Assessing the Mass Sensitivity for Different Electrode Materials Commonly Used in Quartz Crystal Microbalances (QCMs)
Abstract
1. Introduction
2. Theory
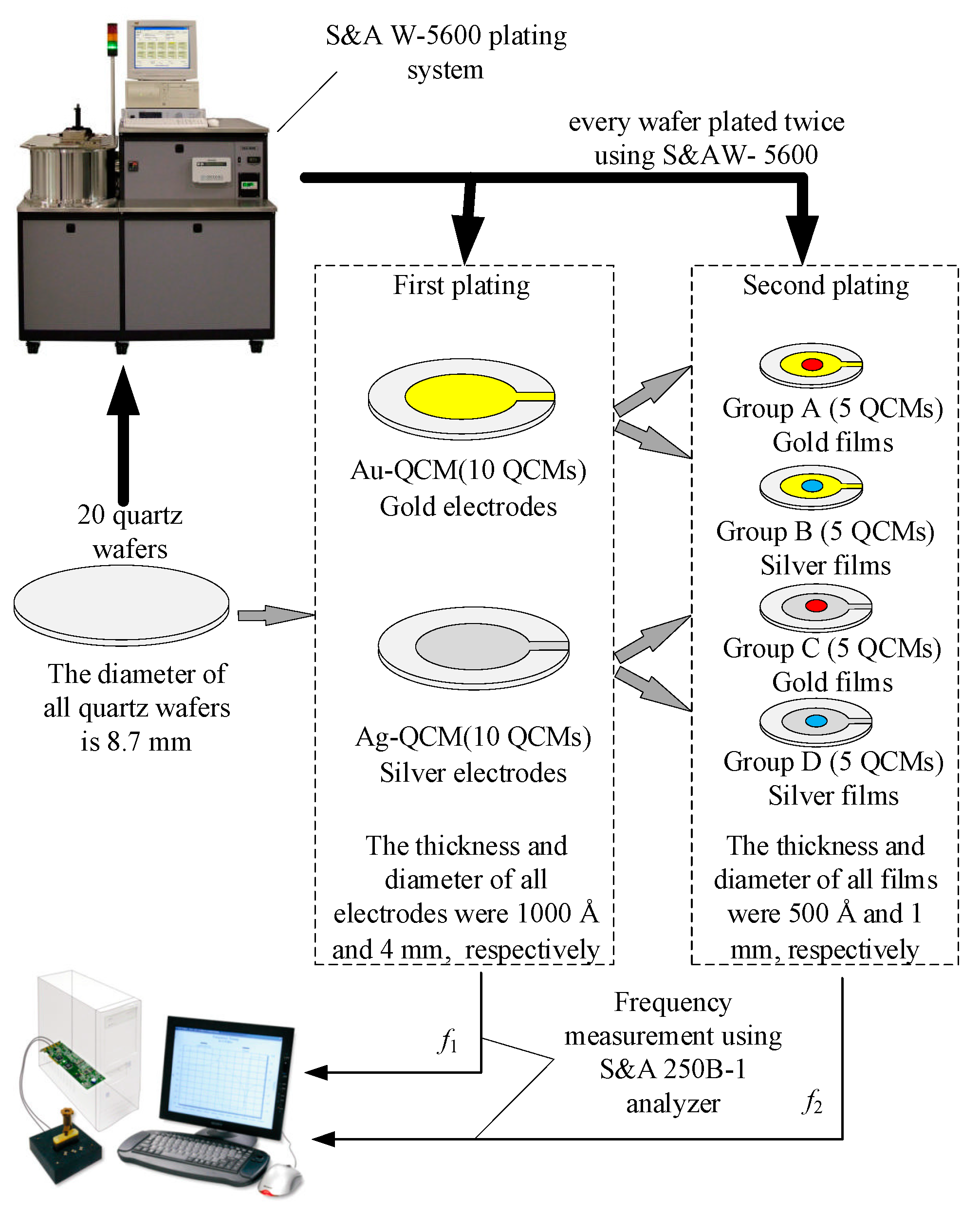
3. Experiment
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dirri, F.; Palomba, E.; Longobardo, A.; Zampetti, E.; Saggin, B.; Scaccabarozzi, D. A review of quartz crystal microbalances for space applications. Sens. Actuators A Phys. 2019, 287, 48–75. [Google Scholar] [CrossRef]
- Vig, J.R.; Filler, R.L.; Kim, Y. Chemical sensor based on quartz microresonators. J. Microelectromech. Syst. 1996, 5, 138–140. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Su, W.N.; Hwang, B.J. In situ analytical techniques for battery interface analysis. Chem. Soc. Rev. 2018, 47, 736–851. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Croce, M.A.; Tiozzo, R.; Facci, P. Monitoring cell-cycle-related viscoelasticity by a quartz crystal microbalance. Appl. Phys. Lett. 2006, 88, 083905. [Google Scholar] [CrossRef]
- Iturri, J.; Vianna, A.C.; Moreno-Cencerrado, A.; Pum, D.; Sleytr, U.B.; Toca-Herrera, J.L. Impact of surface wettability on S-layer recrystallization: A real-time characterization by QCM-D. Beilstein J. Nanotechnol. 2017, 8, 91–98. [Google Scholar] [CrossRef]
- Yang, L.; Huang, X.H.; Sun, L.; Xu, L. A piezoelectric immunosensor for the rapid detection of p16INK4aexpression in liquid-based cervical cytology specimens. Sens. Actuators B Chem. 2016, 224, 863–867. [Google Scholar] [CrossRef]
- Pei, X.M.; Zhang, B.; Tang, J.; Liu, B.Q.; Lai, W.Q.; Tang, D.P. Sandwich-type immunosensors and immunoassays exploiting nanostructure labels: A review. Anal. Chim. Acta 2013, 758, 1–18. [Google Scholar] [CrossRef]
- Karekin, E.D.; Rumiana, G.R.; Georgi, S.S.; Todor, C.A. Superhydrophobic soot coated quartz crystal microbalances: A novel platform for human spermatozoa quality assessment. Sensors 2019, 19, 123. [Google Scholar] [CrossRef]
- Johnson, L.; Gupta, A.K.; Ghafoor, A.; Akin, D.; Bashir, R. Characterization of vaccinia virus particles using microscale silicon cantilever resonators and atomic force microscopy. Sens. Actuators B Chem. 2006, 115, 189–197. [Google Scholar] [CrossRef]
- Ito, T.; Aoki, N.; Tsuchiya, A.; Kaneko, S.; Akiyama, K.; Uetake, K.; Suzuki, K. Detection of stress hormone in the milk for animal welfare using QCM method. J. Sens. 2017, 2017, 7. [Google Scholar] [CrossRef]
- Gupta, A.; Akin, D.; Bashir, R. Single virus particle mass detection using microresonators with nanoscale thickness. Appl. Phys. Lett. 2004, 84, 1976–1978. [Google Scholar] [CrossRef]
- Wang, P.T.; Su, J.W.; Dai, W.; Cernigliaro, G.; Sun, H.W. Ultrasensitive quartz crystal microbalance enabled by micropillar structure. Appl. Phys. Lett. 2014, 104, 043504. [Google Scholar] [CrossRef]
- Chauhan, R.; Solanki, P.R.; Singh, J.; Mukherjee, I.; Basu, T.; Malhotra, B.D. A novel electrochemical piezoelectric label free immunosensor for aflatoxin B1 detection in groundnut. Food Control 2015, 52, 60–70. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Oino, D.; Ohira, H.; Muguruma, H.; Moczko, E.; Piletsky, A.S. Size of heparin-imprinted nanoparticles reflects the matched interactions with the target molecule. Sensors 2019, 19, 2415. [Google Scholar] [CrossRef]
- Sauerbrey, G. Use of quartz vibration for weighing thin films on a microbalance. J. Phys. 1959, 155, 206–222. [Google Scholar]
- Quan, Z.L.; Wu, X.J.; Chen, S.H.; Zhao, S.; Ma, H. Self-assembled monolayers of Schiff bases on copper surfaces. Corrosion 2001, 57, 195–201. [Google Scholar] [CrossRef]
- Wang, L.Y.; Xu, J.; Wang, X.H.; Cheng, Z.X.; Xu, J.Q. Facile preparation of N-rich functional polymer with porous framework as QCM sensing material for rapid humidity detection. Sens. Actuators B Chem. 2019, 288, 289–297. [Google Scholar] [CrossRef]
- Sato, Y. Bio-and chemical sensors and role of soft interface. In Molecular Soft-Interface Science; Springer: Berlin/Heidelberg, Germany, 2019; pp. 181–198. [Google Scholar]
- Lee, J.; Jang, J.; Akin, D.; Savran, C.A.; Bashir, R. Real-time detection of airborne viruses on a mass-sensitive device. Appl. Phys. Lett. 2008, 93, 013901. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Stereo-regulated Schiff base siloxane polymer coated QCM sensor for amine vapor detection. Mater. Chem. Phys. 2019, 226, 214–219. [Google Scholar] [CrossRef]
- Vig, J.R.; Ballato, A. Comments on the effects of nonuniform mass loading on a quartz crystal microbalance. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998, 45, 1123–1124. [Google Scholar] [CrossRef]
- Ward, M.D.; Delawski, E.J. Radial mass sensitivity of the quartz crystal microbalance in liquid media. Anal. Chem. 1991, 63, 886–890. [Google Scholar] [CrossRef]
- Hillier, A.C.; Ward, M.D. Scanning electrochemical mass sensitivity mapping of the quartz crystal microbalance in liquid media. Anal. Chem. 1992, 64, 2539–2554. [Google Scholar] [CrossRef]
- Huang, X.H.; Pan, W.; Hu, J.G.; Bai, Q.S. The exploration and confirmation of the maximum mass sensitivity of quartz crystal microbalance. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1888–1892. [Google Scholar] [CrossRef]
- Gao, J.Y.; Huang, X.H.; Wang, Y. The modified design of ring electrode quartz crystal resonator for uniform mass sensitivity distribution. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 2031–2034. [Google Scholar]
- Huang, X.H.; Bai, Q.S.; Pan, W.; Hu, J.G. Quartz crystal microbalance with approximately uniform sensitivity distribution. Anal. Chem. 2018, 90, 6367–6370. [Google Scholar] [CrossRef]
- Huang, X.H. A quartz crystal microbalance mass sensor with uniform mass sensitivity. China Patent CN201810308560, 2018. [Google Scholar]
- Chen, Q.; Huang, X.H.; Pan, W.; Xu, Y.; Fan, Z.C. Investigation on mass sensitivity of n-m type electrode quartz crystal microbalance. Sensors 2019, 19, 2125. [Google Scholar] [CrossRef]
- Huang, X.H.; Bai, Q.S.; Hu, J.G.; Hou, D. A practical model of quartz crystal microbalance in actual applications. Sensors 2017, 17, 1785. [Google Scholar] [CrossRef]
- Tiersten, H.F. Linear Piezoelectric Plate Vibrations: Elements of the Linear Theory of Piezoelectricity and the Vibrations Piezoelectric Plates; Springer: Berlin/Heidelberg, Germany, 1969. [Google Scholar]
- Josse, F.; Lee, Y.; Martin, S.J.; Cernosek, R.W. Analysis of the radial dependence of mass sensitivity for modified-electrode quartz crystal resonators. Anal. Chem. 1998, 70, 237–247. [Google Scholar] [CrossRef]
| Groups | Es | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Au-QCM | A | 9,961,970 | 9,959,360 | 2610 | 2662 | 69.79 | 757.91 | 2759 | 3.52% |
| 9,963,680 | 9,960,970 | 2710 | |||||||
| 9,963,990 | 9,961,360 | 2630 | |||||||
| 9,962,450 | 9,959,850 | 2600 | |||||||
| 9,963,490 | 9,960,730 | 2760 | |||||||
| B | 9,962,620 | 9,961,290 | 1330 | 1368 | 50.20 | 412.33 | 1501 | 8.86% | |
| 9,962,550 | 9,961,220 | 1330 | |||||||
| 9,962,080 | 9,960,730 | 1350 | |||||||
| 9,962,740 | 9,961,290 | 1450 | |||||||
| 9,963,580 | 9,962,200 | 1380 | |||||||
| Ag-QCM | C | 10,000,940 | 9,998,460 | 2480 | 2428 | 70.90 | 757.91 | 2251 | 7.86% |
| 10,002,010 | 9,999,590 | 2420 | |||||||
| 10,001,480 | 9,998,960 | 2520 | |||||||
| 9,999,510 | 9,997,140 | 2370 | |||||||
| 9,997,960 | 9,995,610 | 2350 | |||||||
| D | 10,001,480 | 10,000,270 | 1210 | 1222 | 19.24 | 412.33 | 1225 | 0.02% | |
| 10,001,300 | 10,000,080 | 1220 | |||||||
| 10,001,650 | 10,000,450 | 1200 | |||||||
| 9,999,620 | 9,998,390 | 1230 | |||||||
| 9,995,910 | 9,994,660 | 1250 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Chen, Q.; Pan, W.; Hu, J.; Yao, Y. Assessing the Mass Sensitivity for Different Electrode Materials Commonly Used in Quartz Crystal Microbalances (QCMs). Sensors 2019, 19, 3968. https://doi.org/10.3390/s19183968
Huang X, Chen Q, Pan W, Hu J, Yao Y. Assessing the Mass Sensitivity for Different Electrode Materials Commonly Used in Quartz Crystal Microbalances (QCMs). Sensors. 2019; 19(18):3968. https://doi.org/10.3390/s19183968
Chicago/Turabian StyleHuang, Xianhe, Qiao Chen, Wei Pan, Jianguo Hu, and Yao Yao. 2019. "Assessing the Mass Sensitivity for Different Electrode Materials Commonly Used in Quartz Crystal Microbalances (QCMs)" Sensors 19, no. 18: 3968. https://doi.org/10.3390/s19183968
APA StyleHuang, X., Chen, Q., Pan, W., Hu, J., & Yao, Y. (2019). Assessing the Mass Sensitivity for Different Electrode Materials Commonly Used in Quartz Crystal Microbalances (QCMs). Sensors, 19(18), 3968. https://doi.org/10.3390/s19183968





