Mechanochromic Fluorescent Polymers with Aggregation-Induced Emission Features
Abstract
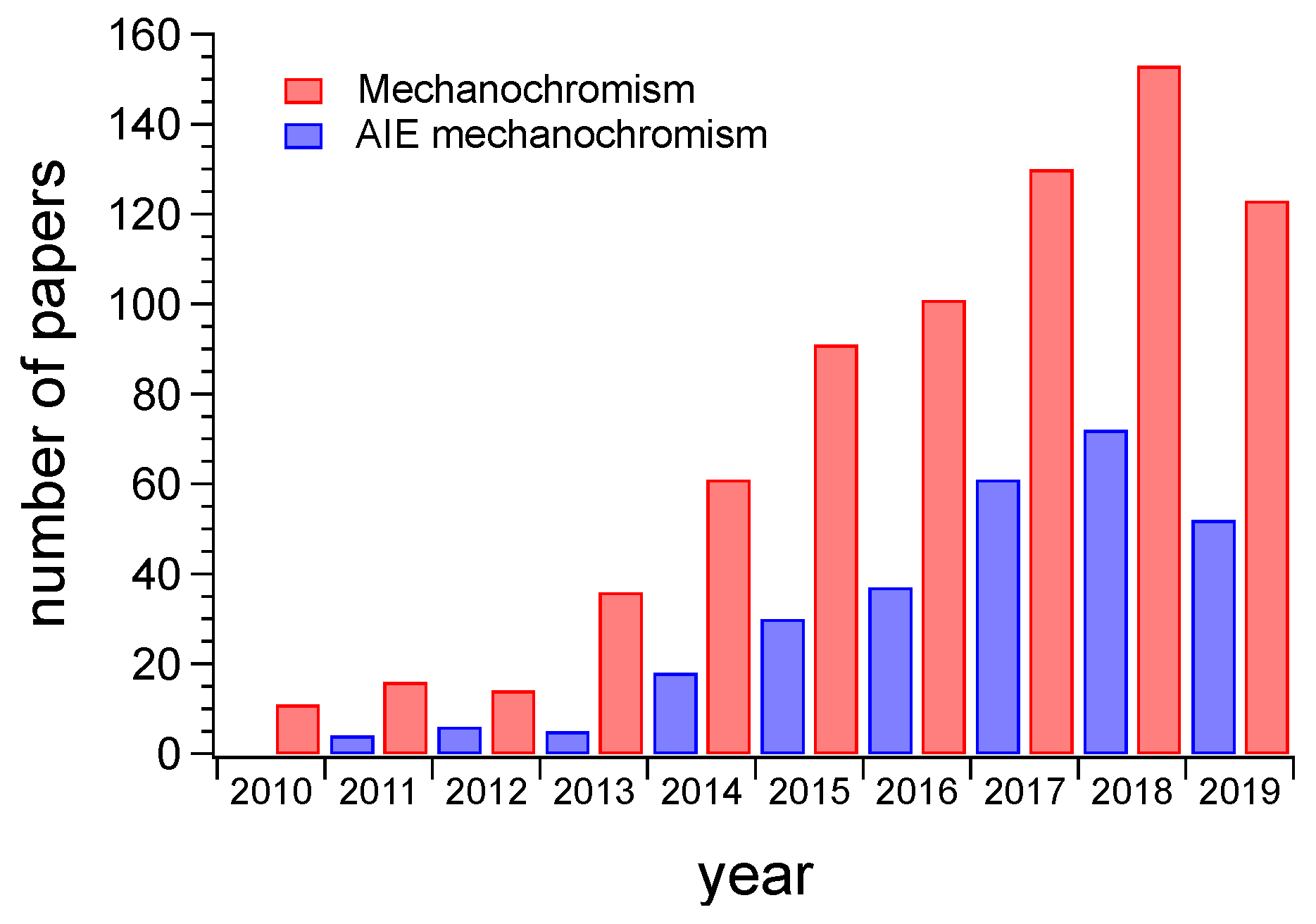
1. Introduction
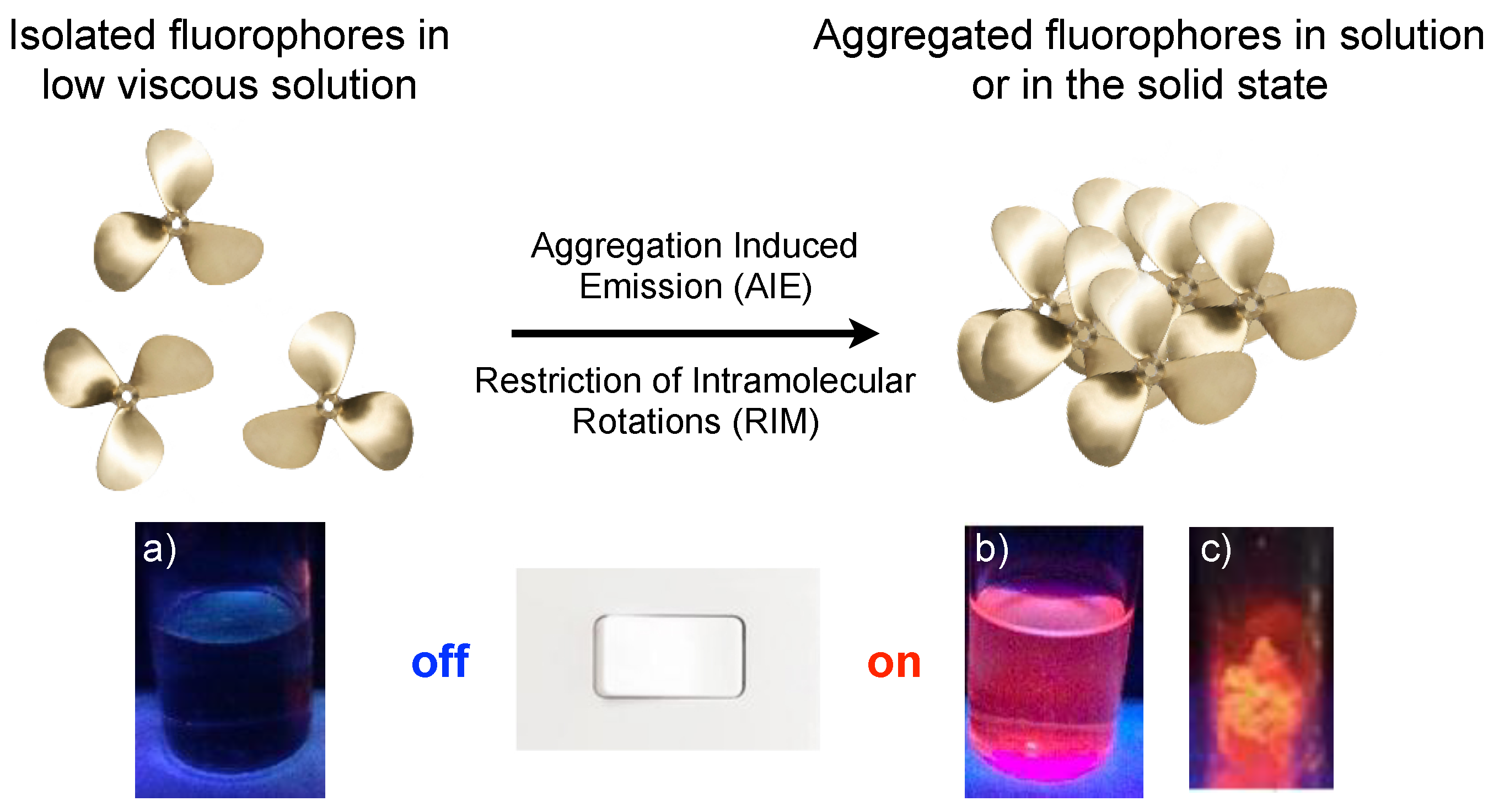
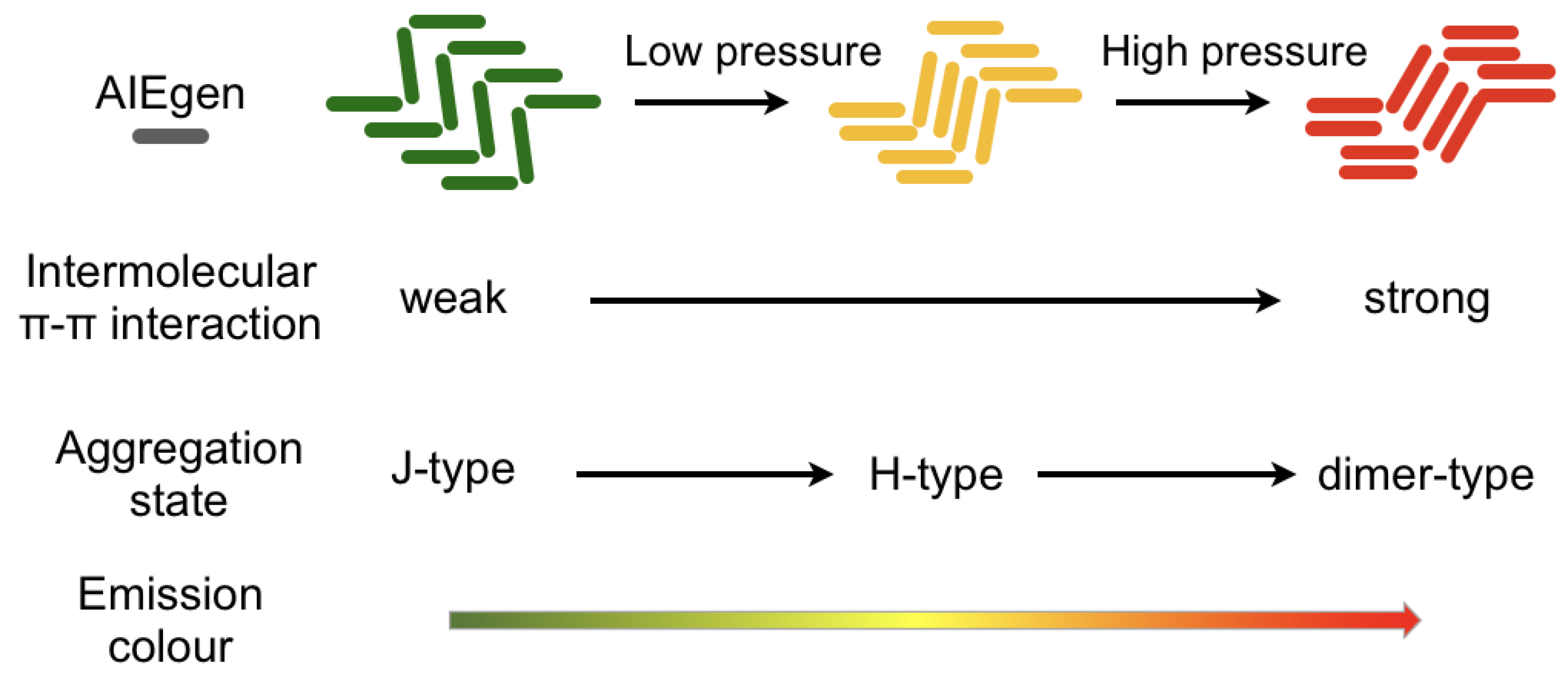
2. AIE and Mechanochromism
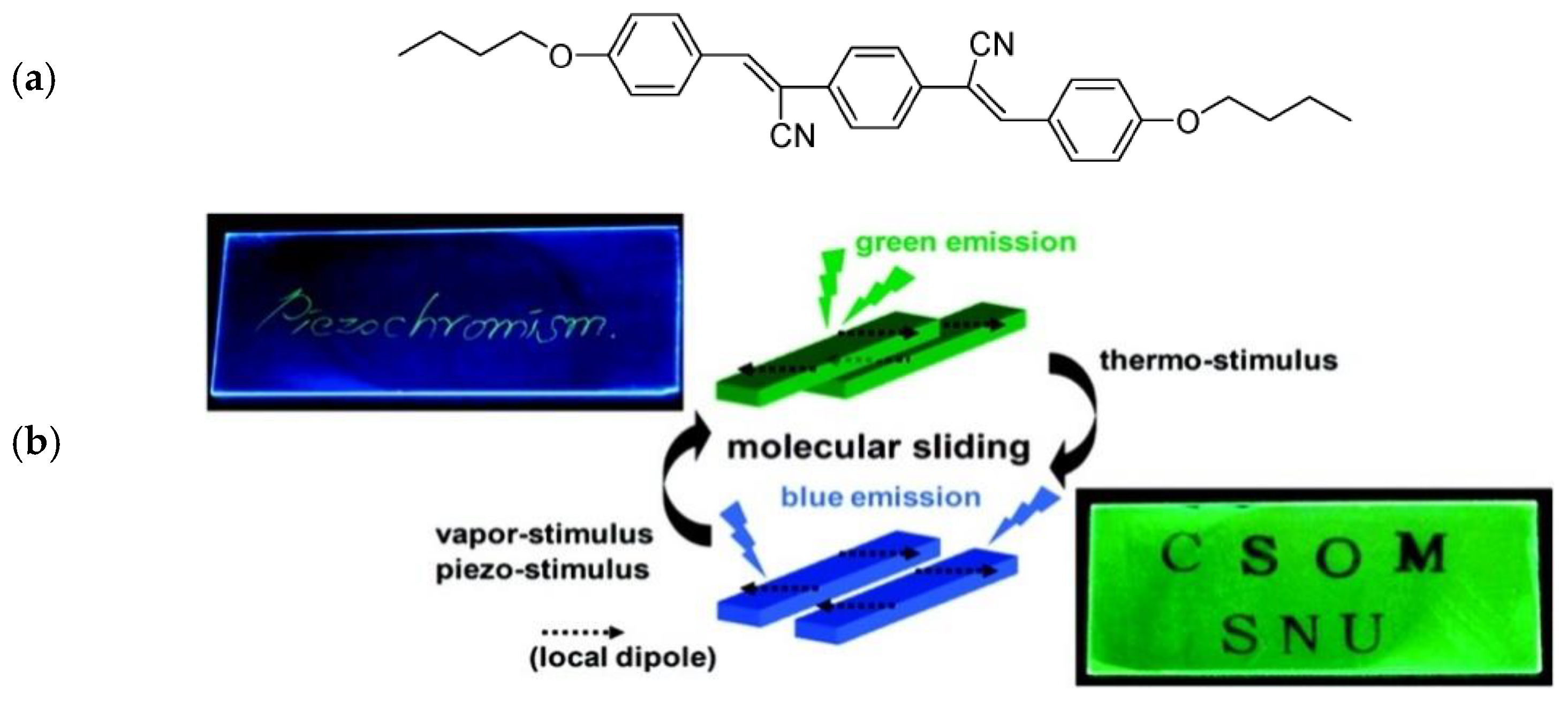
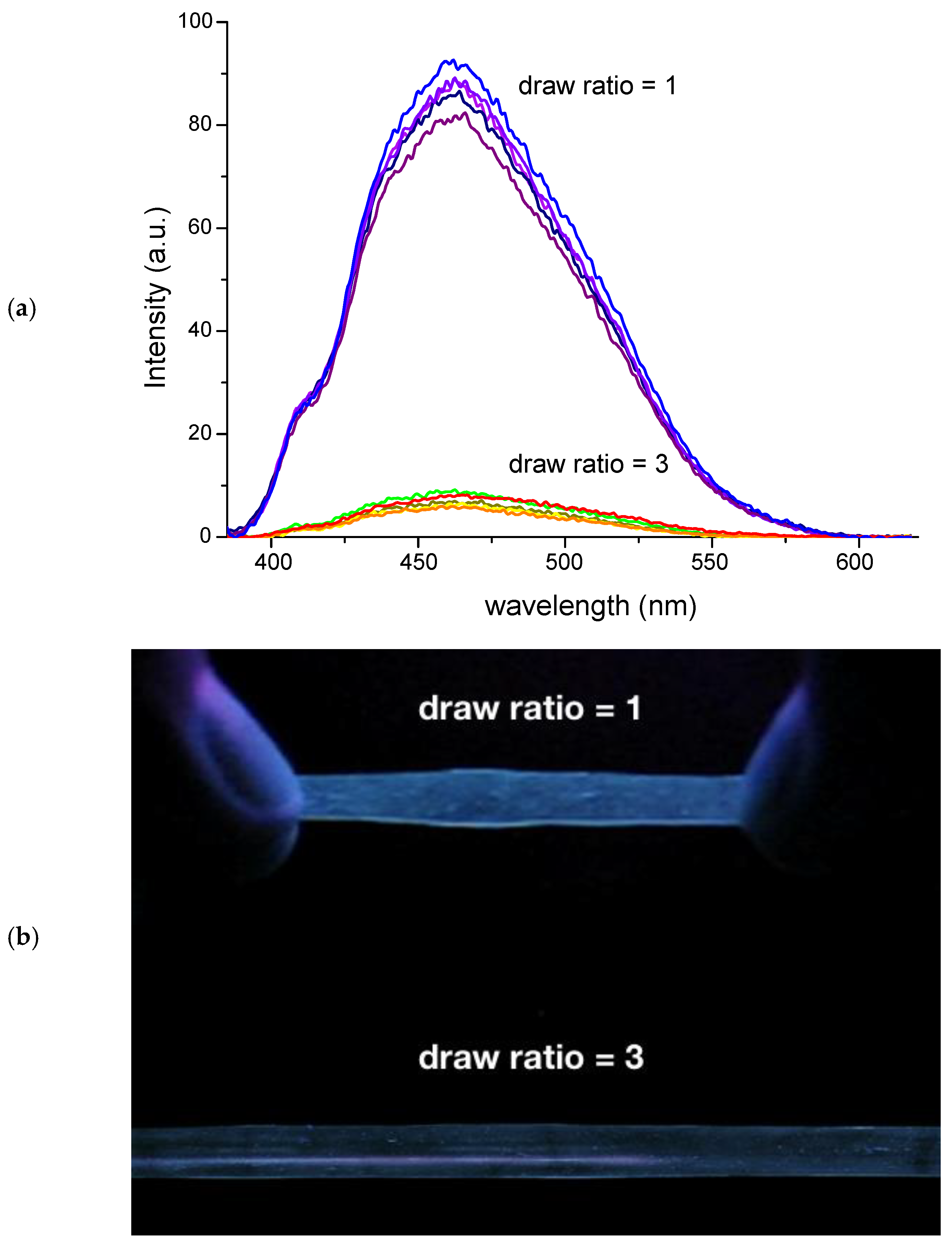
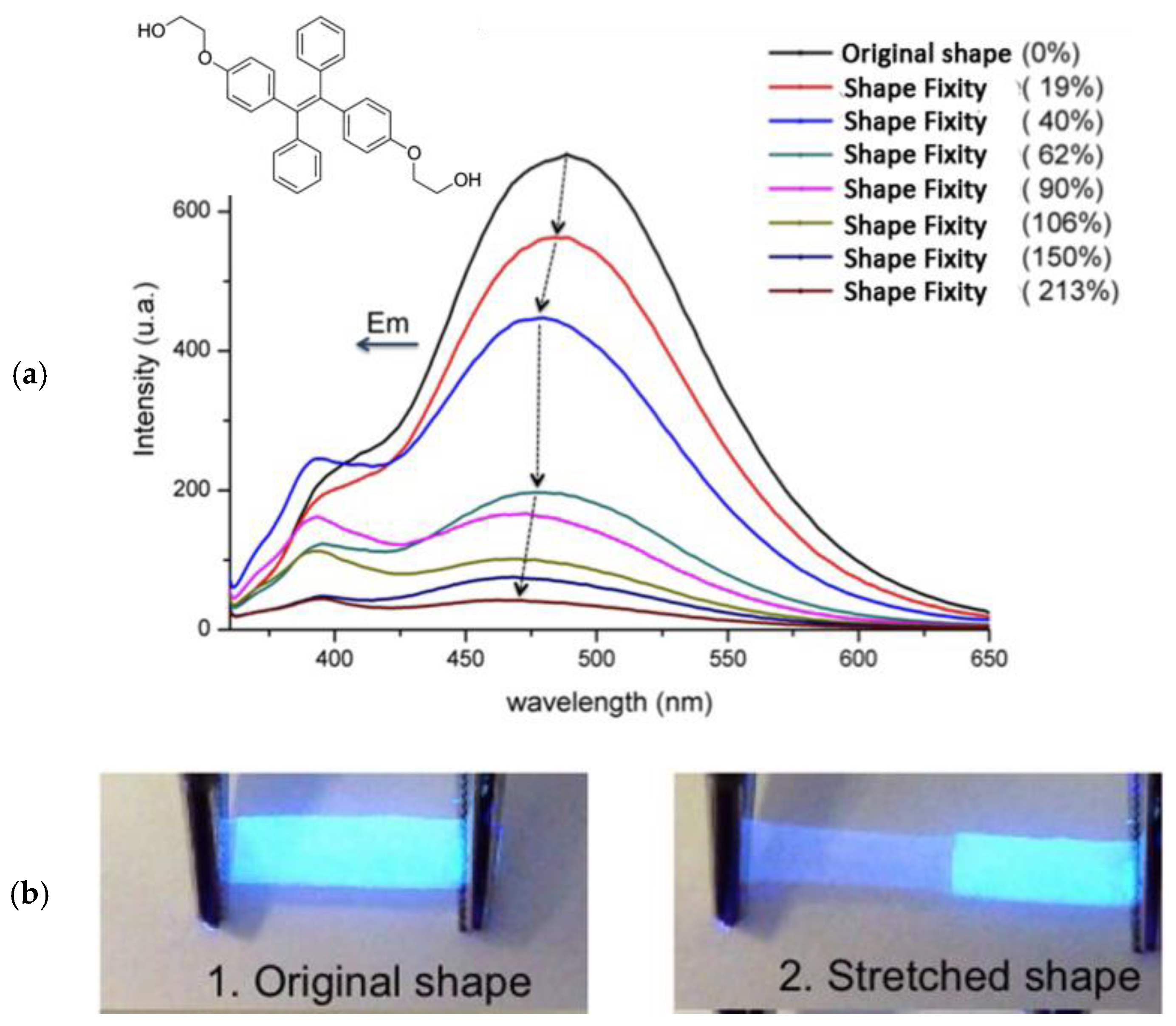
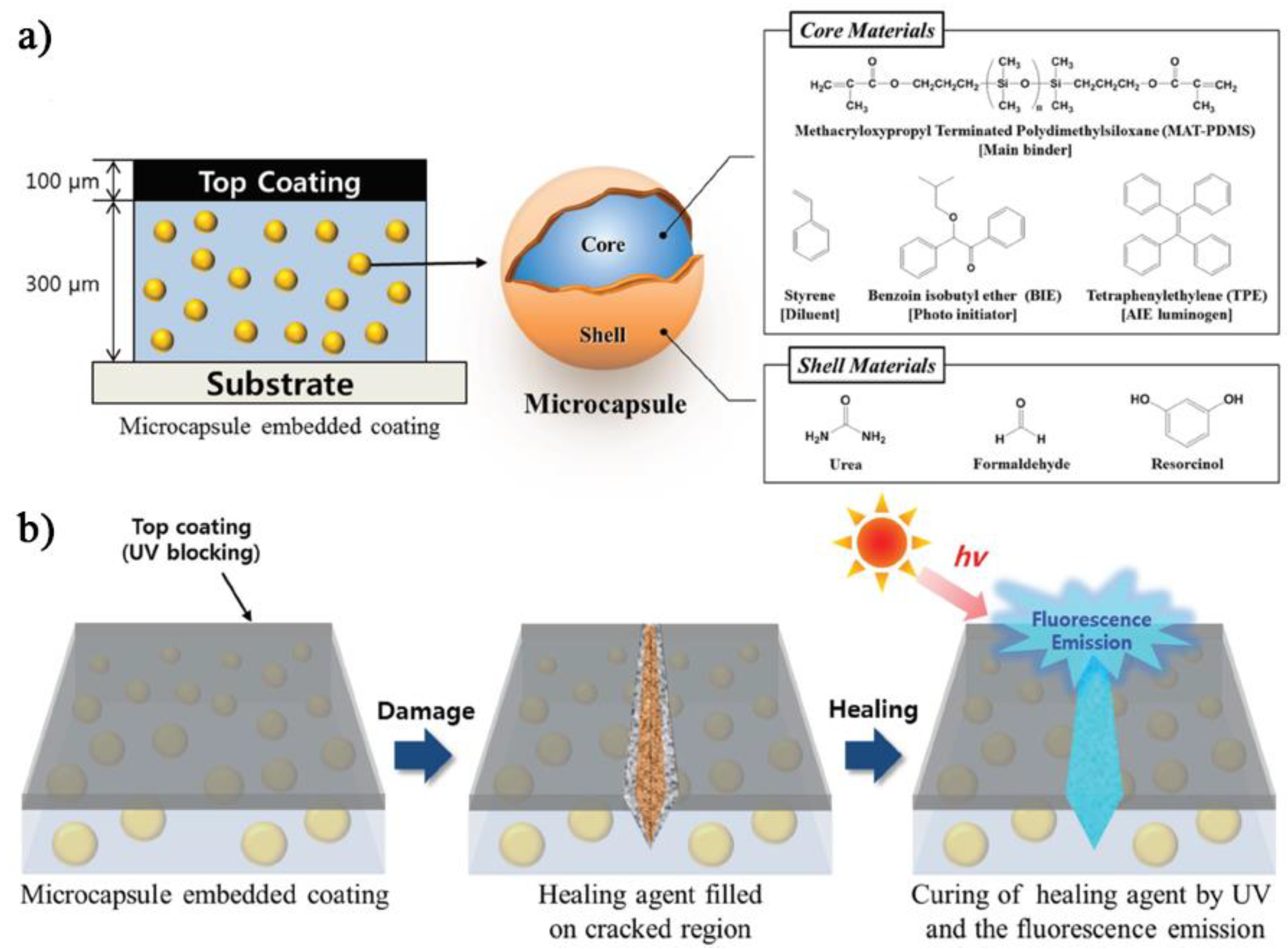
3. AIE and Mechanochromic Fluorescent Polymers
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Demchenko, A.P. Introduction to Fluorescence Sensing; Springer: Cham, Switzerland, 2009. [Google Scholar]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef] [PubMed]
- Basabe-Desmonts, L.; Reinhoudt, D.N.; Crego-Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 2007, 36, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef]
- Schaeferling, M. The art of fluorescence imaging with chemical sensors. Angew. Chem. Int. Ed. 2012, 51, 3532–3554. [Google Scholar] [CrossRef]
- Minei, P.; Pucci, A. Fluorescent vapochromism in synthetic polymers. Polym. Int. 2016, 65, 609–620. [Google Scholar] [CrossRef]
- Pucci, A.; Bizzarri, R.; Ruggeri, G. Polymer composites with smart optical properties. Soft Matter 2011, 7, 3689–3700. [Google Scholar] [CrossRef]
- Pucci, A.; Ruggeri, G. Mechanochromic polymer blends. J. Mater. Chem. 2011, 21, 8282–8291. [Google Scholar] [CrossRef]
- Ciardelli, F.; Ruggeri, G.; Pucci, A. Dye-containing polymers: Methods for preparation of mechanochromic materials. Chem. Soc. Rev. 2013, 42, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Basic Principles. In Introduction to Fluorescence Sensing; Demchenko, A.P., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–37. [Google Scholar]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang Ben, Z. Aggregation-induced emission: the whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- De Nisi, F.; Francischello, R.; Battisti, A.; Panniello, A.; Fanizza, E.; Striccoli, M.; Gu, X.; Leung, N.L.C.; Tang, B.Z.; Pucci, A. Red-emitting AIEgen for luminescent solar concentrators. Mater. Chem. Front. 2017, 1, 1406–1412. [Google Scholar] [CrossRef]
- Geervliet, T.A.; Gavrila, I.; Iasilli, G.; Picchioni, F.; Pucci, A. Luminescent Solar Concentrators Based on Renewable Polyester Matrices. Chem.-Asian J. 2019, 14, 877–883. [Google Scholar] [CrossRef]
- Guidugli, N.; Mori, R.; Bellina, F.; Tang, B.Z.; Pucci, A. Aggregation-Induced Emission: New Emerging Fluorophores for Environmental Sensing. In Principles and Applications of Aggregation-Induced Emission; Tang, Y., Tang, B.Z., Eds.; Springer: Cham, Switzerland, 2019; pp. 335–349. [Google Scholar]
- Liu, B.; Pucci, A.; Baumgartner, T. Aggregation induced emission: A land of opportunities. Mater. Chem. Front. 2017, 1, 1689–1690. [Google Scholar] [CrossRef]
- Mori, R.; Iasilli, G.; Lessi, M.; Muñoz-García, A.B.; Pavone, M.; Bellina, F.; Pucci, A. Luminescent solar concentrators based on PMMA films obtained from a red-emitting ATRP initiator. Polym. Chem. 2018, 9, 1168–1177. [Google Scholar] [CrossRef]
- Pucci, A. Luminescent Solar Concentrators Based on Aggregation Induced Emission. Isr. J. Chem. 2018, 58, 837–844. [Google Scholar] [CrossRef]
- Sorgi, C.; Martinelli, E.; Galli, G.; Pucci, A. Julolidine-labelled fluorinated block copolymers for the development of two-layer films with highly sensitive vapochromic response. Sci. China Chem. 2018, 61, 947–956. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ke, C.; Tang, B.Z. Journey of Aggregation-Induced Emission Research. ACS Omega 2018, 3, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Chen, C.; Ding, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens: Union Is Strength, Gathering Illuminates Healthcare. Adv. Healthcare Mater. 2018, 7, 1800477. [Google Scholar] [CrossRef]
- Yao, W.; Tebyetekerwa, M.; Bian, X.; Li, W.; Yang, S.; Zhu, M.; Hu, R.; Wang, Z.; Qin, A.; Tang, B.Z. Materials interaction in aggregation-induced emission (AIE)-based fluorescent resin for smart coatings. J. Mater. Chem. C 2018, 6, 12849–12857. [Google Scholar] [CrossRef]
- Zhu, C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: A Trailblazing Journey to the Field of Biomedicine. ACS Appl. Bio Mater. 2018, 1, 1768–1786. [Google Scholar] [CrossRef]
- Hu, Y.B.; Lam, J.W.Y.; Tang, B.Z. Recent Progress in AIE-active Polymers. Chin. J. Polym. Sci. 2019, 37, 289–301. [Google Scholar] [CrossRef]
- Huang, W.; Bender, M.; Seehafer, K.; Wacker, I.; Schroeder, R.R.; Bunz, U.H.F. Novel Functional TPE Polymers: Aggregation-Induced Emission, pH Response, and Solvatochromic Behavior. Macromol. Rapid Commun. 2019, 40, 1800774. [Google Scholar] [CrossRef]
- Mao, L.; Liu, Y.; Yang, S.; Li, Y.; Zhang, X.; Wei, Y. Recent advances and progress of fluorescent bio-chemosensors based on aggregation-induced emission molecules. Dyes Pigm. 2019, 162, 611–623. [Google Scholar] [CrossRef]
- Tian, M.; Ma, Y.; Lin, W. Fluorescent Probes for the Visualization of Cell Viability. Acc. Chem. Res. 2019, 52, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens for Activity-Based Sensing. Acc. Chem. Res. 2019, 52, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chi, Z.; Zhu, W.; Tang, B.Z.; Li, Z. Aggregation-induced emission: a coming-of-age ceremony at the age of eighteen. Sci. China Chem. 2019, 62, 1090–1098. [Google Scholar] [CrossRef]
- Zhou, H.; Chua, M.H.; Tang, B.Z.; Xu, J. Aggregation-induced emission (AIE)-active polymers for explosive detection. Polym. Chem. 2019, 10, 3822–3840. [Google Scholar] [CrossRef]
- Xu, S.; Duan, Y.; Liu, B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2019, 0, 1903530. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, L.; Zhao, R.; Li, Z.; Liu, Y.; Duan, Y.; Han, T. A mechanochromic luminescent material with aggregation-induced emission: Application for pressure sensing and mapping. Spectrochim. Acta Part A 2019, 220, 117125. [Google Scholar] [CrossRef]
- Chi, Z.; Xu, J. Mechanochromic Aggregation-Induced Emission Materials. Aggregation-Induced Emission Fundam. Appl. 2013, 1–2, 61–86. [Google Scholar]
- Toma, O.; Allain, M.; Meinardi, F.; Forni, A.; Botta, C.; Mercier, N. Bismuth-Based Coordination Polymers with Efficient Aggregation-Induced Phosphorescence and Reversible Mechanochromic Luminescence. Angew. Chem. Int. Ed. 2016, 55, 7998–8002. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Tang, Q.; Yang, H.; Zhou, Q.; Zhang, X. Rhodamine-functionalized mechanochromic and mechanofluorescent hydrogels with enhanced mechanoresponsive sensitivity. Polymers 2018, 10, 994. [Google Scholar] [CrossRef]
- Yang, W.; Liu, C.; Lu, S.; Du, J.; Gao, Q.; Zhang, R.; Liu, Y.; Yang, C. AIE-active smart cyanostyrene luminogens: polymorphism-dependent multicolor mechanochromism. J. Mater. Chem. C 2018, 6, 290–298. [Google Scholar] [CrossRef]
- Calvino, C.; Sagara, Y.; Buclin, V.; Haehnel, A.P.; del Prado, A.; Aeby, C.; Simon, Y.C.; Schrettl, S.; Weder, C. Mechanoresponsive, Luminescent Polymer Blends Based on an Excimer-Forming Telechelic Macromolecule. Macromol. Rapid Commun. 2019, 40, 1800705. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.P.; Hwang, H.; Chae, S.; Kim, J.-M. A reversibly mechanochromic conjugated polymer. Chem. Commun. 2019, 55, 9395–9398. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Karman, M.; Seki, A.; Pannipara, M.; Tamaoki, N.; Weder, C. Rotaxane-Based Mechanophores Enable Polymers with Mechanically Switchable White Photoluminescence. ACS Cent. Sci. 2019, 5, 874–881. [Google Scholar] [CrossRef]
- Vidavsky, Y.; Yang, S.J.; Abel, B.A.; Agami, I.; Diesendruck, C.E.; Coates, G.W.; Silberstein, M.N. Enabling Room-Temperature Mechanochromic Activation in a Glassy Polymer: Synthesis and Characterization of Spiropyran Polycarbonate. J. Am. Chem. Soc. 2019, 141, 10060–10067. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, L.; Zhang, M.; Li, Z.; Liu, Y.; Han, T.; Duan, Y.; Gao, K. Self-recovering mechanochromic luminescent material with aggregation-induced emission: Implication for pressure sensor. Dyes Pigm. 2019, 167, 181–188. [Google Scholar] [CrossRef]
- Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B. Stimuli-chromism of photoswitches in smart polymers: Recent advances and applications as chemosensors. Prog. Polym. Sci. 2019, 98, 101149. [Google Scholar] [CrossRef]
- McFadden, M.E.; Robb, M.J. Force-Dependent Multicolor Mechanochromism from a Single Mechanophore. J. Am. Chem. Soc. 2019, 141, 11388–11392. [Google Scholar] [CrossRef]
- Calvino, C.; Neumann, L.; Weder, C.; Schrettl, S. Approaches to polymeric mechanochromic materials. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 640–652. [Google Scholar] [CrossRef]
- Gon, M.; Kato, K.; Tanaka, K.; Chujo, Y. Elastic and mechanofluorochromic hybrid films with POSS-capped polyurethane and polyfluorene. Mater. Chem. Front. 2019, 3, 1174–1180. [Google Scholar] [CrossRef]
- Suenaga, K.; Tanaka, K.; Chujo, Y. Heat-Resistant Mechanoluminescent Chromism of the Hybrid Molecule Based on Boron Ketoiminate Modified Octasubstituted Polyhedral Oligomeric Silsesquioxane. Chem. Eur. J. 2017, 23, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Yoshioka, S. Structural Colors in Nature: The Role of Regularity and Irregularity in the Structure. ChemPhysChem 2005, 6, 1442–1459. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.L. Animal Biochromes and Structural Colors; Cambridge University Press: Cambridge, UK, 1953. [Google Scholar]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Winnik, F.M.; Whitten, D.G.; Urban, M.W.; Lopez, G. Stimuli-Responsive Materials: Polymers, Colloids, and Multicomponent Systems. Langmuir 2007, 23, 1–2. [Google Scholar]
- Yerushalmi, R.; Scherz, A.; van der Boom, M.E.; Kraatz, H.-B. Stimuli responsive materials: new avenues toward smart organic devices. J. Mater. Chem. 2005, 15, 4480–4487. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z. Mechanoluminescence Materials with the Characteristic of Aggregation-Induced Emission (AIE). In Principles and Applications of Aggregation-Induced Emission; Tang, Y., Tang, B.Z., Eds.; Springer: Cham, Switzerland, 2019; pp. 141–162. [Google Scholar]
- Yang, Z.; Chi, Z.; Mao, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Aldred, M.P.; Chi, Z. Recent advances in mechano-responsive luminescence of tetraphenylethylene derivatives with aggregation-induced emission properties. Mater. Chem. Front. 2018, 2, 861–890. [Google Scholar] [CrossRef]
- Yang, Z.; Mao, Z.; Yu, T.; Zhang, Y.; Liu, S.; Xu, J.; Chi, Z. Mechano-responsive AIE luminogens. ACS Symp. Ser. 2016, 1226, 221–259. [Google Scholar]
- Prampolini, G.; Bellina, F.; Biczysko, M.; Cappelli, C.; Carta, L.; Lessi, M.; Pucci, A.; Ruggeri, G.; Barone, V. Computational design, synthesis, and mechanochromic properties of new thiophene-based π-conjugated chromophores. Chem. Eur. J. 2013, 19, 1996–2004. [Google Scholar] [CrossRef]
- Battisti, A.; Minei, P.; Pucci, A.; Bizzarri, R. Hue-based quantification of mechanochromism towards a cost-effective detection of mechanical strain in polymer systems. Chem. Commun. 2017, 53, 248–251. [Google Scholar] [CrossRef]
- Herbert, K.M.; Schrettl, S.; Rowan, S.J.; Weder, C. 50th Anniversary Perspective: Solid-State Multistimuli, Multiresponsive Polymeric Materials. Macromolecules 2017, 50, 8845–8870. [Google Scholar] [CrossRef]
- Chang, Z.-F.; Jing, L.-M.; Wei, C.; Dong, Y.-P.; Ye, Y.-C.; Zhao, Y.S.; Wang, J.-L. Hexaphenylbenzene-based, π-conjugated snowflake-shaped luminophores: Tunable aggregation-induced emission effect and piezofluorochromism. Chem. Eur. J. 2015, 21, 8504–8510. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-B.; Chen, Y.-C.; Liu, J.-L.; Jia, J.-H.; Wang, L.-F.; Li, Q.-W.; Tong, M.-L. A Piezochromic Dysprosium(III) Single-Molecule Magnet Based on an Aggregation-Induced-Emission-Active Tetraphenylethene Derivative Ligand. Inorg. Chem. 2017, 56, 8730–8734. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Qiu, Z.; Han, T.; Liu, Y.; Chen, M.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. 1 + 1 >> 2: Dramatically Enhancing the Emission Efficiency of TPE-Based AIEgens but Keeping their Emission Color through Tailored Alkyl Linkages. Adv. Funct. Mater. 2018, 28, 1707210. [Google Scholar] [CrossRef]
- Duraimurugan, K.; Sivamani, J.; Sathiyaraj, M.; Thiagarajan, V.; Siva, A. Piezoflurochromism and Aggregation Induced Emission Properties of 9,10-bis (trisalkoxystyryl) Anthracene Derivatives. J. Fluoresc. 2016, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, G.; Zhu, D.; Su, Z.; Bryce, M.R. An AIE-active phosphorescent Ir(III) complex with piezochromic luminescence (PCL) and its application for monitoring volatile organic compounds (VOCs). J. Mater. Chem. C 2017, 5, 12189–12193. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, D.; Hua, H.; Dai, C.; Wang, L.; Liu, M.; Huang, X.; Guo, Y.; Cheng, Y.; Wu, H. Piezochromism, acidochromism, solvent-induced emission changes and cell imaging of D-π-A 1,4-dihydropyridine derivatives with aggregation-induced emission properties. Dyes Pigm. 2016, 133, 261–272. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, Y.; Yang, X.; Wang, K.; Zou, B.; Yan, D. Piezochromic luminescence of AIE-active molecular co-crystals: Tunable multiple hydrogen bonding and molecular packing. J. Mater. Chem. C 2018, 6, 9660–9666. [Google Scholar] [CrossRef]
- Ma, C.; He, J.; Xu, B.; Xie, G.; Xie, Z.; Mao, Z.; Chi, Z. A TPE-benzothiazole piezochromic and acidichromic molecular switch with high solid state luminescent efficiency. RSC Adv. 2018, 8, 6252–6258. [Google Scholar] [CrossRef]
- Qi, C.; Ma, H.; Fan, H.; Yang, Z.; Cao, H.; Wei, Q.; Lei, Z. Study of Red-Emission Piezochromic Materials Based on Triphenylamine. ChemPlusChem 2016, 81, 637–645. [Google Scholar] [CrossRef]
- Rananaware, A.; Duc La, D.; Bhosale, S.V. Aggregation-induced emission of a star-shape luminogen based on cyclohexanehexone substituted with AIE active tetraphenylethene functionality. RSC Adv. 2015, 5, 56270–56273. [Google Scholar] [CrossRef]
- Sun, Q.K.; Liu, W.; Ying, S.A.; Wang, L.L.; Xue, S.F.; Yang, W.J. 9,10-Bis(N-alkylindole-3-yl-vinyl-2)anthracenes as a new series of alkyl length-dependent piezofluorochromic aggregation-induced emission homologues. RSC Adv. 2015, 5, 73046–73050. [Google Scholar] [CrossRef]
- Teng, X.-Y.; Wu, X.-C.; Cao, Y.-Q.; Jin, Y.-H.; Li, Y.; Yan, X.-L.; Wang, B.-W.; Chen, L.-G. Piezochromic luminescence and aggregation induced emission of 9,10-bis[2-(2-alkoxynaphthalen-1-yl)vinyl]anthracene derivatives. Chin. Chem. Lett. 2017, 28, 1485–1491. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Yang, S.; Lin, Y.; Lin, Z.; Ling, Q. Large Changes in Fluorescent Color and Intensity of Symmetrically Substituted Arylmaleimides Caused by Subtle Structure Modifications. Chem. Eur. J. 2018, 24, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, S.; Wang, B.; Yang, X.; Zou, B.; Yang, B.; Lu, S. Pressure-triggered aggregation-induced emission enhancement in red emissive amorphous carbon dots. Nanoscale Horiz. 2019, 4, 1227–1231. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Liu, X.; Li, G.; Che, W.; Zhu, D.; Su, Z. New cationic Ir(III) complexes without “any soft substituents”: Aggregation-induced emission and piezochromic luminescence. J. Mater. Chem. C 2018, 6, 12217–12223. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, K.; Yao, Z.; Zou, B.; Xu, J.; Bu, X.-H. Multi-stimuli-responsive fluorescence switching from a pyridine-functionalized tetraphenylethene AIEgen. ACS Appl. Mater. Interfaces 2018, 10, 5819–5827. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, J.; Tian, W. Aggregation-Induced Emission of 9,10-Distyrylanthracene Derivatives and Their Applications. In Aggregation-Induced Emission: Fundamentals and Applications; Qin, A.J., Tang, B.Z., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2014; pp. 61–82. [Google Scholar]
- Yadav, P.; Singh, A.K.; Upadhyay, C.; Singh, V.P. Photoluminescence behavior of a stimuli responsive Schiff base: Aggregation induced emission and piezochromism. Dyes Pigm. 2019, 160, 731–739. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Li, Q.; Li, Z. Aggregation-induced emission materials: The art of conjugation and rotation. ACS Symp. Ser. 2016, 1226, 61–83. [Google Scholar]
- Yao, X.; Chi, Z. Piezochromic aggregation-induced emission materials. Zhongguo Kexue Huaxue 2013, 43, 1090–1104. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Lam, J.W.Y.; Tang, B.Z. Mechanochromic Luminescence of Aggregation-Induced Emission Luminogens. J. Phys. Chem. Lett. 2015, 6, 3429–3436. [Google Scholar] [CrossRef] [PubMed]
- Donati, F.; Pucci, A.; Cappelli, C.; Mennucci, B.; Ruggeri, G. Modulation of the optical response of polyethylene films containing luminescent perylene chromophores. J. Phys. Chem. B 2008, 112, 3668–3679. [Google Scholar] [CrossRef] [PubMed]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. Exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Birks, J.B. Excimers. Rep. Prog. Phys. 1975, 38, 903–974. [Google Scholar] [CrossRef]
- Pucci, A.; Ruggeri, G.; Bronco, S.; Bertoldo, M.; Cappelli, C.; Ciardelli, F. Conferring dichroic properties and optical responsiveness to polyolefins through organic chromophores and metal nanoparticles. Prog. Org. Coat. 2007, 58, 105–116. [Google Scholar] [CrossRef]
- Pucci, A.; Ruggeri, G.; Bronco, S.; Signori, F.; Donati, F.; Bernabò, M.; Ciardelli, F. Color responsive smart polymers and biopolymers films through nanodispersion of organic chromophores and metal particles. Prog. Org. Coat. 2011, 72, 21–25. [Google Scholar] [CrossRef]
- Gu, J.; Qin, A.; Tang, B.Z. Polymers with Aggregation-Induced Emission Characteristics. In Principles and Applications of Aggregation-Induced Emission; Tang, Y., Tang, B.Z., Eds.; Springer: Cham, Switzerland, 2019; pp. 77–108. [Google Scholar]
- Yoon, S.-J.; Chung, J.W.; Gierschner, J.; Kim, K.S.; Choi, M.-G.; Kim, D.; Park, S.Y. Multistimuli Two-Color Luminescence Switching via Different Slip-Stacking of Highly Fluorescent Molecular Sheets. J. Am. Chem. Soc. 2010, 132, 13675–13683. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216. [Google Scholar] [CrossRef]
- Iasilli, G.; Battisti, A.; Tantussi, F.; Fuso, F.; Allegrini, M.; Ruggeri, G.; Pucci, A. Aggregation-induced emission of tetraphenylethylene in styrene-based polymers. Macromol. Chem. Phys. 2014, 215, 499–506. [Google Scholar] [CrossRef]
- Lott, J.; Weder, C. Macromol. Chem. Phys. 1/2010. Macromol. Chem. Phys. 2010, 211. [Google Scholar] [CrossRef]
- Taniguchi, R.; Yamada, T.; Sada, K.; Kokado, K. Stimuli-Responsive Fluorescence of AIE Elastomer Based on PDMS and Tetraphenylethene. Macromolecules 2014, 47, 6382–6388. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, J.; Huang, H.; Li, J.; Zhu, Y.; Tang, B.; Han, J.; Li, L. Memory chromic polyurethane with tetraphenylethylene. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 104–110. [Google Scholar] [CrossRef]
- Robb, M.J.; Li, W.; Gergely, R.C.R.; Matthews, C.C.; White, S.R.; Sottos, N.R.; Moore, J.S. A Robust Damage-Reporting Strategy for Polymeric Materials Enabled by Aggregation-Induced Emission. ACS Cent. Sci. 2016, 2, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.M.; Blaiszik, B.J.; Jin, H.; Schelkopf, S.R.; Stradley, D.S.; Sottos, N.R.; White, S.R.; Moore, J.S. Robust, Double-Walled Microcapsules for Self-Healing Polymeric Materials. ACS Appl. Mater. Interfaces 2010, 2, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Calvino, C.; Guha, A.; Weder, C.; Schrettl, S. Self-Calibrating Mechanochromic Fluorescent Polymers Based on Encapsulated Excimer-Forming Dyes. Adv. Mater. 2018, 30, 1704603. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Kim, B.; Lee, T.H.; Kim, J.C.; Nam, J.H.; Noh, S.M.; Park, Y.I. Fluorescence Detection of Microcapsule-Type Self-Healing, Based on Aggregation-Induced Emission. Macromol. Rapid Commun. 2017, 38, 1600657. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Kim, B.; Lee, T.H.; Kim, S.Y.; Kim, J.C.; Noh, S.M.; Park, Y.I. Monitoring Fluorescence Colors to Separately Identify Cracks and Healed Cracks in Microcapsule-containing Self-healing Coating. Sens. Actuators B 2018, 257, 1001–1008. [Google Scholar] [CrossRef]
- Zhao, W.; He, Z.; Peng, Q.; Lam, J.W.Y.; Ma, H.; Qiu, Z.; Chen, Y.; Zhao, Z.; Shuai, Z.; Dong, Y.; et al. Highly sensitive switching of solid-state luminescence by controlling intersystem crossing. Nat. Commun. 2018, 9, 3044. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, W.; Cao, M.; Wang, Y.; Lam, J.W.Y.; Zhang, Z.; Chen, X.; Tang, B.Z. Dynamic Visualization of Stress/Strain Distribution and Fatigue Crack Propagation by an Organic Mechanoresponsive AIE Luminogen. Adv. Mater. 2018, 30, 1803924. [Google Scholar] [CrossRef]
- Lowe, C.; Weder, C. Oligo(p-phenylene vinylene) excimers as molecular probes: deformation-induced color changes in photoluminescent polymer blends. Adv. Mater. 2002, 14, 1625–1629. [Google Scholar] [CrossRef]




© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucci, A. Mechanochromic Fluorescent Polymers with Aggregation-Induced Emission Features. Sensors 2019, 19, 4969. https://doi.org/10.3390/s19224969
Pucci A. Mechanochromic Fluorescent Polymers with Aggregation-Induced Emission Features. Sensors. 2019; 19(22):4969. https://doi.org/10.3390/s19224969
Chicago/Turabian StylePucci, Andrea. 2019. "Mechanochromic Fluorescent Polymers with Aggregation-Induced Emission Features" Sensors 19, no. 22: 4969. https://doi.org/10.3390/s19224969
APA StylePucci, A. (2019). Mechanochromic Fluorescent Polymers with Aggregation-Induced Emission Features. Sensors, 19(22), 4969. https://doi.org/10.3390/s19224969




