Quantitative Assessment of Head Tremor in Patients with Essential Tremor and Cervical Dystonia by Using Inertial Sensors
Abstract
:1. Introduction
1.1. Contributions
- We focus on the head tremor, in contrast to [19], where limb tremor is analyzed, and consider a number of features for differentiation of different types of tremors.
- An analysis of triaxial gyroscope signals for patients with cervical dystonia with and without head tremor.
- An analysis of triaxial gyroscope signals for patients diagnosed with essential tremor and cervical dystonia with head tremor.
2. Materials and Methods
2.1. Subjects
- Group A1: Cervical dystonia without tremor vs. Group B1: Cervical dystonia with tremor present either on head or both head and hands
- Group A2: Essential tremor on head vs. Group B2: Cervical dystonia with tremor present either on head or both head and hands
2.2. Data Acquisition
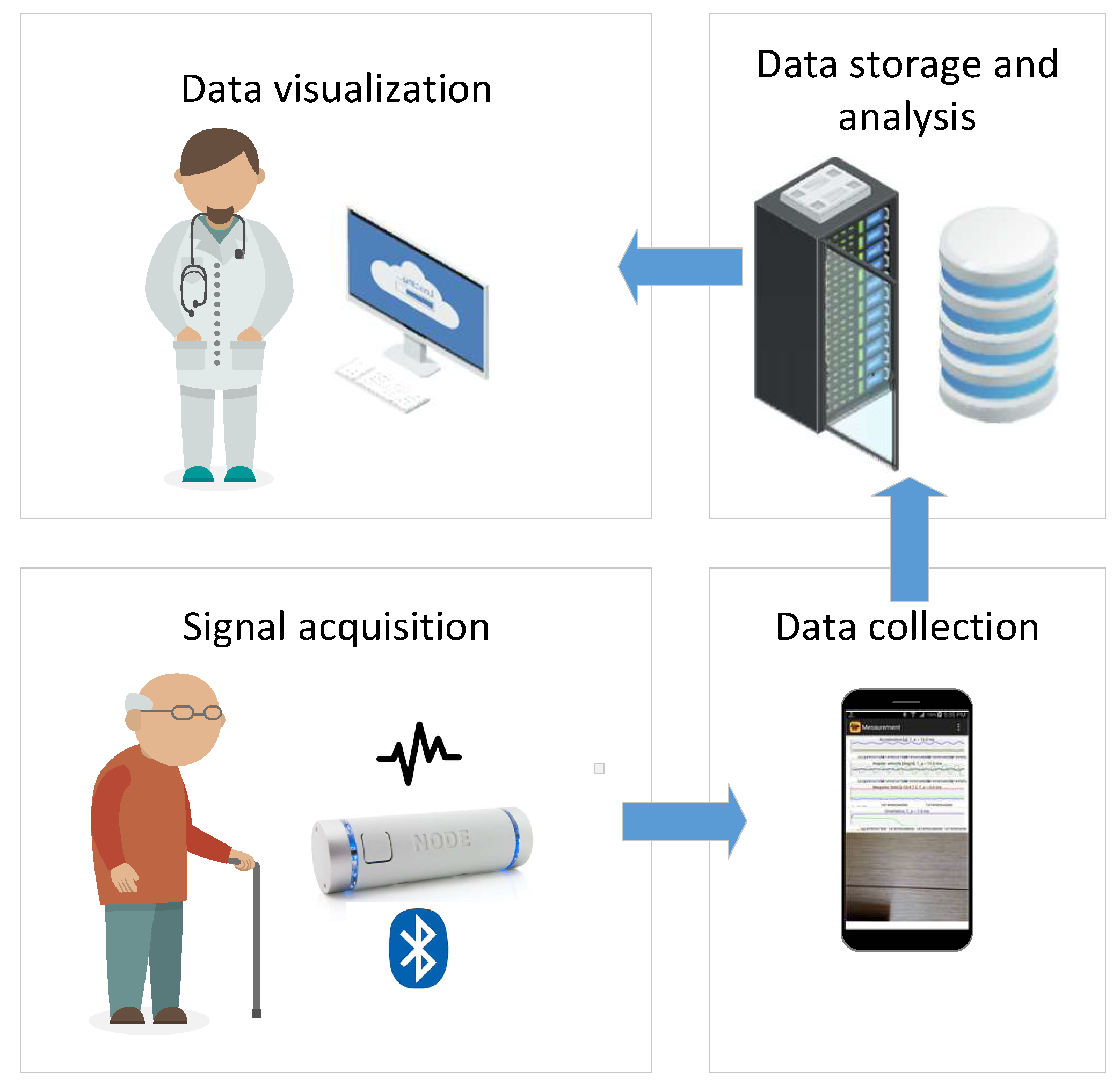
- Signal acquisition: which aims to collect bio-medical and other relevant environmental data and send them to the subsequent data collection block. It consists of one or more sensors (biomedical sensors, occupancy and movement sensors, etc.) that are usually connected wirelessly with the rest of the system.
- Data collection: whose goal is to collect the measurements from different sensors. In our study, an Android smart phone device performs this task, since it has enough computational capabilities and storage, while also supporting different network technologies (3G, 4G, WiFi) that enable seamless connectivity towards the internet and the cloud server where the data are stored and analyzed. In addition, it is possible to implement this unit on an inexpensive computer board serving as an edge node (e.g., Raspberry Pi 4) by using e.g., AndroidThings operating system as the development platform.
- Data storage and analysis block configured as a cloud server. The purpose of this block is to get data from different data collection units deployed in patients’ homes, their storage in the local database and analysis of raw data by using different signal processing and ML algorithms.
- Data visualization implemented as a desktop or web application which enables access to the raw and processed data stored in the data storage and analysis block.
- Signal acquisition: In this study, we employed Node+ sensor platform to measure tremor signals [20]. Node+ consists of a Bluetooth enabled main board with 3 different sensors: accelerometer, gyroscope and magnetometer that are able to measure the corresponding physical phenomena in 3 dimensions. Node+ is capable of real-time streaming of measurements with sampling frequency up to 70 Hz. The accelerometer can measure up to ±2, 4, 8 and 16 g (with resolution 61 g, 122 g, 244 g, and 488 g) along all three axes, whereas the gyroscope has angular velocity range of up to ±250, 500, 1000 and 2000 degrees per second (with resolution 0.00763, 0.015, 0.03 and 0.06 degrees per second). Finally, magnetometer’s range is ±1200 T. For the purpose of measuring tremor in the patient’s head, Node+ is attached to the support as it has been shown in Figure 2.
- Data collection software are implemented as a standalone Android application TremorSense, which is capable of connecting to Node+ sensor platform via Bluetooth, as it is shown in Figure 3. Once TremorSense application is started, a medical practitioner sets the Patient ID number (see Figure 3a). In the next step, she is provided with the list of nearby Bluetooth devices, and prompted to choose the one used for data acquisition (as shown in Figure 3b). Finally, once the Node+ device is connected, a signal acquisition starts and the raw data measurements are shown on the screen, as can be seen in Figure 3c. In the future version of the mobile app, our aim is to perform the signal analysis in the app itself, so that the user can get instant feedback about the results of analysis. In the context of AAL, a user would be the patient itself, and she would be allowed to log into the application with previously provided credentials.
- Data storage and analysis block are implemented as a web application capable of storing uploaded measured data (in .csv format) in the local server file system, and allowing an authorized user to download them to its PC computer. Currently, the data analysis is performed by using Matlab software. The aim is to further improve the web application with analysis capabilities deployed on the server, once the optimal signal processing algorithms have been identified.
- Data visualization is enabled by using Matlab visualization functions. The future work will involve development of standalone desktop or web application, which will be customized according to the requirements provided by medical practitioners.
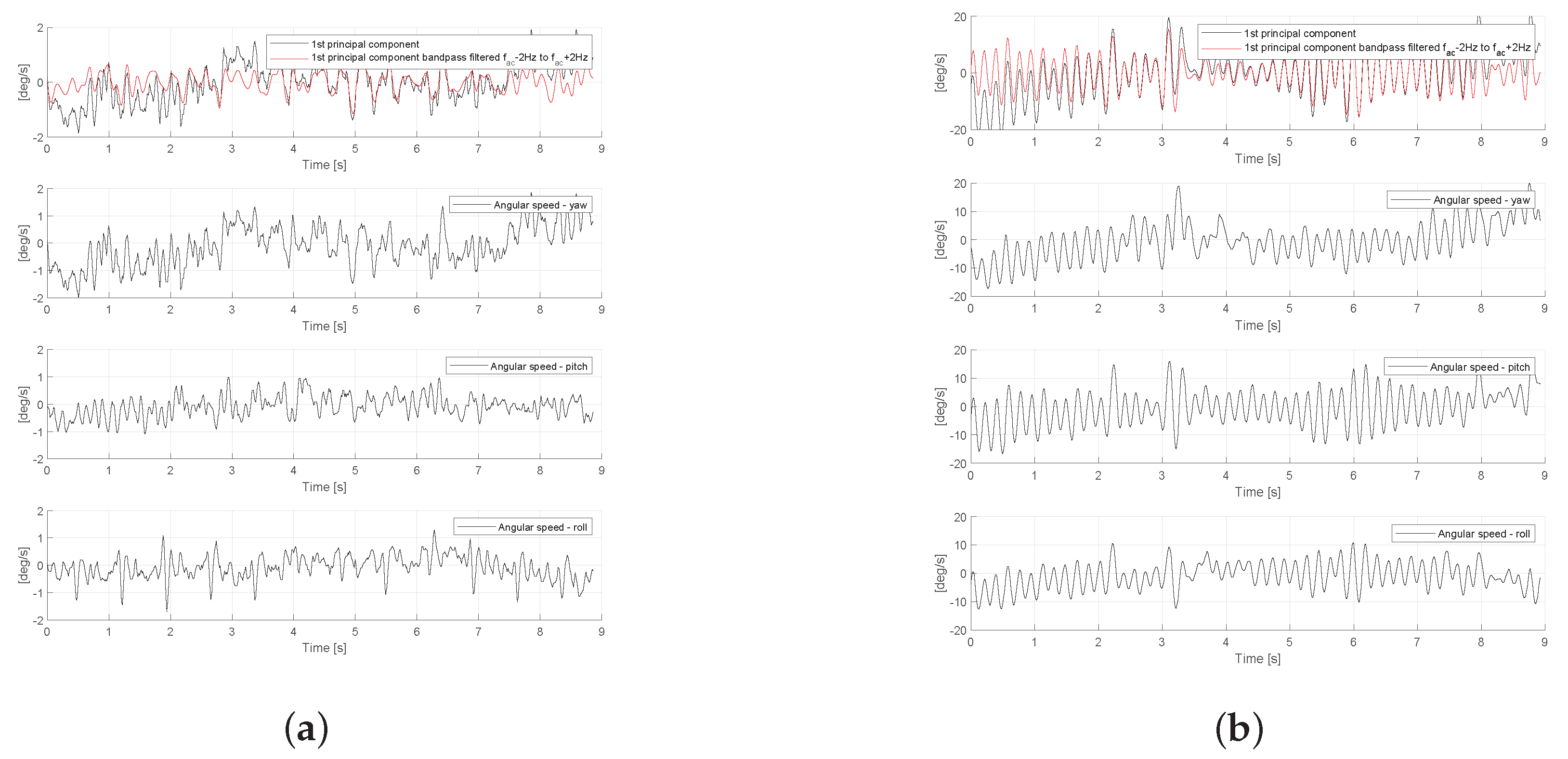
2.3. Data Analysis
- Peak frequency of the Fast Fourier Transform (FFT) spectrum which is calculated by identifying the maximum peak in FFT spectrum of the signal , as it is shown in Figure 6.
- Amplitude of the signal with the corresponding mean, standard deviation (std) and interquartile range (iqr). The amplitude of the signal is calculated as it is shown in Figure 7, according to the following equations:
- Signal regularity with the corresponding mean, standard deviation and interquartile range. This metric is used to describe the regularity of signal period , as it is shown in Figure 7 and calculated as follows:
- Inter-cycle variation of frequency (ICVF) of the signal with the corresponding mean, standard deviation and interquartile range. It is calculated similarly to the signal regularity and it is related to signal regularity through , as follows:
- Signal power concentration ratio (SPCR), which represents the ratio of signal power in range ( Hz, Hz) to the signal power in range ( Hz, Hz), as follows:
3. Results and Discussion
3.1. Cervical Dystonia with and without Head Tremor (A1 vs. B1)
3.2. Essential Head Tremor vs. Cervical Dystonia with Head Tremor (A2 vs. B2)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Population Prospects. 2019. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 6 August 2019).
- Wolff, J.L.; Jacobs, B.J. Chapter 7—Chronic Illness Trends and the Challenges to Family Caregivers: Organizational and Health System Barriers. In Family Caregiving in the New Normal; Gaugler, J.E., Kane, R.L., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 79–103. [Google Scholar] [CrossRef]
- Louis, E.; Ferreira, J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 2010, 25, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Benito-León, J.; Bermejo-Pareja, F.; Louis, E.D. Incidence of essential tremor in three elderly populations of central Spain. Neurology 2005, 64, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.; Bain, P.; Bajaj, N.; Elble, R.; Hallett, M.; Louis, E.; Raethjen, J.; Stamelou, M.; Testa, C.; Deuschl, G. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Lo, S.; Louis, E. Common misdiagnosis of a common neurological disorder: How are we misdiagnosing essential tremor? Arch. Neurol. 2006, 63, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Manto, M. Neurological Tremor: Sensors, Signal Processing and Emerging Applications. Sensors 2010, 10, 1399–1422. [Google Scholar] [CrossRef] [PubMed]
- Gallego, J.; Rocon, E.; Roa, J.; Moreno, J.; Pons, J. Real-Time Estimation of Pathological Tremor Parameters from Gyroscope Data. Sensors 2010, 10, 2129–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veluvolu, K.; Ang, W. Estimation of Physiological Tremor from Accelerometers for Real-Time Applications. Sensors 2011, 11, 3020–3036. [Google Scholar] [CrossRef] [PubMed]
- Teskey, W.; Elhabiby, M.; El-Sheimy, N. Inertial Sensing to Determine Movement Disorder Motion Present before and after Treatment. Sensors 2012, 12, 3512–3527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzallas, A.; Tsipouras, M.; Rigas, G.; Tsalikakis, D.; Karvounis, E.; Chondrogiorgi, M.; Psomadellis, F.; Cancela, J.; Pastorino, M.; Waldmeyer, M.; et al. PERFORM: A System for Monitoring, Assessment and Management of Patients with Parkinson’s Disease. Sensors 2014, 14, 21329–21357. [Google Scholar] [CrossRef] [PubMed]
- Kostikis, N.; Hristu-Varsakelis, D.; Arnaoutoglou, M.; Kotsavasiloglou, C. A Smartphone-Based Tool for Assessing Parkinsonian Hand Tremor. IEEE J. Biomed. Health Inform. 2015, 19, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Salarian, A.; Russmann, H.; Wider, C.; Burkhard, P.R.; Vingerhoets, F.J.G.; Aminian, K. Quantification of Tremor and Bradykinesia in Parkinson’s Disease Using a Novel Ambulatory Monitoring System. IEEE Trans. Biomed. Eng. 2007, 54, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Rigas, G.; Tzallas, A.T.; Tsipouras, M.G.; Bougia, P.; Tripoliti, E.E.; Baga, D.; Fotiadis, D.I.; Tsouli, S.G.; Konitsiotis, S. Assessment of Tremor Activity in the Parkinson’s Disease Using a Set of Wearable Sensors. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jenkins, M.E.; Naish, M.D.; Trejos, A.L. Characterization of Parkinsonian Hand Tremor and Validation of a High-Order Tremor Estimator. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhang, P.; Lueth, T. Quantitative Assessment of Parkinsonian Tremor Based on an Inertial Measurement Unit. Sensors 2015, 15, 25055–25071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, H.; Lee, W.; Park, H.; Lee, H.; Kim, S.; Kim, H.; Jeon, B.; Park, K. Automatic Classification of Tremor Severity in Parkinson’s Disease Using a Wearable Device. Sensors 2017, 17, 2067. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Brittain, J.; Shah, S.; Pedrosa, D.; Cagnan, H.; Mathy, A.; Chen, C.; Martín-Rodríguez, J.; Mir, P.; Timmerman, L.; et al. Tremor stability index: A new tool for differential diagnosis in tremor syndromes. Brain 2017, 140, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.; Jinnah, H.; Tripp, R.; Optican, L.; Ramat, S.; Lenz, F.; Zee, D. Irregularity distinguishes limb tremor in cervical dystonia from essential tremor. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Node+ Sensor Platform. Available online: https://support.variableinc.com/hc/en-us/article_attachments/201266043/NODE-Getting_Started_Guide_iOS-v1.1.pdf (accessed on 13 August 2019).
- Elble, R.; Hellriegel, H.; Raethjen, J.; Deuschl, G. Assessment of Head Tremor with Accelerometers Versus Gyroscopic Transducers. Mov. Disord. 2016, 4, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Characteristic | Group A1 | Group B1 | p-Value |
|---|---|---|---|
| Number of patients | 75 | 96 | |
| Age * | 49.9 ± 11.60 | 55.9 ± 12.29 | 0.01 |
| Sex (M/F) | 28/47 (36.33%/62.67%) | 31/65 (32.3%/67.7%) | 0.41 |
| Dominant hand (R/L) | 73/2 (97.33%/2.67%) | 92/4 (95.83%/4.17%) | 0.82 |
| Age at disease onset * | 41.3 ± 11.27 | 42.9 ± 13.05 | 0.04 |
| Disease duration * | 8.8 ± 6.01 | 12.7 ± 10.08 | 0.007 |
| Characteristic | Group A2 | Group B2 | p-Value |
|---|---|---|---|
| Number of patients | 78 | 96 | |
| Age * | 62.26 ± 13.01 | 55.9 ± 12.29 | 0.004 |
| Sex (M/F) | 18/60 (23.1%/76.9%) | 31/65 (32.3%/67.7%) | 0.18 |
| Dominant hand (R/L) | 77/1 (98.7%/1.3%) | 92/4 (95.83%/4.17%) | 0.26 |
| Age at disease onset * | 50.81 ± 15.82 | 42.9 ± 13.05 | 0.001 |
| Disease duration * | 12.09 ± 11.12 | 12.7 ± 10.08 | 1.00 |
| Feature | Group A1 | Group B1 | p-Value |
|---|---|---|---|
| Peak frequency | 4.92 ± 1.62 | 4.37 ± 1.22 | 0.0408 |
| Amplitude mean (deg/s) | 1.93 ± 2.57 | 7.77 ± 6.72 | 1.2434 × 10 |
| Amplitude std (deg/s) | 1.19 ± 0.969 | 5.26 ± 5.27 | 1.0782 × 10 |
| Amplitude iqr (deg/s) | 1.93 ± 2.57 | 7.77 ± 6.72 | 1.2434 × 10 |
| Regularity mean (s) | 0.192 ± 0.0428 | 0.213 ± 0.0455 | 0.0067156 |
| Regularity std (s) | 0.0529 ± 0.0241 | 0.0533 ± 0.0237 | 0.93321 |
| Regularity iqr (s) | 0.0681 ± 0.04 | 0.0643 ± 0.0381 | 0.58669 |
| ICVF mean (Hz) | 0.00649 ± 0.0702 | −0.00285 ± 0.0696 | 0.45574 |
| ICVF std (Hz) | 2.68 ± 1.25 | 1.97 ± 1.03 | 0.0011396 |
| ICVF iqr (Hz) | 2.53 ± 0.953 | 2.07 ± 0.919 | 0.0072191 |
| SPCR | 45.9 ± 15.4 | 52 ± 18.6 | 0.043076 |
| Feature | Group A2 | Group B2 | p-Value |
|---|---|---|---|
| Peak frequency | 4.25 ± 0.883 | 4.37 ± 1.22 | 0.48278 |
| Amplitude mean (deg/s) | 12.3 ± 15.3 | 7.77 ± 6.72 | 0.024072 |
| Amplitude std (deg/s) | 5.83 ± 8.88 | 5.26 ± 5.27 | 0.63558 |
| Amplitude iqr (deg/s) | 12.3 ± 15.3 | 7.77 ± 6.72 | 0.024072 |
| Regularity mean (s) | 0.225 ± 0.0371 | 0.213 ± 0.0455 | 0.090724 |
| Regularity std (s) | 0.0446 ± 0.0224 | 0.0533 ± 0.0237 | 0.021083 |
| Regularity iqr (s) | 0.0544 ± 0.0356 | 0.0643 ± 0.0381 | 0.10118 |
| ICVF mean (Hz) | −0.005 ± 0.0433 | −0.00285 ± 0.0696 | 0.8165 |
| ICVF std (Hz) | 1.61 ± 1.18 | 1.97 ± 1.03 | 0.041874 |
| ICVF iqr (Hz) | 1.56 ± 1 | 2.07 ± 0.919 | 0.001199 |
| SPCR | 62.8 ± 21 | 49.3 ± 20.2 | 7.9995 × 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berbakov, L.; Jovanović, Č.; Svetel, M.; Vasiljević, J.; Dimić, G.; Radulović, N. Quantitative Assessment of Head Tremor in Patients with Essential Tremor and Cervical Dystonia by Using Inertial Sensors. Sensors 2019, 19, 4246. https://doi.org/10.3390/s19194246
Berbakov L, Jovanović Č, Svetel M, Vasiljević J, Dimić G, Radulović N. Quantitative Assessment of Head Tremor in Patients with Essential Tremor and Cervical Dystonia by Using Inertial Sensors. Sensors. 2019; 19(19):4246. https://doi.org/10.3390/s19194246
Chicago/Turabian StyleBerbakov, Lazar, Čarna Jovanović, Marina Svetel, Jelena Vasiljević, Goran Dimić, and Nenad Radulović. 2019. "Quantitative Assessment of Head Tremor in Patients with Essential Tremor and Cervical Dystonia by Using Inertial Sensors" Sensors 19, no. 19: 4246. https://doi.org/10.3390/s19194246
APA StyleBerbakov, L., Jovanović, Č., Svetel, M., Vasiljević, J., Dimić, G., & Radulović, N. (2019). Quantitative Assessment of Head Tremor in Patients with Essential Tremor and Cervical Dystonia by Using Inertial Sensors. Sensors, 19(19), 4246. https://doi.org/10.3390/s19194246




