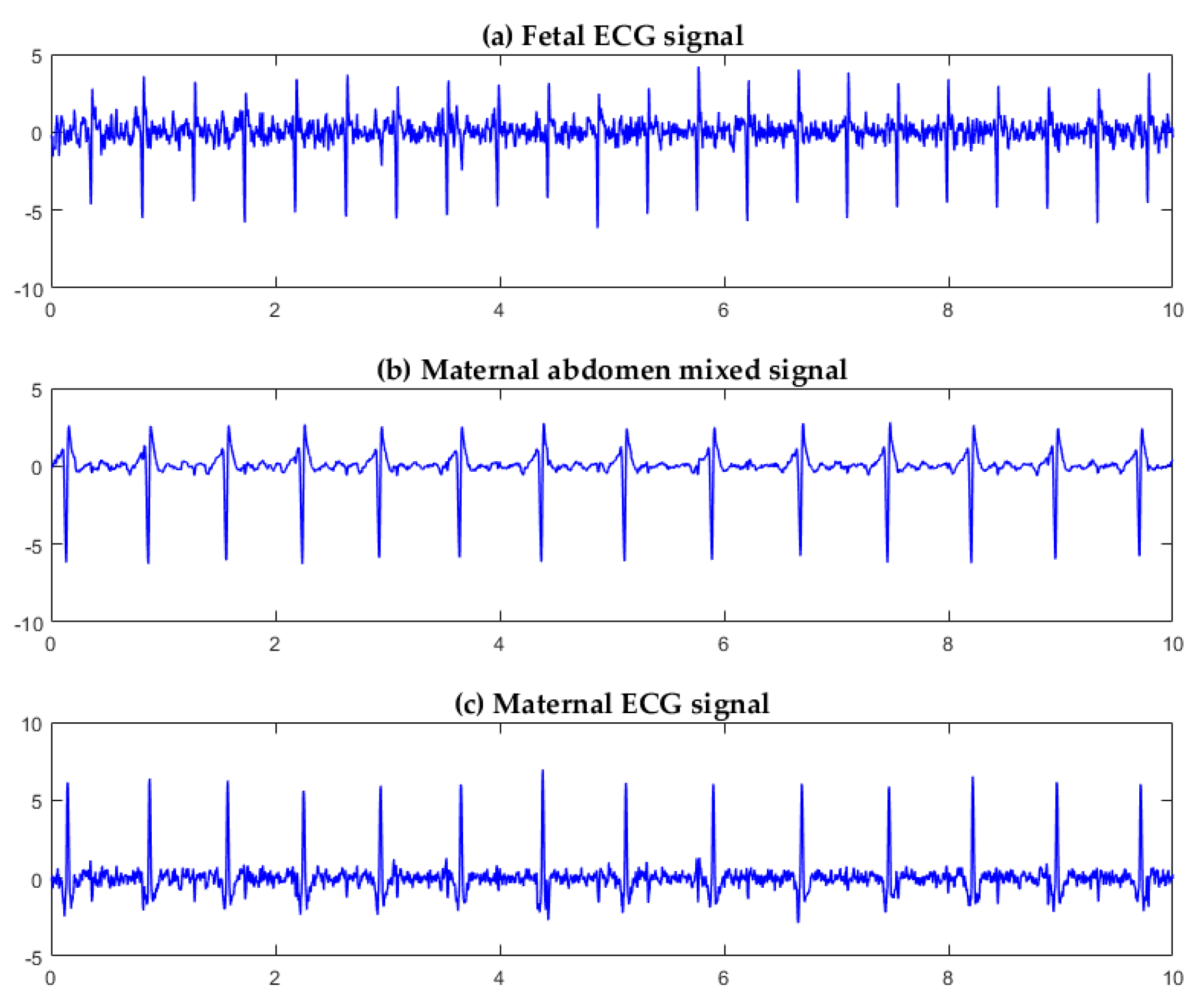
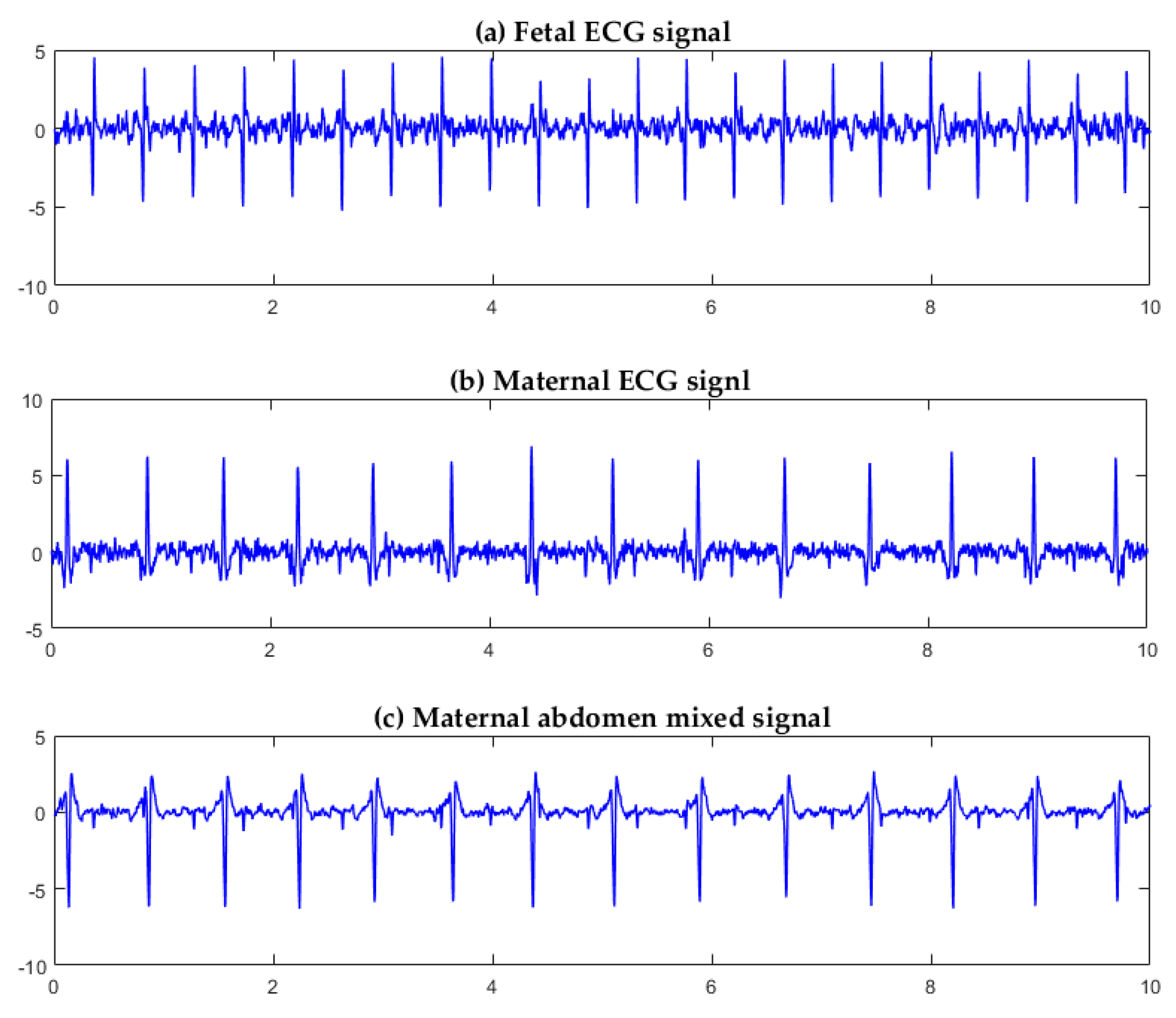
3.1. Algorithm Verification
3.2. Fetal ECG Collector Performance Testing
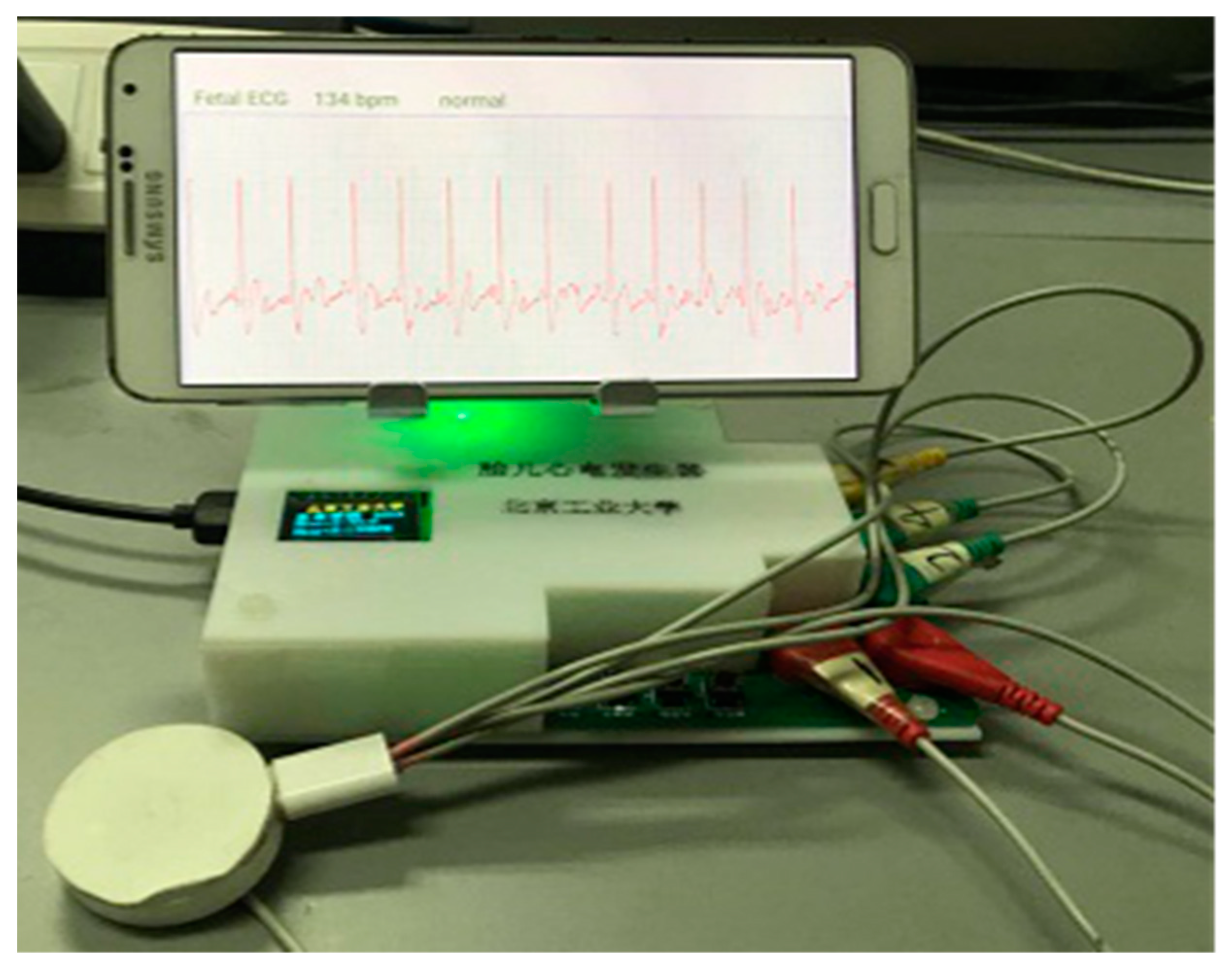
3.3. System Verification
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, L.; Zhou, Z.; Yuan, Y.; Wu, S. An improved FastICA method for fetal ECG extraction. Comput. Math. Methods Med. 2018, 2018, 7061456. [Google Scholar] [CrossRef] [PubMed]
- Varanini, M.; Tartarisco, G.; Balocchi, R.; Macerata, A.; Pioggia, G.; Billeci, L. A new method for QRS complex detection in multichannel ECG: Application to self-monitoring of fetal health. Comput. Biol. Med. 2016, 85, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, G.M.; Bergmans, J.W.M.; Oei, S.G.; Ungureanu, A.; Wolf, W. The event synchronous canceller algorithm removes maternal ECG from abdominal signals without affecting the fetal ECG. Comput. Biol. Med. 2009, 39, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Martinek, R.; Kahankova, R.; Nazeran, H.; Konecny, J.; Jezewski, J.; Janku, P.; Bilik, P.; Zidek, J.; Nedoma, J.; Fajkus, M. Non-invasive fetal monitoring: A maternal surface ECG electrode placement-based novel approach for optimization of adaptive filter control parameters using the LMS and RLS algorithms. Sensors 2017, 17, 1154. [Google Scholar] [CrossRef] [PubMed]
- Behar, J.; Johnson, A.; Clifford, G.D.; Oster, J. A Comparison of single channel fetal ECG extraction methods. Ann. Biomed. Eng. 2014, 42, 1340–1353. [Google Scholar] [CrossRef]
- Wu, S.; Shen, Y.; Zhou, Z.; Lin, L.; Zeng, Y.; Gao, X. Research of fetal ECG extraction using wavelet analysis and adaptive filtering. Comput. Biol. Med. 2013, 43, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Dessì, A.; Pani, D.; Raffo, L. An advanced algorithm for fetal heart rate estimation from non-invasive low electrode density recordings. Physiol. Meas. 2014, 35, 1621–1636. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yi, Z. An algorithm for extracting fetal electrocardiogram. Neurocomputing 2008, 71, 1538–1542. [Google Scholar] [CrossRef]
- Sato, M.; Kimura, Y.; Chida, S.; Ito, T.; Katayama, N.; Okamura, K.; Nakao, M. A novel extraction method of fetal electrocardiogram from the composite abdominal signal. IEEE Trans. Biomed. Eng. 2006, 54, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Luan, Y. An adaptive integrated algorithm for noninvasive fetal ECG separation and noise reduction based on ICA-EEMD-WS. Med. Biol. Eng. Comput. 2015, 53, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.P.; Xiao, Y.G.; Wei, G.; Sun, J.W. A multichannel nonlinear adaptive noise canceller based on generalized FLANN for fetal ECG extraction. Meas. Sci. Technol. 2016, 27, 015703. [Google Scholar] [CrossRef]
- Zhang, N.N.; Zhang, J.Y.; Li, H.; Mumini, O.O.; Samuel, O.W.; Ivanov, K.; Wang, L. A novel technique for fetal ECG extraction using single-channel abdominal recording. Sensors 2017, 17, 457. [Google Scholar] [CrossRef] [PubMed]
- Hyvarinen, A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 2002, 10, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Torti, E.; Koliopoulos, D.; Matraxia, M.; Danese, G.; Leporati, F. Custom FPGA processing for real-time fetal ECG extraction and identification. Comput. Biol. Med. 2016, 80, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.D.; Silva, I.; Behar, J.; Moody, G.B. Noninvasive fetal ECG analysis. Physiol. Meas. 2014, 35, 1521–1536. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, R.; Rieta, J.J. A review on sample entropy applications for the non-invasive analysis of atrial fibrillation electrocardiograms. Biomed. Signal. Process. Control. 2010, 5, 1–14. [Google Scholar] [CrossRef]
- Jia, Y.F.; Yang, X.D. A fetal electrocardiogram signal extraction algorithm based on fast one-unit independent component analysis with reference. Comput. Math. Methods Med. 2016, 2016, 5127978. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.A.; Kreutz-Delgado, K.; Makeig, S. Strong sub-and super-gaussianity. In Proceedings of the 2010 International Conference on Latent Variable Analysis and Signal Separation, St. Malo, France, 27–30 September 2010; pp. 303–310. [Google Scholar]
- Lathauwer, L. DaISy: Database for the Identification of Systems. Available online: http:// www.esat.kuleu-ven.ac.be./sista/daisy (accessed on 19 December 2018).
- Fatemi, M.; Ogburn, P.L.; Greenleaf, J.F. Fetal stimulation by pulsed diagnostic ultrasound. J. Ultrasound Med. 2001, 20, 883–889. [Google Scholar] [CrossRef]
- Piéri, J.F.; Crowe, J.A.; Hayesgill, B.R.; Spencer, C.J.; Bhogal, K.; James, D.K. Compact long-term recorder for the transabdominal foetal and maternal electrocardiogram. Med. Biol. Eng. Comput. 2001, 39, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Cohen, W.R.; Ommani, S.; Hassan, S.; Mirza, F.G.; Solomon, M.; Brown, R.; Schifrin, B.S.; Himsworth, J.M.; Hayes-Gill, B.R. Accuracy and reliability of fetal heart rate monitoring using maternal abdominal surface electrodes. Acta Obstet. Gynecol. Scand. 2012, 91, 1306–1313. [Google Scholar] [CrossRef]
- Zhao, Z.D.; Tong, H.L.; Deng, Y.J.; Xu, W.; Zhang, Y.F.; Ye, H.H. Single-lead fetal ECG extraction based on a parallel marginalized particle filter. Sensors 2017, 6, 1456. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.J.; Han, L.; Liu, Q.; Jiang, A.Y. FECG extraction based on least square support vector machine combined with FastICA. J. Inf. Sci. Eng. 2017, 33, 1595–1609. [Google Scholar]
- Vullings, R.; Mischi, M. Probabilistic source separation for robust fetal electrocardiography. Comput. Math. Methods Med. 2013, 2013, 109756. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.; Anyadi, P.; Stephens, L.; Thomas, S.L.; Reid, H.; Higgins, L.E.; Warrander, L.K.; Johnstone, E.D.; Heazell, A.E.P. A mixed-methods evaluation of continuous electronic fetal monitoring for an extended period. Acta Obstet. Gynecol. Scand. 2018, 97, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.S. Continuously monitoring foetal ECG using mobile telemedicine sensor network. Engineering 2013, 5, 63–66. [Google Scholar] [CrossRef]
- Greene, K.R.; Dawes, G.S.; Lilja, H.; Rosén, K.G. Changes in the ST waveform of the fetal lamb electrocardiogram with hypoxemia. Am. J. Obstet. Gynecol. 1982, 144, 950–958. [Google Scholar] [CrossRef]
- Slama, A.B.; Lentka, Ł.; Mouelhi, A.; Diouani, M.F.; Sayadi, M.; Smulko, J. Application of statistical features and multilayer neural network to automatic diagnosis of arrhythmia by ECG signals. Metrol. Meas. Syst. 2018, 25, 87–101. [Google Scholar]
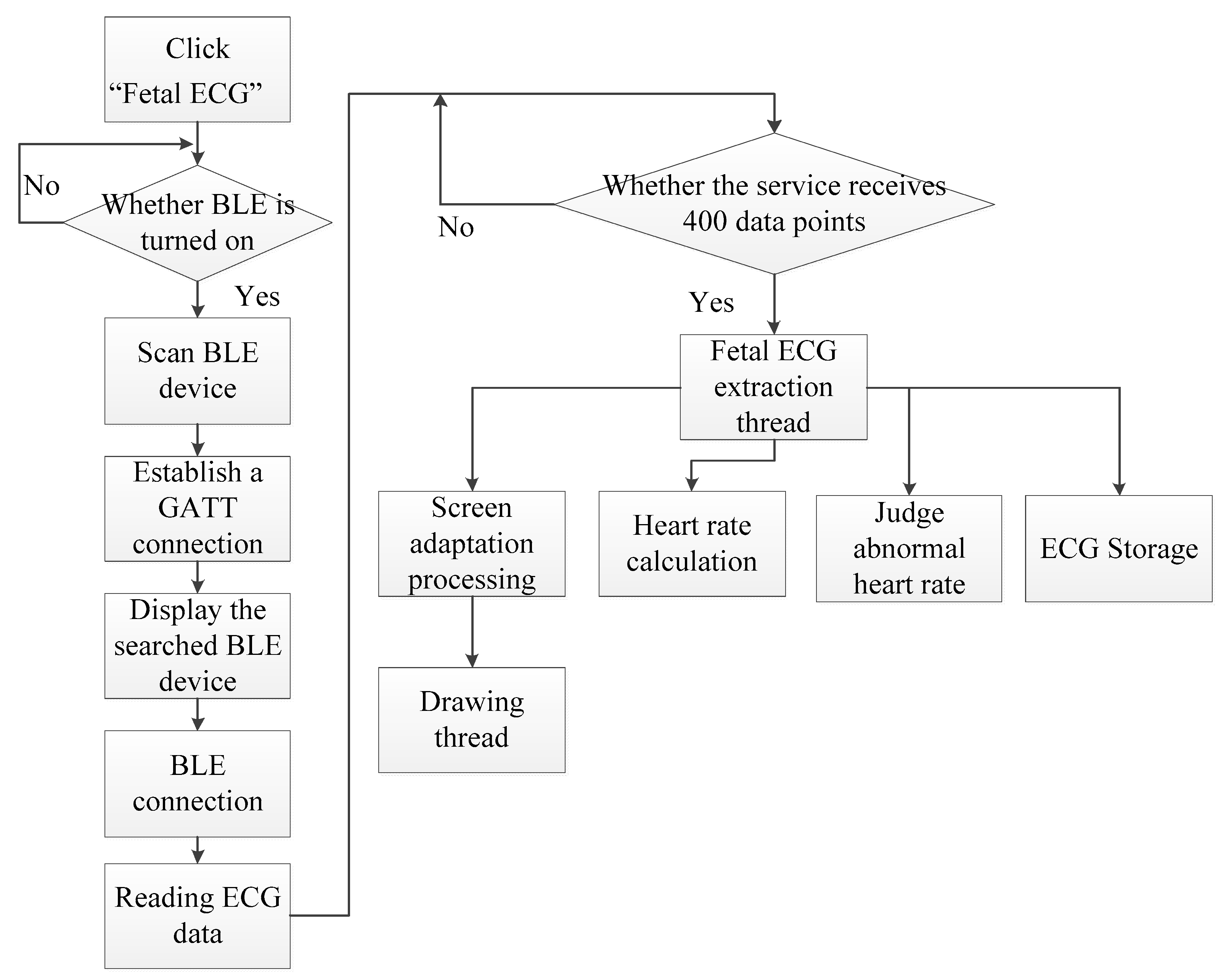
| Module | Functional Description |
|---|---|
| Registration/login module | User registration and login |
| Communication module | Communicate with the fetal ECG collector via Bluetooth to receive data in real time |
| Display module | Display real-time ECG waveform, heart rate, and analysis results on the smartphone |
| Data processing module | Using FastICA to separate source components, sample entropy to identify fetal ECG signals, and thresholding to calculate fetal heart rate |
| Storage module | Save fetal ECG data to “.txt” format locally |
| Cloud interaction | Upload fetal ECG data to the cloud |
| Abdomen Three-lead Signal | Entropy of Fetal ECG | Entropy of Mixed ECG | Entropy of Maternal ECG |
|---|---|---|---|
| Ch1, Ch2, Ch3 | 1.32 | 0.3 | 0.92 |
| Ch1, Ch2, Ch4 | 1.10 | 0.29 | 0.47 |
| Ch1, Ch2, Ch5 | 1.56 | 0.22 | 0.61 |
| Ch1, Ch3, Ch4 | 1.02 | 0.35 | 0.61 |
| Ch1, Ch3, Ch5 | 1.03 | 0.26 | 0.90 |
| Ch1, Ch4, Ch5 | 1.14 | 0.26 | 0.61 |
| Ch2, Ch3, Ch4 | 1.54 | 0.33 | 0.88 |
| Ch2, Ch3, Ch5 | 1.12 | 0.43 | 0.96 |
| Ch2, Ch4, Ch5 | 1.14 | 0.25 | 0.61 |
| Ch3, Ch4, Ch5 | 1.27 | 0.25 | 0.75 |
| Mean | 1.22 | 0.29 | 0.73 |
| Index | Testing Result | Requirement by the China National Standard |
|---|---|---|
| Dynamic input range | 403 mV | 300 mV |
| Input resistance | 500 MΩ | >10 MΩ |
| Common-mode rejection ratio | 95 dB | >45 dB |
| Gain accuracy | 0.0% | 10% |
| System noise | 7 μV | 50 μV |
| Frequency response | −0.2 to 0.9 dB | −3 to 3 dB |
| Minimum detectable signal | 2 μV | 50 μV |
| Timing accuracy | 1 s | ≤30 s |
| Duration of monitoring | 34 h | >24 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Yuan, Y.; Zhou, Z.; Bai, Y.; Wu, S. A Fetal ECG Monitoring System Based on the Android Smartphone. Sensors 2019, 19, 446. https://doi.org/10.3390/s19030446
Yuan L, Yuan Y, Zhou Z, Bai Y, Wu S. A Fetal ECG Monitoring System Based on the Android Smartphone. Sensors. 2019; 19(3):446. https://doi.org/10.3390/s19030446
Chicago/Turabian StyleYuan, Li, Yanchao Yuan, Zhuhuang Zhou, Yanping Bai, and Shuicai Wu. 2019. "A Fetal ECG Monitoring System Based on the Android Smartphone" Sensors 19, no. 3: 446. https://doi.org/10.3390/s19030446






