Application of Electrochemical Aptasensors toward Clinical Diagnostics, Food, and Environmental Monitoring: Review
Abstract
1. Introduction
1.1. Aptasensors Electrochemical Detection Strategies
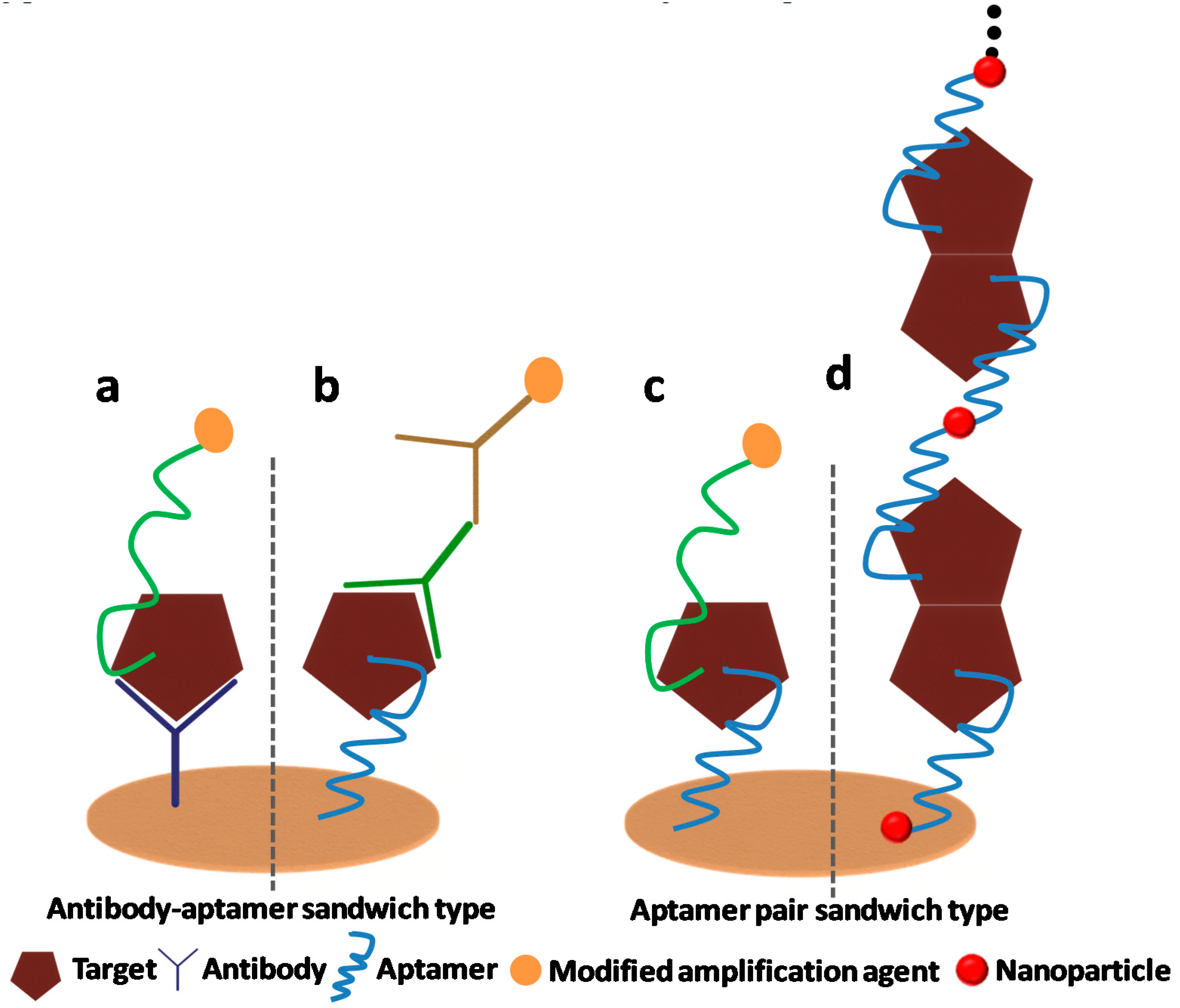
1.1.1. Sandwich Sensors Combining Aptamer and Antibody
1.1.2. Electrochemical Impedance Spectroscopy Aptasensors
2. Electrochemical Aptasensors for Clinical Diagnostic Applications
2.1. Application of Aptasensors for Biomarker Detection
2.1.1. Aptasensors for Cancer Biomarkers
2.1.2. Aptasensors for CVD Biomarkers
2.1.3. Aptasensors for Neurotransmitters and Alzheimer’s Biomarker
3. Electrochemical Aptasensors for Environmental Sample Application
3.1. Aptasensors for Heavy Metal Detection
3.2. Aptasensors for Pesticide Detection
4. Electrochemical Aptasensors for Food Sample Applications
4.1. Aptasensors for Food Toxins
4.2. Aptasensors for Antibiotic Residues
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blind, M.; Blank, M. Aptamer Selection Technology and Recent Advances. Mol. Ther. Nucleic Acids 2015, 4, e223. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef]
- Civit, L.; Taghdisi, S.M.; Jonczyk, A.; Haßel, S.K.; Gröber, C.; Blank, M.; Stunden, H.J.; Beyer, M.; Schultze, J.; Latz, E.; et al. Systematic evaluation of cell-SELEX enriched aptamers binding to breast cancer cells. Biochimie 2018, 145, 53–62. [Google Scholar] [CrossRef]
- Mishra, R.K.; Hayat, A.; Catanante, G.; Istamboulie, G.; Marty, J.-L. Sensitive quantitation of Ochratoxin A in cocoa beans using differential pulse voltammetry based aptasensor. Food Chem. 2016, 192, 799–804. [Google Scholar] [CrossRef]
- Mishra, R.K.; Hayat, A.; Catanante, G.; Ocaña, C.; Marty, J.-L. A label free aptasensor for Ochratoxin A detection in cocoa beans: An application to chocolate industries. Anal. Chim. Acta 2015, 889, 106–112. [Google Scholar] [CrossRef]
- McCauley, T.G.; Hamaguchi, N.; Stanton, M. Aptamer-based biosensor arrays for detection and quantification of biological macromolecules. Anal. Biochem. 2003, 319, 244–250. [Google Scholar] [CrossRef]
- Willner, I.; Zayats, M. Electronic Aptamer-Based Sensors. Angew. Chem. Int. Ed. 2007, 46, 6408–6418. [Google Scholar] [CrossRef]
- Najafabadi, M.E.; Khayamian, T.; Hashemian, Z. Aptamer-conjugated magnetic nanoparticles for extraction of adenosine from urine followed by electrospray ion mobility spectrometry. J. Pharm. Biomed. Anal. 2015, 107, 244–250. [Google Scholar] [CrossRef]
- Drabik, A.; Ner-Kluza, J.; Mielczarek, P.; Civit, L.; Mayer, G.; Silberring, J. Advances in the Study of Aptamer–Protein Target Identification Using the Chromatographic Approach. J. Proteome Res. 2018, 17, 2174–2181. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef]
- Davis, K.A.; Abrams, B.; Lin, Y.; Jayasena, S.D. Use of a High Affinity DNA Ligand in Flow Cytometry. Nucleic Acids Res. 1996, 24, 702–706. [Google Scholar] [CrossRef]
- Bakker, E.; Qin, Y. Electrochemical sensors. Anal. Chem. 2006, 78, 3965–3984. [Google Scholar] [CrossRef]
- Salimi, A.; Kurd, M.; Teymourian, H.; Hallaj, R. Highly sensitive electrocatalytic detection of nitrite based on SiC nanoparticles/amine terminated ionic liquid modified glassy carbon electrode integrated with flow injection analysis. Sens. Actuators B Chem. 2014, 205, 136–142. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Parker, S.G.; Barfidokht, A.; Alam, M.T.; Walker, D.B.; Messerle, B.A.; Gooding, J.J. A Ruthenium Based Organometallic Complex for Biosensing that is both a Stable Redox Label and a Homobifunctional Linker. Electroanalysis 2015, 27, 1078–1085. [Google Scholar] [CrossRef]
- Taufik, S.; Barfidokht, A.; Alam, M.T.; Jiang, C.; Parker, S.G.; Gooding, J.J. An antifouling electrode based on electrode–organic layer–nanoparticle constructs: Electrodeposited organic layers versus self-assembled monolayers. J. Electroanal. Chem. 2016, 779, 229–235. [Google Scholar] [CrossRef]
- Mehrgardi, M.A.; Barfidokht, A. Electrocatalytic activity of thianthrene toward one-electron oxidation of guanosine and DNA in a non-aqueous medium. J. Electroanal. Chem. 2010, 644, 44–49. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Martin, A.; García-Carmona, L.; Barfidokht, A.; Kurniawan, J.F.; Moreto, J.R.; Tang, G.; Shin, A.; Liu, X.; Escarpa, A.; et al. Skin-Worn Soft Microfluidic Potentiometric Detection System. Electroanalysis 2018. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef]
- Barfidokht, A.; Ciampi, S.; Luais, E.; Darwish, N.; Gooding, J.J. Distance-Dependent Electron Transfer at Passivated Electrodes Decorated by Gold Nanoparticles. Anal. Chem. 2013, 85, 1073–1080. [Google Scholar] [CrossRef]
- Barfidokht, A.; Ciampi, S.; Luais, E.; Darwish, N.; Gooding, J.J. The Influence of Organic-Film Morphology on the Efficient Electron Transfer at Passivated Polymer-Modified Electrodes to which Nanoparticles are Attached. ChemPhysChem 2013, 14, 2190–2197. [Google Scholar] [CrossRef]
- Kashi, M.B.; Silva, S.M.; Yang, Y.; Gonçales, V.R.; Parker, S.G.; Barfidokht, A.; Ciampi, S.; Gooding, J.J. Light-activated electrochemistry without surface-bound redox species. Electrochim. Acta 2017, 251, 250–255. [Google Scholar] [CrossRef]
- Carter, L.; Chuah, K.; Tavallaie, R.; Barfidokht, A.; Parker, S.G.; Gooding, J.J. Switching “on and off” faradaic electrochemistry at an otherwise passivated electrode using gold-coated magnetic nanoparticles. Electrochem. Commun. 2015, 61, 93–96. [Google Scholar] [CrossRef]
- Wang, J. Nanomaterial-based electrochemical biosensors. Analyst 2005, 130, 421. [Google Scholar] [CrossRef]
- Khodadadi, M.; Malekpour, A.; Mehrgardi, M.A. Aptamer functionalized magnetic nanoparticles for effective extraction of ultratrace amounts of aflatoxin M1 prior its determination by HPLC. J. Chromatogr. A 2018, 1564, 85–93. [Google Scholar] [CrossRef]
- Urban, G.; Jobst, G.; Kohl, F.; Jachimowicz, A.; Olcaytug, F.; Tilado, O.; Goiser, P.; Nauer, G.; Pittner, F.; Schalkhammer, T.; et al. Miniaturized thin-film biosensors using covalently immobilized glucose oxidase. Biosens. Bioelectron. 1991, 6, 555–562. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci. 2018, 1800880. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Barfidokht, A.; Park, J.; Wang, J.; Mercier, P.P. A Battery-Powered Wireless Ion Sensing System Consuming 5.5 nW of Average Power. IEEE J. Solid State Circuits 2018, 53, 2043–2053. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Park, J.; Barfidokht, A.; Wang, J.; Mercier, P.P. A 5.5nW Battery-Powered Wireless Ion Sensing System. In Proceedings of the IEEE Eur. Solid State Circuits Confrence, Leuven, Belgium, 11–14 September 2017. [Google Scholar] [CrossRef]
- Mishra, R.K.; Hubble, L.J.; Martín, A.; Kumar, R.; Barfidokht, A.; Kim, J.; Musameh, M.M.; Kyratzis, I.L.; Wang, J. Wearable Flexible and Stretchable Glove Biosensor for On-Site Detection of Organophosphorus Chemical Threats. ACS Sens. 2017, 2, 553–561. [Google Scholar] [CrossRef]
- Mishra, R.K.; Barfidokht, A.; Karajic, A.; Sempionatto, J.R.; Wang, J.; Wang, J. Wearable potentiometric tattoo biosensor for on-body detection of G-type nerve agents simulants. Sens. Actuators B Chem. 2018, 273, 966–972. [Google Scholar] [CrossRef]
- Mishra, R.K.; Martín, A.; Nakagawa, T.; Barfidokht, A.; Lu, X.; Sempionatto, J.R.; Lyu, K.M.; Karajic, A.; Musameh, M.M.; Kyratzis, I.L.; et al. Detection of vapor-phase organophosphate threats using wearable conformable integrated epidermal and textile wireless biosensor systems. Biosens. Bioelectron. 2018, 101, 227–234. [Google Scholar] [CrossRef]
- Radi, A.-E.; Acero Sánchez, J.L.; Baldrich, E.; O’Sullivan, C.K. Reagentless, Reusable, Ultrasensitive Electrochemical Molecular Beacon Aptasensor. J. Am. Chem. Soc. 2006, 128, 117–124. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A. Aptamer-conjugated silver nanoparticles for electrochemical detection of adenosine triphosphate. Biosens. Bioelectron. 2012, 37, 94–98. [Google Scholar] [CrossRef]
- Yugender Goud, K.; Catanante, G.; Hayat, A.; Satyanarayana, M.; Vengatajalabathy Gobi, K.; Marty, J.L. Disposable and portable electrochemical aptasensor for label free detection of aflatoxin B1 in alcoholic beverages. Sens. Actuators B Chem. 2016, 235, 466–473. [Google Scholar] [CrossRef]
- Goud, K.Y.; Hayat, A.; Catanante, G.; Satyanarayana, M.; Gobi, K.V.; Marty, J.L. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim. Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
- Ikebukuro, K.; Kiyohara, C.; Sode, K. Electrochemical Detection of Protein Using a Double Aptamer Sandwich. Anal. Lett. 2004, 37, 2901–2909. [Google Scholar] [CrossRef]
- Mishra, G.; Sharma, V.; Mishra, R. Electrochemical Aptasensors for Food and Environmental Safeguarding: A Review. Biosensors 2018, 8, 28. [Google Scholar] [CrossRef]
- Kang, Y.; Feng, K.J.; Chen, J.W.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Electrochemical detection of thrombin by sandwich approach using antibody and aptamer. Bioelectrochemistry 2008, 73, 76–81. [Google Scholar] [CrossRef]
- Guo, L.; Kim, D.H. LSPR biomolecular assay with high sensitivity induced by aptamer–antigen–antibody sandwich complex. Biosens. Bioelectron. 2012, 31, 567–570. [Google Scholar] [CrossRef]
- Huang, Y.; Nie, X.M.; Gan, S.L.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Electrochemical immunosensor of platelet-derived growth factor with aptamer-primed polymerase amplification. Anal. Biochem. 2008, 382, 16–22. [Google Scholar] [CrossRef]
- Ocaña, C.; Hayat, A.; Mishra, R.; Vasilescu, A.; del Valle, M.; Marty, J.L. A novel electrochemical aptamer–antibody sandwich assay for lysozyme detection. Analyst 2015, 140, 4148–4153. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M.; Li, Z. A highly selective electrochemical impedance spectroscopy-based aptasensor for sensitive detection of acetamiprid. Biosens. Bioelectron. 2013, 43, 12–18. [Google Scholar] [CrossRef]
- Ceylan, O.; Mishra, G.K.; Yazici, M.; Cakmakci, R.C.; Niazi, J.H.; Qureshi, A.; Gurbuz, Y. Development of Hand-Held Point-of-Care Diagnostic Device for Detection of Multiple Cancer and Cardiac Disease Biomarkers. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018; IEEE: Piscatway, NJ, USA, 2018. [Google Scholar]
- Allender, S.; Scarborough, P.; Peto, V.; Rayner, M.; Leal, J.; Fernandez, R.L.; Gray, A. European Cardiovascular Disease Statistics; University of Oxford: Oxford, UK, 2008; ISBN 9782953789812. [Google Scholar]
- Mitsakakis, K.; Gizeli, E. Detection of multiple cardiac markers with an integrated acoustic platform for cardiovascular risk assessment. Anal. Chim. Acta 2011, 699, 1–5. [Google Scholar] [CrossRef]
- Blennow, K. Biomarkers in Alzheimer’s disease drug development. Nat. Med. 2010, 16, 1218–1222. [Google Scholar] [CrossRef]
- Cummings, J.L. Biomarkers in Alzheimer’s disease drug development. Alzheimer’s Dement. 2011, 7, e13–e44. [Google Scholar] [CrossRef]
- Mayeux, R.; Schupf, N. Blood-based biomarkers for Alzheimer’s disease: Plasma Aβ40 and Aβ42, and genetic variants. Neurobiol. Aging 2011, 32, S10–S19. [Google Scholar] [CrossRef]
- Sett, A.; Das, S.; Sharma, P.; Bora, U. Aptasensors in Health, Environment and Food Safety Monitoring. Open J. Appl. Biosens. 2012, 1, 9–19. [Google Scholar] [CrossRef]
- Jolly, P.; Formisano, N.; Tkáč, J.; Kasák, P.; Frost, C.G.; Estrela, P. Label-free impedimetric aptasensor with antifouling surface chemistry: A prostate specific antigen case study. Sens. Actuators B Chem. 2015, 209, 306–312. [Google Scholar] [CrossRef]
- Liu, B.; Lu, L.; Hua, E.; Jiang, S.; Xie, G. Detection of the human prostate-specific antigen using an aptasensor with gold nanoparticles encapsulated by graphitized mesoporous carbon. Microchim. Acta 2012, 178, 163–170. [Google Scholar] [CrossRef]
- Diaconu, I.; Cristea, C.; Hârceagă, V.; Marrazza, G.; Berindan-Neagoe, I.; Săndulescu, R. Electrochemical immunosensors in breast and ovarian cancer. Clin. Chim. Acta 2013, 425, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Ludovini, V.; Gori, S.; Colozza, M.; Pistola, L.; Rulli, E.; Floriani, I.; Pacifico, E.; Tofanetti, F.R.; Sidoni, A.; Basurto, C.; et al. Evaluation of serum HER2 extracellular domain in early breast cancer patients: Correlation with clinicopathological parameters and survival. Ann. Oncol. 2008, 19, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Windmiller, J.R.; Zhou, N.; Chuang, M.-C.; Valdés-Ramírez, G.; Santhosh, P.; Miller, P.R.; Narayan, R.; Wang, J. Microneedle array-based carbon paste amperometric sensors and biosensors. Analyst 2011, 136, 1846. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Capacitive aptamer–antibody based sandwich assay for the detection of VEGF cancer biomarker in serum. Sens. Actuators B Chem. 2015, 209, 645–651. [Google Scholar] [CrossRef]
- Jarczewska, M.; Sheelam, S.R.; Ziółkowski, R.; Górski, Ł. A Label-Free Electrochemical DNA Aptasensor for the Detection of Dopamine. J. Electrochem. Soc. 2016, 163, B26–B31. [Google Scholar] [CrossRef]
- Meirinho, S.G.; Dias, L.G.; Peres, A.M.; Rodrigues, L.R. Electrochemical aptasensor for human osteopontin detection using a DNA aptamer selected by SELEX. Anal. Chim. Acta 2017, 987, 25–37. [Google Scholar] [CrossRef]
- Meirinho, S.G.; Dias, L.G.; Peres, A.M.; Rodrigues, L.R. Development of an electrochemical RNA-aptasensor to detect human osteopontin. Biosens. Bioelectron. 2015, 71, 332–341. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Biosensors for cardiac biomarkers detection: A review. Sens. Actuator B Chem. 2012, 171–172, 62–76. [Google Scholar] [CrossRef]
- Centi, S.; Bonel Sanmartin, L.; Tombelli, S.; Palchetti, I.; Mascini, M. Detection of C Reactive Protein (CRP) in Serum by an Electrochemical Aptamer-Based Sandwich Assay. Electroanalysis 2009, 21, 1309–1315. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Kallempudi, S.; Niazi, J.H. Label-free RNA aptamer-based capacitive biosensor for the detection of C-reactive protein. Phys. Chem. Chem. Phys. 2010, 12, 9176. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Zhang, J.; Zhang, W.; Zhang, Y. RNA aptamer-based electrochemical aptasensor for C-reactive protein detection using functionalized silica microspheres as immunoprobes. Biosens. Bioelectron. 2017, 95, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tertiş, M.; Ciui, B.; Suciu, M.; Săndulescu, R.; Cristea, C. Label-free electrochemical aptasensor based on gold and polypyrrole nanoparticles for interleukin 6 detection. Electrochim. Acta 2017, 258, 1208–1218. [Google Scholar] [CrossRef]
- Perry, M.; Li, Q.; Kennedy, R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta 2009, 653, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Farjami, E.; Campos, R.; Nielsen, J.S.; Gothelf, K.V.; Kjems, J.; Ferapontova, E.E. RNA Aptamer-Based Electrochemical Biosensor for Selective and Label-Free Analysis of Dopamine. Anal. Chem. 2013, 85, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, W.; Yu, P.; Xiong, E.; Zhang, X.; Chen, J. A simple label-free electrochemical aptasensor for dopamine detection. RSC Adv. 2014, 4, 52250–52255. [Google Scholar] [CrossRef]
- Álvarez-Martos, I.; Ferapontova, E.E. Electrochemical Label-Free Aptasensor for Specific Analysis of Dopamine in Serum in the Presence of Structurally Related Neurotransmitters. Anal. Chem. 2016, 88, 3608–3616. [Google Scholar] [CrossRef]
- Shui, B.; Tao, D.; Cheng, J.; Mei, Y.; Jaffrezic-Renault, N.; Guo, Z. A novel electrochemical aptamer–antibody sandwich assay for the detection of tau-381 in human serum. Analyst 2018, 143, 3549–3554. [Google Scholar] [CrossRef]
- Jarczewska, M.; Rębiś, J.; Górski, L.; Malinowska, E. Development of DNA aptamer-based sensor for electrochemical detection of C-reactive protein. Talanta 2018, 189, 45–54. [Google Scholar] [CrossRef]
- Raouafi, A.; Sánchez, A.; Raouafi, N.; Villalonga, R. Electrochemical aptamer-based bioplatform for ultrasensitive detection of prostate specific antigen. Sens. Actuators B Chem. 2019, 297, 126762. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, L.; Sun, Y.; Zheng, F.; Ke, W. Ag/CdO NP-Engineered Magnetic Electrochemical Aptasensor for Prostatic Specific Antigen Detection. ACS Appl. Mater. Interfaces 2019, 11, 3474–3481. [Google Scholar] [CrossRef]
- Nguyen, P.-L.; Sekhon, S.S.; Ahn, J.-Y.; Ko, J.H.; Lee, L.; Cho, S.-J.; Min, J.; Kim, Y.-H. Aptasensor for environmental monitoring. Toxicol. Environ. Health Sci. 2017, 9, 89–101. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, C.; Huang, D.; Lai, C.; Tang, L.; Zhou, Y.; Xu, P.; Wang, H.; Qin, L.; Cheng, M. Practical and regenerable electrochemical aptasensor based on nanoporous gold and thymine-Hg 2+ -thymine base pairs for Hg 2+ detection. Biosens. Bioelectron. 2017, 90, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, K.; Xu, L.; Yan, X.; Zhang, K.; Chen, X.; Wang, Q.; Zhang, L.; Pei, R. N-doped TiO 2 based visible light activated label-free photoelectrochemical biosensor for detection of Hg 2+ through quenching of photogenerated electrons. Analyst 2015, 140, 4143–4147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xue, Y.; Wang, W. Femtomole level photoelectrochemical aptasensing for mercury ions using quercetin–copper(II) complex as the DNA intercalator. Biosens. Bioelectron. 2014, 54, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, X.; Gao, J.; Xue, S.; Zhao, J. Label-free and enzyme-free strategy for sensitive electrochemical lead aptasensor by using metal-organic frameworks loaded with AgPt nanoparticles as signal probes and electrocatalytic enhancers. Electrochim. Acta 2017, 251, 25–31. [Google Scholar] [CrossRef]
- Gao, F.; Gao, C.; He, S.; Wang, Q.; Wu, A. Label-free electrochemical lead (II) aptasensor using thionine as the signaling molecule and graphene as signal-enhancing platform. Biosens. Bioelectron. 2016, 81, 15–22. [Google Scholar] [CrossRef]
- Madianos, L.; Tsekenis, G.; Skotadis, E.; Patsiouras, L.; Tsoukalas, D. A highly sensitive impedimetric aptasensor for the selective detection of acetamiprid and atrazine based on microwires formed by platinum nanoparticles. Biosens. Bioelectron. 2018, 101, 268–274. [Google Scholar] [CrossRef]
- Jiao, Y.; Jia, H.; Guo, Y.; Zhang, H.; Wang, Z.; Sun, X.; Zhao, J. An ultrasensitive aptasensor for chlorpyrifos based on ordered mesoporous carbon/ferrocene hybrid multiwalled carbon nanotubes. RSC Adv. 2016, 6, 58541–58548. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Fan, M.; Liu, X. Isolation and Identification of the DNA Aptamer Target to Acetamiprid. J. Agric. Food Chem. 2011, 59, 1582–1586. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Zhang, Q.; Zhang, C.; Liu, Y.; Tu, K.; Tu, J. Selection of DNA aptamers that bind to four organophosphorus pesticides. Biotechnol. Lett. 2012, 34, 869–874. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Abnous, K. Electrochemical aptamer based assay for the neonicotinoid insecticide acetamiprid based on the use of an unmodified gold electrode. Microchim. Acta 2016, 184, 499–505. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. Selection and Characterization of DNA Aptamers for Electrochemical Biosensing of Carbendazim. Anal. Chem. 2017, 89, 3138–3145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, G.; Zhang, Q.; Wang, H.; Zhang, Y.; Cao, W.; Zhang, N.; Du, B.; Wei, Q. Electrochemical aptasensor based on gold modified graphene nanocomposite with different morphologies for ultrasensitive detection of Pb2. Sens. Actuators B Chem. 2019, 288, 325–331. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, S.; Li, H.; Liu, H.; Pang, P.; Wang, H.; Wu, Z.; Yang, W. A Pb2+-ion electrochemical biosensor based on single-stranded DNAzyme catalytic beacon. Sens. Actuators B Chem. 2016, 222, 1083–1089. [Google Scholar] [CrossRef]
- Fu, J.; An, X.; Yao, Y.; Guo, Y.; Sun, X. Electrochemical aptasensor based on one step co-electrodeposition of aptamer and GO-CuNPs nanocomposite for organophosphorus pesticide detection. Sens. Actuators B Chem. 2019, 287, 503–509. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K. An electrochemical aptasensor based on gold nanoparticles, thio-nine and hairpin structure of complementary strand of aptamer for ultrasensitive detection of lead. Sens. Actuators B Chem. 2016, 234, 462–469. [Google Scholar] [CrossRef]
- Diaz-Amaya, S.; Lin, L.K.; DiNino, R.E.; Ostos, C.; Stanciu, L.A. Inkjet printed electrochemical aptasensor for detection of Hg2+ in organic solvents. Electrochimica Acta 2019, 316, 33–42. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Liu, Q.; Zhou, L.; Dai, L.; Qian, J.; Wang, K. Silver nanoparticles anchored on nitrogen-doped graphene as a novel electrochemical biosensing platform with enhanced sensitivity for aptamer-based pesticide assay. Analyst 2015, 140, 6404–6411. [Google Scholar] [CrossRef]
- Mishra, G.; Barfidokht, A.; Tehrani, F.; Mishra, R. Food Safety Analysis Using Electrochemical Biosensors. Foods 2018, 7, 141. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin B1 by aptamer-based biosensor using PAMAM dendrimers as immobilization platform. Food Cont. 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Tran, L.D.; Do, Q.P.; Nguyen, H.L.; Tran, N.H.; Nguyen, P.X. Label-free detection of aflatoxin M1 with electrochemical Fe3O4/polyaniline-based aptasensor. Mater. Sci. Eng. C 2013, 33, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Catanante, G.; Mishra, R.K.; Hayat, A.; Marty, J.-L. Sensitive analytical performance of folding based biosensor using methylene blue tagged aptamers. Talanta 2016, 153, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Fetter, L.; Richards, J.; Daniel, J.; Roon, L.; Rowland, T.J.; Bonham, A.J. Electrochemical aptamer scaffold biosensors for detection of botulism and ricin toxins. Chem. Comm. 2015, 51, 15137–15140. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Zheng, Y.T.; Zhang, H.B.; He, C.H.; Wu, W.D.; Zhang, H.B. DNA electrochemical aptasensor for detecting fumonisins B1 based on graphene and thionine nanocomposite. Electroanalysis 2015, 27, 1097–1103. [Google Scholar] [CrossRef]
- Mohammad Danesh, N.; Ramezani, M.; Sarreshtehdar Emrani, A.; Abnous, K.; Taghdisi, S.M. A novel electrochemical aptasensor based on arch-shape structure of aptamer-complimentary strand conjugate and exonuclease I for sensitive detection of streptomycin. Biosens. Bioelectron. 2016, 75, 123–128. [Google Scholar] [CrossRef]
- Zhou, N.; Luo, J.; Zhang, J.; You, Y.; Tian, Y. A label-free electrochemical aptasensor for the detection of kanamycin in milk. Anal. Methods 2015, 7, 1991–1996. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Xu, W.; Leng, X.; Wang, H.; Guo, Y.; Huang, J. A novel sandwich-type electrochemical aptasensor based on GR-3D Au and aptamer-AuNPs-HRP for sensitive detection of oxytetracycline. Biosens. Bioelectron. 2017, 88, 181–187. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, Y.S.; Niazi, J.H.; Gu, M.B. Electrochemical aptasensor for tetracycline detection. Bioprocess. Biosyst. Eng. 2010, 33, 31–37. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Taghdisi, S.M.; Emrani, A.S. A novel electrochemical aptasensor based on H-shape structure of aptamer-complimentary strands conjugate for ultrasensitive detection of cocaine. Sens. Actuators B Chem. 2016, 224, 351–355. [Google Scholar] [CrossRef]
- Hu, X.; Goud, Y.K.; Kumar, V.S.; Catanante, G.; Li, Z.; Zhu, Z.; Marty, J.L. Disposable electrochemical aptasensor based on carbon nanotubes-V2O5-chitosan nanocomposite for detection of ciprofloxacin. Sens. Actuators B Chem. 2018, 268, 278–286. [Google Scholar] [CrossRef]
- Hu, X.; Wei, P.; Catanante, G.; Li, Z.; Marty, J.L.; Zhu, Z. Ultrasensitive ciprofloxacin assay based on the use of a fluorescently labeled aptamer and a nanocomposite prepared from carbon nanotubes and MoSe2. Microchim. Acta 2019, 186, 507. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Y.; Ma, X.; Jia, F.; Guo, X.; Wang, Z. Impedimetric aptamer-based determination of the mold toxin fumonisin B1. Microchim. Acta 2015, 182, 1709–1714. [Google Scholar] [CrossRef]
- Wang, C.; Qian, J.; An, K.; Huang, X.; Zhao, L.; Liu, Q.; Hao, N.; Wang, K. Magneto-controlled aptasensor for simultaneous electrochemical detection of dual mycotoxins in maize using metal sulfide quantum dots coated silica as labels. Biosens. Bioelectron. 2017, 89, 802–809. [Google Scholar] [CrossRef]
- Geleta, G.S.; Zhao, Z.; Wang, Z. Novel reduced graphene oxide/molybdenum disulfide/polyaniline nanocomposite based electrochemical aptasensor for detection of aflatoxin B1. Analyst 2018, 143, 1644–1649. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Sitdikov, R.; Evtugyn, V.; Stoikov, I.; Antipin, I.; Hianik, T. Electrochemical Aptasensor for the Determination of Ochratoxin A at the Au Electrode Modified with Ag Nanoparticles Decorated with Macrocyclic Ligand. Electroanalysis 2013, 25, 1847–1854. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Stepanova, V.; Sitdikov, R.; Stoikov, I.; Nikolelis, D.; Hianik, T. Electrochemical Aptasensor Based on Polycarboxylic Macrocycle Modified with Neutral Red for Aflatoxin B1 Detection. Electroanalysis 2014, 26, 2100–2109. [Google Scholar] [CrossRef]
- Prabhakar, N.; Matharu, Z.; Malhotra, B.D. Polyaniline Langmuir–Blodgett film based aptasensor for ochratoxin A detection. Biosens. Bioelectron. 2011, 26, 4006–4011. [Google Scholar] [CrossRef]
- Ma, L.; Bai, L.; Zhao, M.; Zhou, J.; Chen, Y.; Mu, Z. An electrochemical aptasensor for highly sensitive detection of zearalenone based on PEI-MoS2-MWCNTs nanocomposite for signal enhancement. Anal. Chim. Act. 2019, 1060, 71–78. [Google Scholar] [CrossRef]


| S.N | Analyte | Detection Method | LOD/Range | System | Reference |
|---|---|---|---|---|---|
| 1 | PSA | EIS | 1 ng/mL | Thiol terminated sulfo-betaine | [52] |
| 2 | PSA | DPV | 0.25 ng mL−1 | Graphitized meso-porous carbon nanoparticles | [53] |
| 3 | OPN | SWV | 0.4–4.5 nM | Gold/DPA | [59] |
| 4 | OPN | SWV | 3.7 nM | Biotinylated RNA aptamer | [60] |
| 5 | CRP | DPV | 0.2 mg/L | Mangnetic nanoparticles on SPCE | [62] |
| 6 | CRP | SWV | 0.0017 ng mL−1 | Functionalized silica–Au nanoparticles | [64] |
| 7 | IL-6 | CV/EIS | 0.33 pg mL−1 | SPCE-polypyrole | [65] |
| 8 | Tau-381 | DPV | 0.42 pM | Cysteamine-stabilized gold nanoparticles (AuNPs) | [70] |
| 9 | CRP | SWV/EIS | 100 pM | Thiolated aptamers-Au surface | [71] |
| 10 | PSA | Potentio-amperometric | 0.064 pg mL−1 | Functionalized graphene-modified carbon screen-printed electrodes as tr | [72] |
| 11 | PSA | DPV | 28 pg/mL | Ag/CdO nanoparticles-graphene oxide nanosheet | [73] |
| SN | Analyte | Detection Method | LOD/Range | System | Reference |
|---|---|---|---|---|---|
| 1 | Hg2+ | DPV | 0.0036 nM | Thymine- Hg2+ -Thymine | [75] |
| 2 | Hg2+ | Photoelectrochemical | 2–6 µM | N-doped-TiO2 | [76] |
| 3 | Hg2+ | Photoelectrochemical | 3.33 fmol/L | PCTA/GO | [77] |
| 4 | Pb2+ | DPV | 0.032 pM | Ag/Pt nanoparticle | [78] |
| 5 | Pb2+ | DPV | 3.2 × 10−14 M | Graphene/Thionine | [79] |
| 6 | Pb2+ | EIS | 1.67 pmol/L | Au@p-rGO | [86] |
| 7 | Pb2+ | SWV/EIS | 32 pM | hemin/G-quadruplex -based DNAzym | [87] |
| 8 | Profenofos, Phorate, Isocarbophos, Omethoate | DPV | 0.003 nM, 0.3 nM, 0.03 nM and 0.3 nM | GO-CuNPs | [88] |
| 9 | Pb2+ | 312 pM | AuNPs- hairpin-aptamer and thionine. | [89] | |
| 10 | Hg2+ | EIS | 0.005 ppm | ink-jet printed gold electrodes | [90] |
| 11 | Acetamiprid | DPV | 153 pM | SiNP-streptavidin conjugate modified MB-dsDNA | [84] |
| 12 | Acetamiprid | EIS | 3.3 × 10−14 | Ag nanoparticle decorated nitrogen doped graphene | [91] |
| S.N | Analyte | Detection Method | LOD/Range | System | Reference |
|---|---|---|---|---|---|
| 1 | AFB1 | CV/EIS | 0.03 nM | Poly(amidoamine) dendrimers | [94] |
| 2 | AFM1 | SWV | 1.98 ng·L−1 | Polyaniline (Fe3O4/PANi)film | [95] |
| 3 | OTA | DPV | 0.01 ng/mL | HMDA-MB system | [96] |
| 4 | FB1 | CV | 1 pg/mL | AuNPs)and graphene/thionine nanocomposites | [97] |
| 5 | Streptomycin | CV/DPV | 11.4 nM | Aptamer-on gold electrode | [99] |
| 6 | Kanamycin | SWV | 10–2000 nM | Aptamer-on gold electrode | [100] |
| 7 | Oxytetracycline | 4.98 × 10−10 g L−1 | Graphene three dimensional nanostructure gold nanocomposite | [101] | |
| 8 | Tetracycline | SWV | 10 nM | Streptavidin-modified screen-printed gold electrode | [102] |
| 9 | Ciprofloxacin | EIS | 0.5 ng mL−1 | CNT- V2O5-chitosan | [104] |
| 10 | FB1 | EIS | 2 pM | Thiolated aptamers on AuNP | [106] |
| 11 | OTA | EIS | 0.15 ng/m | Di-azonium coupled reaction | [5] |
| 12 | OTA | DPV | 0.07 ng/mL | APL-pNPP based | [4] |
| 13 | OTA /FB1 | SWV | 10 pg mL−1 to 10 ng mL−1 and 50 pg mL−1 to 50 ng mL−1 | Magneto-controlled aptasensor | [107] |
| 14 | AB1 | DPV | 0.002 fg/mL | Reduced graphene oxide/molybdenum disulfide/polyaniline@gold nanopa | [108] |
| 15 | OTA | CV | 0.05 nM | Gold electrode covered with electropolymerized neutral red and silver nanoparticles | [109] |
| 16 | AFB1 | CV/EIS | 0.1 nM and 0.05 nM | Glassy carbon electrodes modified with electropolymerized Neutral red and polycarboxylated macrocyclic ligands | [110] |
| 17 | OTA | EIS/CV | 0.1 ng/mL in | A Langmuir–Blodgett (polyaniline (PANI)–stearic acid (SA)) film | [111] |
| 18 | Zearalenone | CV | 0.17 pg mL | Molybdenum disulfide (MoS2) doped multi-walled carbon nanotubes (PEI-MoS2-MWCNTs) nanohybrid | [112] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Mohamed, M.A.; Vinu Mohan, A.M.; Zhu, Z.; Sharma, V.; Mishra, G.K.; Mishra, R.K. Application of Electrochemical Aptasensors toward Clinical Diagnostics, Food, and Environmental Monitoring: Review. Sensors 2019, 19, 5435. https://doi.org/10.3390/s19245435
Li Z, Mohamed MA, Vinu Mohan AM, Zhu Z, Sharma V, Mishra GK, Mishra RK. Application of Electrochemical Aptasensors toward Clinical Diagnostics, Food, and Environmental Monitoring: Review. Sensors. 2019; 19(24):5435. https://doi.org/10.3390/s19245435
Chicago/Turabian StyleLi, Zhanhong, Mona A. Mohamed, A. M. Vinu Mohan, Zhigang Zhu, Vinay Sharma, Geetesh K. Mishra, and Rupesh K. Mishra. 2019. "Application of Electrochemical Aptasensors toward Clinical Diagnostics, Food, and Environmental Monitoring: Review" Sensors 19, no. 24: 5435. https://doi.org/10.3390/s19245435
APA StyleLi, Z., Mohamed, M. A., Vinu Mohan, A. M., Zhu, Z., Sharma, V., Mishra, G. K., & Mishra, R. K. (2019). Application of Electrochemical Aptasensors toward Clinical Diagnostics, Food, and Environmental Monitoring: Review. Sensors, 19(24), 5435. https://doi.org/10.3390/s19245435






