Capturing Electrocardiogram Signals from Chairs by Multiple Capacitively Coupled Unipolar Electrodes
Abstract
1. Introduction
2. Materials and Methods
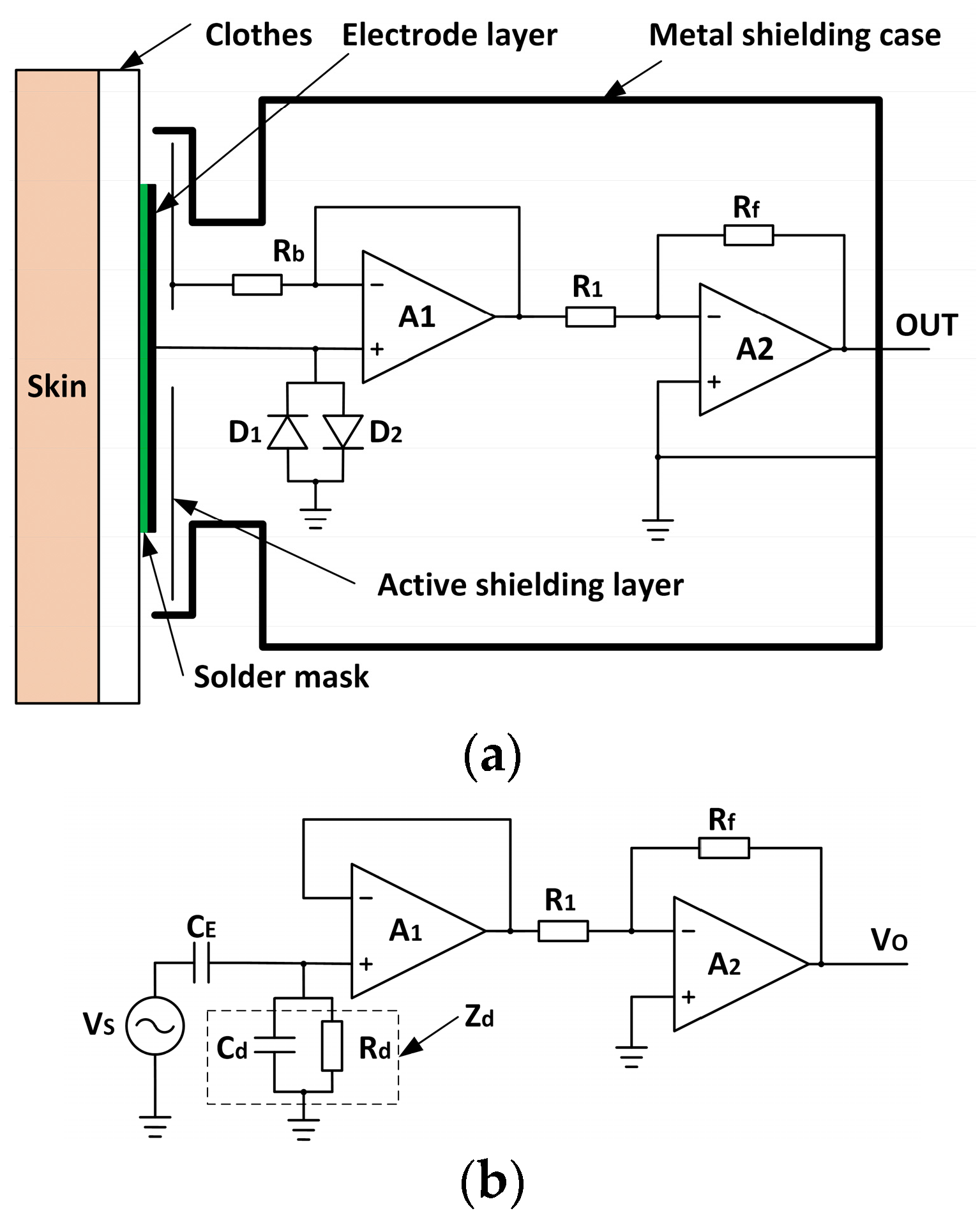
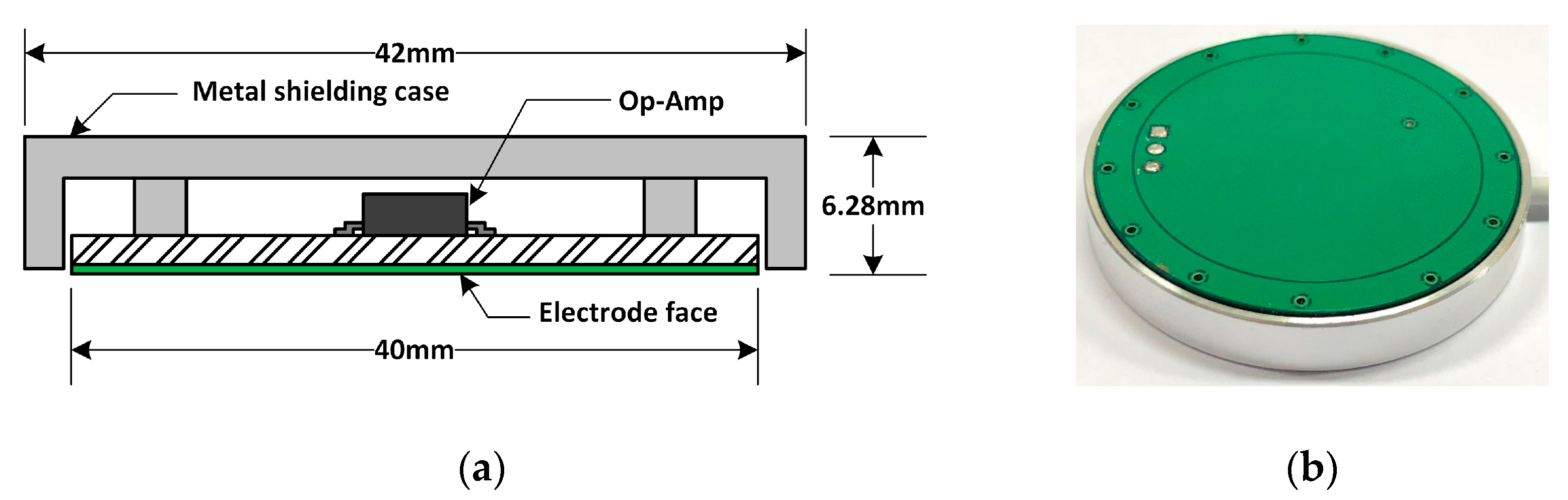
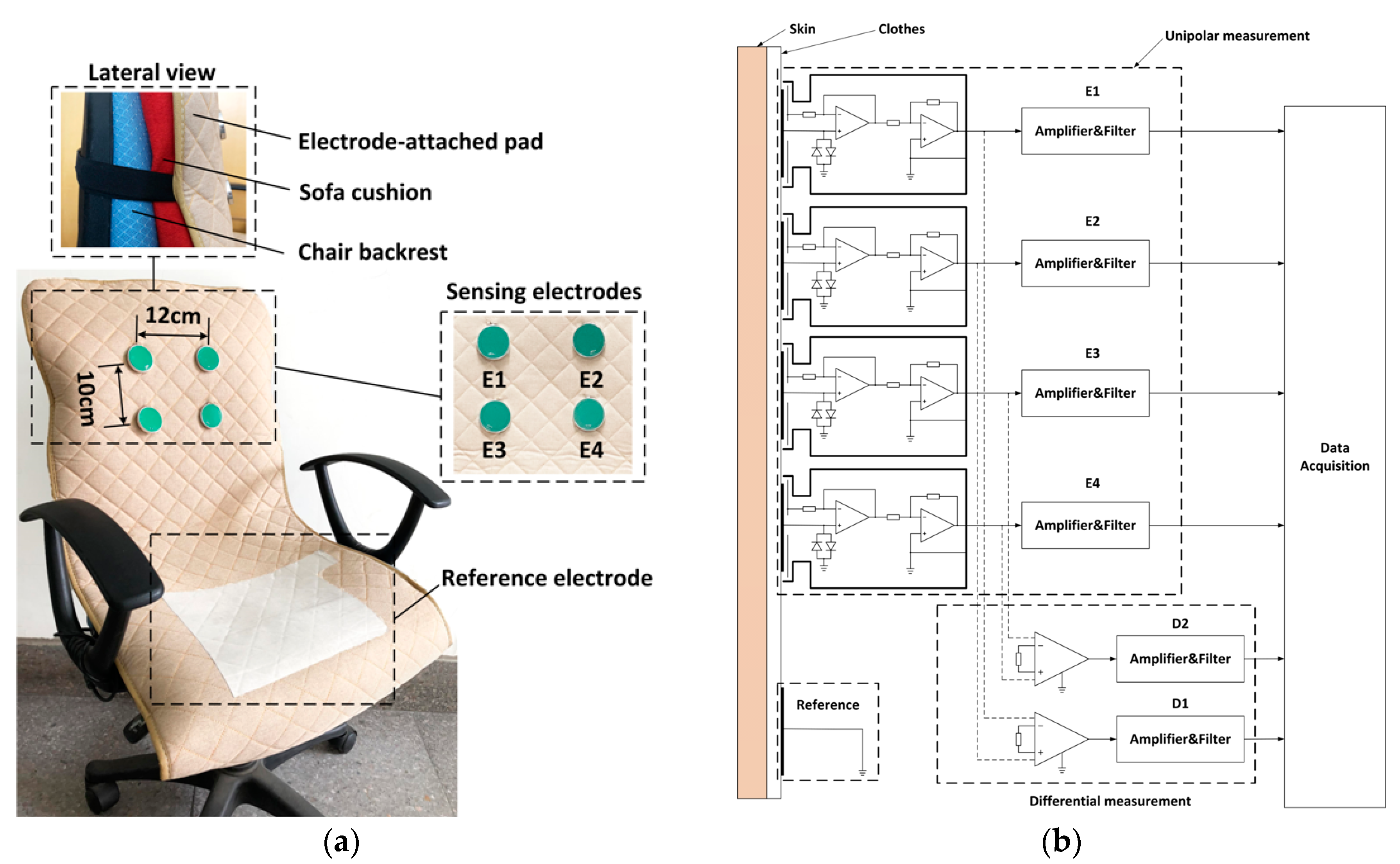
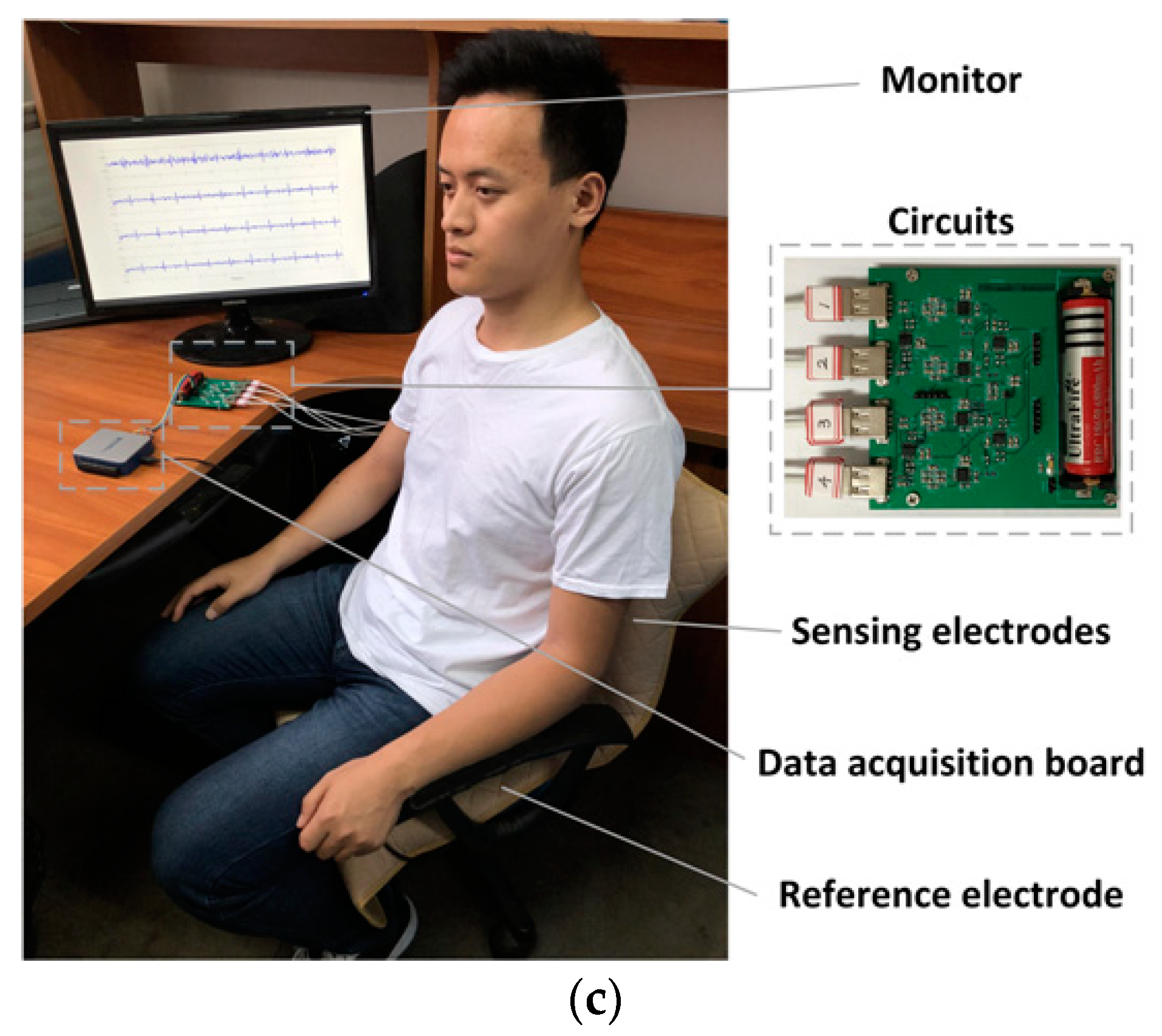
2.1. Experimental Setup
- (1)
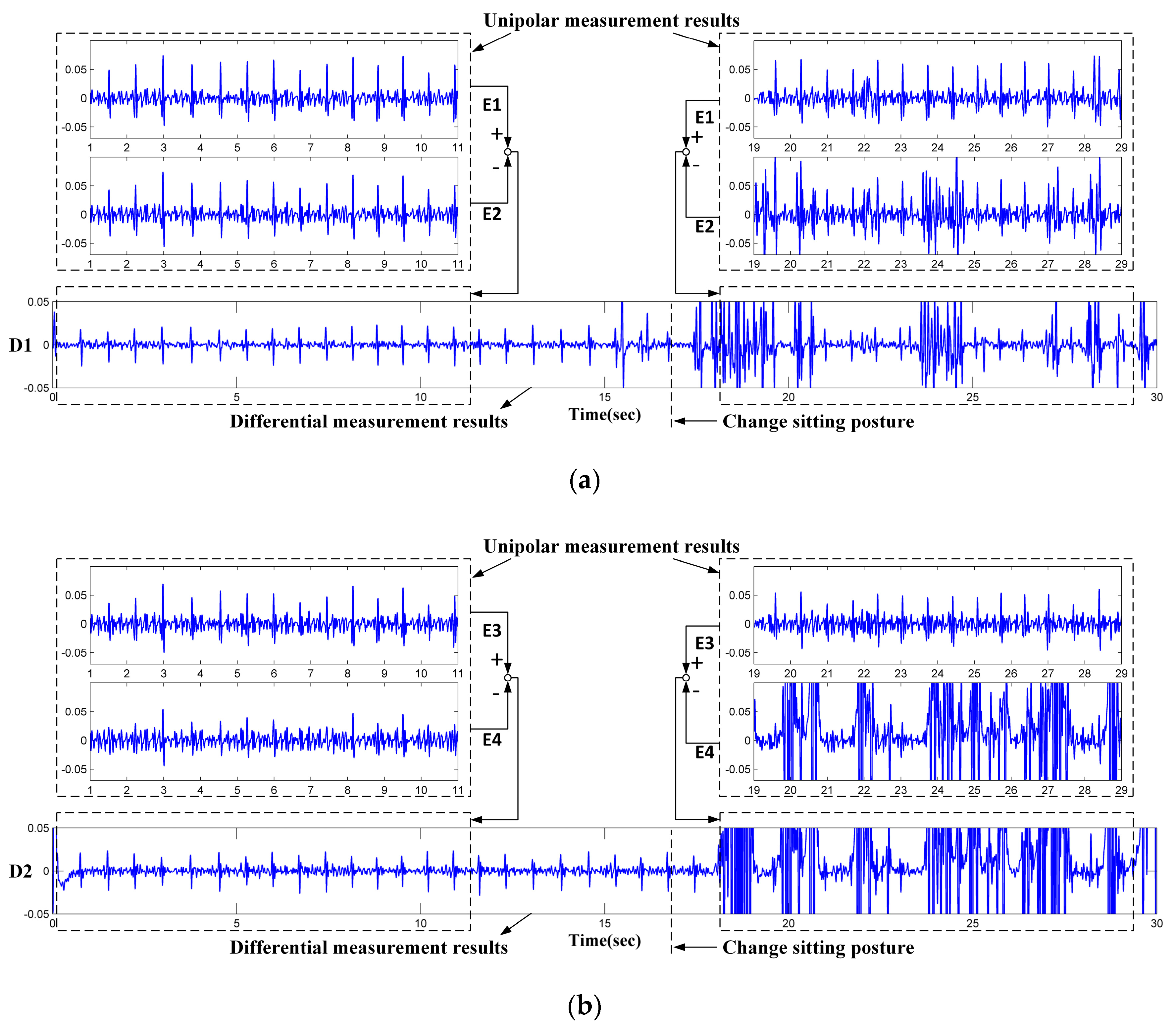
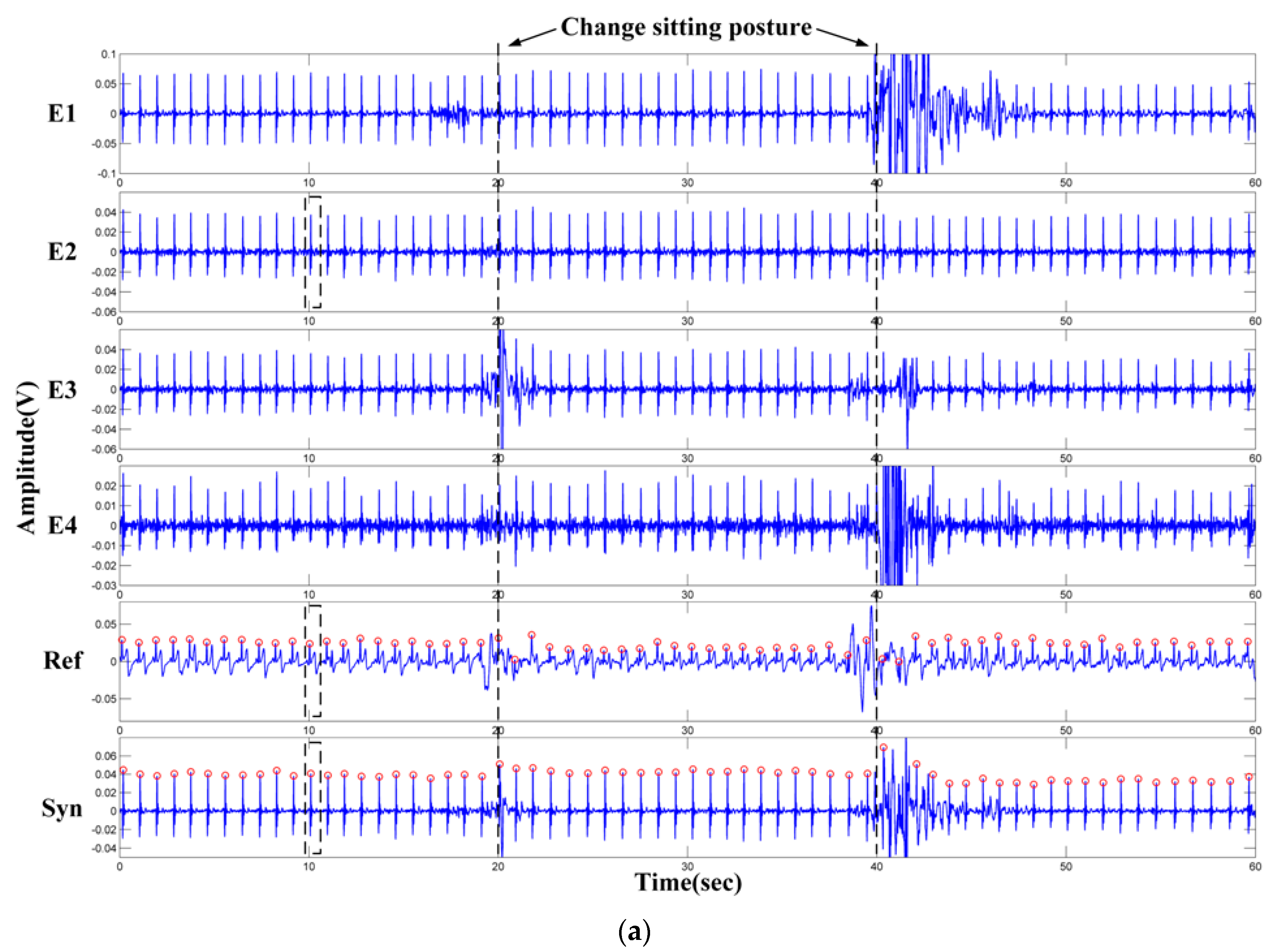
- The electrodes are fixed on the chair. During the measurement, the subjects may perform slight changes in sitting postures and do some daily work. The capacitive electrode is hard and can slip over the clothes, the output signal is therefore susceptible to artifacts because of the poor contact and charging effects [29]. In addition, since the biopotential is picked up in a capacitive-coupling way, the electrode placed on different positions will result in different ECG signal quality [30].
- (2)
- Most clothes are soft and flexible. It is easy to produce random cloth folds when subjects are sitting on the chair with their back leaning on the backrest. The uneven distribution of clothes wrinkles may cause inconsistent distances between differential measurement electrodes and the skin.
- (3)
- Because the shape of the human back is irregular, during the measurement, the electrodes at different positions are subjected to different pressures. The pressure on the electrodes affects the contact conditions and therefore the impedances between the electrodes and the skin. The quality of the measured ECG signal is then be affected [31].
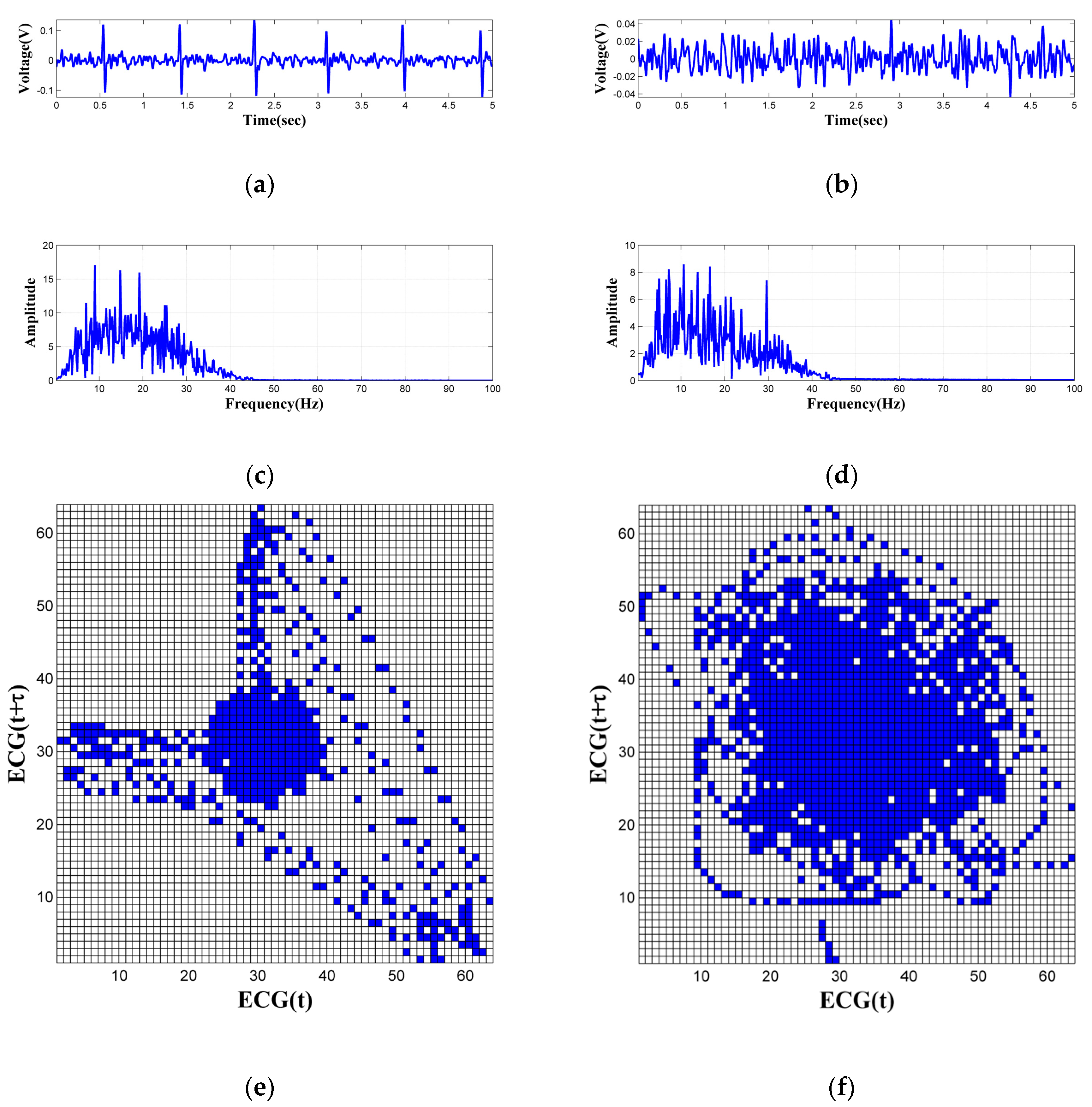
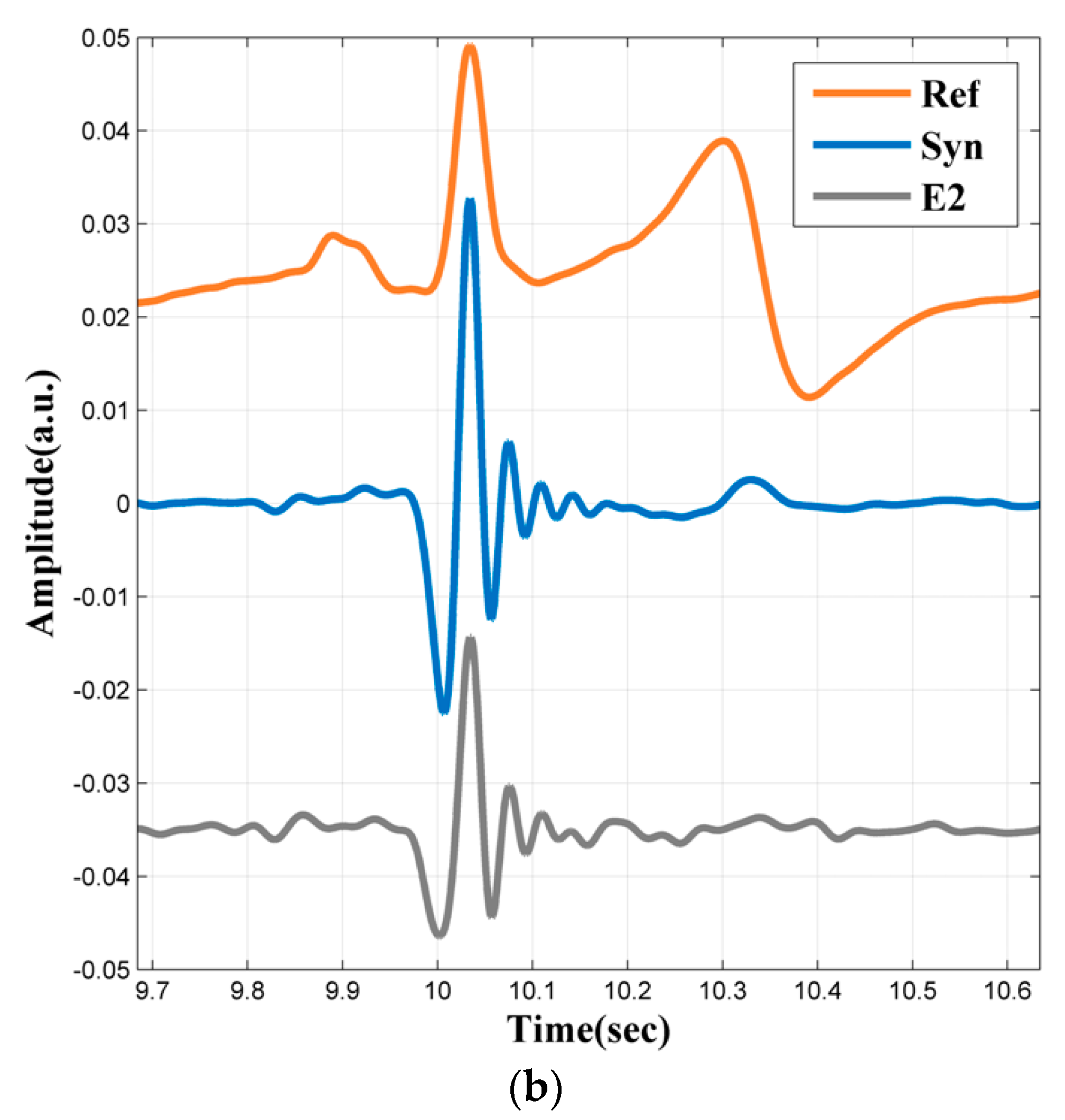
2.2. Signal Acquisition and Processing
3. Evaluation and Results
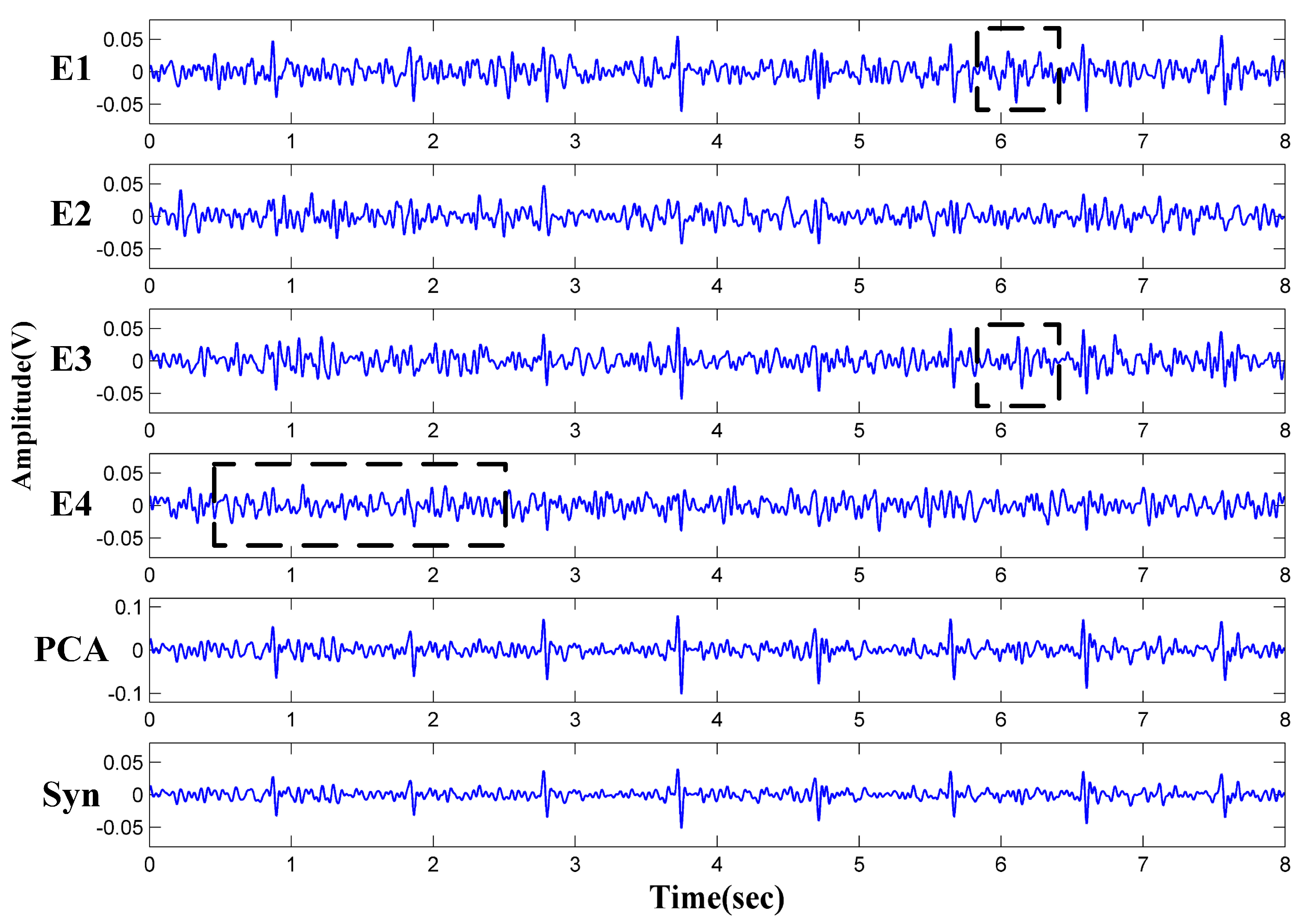
3.1. Backrest Softness and Electrode Positions
3.2. The Influence of Clothing Thickness
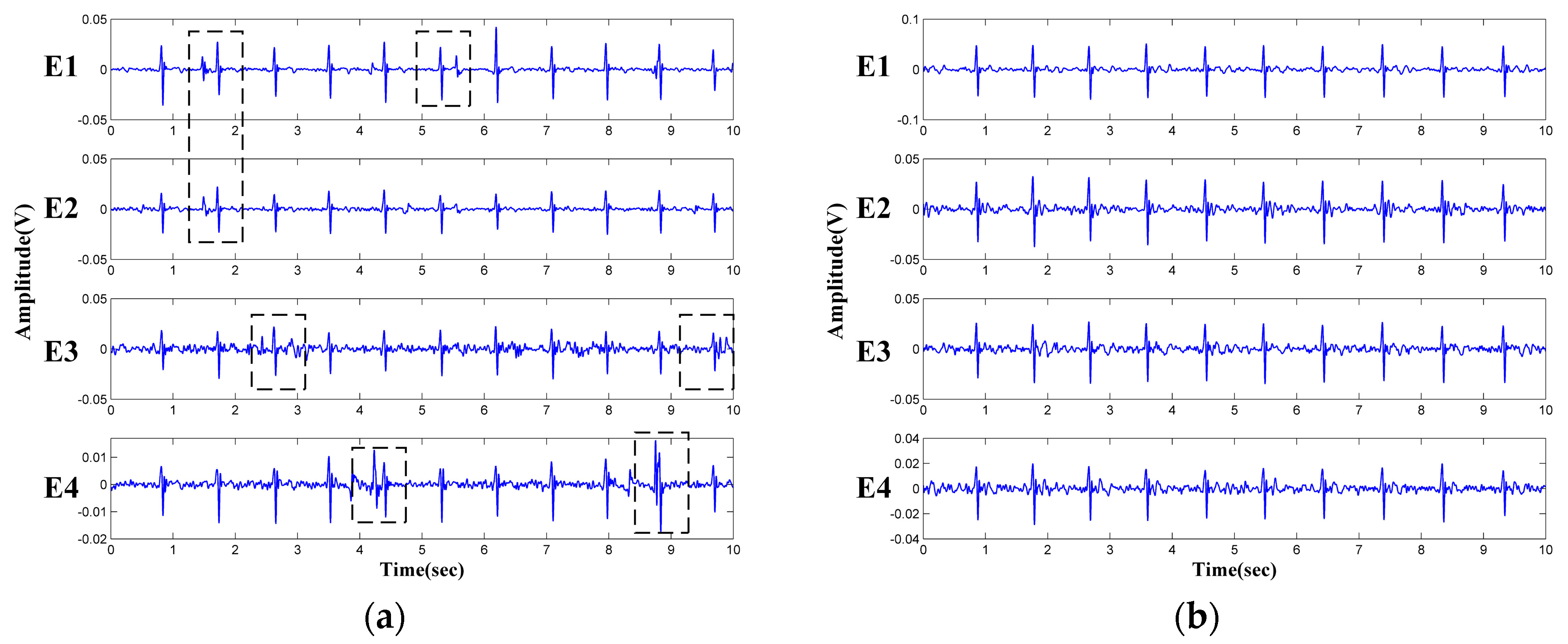
3.3. Performance Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, M.; Ma, Y.J.; Li, Y.; Wu, D.; Zhang, Y.; Youn, C.H. Wearable 2.0: Enabling human-cloud integration in next generation healthcare systems. IEEE Commun. Mag. 2017, 55, 54–61. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, W.; Park, H.; Lee, H.J.; Kim, S.K.; Kim, H.B.; Jeon, B.; Park, K.S. Automatic classification of tremor severity in Parkinson’s disease using a wearable device. Sensors 2017, 17, 2067. [Google Scholar] [CrossRef] [PubMed]
- Alshurafa, N.; Kalantarian, H.; Pourhomayoun, M.; Liu, J.J.; Sarin, S.; Shahbazi, B.; Sarrafzadeh, M. Recognition of nutrition intake using time-frequency decomposition in a wearable necklace using a piezoelectric sensor. IEEE Sens. J. 2015, 15, 3909–3916. [Google Scholar] [CrossRef]
- Bayo-Monton, J.-L.; Martinez-Millana, A.; Han, W.; Fernandez-Llatas, C.; Sun, Y.; Traver, V. Wearable Sensors Integrated with Internet of Things for Advancing eHealth Care. Sensors 2018, 18, 1851. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yu, C.Y.; Dong, Y.G. Capacitively Coupled Electrocardiogram Measuring System and Noise Reduction by Singular Spectrum Analysis. IEEE Sens. J. 2016, 16, 3802–3810. [Google Scholar] [CrossRef]
- Lee, H.J.; Hwang, S.H.; Yoon, H.N.; Lee, W.K.; Park, K.S. Heart Rate Variability Monitoring during Sleep Based on Capacitively Coupled Textile Electrodes on a Bed. Sensors 2015, 15, 11295–11311. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Heggen, H.; Wichert, R. Cap floor—A Flexible Capacitive Indoor Localization System. In Communications in Computer and Information Science; Springer: Berlin, Germany, 2012; Volume 309, pp. 26–35. [Google Scholar]
- La, H.J. A Conceptual Framework for Trajectory-Based Medical Analytics with IoT Contexts. J. Comput. Syst. Sci. 2016, 82, 610–626. [Google Scholar] [CrossRef]
- Leon-Munoz, L.M.; Martinez-Gomez, D.; Balboa-Castillo, T.; Lopez-Garcia, E.; Guallar-Castillon, P.; Rodriguez-Artalejo, F. Continued Sedentariness, Change in Sitting Time, and Mortality in Older Adults. Med. Sci. Sports Exerc. 2013, 45, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.; Saponas, T.S.; Brush, A.J.B. Health chair: Implicitly Sensing Heart and Respiratory Rate. In Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing, Seattle, WA, USA, 13–17 September 2014; pp. 661–671. [Google Scholar]
- Hesse, M.; Krause, A.F.; Vogel, L.; Chamadiya, H.; Schilling, M.; Schack, T.; Jungeblut, T. A Connected Chair as Part of a Smart Home Environment. In Proceedings of the 2017 IEEE 14th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Eindhoven, The Netherlands, 9–12 May 2017; pp. 47–50. [Google Scholar]
- Kumar, R.; Bayliff, A.; De, D.; Evans, A.; Das, S.K.; Makos, M. Care-chair: Sedentary Activities and Behavior Assessment with Smart Sensing on Chair Backrest. In Proceedings of the 2016 IEEE International Conference on Smart Computing (SMARTCOMP), St. Louis, MO, USA, 18–20 May 2016; pp. 31–38. [Google Scholar]
- Liang, T.; Yuan, Y.J. Wearable Medical Monitoring Systems Based on Wireless Networks: A Review. IEEE Sens. J. 2016, 16, 8186–8199. [Google Scholar] [CrossRef]
- Cho, D.; Ham, J.; Oh, J.; Park, J.; Kim, S.; Lee, N.-K.; Lee, B. Detection of Stress Levels from Biosignals Measured in Virtual Reality Environments Using a Kernel-Based Extreme Learning Machine. Sensors 2017, 17, 2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Fu, R. Real-Time ECG-Based Detection of Fatigue Driving Using Sample Entropy. Entropy 2018, 20, 196. [Google Scholar] [CrossRef]
- Baek, H.J.; Chung, G.S.; Kim, K.K.; Park, K.S. A Smart Health Monitoring Chair for Nonintrusive Measurement of Biological Signals. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Carek, A.M.; Mukkamala, R.; Inan, O.T.; Hahn, J.O. Ballistocardiogram as Proximal Timing Reference for Pulse Transit Time Measurement: Potential for Cuffless Blood Pressure Monitoring. IEEE Trans. Biomed. Eng. 2015, 62, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Y.; Tamura, T.; Sekine, M.; Huang, M.; Chen, W.X.; Yoshida, M.; Sakatani, K.; Kobayashi, H.; Kanaya, S. A Chair-Based Unobtrusive Cuffless Blood Pressure Monitoring System Based on Pulse Arrival Time. IEEE J. Biomed. Health 2017, 21, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Sarkar, A.; Anoop, C.S. A Health Monitoring System Using Multiple Non-contact ECG Sensors for Automotive Drivers. In Proceedings of the 2016 IEEE International Instrumentation and Measurement Technology Conference Proceedings, Taipei, Taiwan, 23–26 May 2016; pp. 1201–1206. [Google Scholar]
- Choi, M.; Jeong, J.J.; Kim, S.H.; Kim, S.W. Reduction of Motion Artifacts and Improvement of R Peak Detecting Accuracy Using Adjacent Non-Intrusive ECG Sensors. Sensors 2016, 16, 715. [Google Scholar] [CrossRef] [PubMed]
- Schommartz, A.; Eilebrecht, B.; Wartzek, T.; Walter, M.; Leonhardt, S. Advances in Modern Capacitive ECG Systems for Continuous Cardiovascular Monitoring. Acta Polytech. 2011, 51, 100–105. [Google Scholar]
- Gargiulo, G.D.; Tapson, J.; van Schaik, A.; McEwan, A.; Thiagalingam, A. Unipolar ECG Circuits: Towards More Precise Cardiac Event Identification. In Proceedings of the 2013 IEEE International Symposium on Circuits and Systems (ISCAS2013), Beijing, China, 19–23 May 2013; pp. 662–665. [Google Scholar]
- Mizumaki, K.; Fujiki, A.; Tsuneda, T.; Sakabe, M.; Nishida, K.; Sugao, M.; Inoue, H. Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada Syndrome. J. Cardiovasc. Electrophysiol. 2004, 15, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.D.; McEwan, A.L.; Bifulco, P.; Cesarelli, M.; Jin, C.; Tapson, J.; Thiagalingam, A.; van Schaik, A. Towards true unipolar bio-potential recording: A preliminary result for ECG. Physiol. Meas. 2013, 34, N1–N7. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.D.; Varaki, E.S.; Hamilton, T.J.; Bifulco, P.; Cesarelli, M.; Romano, M. A 9-independent-leads ECG system from 10 electrodes. In Proceedings of the 2015 IEEE Biomedical Circuits and Systems Conference (BioCAS), Atlanta, GA, USA, 22–24 October 2015; pp. 89–92. [Google Scholar]
- Gargiulo, G.D. True unipolar ECG machine for Wilson Central Terminal measurements. BioMed Res. Int. 2015, 2015, 586397. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.E.; Albert, M.H.; Sallee, B.N. Recording unipolar ECG leads using one limb of the Wilson Central Terminal. J. Electrocardiol. 2017, 50, 857. [Google Scholar] [CrossRef]
- Aleksandrowicz, A.; Leonhardt, S. Wireless and Non-contact ECG Measurement System—The “Aachen SmartChair”. In The Oxford Handbook of Modernisms; University of Oxford: Oxford, UK, 2007; pp. 1343–1350. [Google Scholar]
- Meziane, N.; Webster, J.; Attari, M.; Nimunkar, A. Dry electrodes for electrocardiography. Physiol. Meas. 2013, 34, R47–R69. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Eilebrecht, B.; Wartzek, T.; Leonhardt, S. The Smart Car Seat: Personalized Monitoring of Vital Signs in Automotive Applications. Pers. Ubiquitous Comput. 2011, 15, 707–715. [Google Scholar] [CrossRef]
- Meziane, N.; Yang, S.; Shokoueinejad, M.; Webster, J.; Attari, M.; Eren, H. Simultaneous comparison of 1 gel with 4 dry electrode types for electrocardiography. Physiol. Meas. 2015, 36, 513. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Rajagopalan, C.; Clifford, G.D. A Machine Learning Approach to Multi-Level ECG Signal Quality Classification. Comput. Methods Programs Biomed. 2014, 117, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Jekova, I.; Krasteva, V.; Leber, R.; Schmid, R.; Twerenbold, R.; Reichlin, T.; Muller, C.; Abacherli, R. A Real-Time Quality Monitoring System for Optimal Recording of 12-Lead Resting ECG. Biomed. Signal Process. 2017, 34, 126–133. [Google Scholar] [CrossRef]
- Acharya, U.R.; Suri, J.S.; Spaan, J.A.E.; Krishnan, S.M. Advances in Cardiac Signal Processing; Springer: Berlin/Heidelberg, Germany, 2007; pp. 407–422. [Google Scholar]
- Takens, F. Detecting Strange Attractors in Turbulence; Springer: Berlin/Heidelberg, Germany, 1981; pp. 366–381. [Google Scholar]
- Hou, Z.J.; Dong, Y.G.; Xiang, J.X.; Li, X.W.; Yang, B. A Real-Time QRS Detection Method Based on Phase Portraits and Box-Scoring Calculation. IEEE Sens. J. 2018, 18, 3694–3702. [Google Scholar] [CrossRef]
- Moody, G.A.; Mark, R.G. The impact of the MIT-BIH arrhythmia database. IEEE Eng. Med. Biol. 2001, 20, 45–50. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intell. Lab. 1987, 2, 37–52. [Google Scholar] [CrossRef]


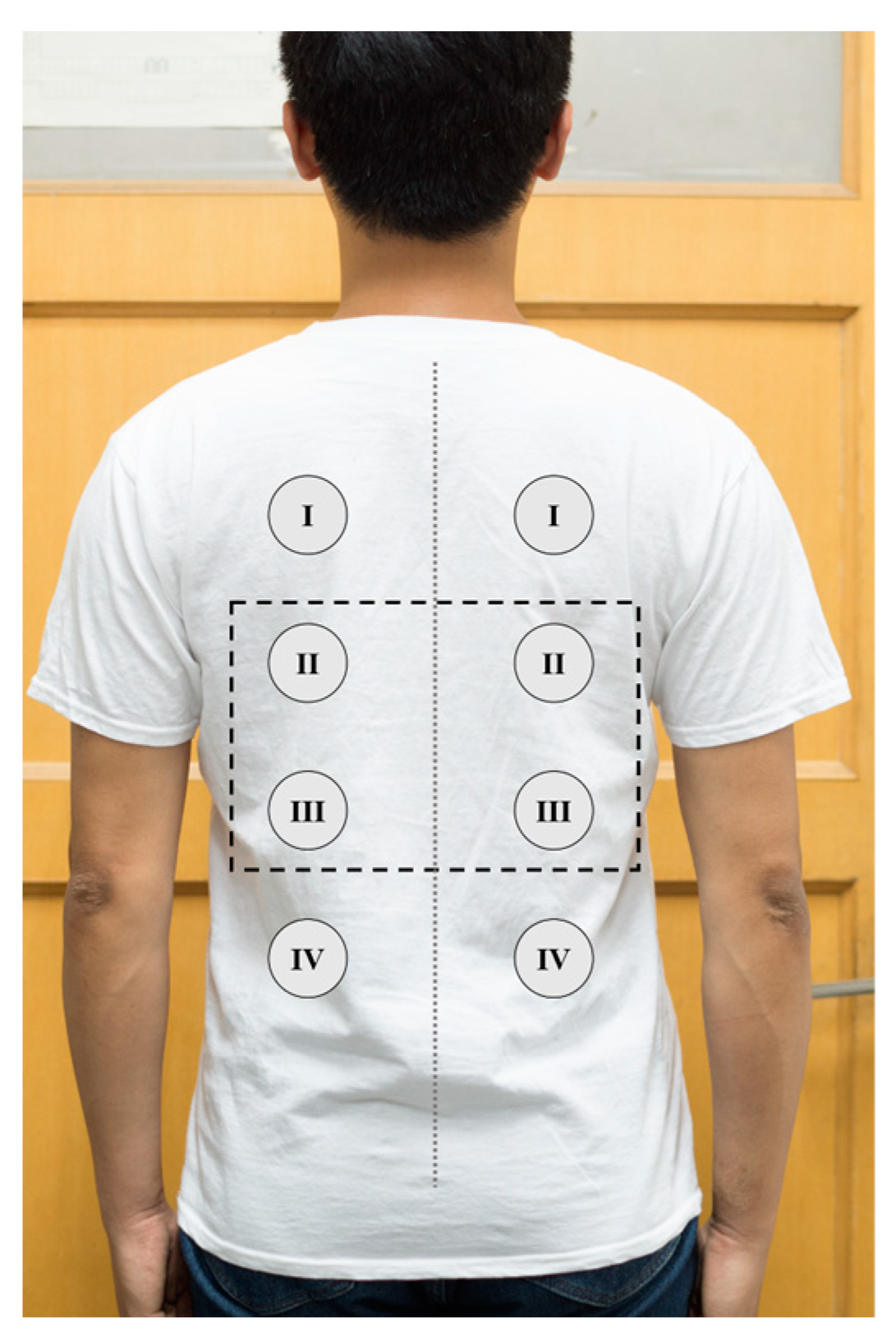

| Left Electrode Position | QRS Peak Amplitude (Mean ± SD mV) | Right Electrode Position | QRS Peak Amplitude (Mean ± SD mV) |
|---|---|---|---|
| I | 71.58 ± 5.38 | I | 80.63 ± 4.20 |
| II | 68.93 ± 4.51 | II | 74.97 ± 5.22 |
| III | 34.04 ± 3.93 | III | 37.55 ± 4.71 |
| IV | 24.35 ± 3.23 | IV | 21.54 ± 3.97 |
| Sub. (BMI [kg/m2], Cloth Thickness [mm]) | TB | TP | FN | FP | Mean HR ± SD (bpm) | Se (%) | P (%) |
|---|---|---|---|---|---|---|---|
| No. 1 (23.60, 0.30) | 198 | 184 | 4 | 3 | 66.0 ± 1.0 | 97.87 | 98.40 |
| No. 2 (25.34, 0.35) | 222 | 219 | 3 | 0 | 74.0 ± 3.0 | 98.65 | 100.00 |
| No. 3 (24.82, 0.30) | 196 | 194 | 2 | 3 | 65.3 ± 2.3 | 98.98 | 98.48 |
| No. 4 (24.80, 0.26) | 214 | 213 | 1 | 0 | 71.33 ± 1.2 | 99.53 | 100.00 |
| No. 5 (25.63, 0.55) | 217 | 215 | 1 | 2 | 72.3 ± 0.6 | 99.54 | 99.08 |
| No. 6 (19.38, 0.40) | 224 | 224 | 0 | 1 | 74.7 ± 2.5 | 100.00 | 99.56 |
| Total | 1271 | 1249 | 11 | 9 | - | 99.13 | 99.28 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Xiang, J.; Dong, Y.; Xue, X.; Xiong, H.; Yang, B. Capturing Electrocardiogram Signals from Chairs by Multiple Capacitively Coupled Unipolar Electrodes. Sensors 2018, 18, 2835. https://doi.org/10.3390/s18092835
Hou Z, Xiang J, Dong Y, Xue X, Xiong H, Yang B. Capturing Electrocardiogram Signals from Chairs by Multiple Capacitively Coupled Unipolar Electrodes. Sensors. 2018; 18(9):2835. https://doi.org/10.3390/s18092835
Chicago/Turabian StyleHou, Zhongjie, Jinxi Xiang, Yonggui Dong, Xiaohui Xue, Hao Xiong, and Bin Yang. 2018. "Capturing Electrocardiogram Signals from Chairs by Multiple Capacitively Coupled Unipolar Electrodes" Sensors 18, no. 9: 2835. https://doi.org/10.3390/s18092835
APA StyleHou, Z., Xiang, J., Dong, Y., Xue, X., Xiong, H., & Yang, B. (2018). Capturing Electrocardiogram Signals from Chairs by Multiple Capacitively Coupled Unipolar Electrodes. Sensors, 18(9), 2835. https://doi.org/10.3390/s18092835





