Long-Term Home-Monitoring Sensor Technology in Patients with Parkinson’s Disease—Acceptance and Adherence
Abstract
:1. Introduction
2. Results
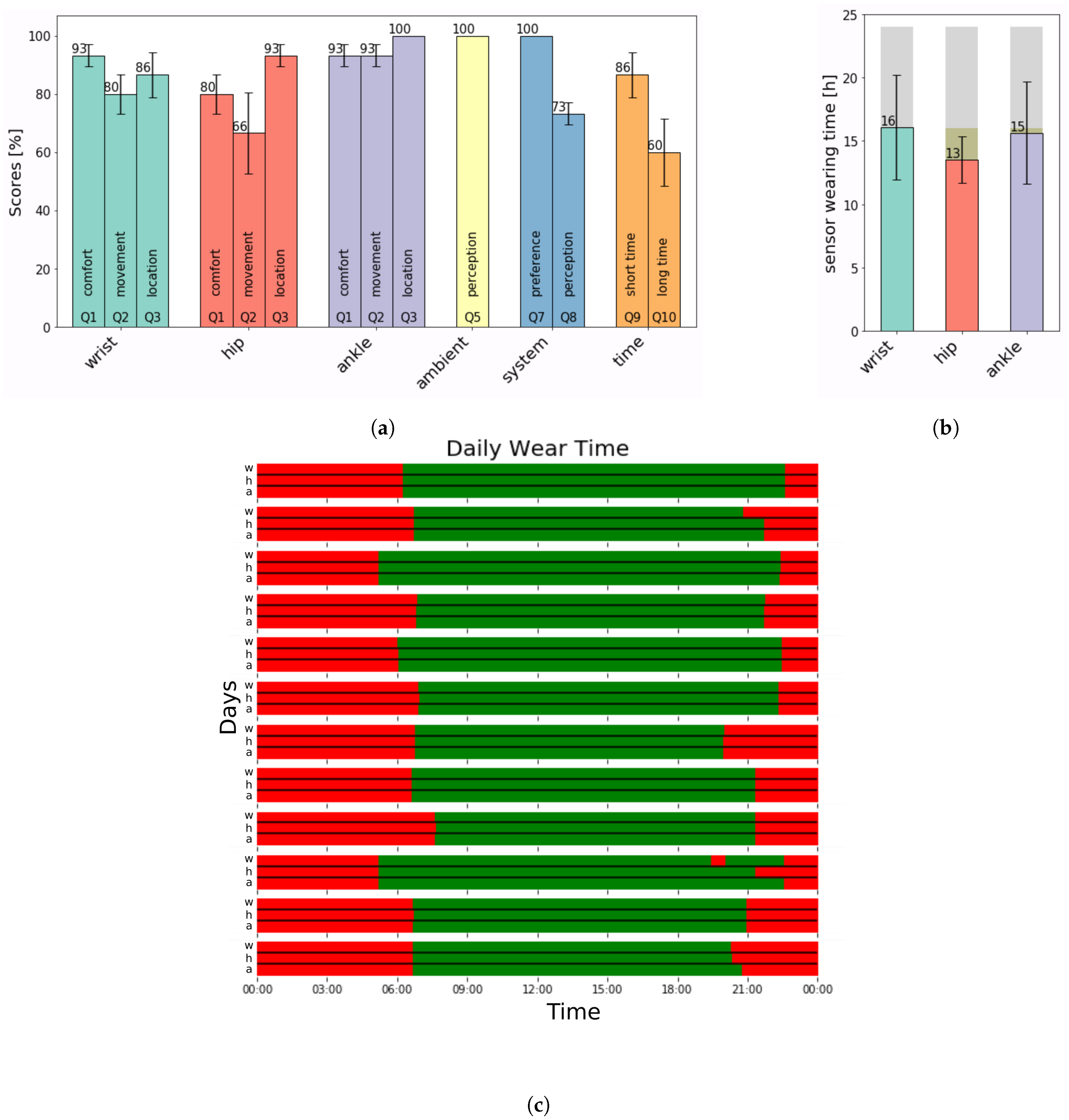
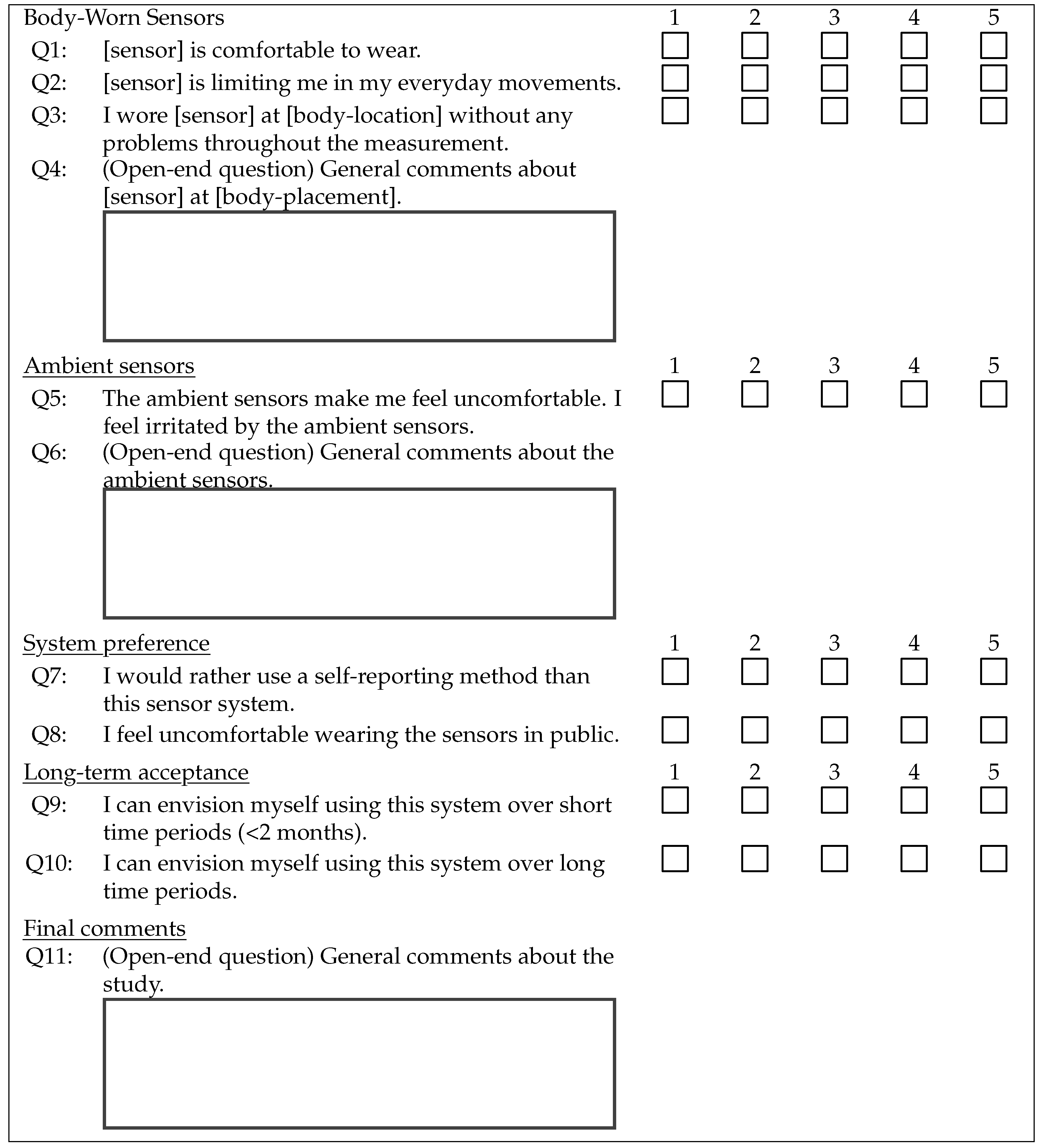
2.1. Outcome Measure–Questionnaire
2.2. Analysis–Adherence
2.3. Data Validity and Usability
3. Discussion
4. Limitations
5. Methods
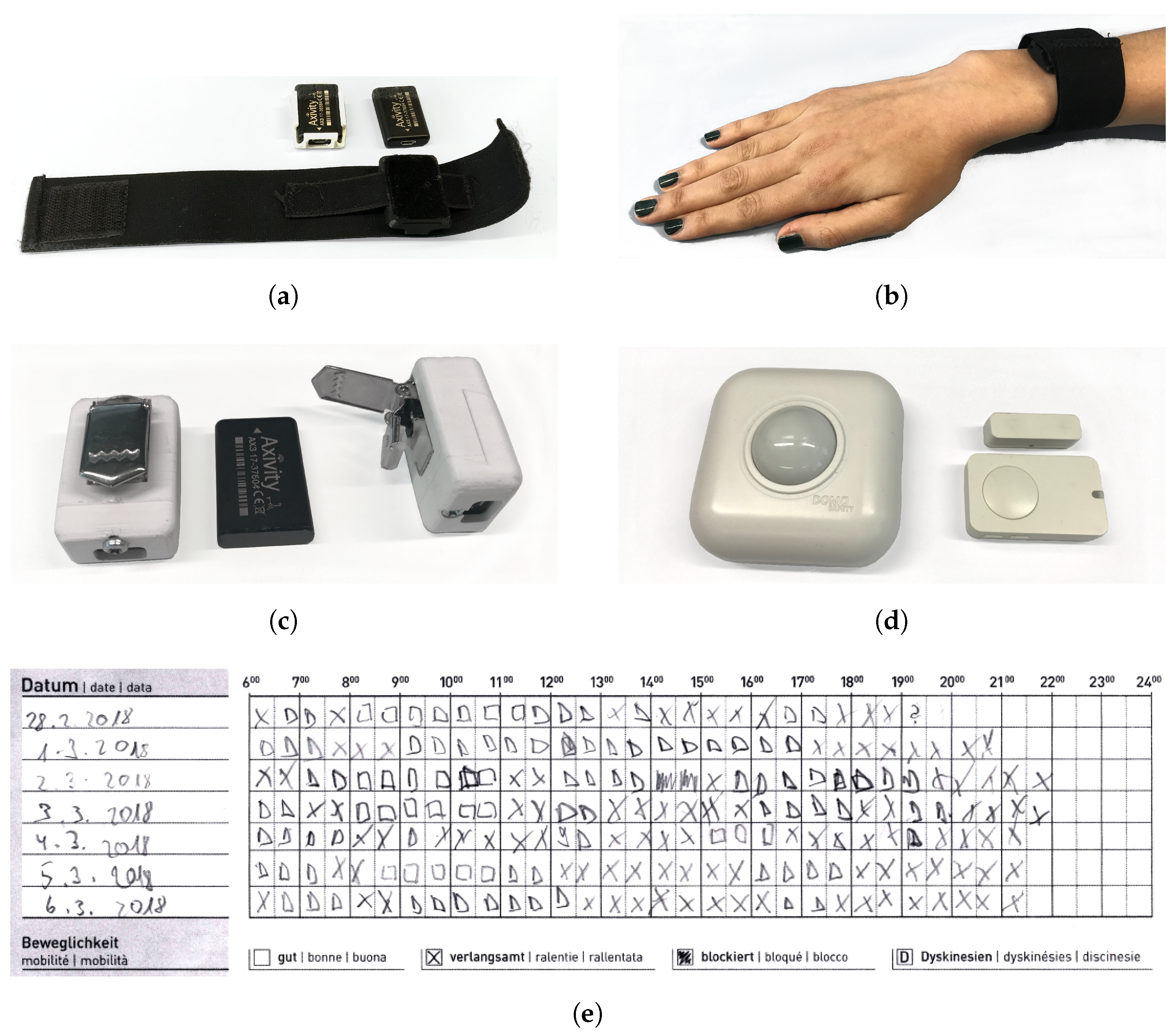
5.1. Materials
5.2. User Focus Meetings
5.3. Subject Recruitment
5.4. Data Collection
5.4.1. Analysis
5.4.2. Adherence
5.4.3. Statistical Evaluations
5.4.4. Data Validity and Usability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| UPDRS | Unified Parkinson Disease Rating Scale |
| H&Y | Hoen & Yahr |
| SD | Standard deviation |
| PIR-sensor | Passive Infrared Sensor |
| FoG | Freezing of Gait |
| SUS | System Usability Scale |
References
- De Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Samii, A.; Nutt, J.G.; Ransom, B.R. Parkinson’s disease. Lancet 2004, 363, 1783–1793. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef]
- Marsden, C.D. Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 672–681. [Google Scholar] [CrossRef]
- Yahr, M.D.; Duvoisin, R.C.; Schear, M.J.; Barrett, R.E.; Hoehn, M.M. Treatment of Parkinsonism with Levodopa. Arch. Neurol. 1969, 21, 343–354. [Google Scholar] [CrossRef]
- Marsden, C.D.; Parkes, J.D. Success and problems of long-term levodopa therapy in parkinson’s disease. Lancet 1977, 309, 345–349. [Google Scholar] [CrossRef]
- Antonini, A.; Mancini, F.; Canesi, M.; Zangaglia, R.; Isaias, I.; Manfredi, L.; Pacchetti, C.; Zibetti, M.; Natuzzi, F.; Lopiano, L.; et al. Duodenal Levodopa Infusion Improves Quality of Life in Advanced Parkinson’s Disease. Neurodegener. Dis. 2008, 5, 244–246. [Google Scholar] [CrossRef]
- Katzenschlager, R.; Lees, A.J. Treatment of Parkinson’s disease: Levodopa as the first choice. J. Neurol. 2002, 249, II19–II24. [Google Scholar] [CrossRef]
- Rahman, S.; Griffin, H.J.; Quinn, N.P.; Jahanshahi, M. The Factors that Induce or Overcome Freezing of Gait in Parkinson’s Disease. Behav. Neurol. 2008. [Google Scholar] [CrossRef]
- Jankovic, J. Motor fluctuations and dyskinesias in Parkinson’s disease: Clinical manifestations. Mov. Dis. 2005, 20, S11–S16. [Google Scholar] [CrossRef]
- Lungu, C.; Kim, B.; Hallet, M.; Lukasiewicz, K.; Harrington, J.; Altemus, A.; Baldwin, J. iPad App for Daily Parkinson Diary (P1.193); Wolters Kluwer Health, Inc.: Philadelphia, PA, USA, 2015; Volume 84. [Google Scholar]
- Vega, J.; Couth, S.; Poliakoff, E.; Kotz, S.; Sullivan, M.; Jay, C.; Vigo, M.; Harper, S. Back to Analogue: Self-Reporting for Parkinson’s Disease. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems—CHI’18, Montreal, QC, Canada, 21–26 April 2018; ACM Press: Montreal, QC, Canada, 2018; pp. 1–13. [Google Scholar] [CrossRef]
- Papapetropoulos, S.S. Patient Diaries As a Clinical Endpoint in Parkinson’s Disease Clinical Trials. CNS Neurosci. Ther. 2012, 18, 380–387. [Google Scholar] [CrossRef]
- Willemsen, A.T.M.; van Alsté, J.A.; Boom, H.B.K. Real-time gait assessment utilizing a new way of accelerometry. J. Biomech. 1990, 23, 859–863. [Google Scholar] [CrossRef]
- Kemp, B.; Janssen, A.J.M.W.; van der Kamp, B. Body position can be monitored in 3D using miniature accelerometers and earth-magnetic field sensors. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Mot. Control 1998, 109, 484–488. [Google Scholar] [CrossRef]
- Bachlin, M.; Plotnik, M.; Roggen, D.; Maidan, I.; Hausdorff, J.; Giladi, N.; Troster, G. Wearable Assistant for Parkinson’s Disease Patients With the Freezing of Gait Symptom. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Rigas, G.; Tzallas, A.T.; Tsipouras, M.G.; Bougia, P.; Tripoliti, E.E.; Baga, D.; Fotiadis, D.I.; Tsouli, S.G.; Konitsiotis, S. Assessment of Tremor Activity in the Parkinson’s Disease Using a Set of Wearable Sensors. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 478–487. [Google Scholar] [CrossRef]
- Samà, A.; Pérez-Lopez, C.; Romagosa, J.; Rodríguez-Martín, D.; Català, A.; Cabestany, J.; Pérez-Martínez, D.A.; Rodríguez-Molinero, A. Dyskinesia and motor state detection in Parkinson’s Disease patients with a single movement sensor. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1194–1197. [Google Scholar] [CrossRef]
- Salarian, A.; Russmann, H.; Wider, C.; Burkhard, P.R.; Vingerhoets, F.J.G.; Aminian, K. Quantification of Tremor and Bradykinesia in Parkinson’s Disease Using a Novel Ambulatory Monitoring System. IEEE Trans. Biomed. Eng. 2007, 54, 313–322. [Google Scholar] [CrossRef]
- Weiss, A.; Sharifi, S.; Plotnik, M.; van Vugt, J.P.P.; Giladi, N.; Hausdorff, J.M. Toward Automated, At-Home Assessment of Mobility Among Patients With Parkinson Disease, Using a Body-Worn Accelerometer. Neurorehabil. Neural Repair. 2011, 25, 810–818. [Google Scholar] [CrossRef]
- Chen, B.; Patel, S.; Buckley, T.; Rednic, R.; McClure, D.J.; Shih, L.; Tarsy, D.; Welsh, M.; Bonato, P. A Web-Based System for Home Monitoring of Patients With Parkinson’s Disease Using Wearable Sensors. IEEE Trans. Biomed. Eng. 2011, 58, 831–836. [Google Scholar] [CrossRef]
- Patel, S.; Chen, B.; Buckley, T.; Rednic, R.; McClure, D.; Tarsy, D.; Shih, L.; Dy, J.; Welsh, M.; Bonato, P. Home monitoring of patients with Parkinson’s disease via wearable technology and a web-based application. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 4411–4414. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Movement Disorders Society Task Force on Technology. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- Tzallas, A.T.; Tsipouras, M.G.; Rigas, G.; Tsalikakis, D.G.; Karvounis, E.C.; Chondrogiorgi, M.; Psomadellis, F.; Cancela, J.; Pastorino, M.; Waldmeyer, M.T.A.; et al. PERFORM: A System for Monitoring, Assessment and Management of Patients with Parkinson’s Disease. Sensors 2014, 14, 21329–21357. [Google Scholar] [CrossRef] [PubMed]
- Cancela, J.; Pastorino, M.; Tzallas, A.T.; Tsipouras, M.G.; Rigas, G.; Arredondo, M.T.; Fotiadis, D.I. Wearability Assessment of a Wearable System for Parkinson’s Disease Remote Monitoring Based on a Body Area Network of Sensors. Sensors 2014, 14, 17235–17255. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.M.; Hammerla, N.Y.; Rochester, L.; Andras, P.; Walker, R.W. Body-Worn Sensors in Parkinson’s Disease: Evaluating Their Acceptability to Patients. Telemed. e-Health 2015, 22, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.M.; Hammerla, N.Y.; Ploetz, T.; Andras, P.; Rochester, L.; Walker, R.W. Unsupervised home monitoring of Parkinson’s disease motor symptoms using body-worn accelerometers. Parkinsonism Relat. Dis. 2016, 33, 44–50. [Google Scholar] [CrossRef]
- Stucki, R.A.; Urwyler, P.; Rampa, L.; Müri, R.; Mosimann, U.P.; Nef, T. A Web-Based Non-Intrusive Ambient System to Measure and Classify Activities of Daily Living. J. Med. Internet Res. 2014, 16, e175. [Google Scholar] [CrossRef]
- Schütz, N.; Saner, H.; Rudin, B.; Botros, A.; Pais, B.; Santschi, V.; Buluschek, P.; Gatica-Perez, D.; Urwyler, P.; Marchal-Crespo, L.; et al. Validity of pervasive computing based continuous physical activity assessment in community-dwelling old and oldest-old. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- ElHelw, M.; Pansiot, J.; McIlwraith, D.; Ali, R.; Lo, B.; Atallah, L. An integrated multi-sensing framework for pervasive healthcare monitoring. In Proceedings of the 2009 3rd International Conference on Pervasive Computing Technologies for Healthcare, London, UK, 1–3 April 2009; pp. 1–7. [Google Scholar] [CrossRef]
- Doherty, A.; Jackson, D.; Hammerla, N.; Plötz, T.; Olivier, P.; Granat, M.H.; White, T.; Hees, V.T.V.; Trenell, M.I.; Owen, C.G.; et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS ONE 2017, 12, e0169649. [Google Scholar] [CrossRef]
- Bangor, A.; Kortum, P.; Miller, J. Determining What Individual SUS Scores Mean: Adding an Adjective Rating Scale. J. Usability Stud. 2009, 4, 114–123. [Google Scholar]
- Galea, S.; Tracy, M. Participation Rates in Epidemiologic Studies. Ann. Epidemiol. 2007, 17, 643–653. [Google Scholar] [CrossRef]
- Axivity. AX3. 2019. Available online: http://www.axivity.com/product/1 (accessed on 25 November 2019).
- Domosafety. DomoCare/DomoMedical. 2019. Available online: http://www.domo-safety.com/en/for-seniors/ (accessed on 25 November 2019).
- Rogers, E.M. Diffusion of Innovations, 3rd ed.; Free Press: New York, NY, USA; Collier Macmillan: London, UK, 1983. [Google Scholar]
- Brooke, J.; Jordan, P.; Thomas, B.; Weerdmeester, B.; McClelland, I. Usability evaluation in industriy. In SUS: A ‘Quick and Dirty’ Usability Scale; Redhatch Consulting Ltd.: Reading, UK, 1996; pp. 184–194. [Google Scholar]
- Reinhard, W.; Ruegenhagen, E.; Rummel, B. System Usability Scale—Jetzt auch auf Deutsch. 2015. Available online: https://experience.sap.com/skillup/system-usability-scale-jetzt-auch-auf-deutsch/ (accessed on 12 January 2019).
- Khandelwal, S.; Wickström, N. Gait Event Detection in Real-World Environment for Long-Term Applications: Incorporating Domain Knowledge Into Time-Frequency Analysis. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1363–1372. [Google Scholar] [CrossRef]
| Open-End Questions | |
|---|---|
| Q4 (wrist): | “When changing clothes, like jumpers or jackets, the [wrist] sensor was a bit disturbing”. |
| Q4 (ankle): | “When changing trousers, the [ankle] sensor was a bit disturbing”. |
| Q4 (hip): | “In winter, the sensor is less disturbing as compared to when light clothes are worn. |
| I wore the [hip] sensor at the front. On the back it was more disturbing”. | |
| “When using the toilet, it was bothersome”. | |
| Q6: | “I forgot about the [ambient] sensors”. |
| Q11: | “Not in summer”. |
| “I enjoyed participating in this study”. | |
| p-Values | P1 | P2 | P3 | P4 |
|---|---|---|---|---|
| wrist-hip | 0.07 | 0.46 | <0.01 | 0.02 |
| wrist-ankle | 0.30 | 0.14 | 0.01 | 0.04 |
| hip-ankle | 0.82 | 0.55 | <0.01 | 0.13 |
| Motor Symptom | Sensitivity | Specificity | Precision |
|---|---|---|---|
| Bradykinesia | 69.8% | 68.3% | 70.1% |
| Dyskinesia | 69.0% | 72.2% | 72.5% |
| Tremor | 74.2% | 69.7% | 70.8% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botros, A.; Schütz, N.; Camenzind, M.; Urwyler, P.; Bolliger, D.; Vanbellingen, T.; Kistler, R.; Bohlhalter, S.; Müri, R.M.; Mosimann, U.P.; et al. Long-Term Home-Monitoring Sensor Technology in Patients with Parkinson’s Disease—Acceptance and Adherence. Sensors 2019, 19, 5169. https://doi.org/10.3390/s19235169
Botros A, Schütz N, Camenzind M, Urwyler P, Bolliger D, Vanbellingen T, Kistler R, Bohlhalter S, Müri RM, Mosimann UP, et al. Long-Term Home-Monitoring Sensor Technology in Patients with Parkinson’s Disease—Acceptance and Adherence. Sensors. 2019; 19(23):5169. https://doi.org/10.3390/s19235169
Chicago/Turabian StyleBotros, Angela, Narayan Schütz, Martin Camenzind, Prabitha Urwyler, Daniel Bolliger, Tim Vanbellingen, Rolf Kistler, Stephan Bohlhalter, Rene M. Müri, Urs P. Mosimann, and et al. 2019. "Long-Term Home-Monitoring Sensor Technology in Patients with Parkinson’s Disease—Acceptance and Adherence" Sensors 19, no. 23: 5169. https://doi.org/10.3390/s19235169
APA StyleBotros, A., Schütz, N., Camenzind, M., Urwyler, P., Bolliger, D., Vanbellingen, T., Kistler, R., Bohlhalter, S., Müri, R. M., Mosimann, U. P., & Nef, T. (2019). Long-Term Home-Monitoring Sensor Technology in Patients with Parkinson’s Disease—Acceptance and Adherence. Sensors, 19(23), 5169. https://doi.org/10.3390/s19235169





