Dopamine/2-Phenylethylamine Sensitivity of Ion-Selective Electrodes Based on Bifunctional-Symmetrical Boron Receptors
Abstract
1. Introduction
2. Materials and Methods
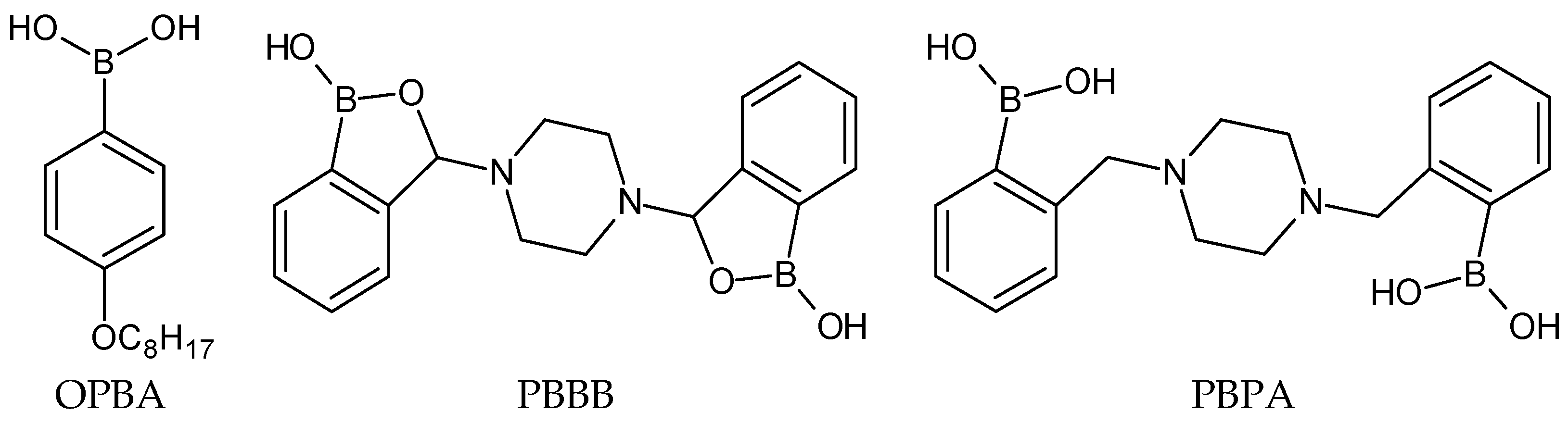
2.1. Chemicals and Membrane Materials
2.2. Ion-Selective Electrode Preparation and Electromotive Force (EMF) Measurements
2.3. Computational Methods
3. Results and Discussion
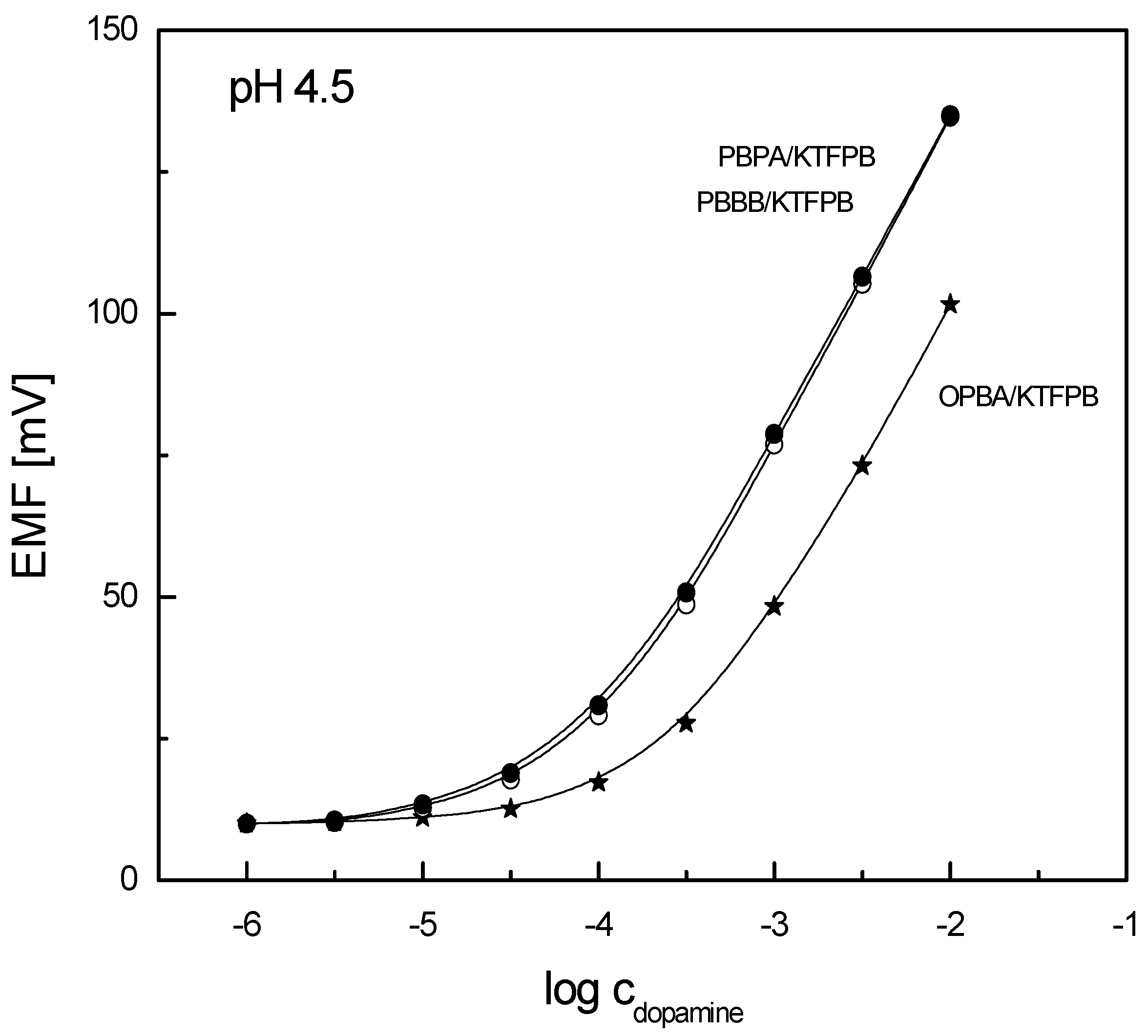
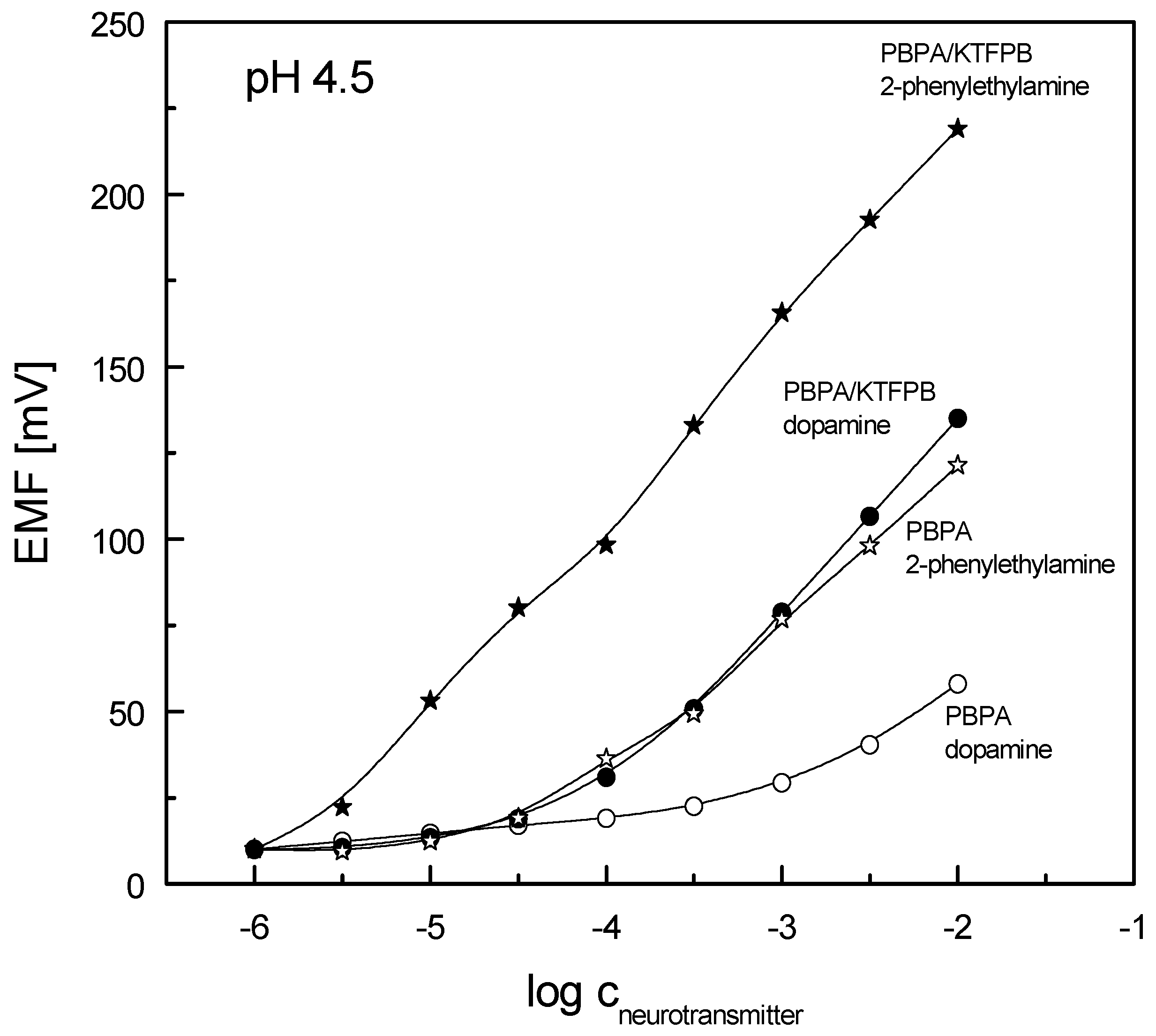
3.1. Sensitivity and Selectivity of Potentiometric Sensors
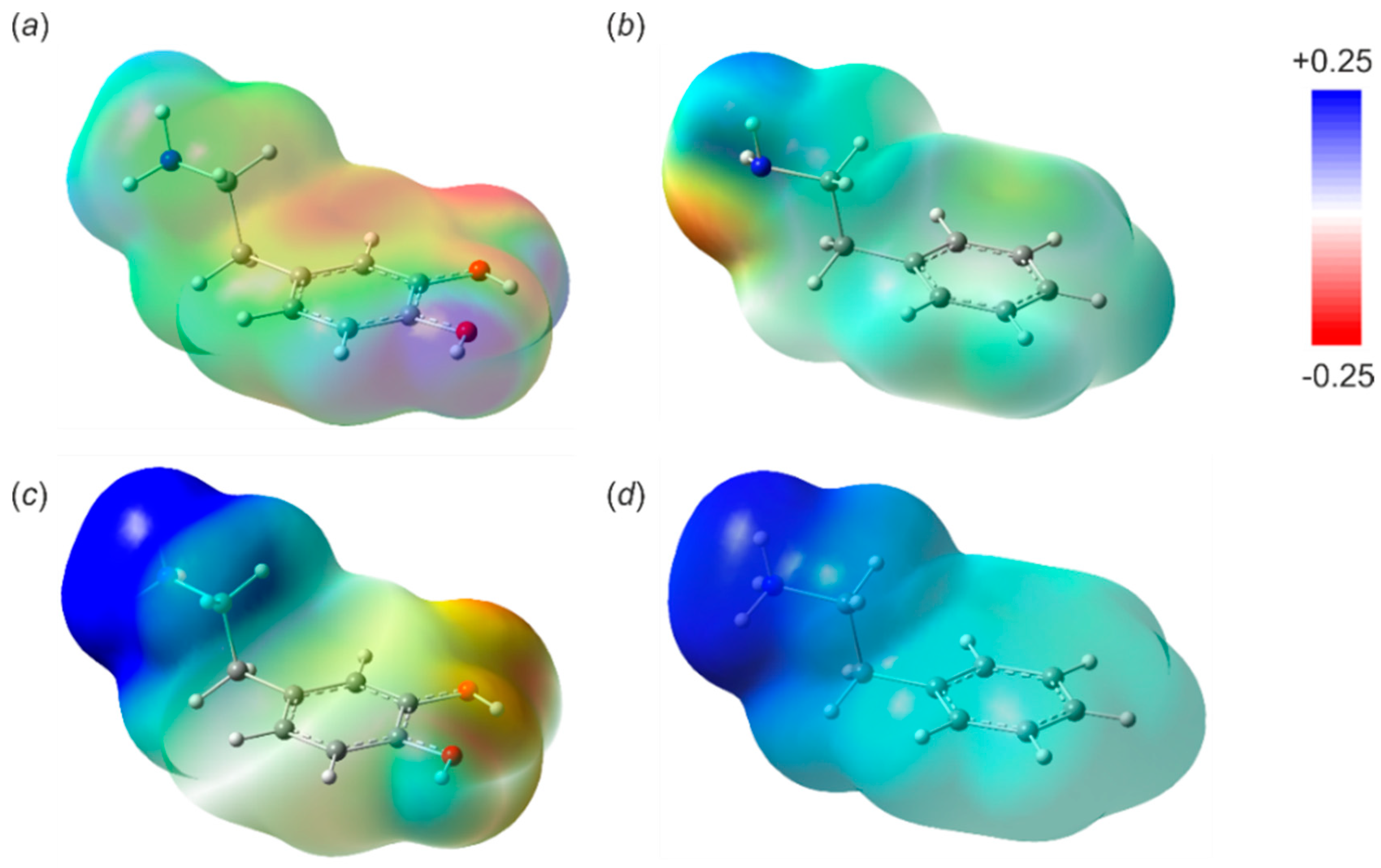
3.2. Receptor–Analyte Interaction—A Computational Approach
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Ferrier, R.J. Carbohydrate boronates. Adv. Carbohydr. Chem. Biochem. 1978, 35, 31–80. [Google Scholar]
- Yu, H.; Wang, B. Phenylboronic acids facilitated selective reduction of aldehydes by tributyltin hydride. Synth. Commun. 2001, 31, 163–169. [Google Scholar] [CrossRef]
- Myung, J.; Kim, K.B.; Crews, C.M. The ubiquitin-proteasome pathway and proteasome inhibitors. Med. Res. Rev. 2001, 21, 245–273. [Google Scholar] [CrossRef] [PubMed]
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.-G.; Barth, R.F.; Codogni, I.M.; Wilson, G. The chemistry of neutron capture therapy. Chem. Rev. 1998, 98, 1515–1562. [Google Scholar] [CrossRef] [PubMed]
- Kitano, S.; Koyama, Y.; Kataoka, K.; Okano, T.; Sakurai, Y. A novel drug delivery system utilizing a glucose responsive polymer complex between poly(vinyl alcohol) and poly(N-vinyl-2-pyrrolidone) with a phenylboronic acid moiety. J. Control. Release 1992, 19, 162–170. [Google Scholar] [CrossRef]
- James, T.D.; Sandanayake, K.R.A.S.; Shinkai, S. Saccharide sensing with molecular receptors based on boronic acid. Angew. Chem. Int. Ed. 1996, 35, 1911–1922. [Google Scholar] [CrossRef]
- Wang, W.; Gao, X.; Wang, B. Boronic acid-based sensors. Curr. Org. Chem. 2002, 6, 1285–1317. [Google Scholar] [CrossRef]
- Peters, J.A. Interactions between boric acid derivatives and saccharides in aqueous media: Structures and stabilities of resulting esters. Coord. Chem. Rev. 2014, 268, 1–22. [Google Scholar] [CrossRef]
- Hansen, J.S.; Christensen, J.B.; Petersen, J.F.; Hoeg-Jensen, T.; Norrild, J.C. Arylboronic acids: A diabetic eye on glucose sensing. Sens. Actuators B Chem. 2012, 161, 45–79. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Chen, X.-X.; Fossey, J.S.; James, T.D.; Jiang, Y.-B. Selective sensing of saccharides using simple boronic acids and their aggregates. Chem. Soc. Rev. 2013, 42, 8032–8048. [Google Scholar] [CrossRef] [PubMed]
- Anjali Devi, J.S.; Anulekshmi, A.H.; Salini, S.; Aparna, R.S.; George, S. Boronic acid functionalized nitrogen doped carbon dots for fluorescent turn-on detection of dopamine. Microchim. Acta 2017, 184, 4081–4090. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, X.; Song, F.; Wang, C.; Chu, F.; Wu, S. A sensing approach for dopamine determination by boronic acid-functionalized molecularly imprinted graphene quantum dots composite. Appl. Surf. Sci. 2017, 423, 810–816. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Borys, K.M.; Sporzyński, A. Recent developments in the chemistry and biological applications of benzoxaboroles. Chem. Rev. 2015, 115, 5224–5247. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Cyrański, M.K.; Żubrowska, A.; Sporzyński, A. Benzoxaboroles—Old compounds with new applications. J. Organomet. Chem. 2009, 694, 3533–3541. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, M.Y.; Lin, Y.N.; Zhou, H.C. The synthesis of benzoxaboroles and their applications in medicinal chemistry. Sci. China Chem. 2013, 56, 1372–1381. [Google Scholar] [CrossRef]
- Liu, C.T.; Tomsho, J.W.; Benkovic, S.J. The unique chemistry of benzoxaboroles: Current and emerging applications in biotechnology and therapeutic treatments. Bioorg. Med. Chem. 2014, 22, 4462–4473. [Google Scholar] [CrossRef]
- Baker, S.J.; Zhang, Y.-K.; Akama, T.; Lau, A.; Zhou, H.; Hermandez, V.; Mao, W.; Alley, M.R.K.; Sanders, V.; Plattner, J.J. Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (AN2690), for the potential treatment of onychomycosis. J. Med. Chem. 2006, 49, 4447–4450. [Google Scholar] [CrossRef] [PubMed]
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crepin, T.; Zhou, H.C.; Zhang, Y.K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef]
- Seiradake, E.; Mao, W.; Hernandez, V.; Baker, S.J.; Plattner, J.J.; Alley, M.R.K.; Cusack, S. Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. J. Mol. Biol. 2009, 390, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Alley, M.R.; Sader, H.S.; Biedenbach, D.J.; Jones, R.N. Potency and spectrum of activity of AN3365, a novel boron-containing protein synthesis inhibitor, tested against clinical isolates of Enterobacteriaceae and nonfermentative Gram-negative bacilli. Antimicrob. Agents Chemother. 2013, 57, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Dowlut, M.; Hall, D.G. An improved class of sugar-binding boronic acids, soluble and capable of complexing glycosides in neutral water. J. Am. Chem. Soc. 2006, 128, 4226–4227. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, M.; Dowlut, M.; Hall, D.G. Benzoboroxoles as efficient glycopyranoside-binding agents in physiological conditions: Structure and selectivity of complex formation. J. Org. Chem. 2008, 73, 6471–6479. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Berube, M.; Hall, D.G. Design, synthesis, and screening of a library of peptidyl bis(boroxoles) as oligosaccharide receptors in water: Identification of a receptor for the tumor marker TF-antigen disaccharide. Angew. Chem. Int. Ed. 2010, 49, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Borys, K.M.; Madura, I.D.; Pawełko, A.; Tomecka, E.; Zukowski, K. Lewis acidity and sugar receptor activity of 3-amino-substituted benzoxaboroles and their ortho-aminomethylphenylboronic acid analogues. New J. Chem. 2013, 37, 188–194. [Google Scholar] [CrossRef]
- Wulff, G. Selective binding to polymers via covalent bonds. The construction of chiral cavities as specific receptor sites. Pure Appl. Chem. 1982, 54, 2093–2102. [Google Scholar] [CrossRef]
- Wiskur, S.L.; Lavigne, J.J.; Ait-Haddou, H.; Lynch, V.; Chiu, Y.H.; Canary, J.W.; Anslyn, E.V. pKa values and geometries of secondary and tertiary amines complexed to boronic acids. Implications for sensor design. Org. Lett. 2001, 3, 1311–1314. [Google Scholar] [CrossRef]
- Yang, W.; He, H.; Drueckhammer, D.G. Computer-guided design in molecular recognition: Design and synthesis of a glucopyranose receptor. Angew. Chem. Int. Ed. 2001, 40, 1714–1718. [Google Scholar] [CrossRef]
- Cao, H.; Heagy, M.D. Fluorescent chemosensors for carbohydrates: A decade’s worth of bright spies for saccharides in review. J. Fluoresc. 2004, 14, 569–584. [Google Scholar] [CrossRef]
- Phillips, M.D.; James, T.D. Boronic acid based modular fluorescent sensors for glucose. J. Fluoresc. 2004, 14, 549–559. [Google Scholar] [CrossRef]
- Zhao, J.; Davidson, M.G.; Mahon, M.F.; Kociok-Kohn, G.; James, T.D. An enantioselective fluorescent sensor for sugar acids. J. Am. Chem. Soc. 2004, 126, 16179–16186. [Google Scholar] [CrossRef] [PubMed]
- Swamy, K.M.K.; Yun Jang, J.; Park, M.S.; Koh, H.S.; Lee, S.K.; Yoon, Y.J.; Yoon, J. A sorbitol-selective fluorescence sensor. Tetrahedron Lett. 2005, 46, 3453–3456. [Google Scholar] [CrossRef]
- Wieczorek, D.; Lipok, J.; Borys, K.M.; AdamczykWoźniak, A.; Sporzyński, A. Investigation of fungicidal activity of 3-piperazine-bis(benzoxaborole) and its boronic acid analogue. Appl. Organomet. Chem. 2014, 28, 347–350. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Borys, K.M.; Madura, I.D.; Michałek, S.; Pawełko, A. Straightforward synthesis and crystal structures of the 3-piperazine-bisbenzoxaboroles and their boronic acid analogs. Tetrahedron 2013, 69, 8936–8942. [Google Scholar] [CrossRef]
- Hargrove, A.E.; Reyes, R.N.; Riddington, I.; Anslyn, E.V.; Sessler, J.L. Boronic acid porphyrin receptor for ginsenoside sensing. Org. Lett. 2010, 12, 4804–4807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; You, L.; Anslyn, E.V.; Qian, X. Discrimination and classification of ginsenosides and ginsengs using bis-boronic acid receptors in dynamic multicomponent indicator displacement sensor arrays. Chem. Eur. J. 2012, 18, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Birtwistle, J.; Baldwin, D. Role of dopamine in schizophrenia and Parkinson’s disease. Br. J. Nurs. 1998, 7, 832–841. [Google Scholar] [CrossRef]
- Foti, A.; Kimura, S.; DeQuattro, V.; Lee, D. Liquid-chromatographic measurement of catecholamines and metabolites in plasma and urine. Clin. Chem. 1987, 33, 2209–2213. [Google Scholar]
- Gao, Z.; Huang, H. Simultaneous determination of dopamine, uric acid and ascorbic acid at an ultrathin film modified gold electrode. Chem. Commun. 1998, 19, 2107–2108. [Google Scholar] [CrossRef]
- Bath, B.D.; Michael, D.J.; Trafton, B.J.; Joseph, J.D.; Runnels, P.L.; Wightman, R.M. Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Anal. Chem. 2000, 15, 5994–6002. [Google Scholar] [CrossRef]
- Zen, J.-M.; Chen, P.-J. A selective voltammetric method for uric acid and dopamine detection using clay-modified electrodes. Anal. Chem. 1997, 69, 5087–5093. [Google Scholar] [CrossRef]
- Ramesh, P.; Suresh, G.S.; Sampath, S. Selective determination of dopamine using unmodified, exfoliated graphite electrodes. J. Electroanal. Chem. 2004, 561, 173–180. [Google Scholar] [CrossRef]
- Selvaraju, T.; Ramaraj, R. Simultaneous determination of dopamine and serotonin in the presence of ascorbic acid and uric acid at poly(o-phenylenediamine) modified electrode. J. Appl. Electrochem. 2003, 33, 759–762. [Google Scholar] [CrossRef]
- Thiagarajan, S.; Chen, S.-M. Preparation and characterization of PtAu hybrid film modified electrodes and their use in simultaneous determination of dopamine, ascorbic acid and uric acid. Talanta 2007, 74, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, N.; Sun, Q.; Bai, Z.; Zheng, J. Electrochemical sensor for dopamine based on imprinted silica matrix-poly(aniline boronic acid) hybrid as recognition element. Talanta 2016, 159, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Senel, M.; Cevik, E. Novel impedimetric dopamine biosensor based on boronic acid functional polythiophene modified electrodes. Mater. Sci. Eng. C 2017, 72, 641–649. [Google Scholar] [CrossRef]
- Fabre, B.; Hauquier, F. Boronic acid-functionalized oxide-free silicon surfaces for the electrochemical sensing of dopamine. Langmuir 2017, 33, 8693–8699. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, H.; Komada, H.; Goto, K.; Fujimoto, J.; Kiriyama, N.; Tucker, J.H.R. Selective recognition and electrochemical sensing of dopamine using a ferrocene-based heteroditopic receptor. Tetrahedron Lett. 2018, 59, 3853–3857. [Google Scholar] [CrossRef]
- Saijo, R.; Tsunekawa, S.; Murakami, H.; Shirai, N.; Ikeda, S.; Odashima, K. Dopamine-selective potentiometric responses by new ditopic sensory elements based on a hexahomotrioxacalix[3]arene. Bioorg. Med. Chem. Lett. 2007, 17, 767–771. [Google Scholar] [CrossRef]
- Jańczyk, M.; Adamczyk-Woźniak, A.; Sporzyński, A.; Wróblewski, W. Organoboron compounds as Lewis acid receptors of fluoride ions in polymeric membranes. Anal. Chim. Acta 2012, 733, 71–77. [Google Scholar] [CrossRef]
- Jańczyk, M.; Kutyła-Olesiuk, A.; Cetó, X.; del Valle, M.; Ciosek, P.; Wróblewski, W. Resolution of amino acid mixtures by an array of potentiometric sensors based on boronic acid derivative in a SIA flow system. Sens. Actuators B Chem. 2013, 189, 179–186. [Google Scholar] [CrossRef]
- Ćwik, P.; Wawrzyniak, U.E.; Jańczyk, M.; Wróblewski, W. Electrochemical studies of self-assembled monolayers composed of various phenylboronic acids derivatives. Talanta 2014, 119, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Jańczyk, M.; Borys, K.M.; Sporzyński, A.; Wróblewski, W. Ion-selective electrodes based on organoboron compounds as neurotransmitter receptors. Procedia Eng. 2014, 87, 568–571. [Google Scholar] [CrossRef]
- Ishi-i, T.; Murakami, K.; Imai, Y.; Mataka, S. Self-assembled fluorescent hexaazatriphenylenes that act as a light-harvesting antenna. J. Org. Chem. 2006, 71, 5752–5760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Binkley, J.S.; Pople, J.A.; Hehre, W.J. Self-consistent molecular orbital methods. 21. Small split-valence basis sets for first-row elements. J. Am. Chem. Soc. 1980, 102, 939–947. [Google Scholar] [CrossRef]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-consistent molecular-orbital methods. 22. Small split-valence basis sets for second-row elements. J. Am. Chem. Soc. 1982, 104, 2797–2803. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian09; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Cossi, M.; Scalmani, G.; Rega, N.; Barone, V.J. New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J. Chem. Phys. 2002, 117, 43–54. [Google Scholar] [CrossRef]
- Barone, V.; Improta, R.; Rega, N. Computation of protein pK’s values by an integrated density functional theory/Polarizable continuum model approach. Theor. Chem. Accounts 2004, 111, 237–245. [Google Scholar] [CrossRef]
- Nishiyabu, R.; Kubo, Y.; James, T.D.; Fossey, J.S. Boronic acid building blocks: Tools for sensing and separation. Chem. Commun. 2011, 47, 1106–1123. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Shin, I.; Yoon, J. Recognition and sensing of various species using boronic acid derivatives. Chem. Commun. 2012, 48, 5956–5967. [Google Scholar] [CrossRef] [PubMed]
- Bromba, C.; Carrie, P.; Chui, J.K.W.; Fyles, T.M. Phenyl boronic acid complexes of diols and hydroxyacids. Supramol. Chem. 2009, 21, 81–88. [Google Scholar] [CrossRef]
- Tomsho, J.W.; Pal, A.; Hall, D.G.; Benkovic, S.J. Ring structure and aromatic substituent effects on the pKa of the benzoxaborole pharmacophore. ACS Med. Chem. Lett. 2012, 3, 48–52. [Google Scholar] [CrossRef]
| Sensitivity (mV/dec) | Linear Range (M) | Detection Limit (M) | |
|---|---|---|---|
| OPBA (a) | - | - | - |
| OPBA/KTFPB (a) | 53.5 | 10−3 ÷ 10−2 | 2 × 10−4 |
| PBBB (a) | 9.5 | 10−3 ÷ 10−2 | 3 × 10−4 |
| PBBB/KTFPB (a) | 56.5 | 3 × 10−4 ÷ 10−2 | 8 × 10−5 |
| PBPA (a) | 27.5 | 10−3 ÷ 10−2 | 6 × 10−4 |
| PBPA/KTFPB (a) | 56.0 | 3 × 10−4 ÷ 10−2 | 8 × 10−5 |
| PBPA (b) | 47.0 | 3 × 10−4 ÷ 10−2 | 8 × 10−5 |
| PBPA/KTFPB (b) | 56.5 | 10−5 ÷ 10−2 | 3 × 10−6 |
| log K DOP, X | OPBA/KTFPB | PBBB/KTFPB | PBPA/KTFPB | PBPA |
|---|---|---|---|---|
| 2-Phenylethylamine | - | 1.70 | 1.70 | 1.60 |
| Acetylcholine | 0.45 | 0.40 | 0.45 | 0.20 |
| Dopamine | 0.00 | 0.00 | 0.00 | 0.00 |
| Na+ | −1.35 | −1.40 | −1.35 | −1.10 |
| K+ | −1.00 | −0.95 | −0.90 | −0.70 |
| NH4+ | −2.45 | −2.40 | −2.45 | −1.70 |
| Ca2+ | −2.50 | −2.65 | −2.70 | −1.90 |
| ΔE/kJ mol−1 | OPBA | PBBB | PBPA |
|---|---|---|---|
| PhEtNH2 (B–N) | −6.7 | −16.1 | −21.2 |
| Dopamine (B–N) | −6.6 | −15.0 | −18.3 |
| Dopamine (catechol) | −12.9 | −8.1 | −8.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durka, M.; Durka, K.; Adamczyk-Woźniak, A.; Wróblewski, W. Dopamine/2-Phenylethylamine Sensitivity of Ion-Selective Electrodes Based on Bifunctional-Symmetrical Boron Receptors. Sensors 2019, 19, 283. https://doi.org/10.3390/s19020283
Durka M, Durka K, Adamczyk-Woźniak A, Wróblewski W. Dopamine/2-Phenylethylamine Sensitivity of Ion-Selective Electrodes Based on Bifunctional-Symmetrical Boron Receptors. Sensors. 2019; 19(2):283. https://doi.org/10.3390/s19020283
Chicago/Turabian StyleDurka, Martyna, Krzysztof Durka, Agnieszka Adamczyk-Woźniak, and Wojciech Wróblewski. 2019. "Dopamine/2-Phenylethylamine Sensitivity of Ion-Selective Electrodes Based on Bifunctional-Symmetrical Boron Receptors" Sensors 19, no. 2: 283. https://doi.org/10.3390/s19020283
APA StyleDurka, M., Durka, K., Adamczyk-Woźniak, A., & Wróblewski, W. (2019). Dopamine/2-Phenylethylamine Sensitivity of Ion-Selective Electrodes Based on Bifunctional-Symmetrical Boron Receptors. Sensors, 19(2), 283. https://doi.org/10.3390/s19020283






