Portable and Low-Cost Respirometric Microsystem for the Static and Dynamic Respirometry Monitoring of Compost
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Electronic Components
2.3. Oxygen Sensor Design
2.4. Oxygen Sensor Characterizations
2.5. Sensor Lifetime
2.6. Integration to Respirometric Microsystem
2.7. Instrumental Circuit Design
2.8. Static Respirometry Measurements of Compost
2.9. Dynamic Respirometric Measurement of the Compost
2.10. Cost Analysis
2.11. Analytic Hierarchy Process
3. Results and Discussion
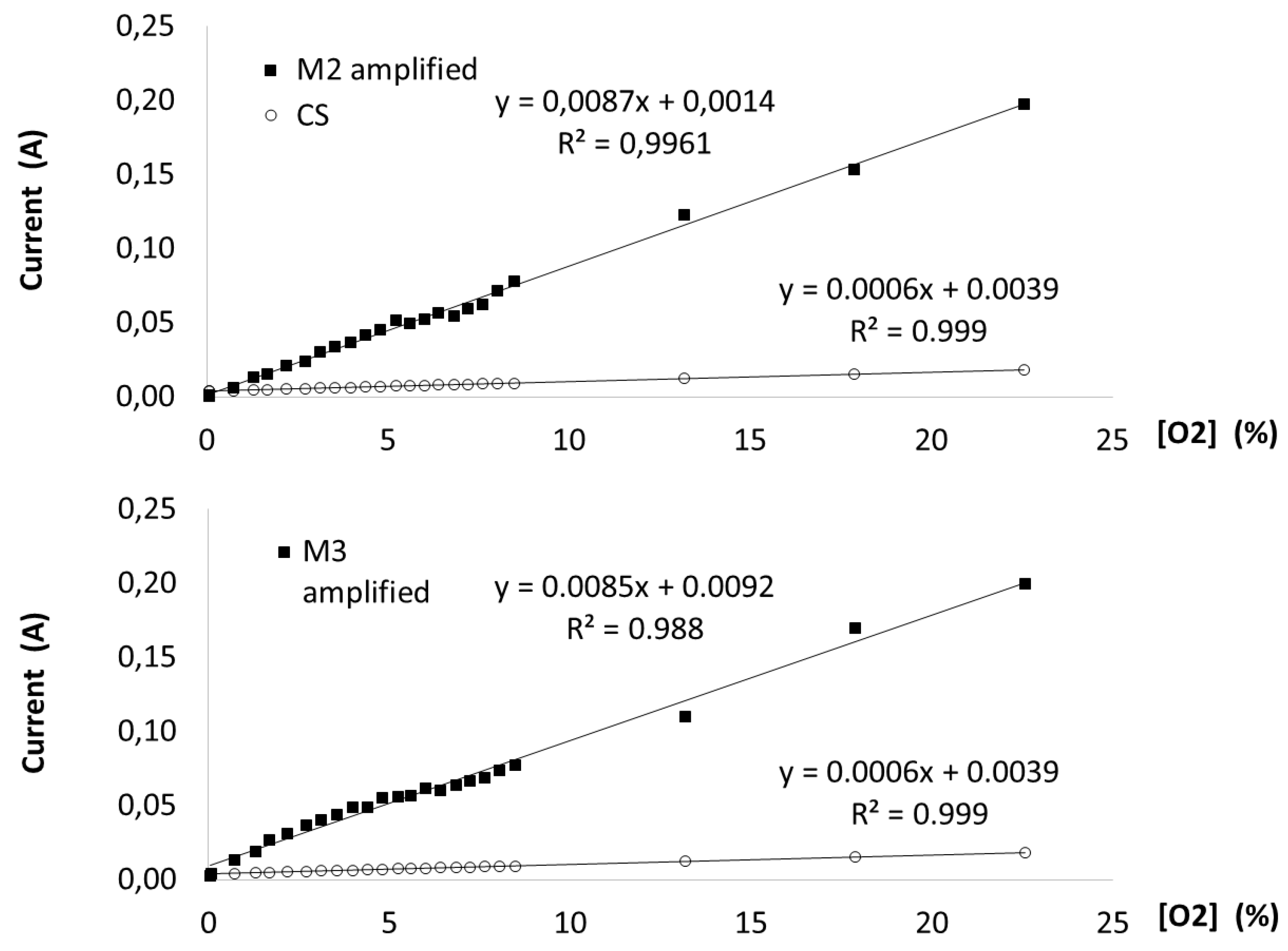
3.1. Oxygen Sensor Design
3.2. Oxygen Sensor Characterization
3.3. Sensor Lifetime
3.4. Static Respirometry Measurements (SRI) of Compost
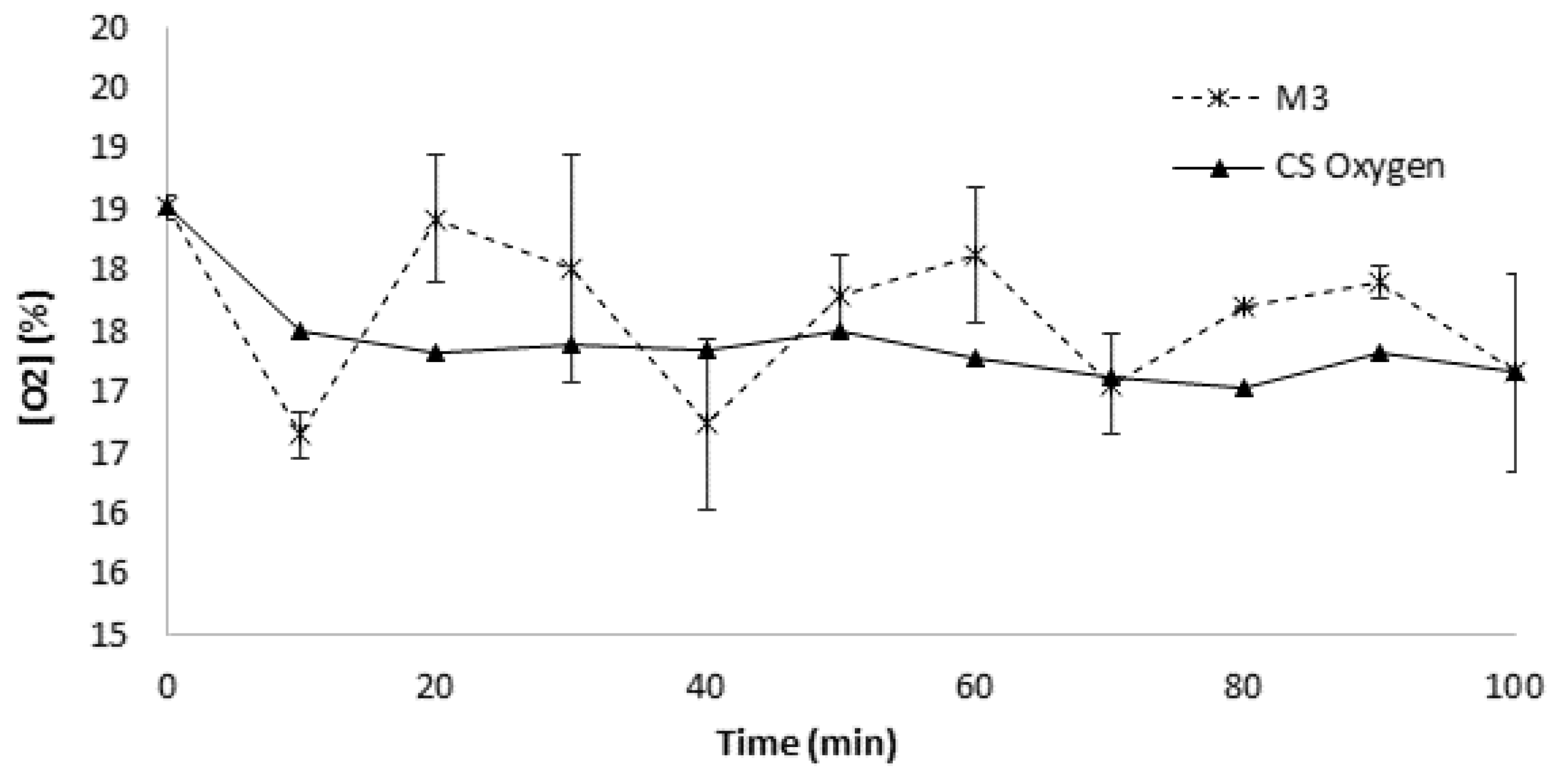
3.5. Dynamic Respirometry Measurement (DRI) of Compost
3.6. Cost Analysis
3.7. Analytic Hierarchy Process (AHP)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morone, P.; Koutinas, A.; Gathergood, N.; Arshadi, M.; Matharu, A. Food waste: Challenges and opportunities for enhancing the emerging. J. Clean. Prod. 2019, 221, 10–16. [Google Scholar] [CrossRef]
- Corrado, S.; Caldeira, C.; Eriksson, M.; Jørgen, O.; Hauser, H.; van Holsteijn, F.; Liu, G.; Östergren, K.; Parry, A.; Secondi, L. Food waste accounting methodologies: Challenges, opportunities, and further advancements. Glob. Food Sec. 2019, 20, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Thi, N.B.D.; Kumar, G.; Lin, C.Y. An overview of food waste management in developing countries: Current status and future perspective. J. Environ. Manag. 2015, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ikhlayel, M. Development of management systems for sustainable municipal solid waste in developing countries: A systematic life cycle thinking approach. J. Clean. Prod. 2018, 180, 571–586. [Google Scholar] [CrossRef]
- Han, Z.; Liu, Y.; Zhong, M.; Shi, G.; Li, Q.; Zeng, D.; Zhang, Y.; Fei, Y.; Xie, Y. Influencing factors of domestic waste characteristics in rural areas of developing countries. Waste Manag. 2018, 72, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Sukholthaman, P.; Sharp, A. A system dynamics model to evaluate effects of source separation of municipal solid waste management: A case of Bangkok, Thailand. Waste Manag. 2016, 52, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Onwosi, C.O.; Igbokwe, V.C.; Odimba, J.N.; Eke, I.E.; Nwankwoala, M.O.; Iroh, I.N.; Ezeogu, L.I. Composting technology in waste stabilization: On the methods, challenges and future prospects. J. Environ. Manag. 2017, 190, 140–157. [Google Scholar] [CrossRef]
- Cerda, A.; Artola, A.; Font, X.; Barrena, R.; Gea, T.; Sánchez, A. Composting of food wastes: Status and challenges. Bioresour. Technol. 2018, 248, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Oliveira, L.S.B.L.; Oliveira, D.S.B.L.; Bezerra, B.S.; Silva Pereira, B.; Battistelle, R.A.G. Environmental analysis of organic waste treatment focusing on composting scenarios. J. Clean. Prod. 2017, 155, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization (FAO). Composting: Let’s Give the Soil Something Back. 20 March 2015. Available online: http://www.fao.org/soils-2015/news/news-detail/en/c/280674/ (accessed on 24 September 2019).
- United States Environmental Protection Agency. Composting at Home. Available online: https://www.epa.gov/recycle/composting-home#benefits (accessed on 15 May 2019).
- Benito, M.; Masaguer, A.; Moliner, A.; Arrigo, N.; Palma, R.M. Chemical and microbiological parameters for the characterisation of the stability and maturity of pruning waste compost. Biol. Fertil. Soils 2003, 37, 184–189. [Google Scholar]
- Li, Z.; Lu, H.; Ren, L.; He, L. Experimental and modeling approaches for food waste composting: A review. Chemosphere 2013, 93, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, X.; Guo, J. Characteristics of municipal solid waste and sewage sludge co-composting. Waste Manag. 2009, 29, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Siles, J.A.; Gutiérrez, M.C.; Martín, M.A. Monitoring of the composting process of different agroindustrial waste: Influence of the operational variables on the odorous impact. Waste Manag. 2018, 76, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Scoton, E.J.; Battistelle, R.A.G.; Bezerra, B.S.; Akutsu, J. A sewage sludge co-composting process using respirometric monitoring method in hermetic rotary reactor. J. Clean. Prod. 2016, 121, 169–175. [Google Scholar] [CrossRef]
- Li, R.; Li, L.; Huang, R.; Sun, Y.; Mei, X.; Shen, B.; Shen, Q. Variations of culturable thermophilic microbe numbers and bacterial communities during the thermophilic phase of composting. World J. Microbiol. Biotechnol. 2014, 30, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, V.; María, A.; Perea, V.; Marcela, L. Influencia de la Concentración del Oxígeno Disuelto y los Nutrientes Sobre la Biodegradabilidad Aerobia de Biorresiduos de Origen Municipal. 20 October 2015. Available online: http://bibliotecadigital.univalle.edu.co/xmlui/bitstream/handle/10893/8978/3754-0505724.pdf?sequence=1 (accessed on 24 September 2019).
- Plessis, C.A.; Barnard, P.; Naldrett, K.; de Kock, S.H. Development of respirometry methods to assess the microbial activity of thermophilic bioleaching archaea. J. Microbiol. Methods 2001, 47, 189–198. [Google Scholar] [CrossRef]
- Barrena, R.; Artola, A.; Font, X.; Gea, T.; Pognani, M.; Ponsá, S.; Colón, J. La respirometría como herramienta para la clasificación de la biodegradabilidad de residuos orgánicos. Resultados de la Experiencia de 2013, 10, 12–19. [Google Scholar]
- Arias, V.S.; Fernández, F.J.; Rodríguez, L.; Villaseñor, J. Respiration indices and stability measurements of compost through electrolytic respirometry. J. Environ. Manag. 2012, 95, S134–S138. [Google Scholar] [CrossRef]
- Scaglia, B.; Tambone, F.; Genevini, P.L.; Adani, F. Respiration Index Determination: Dynamic And Static Approaches. Compost Sci. Util. 2013, 8, 90–98. [Google Scholar] [CrossRef]
- Gea, T.; Sa, A. Respirometric assays at fixed and process temperatures to monitor composting process. Bioresour. Technol. 2005, 96, 1153–1159. [Google Scholar] [Green Version]
- Gómez, R.B. Compostaje de Residuos Sólidos Orgánicos Aplicación de Técnicas Respirométricas en el Seguimiento del Proceso; Autonomous University of Barcelona: Bellaterra, Spain, 2006. [Google Scholar]
- Saldarriaga, J.F.; Gallego, J.L.; López, J.E.; Aguado, R.; Olazar, M. Selecting Monitoring Variables in the Manual Composting of Municipal Solid Waste Based on Principal Component Analysis International Classification for Standards. Waste Biomass V. 2018, 10, 1811–1819. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Wang, J.; Zhang, P.; Zhang, Y.; Miao, Y.; Gao, S. Recent advances in micro fl uidic chip integrated electronic biosensors for multiplexed detection. Biosens. Bioelectron. 2018, 121, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Thai, P.K.; Liu, X.; Asumadu-sakyi, A.; Ayoko, G.; Bartonova, A.; Bedini, A.; Chai, F.; Christensen, B.; Dunbabin, M.; et al. Applications of low-cost sensing technologies for air quality monitoring and exposure assessment: How far have they gone? Environ. Int. 2018, 116, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Mckercher, G.R.; Salmond, J.A.; Vanos, J.K. Characteristics and applications of small, portable gaseous air pollution monitors. Environ. Pollut. 2017, 223, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castell, N.; Dauge, F.R.; Schneider, P.; Vogt, M.; Lerner, U.; Fishbain, B.; Broday, D.; Bartonova, A. Can commercial low-cost sensor platforms contribute to air quality monitoring and exposure estimates? Environ. Int. 2017, 99, 293–302. [Google Scholar] [CrossRef]
- Zhou, F.; Song, X.; Zhou, X.; Gao, J.; Bao, J.; Tian, Z.; An, S. Limiting-current oxygen sensor with LaNi0.6Fe0.4O3−δ dense diffusion barrier and Ce0.8Gd0.15Ca0.05O2−δ electrolyte. Ceram. Int. J. 2019, 45, 12060–12065. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Zhang, X.; Gao, X.; Li, L.; Yu, J.; Yin, X. A limiting current oxygen sensor prepared by a co-pressing and co-sintering technique. Sens. Actuators B Chem. 2018, 277, 216–223. [Google Scholar] [CrossRef]
- Rebelo, R.; Barbosa, A.I.; Caballero, D.; Kwon, I.K.; Oliveira, J.M. 3D biosensors in advanced medical diagnostics of high mortality diseases. Biosens. Bioelectron. 2019, 130, 20–39. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Sepulveda, D.; Aroca, M.A.; Varela, A.; Del Portillo, P.; Osma, J.F. Bioelectrochemical detection of mycobacterium tuberculosis ESAT-6 in an antibody-based biomicrosystem. Sensors 2017, 17, 2178. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, S.; Karnaukh, M.; Panchuk, V.; Andreev, E. Potentiometric multisensor system as a possible simple tool for non-invasive prostate cancer diagnostics through urine analysis. Sens. Actuators B Chem. 2019, 289, 42–47. [Google Scholar] [CrossRef]
- Bunge, F.; van den Driesche, S.; Waespy, M.; Radtke, A.; Belge, G.; Kelm, S.; Waite, A.M.; Mirastschijski, U.; Vellekoop, M.J. Micro fl uidic oxygen sensor system as a tool to monitor the metabolism of mammalian cells. Sens. Actuators B Chem. 2019, 289, 24–31. [Google Scholar] [CrossRef]
- Loo, J.F.C.; Ho, A.H.P.; Turner, A.P.F.; Mak, W.C. Integrated Printed Microfluidic Biosensors. Trends Biotechnol. 2019, 37, 1104–1120. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Zitka, O.; Klimanek, M.; Vrba, R.; Adam, V. Microfluidic electrochemical devices for pollution analysis—A review. Sens. Actuators B Chem. 2017, 246, 578–590. [Google Scholar] [CrossRef]
- Ungerböck, B.; Charwat, V.; Ertlb, P.; Mayr, T. Microfluidic oxygen imaging using integrated optical sensor layers and a color camera. Lab Chip 2013, 13, 1593–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Kumar, A.; Khan, J.; Singh, P.; Wong, J.W.C.; Selvam, A. Bioresource Technology Evaluation of thermophilic fungal consortium for organic municipal solid waste composting. Bioresour. Technol. 2014, 168, 214–221. [Google Scholar]
- Changa, C.M.; Wang, P.; Watson, M.E.; Hoitink, H.A.J.; Michel, F.C.M., Jr. Assessment of the Reliability of a Commercial Maturity Test Kit for Composted Manures. Compost Sci. Util. 2013, 11, 125–143. [Google Scholar] [CrossRef]
- Ponsá, S.; Gea, T.; Sanchez, A. Different Indices to Express Biodegradability in Organic Solid Wastes. J. Environ. Qual. Abstr. Waste Manag. 2010, 39, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Osma, J.F.; Toca-herrera, J.L.; Rodríguez-couto, S. Cost analysis in laccase production. J. Environ. Manag. 2011, 92, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Frikha, A.; Moalla, H. Analytic hierarchy process for multi-sensor data fusion based on belief function theory. Eur. J. Oper. Res. 2015, 241, 133–147. [Google Scholar] [CrossRef]
- Acharya, V.; Sharma, S.K.; Gupta, S.K. Analyzing the factors in industrial automation using analytic hierarchy process R. Comput. Electr. Eng. 2018, 71, 877–886. [Google Scholar] [CrossRef]
- Park, J.; Cho, D. Decision methodology for nitrogen removal process in the LNG plant using analytic hierarchy process. J. Ind. Eng. Chem. 2016, 37, 75–83. [Google Scholar] [CrossRef]
- Al-hawari, T. Selection of Temperature Measuring Sensors Using the Analytic Hierarchy Process. Jordan J. Mech. Ind. Eng. 2011, 5, 451–459. [Google Scholar]
- Wong, J.K.; Li, H. Application of the analytic hierarchy process (AHP) in multi-criteria analysis of the selection of intelligent building systems. Build. Environ. 2008, 43, 108–125. [Google Scholar] [CrossRef]
- Grošelj, P.; Stirn, L.Z. Acceptable consistency of aggregated comparison matrices in analytic hierarchy process. Eur. J. Oper. Res. 2012, 223, 417–420. [Google Scholar] [CrossRef]
- Paletski, W.T.; Young, J.C. Stability Measurement of Biosolids Compost by Aerobic Respirometry. Compost Sci. Util. 1995, 3, 16–24. [Google Scholar] [CrossRef]
- European Committee for Standardization (CEN). Solid Recovered Fuels—Determination of Potential Rate of Microbial Self Heating Using the Real Dynamic Respiration Index; European Committee for Standardization (CEN): Brussels, Belgium, 2007. [Google Scholar]
- Komilis, D.; Kanellos, D. A modified dynamic respiration test to assess compost stability: Effect of sample size and air flowrate. Bioresour. Technol. 2012, 117, 300–309. [Google Scholar] [CrossRef]






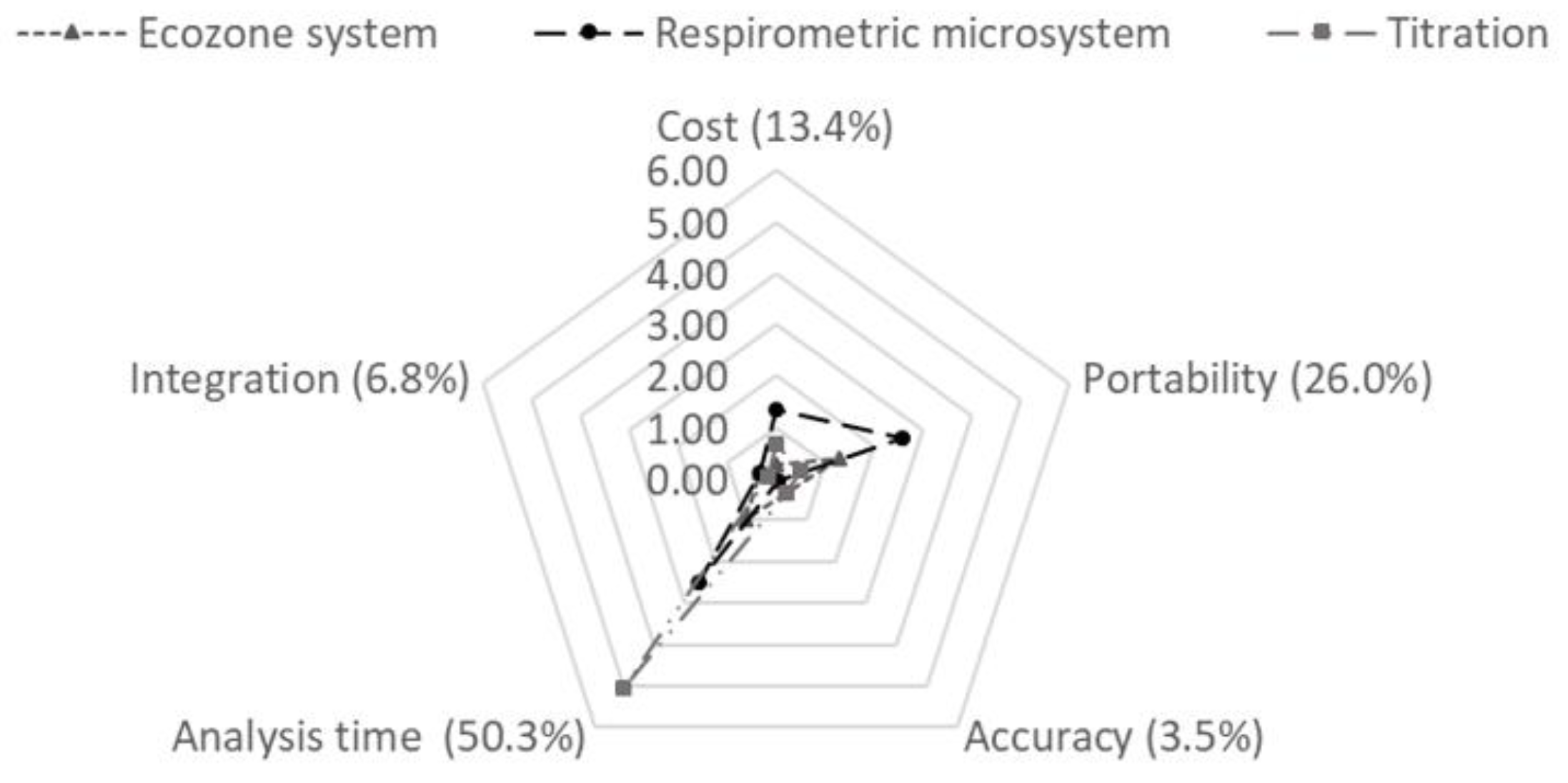
| Oxygen Sensor Design | Cost (USD) |
|---|---|
| MG | 384 |
| MP | 2 |
| CS | 99 |
| Respirometric Methods | Cost (USD) |
|---|---|
| Respirometric microsystem | 6 |
| EcoZone system | 34 |
| Titration | 13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermudez, J.F.; Saldarriaga, J.F.; Osma, J.F. Portable and Low-Cost Respirometric Microsystem for the Static and Dynamic Respirometry Monitoring of Compost. Sensors 2019, 19, 4132. https://doi.org/10.3390/s19194132
Bermudez JF, Saldarriaga JF, Osma JF. Portable and Low-Cost Respirometric Microsystem for the Static and Dynamic Respirometry Monitoring of Compost. Sensors. 2019; 19(19):4132. https://doi.org/10.3390/s19194132
Chicago/Turabian StyleBermudez, Juliette F., Juan F. Saldarriaga, and Johann F. Osma. 2019. "Portable and Low-Cost Respirometric Microsystem for the Static and Dynamic Respirometry Monitoring of Compost" Sensors 19, no. 19: 4132. https://doi.org/10.3390/s19194132
APA StyleBermudez, J. F., Saldarriaga, J. F., & Osma, J. F. (2019). Portable and Low-Cost Respirometric Microsystem for the Static and Dynamic Respirometry Monitoring of Compost. Sensors, 19(19), 4132. https://doi.org/10.3390/s19194132







