Gold Nanocage-Based Electrochemical Sensing Platform for Sensitive Detection of Luteolin
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Apparatus
2.3. Fabrication of AuNC/CILE
3. Results and Discussion
3.1. Material Characterization
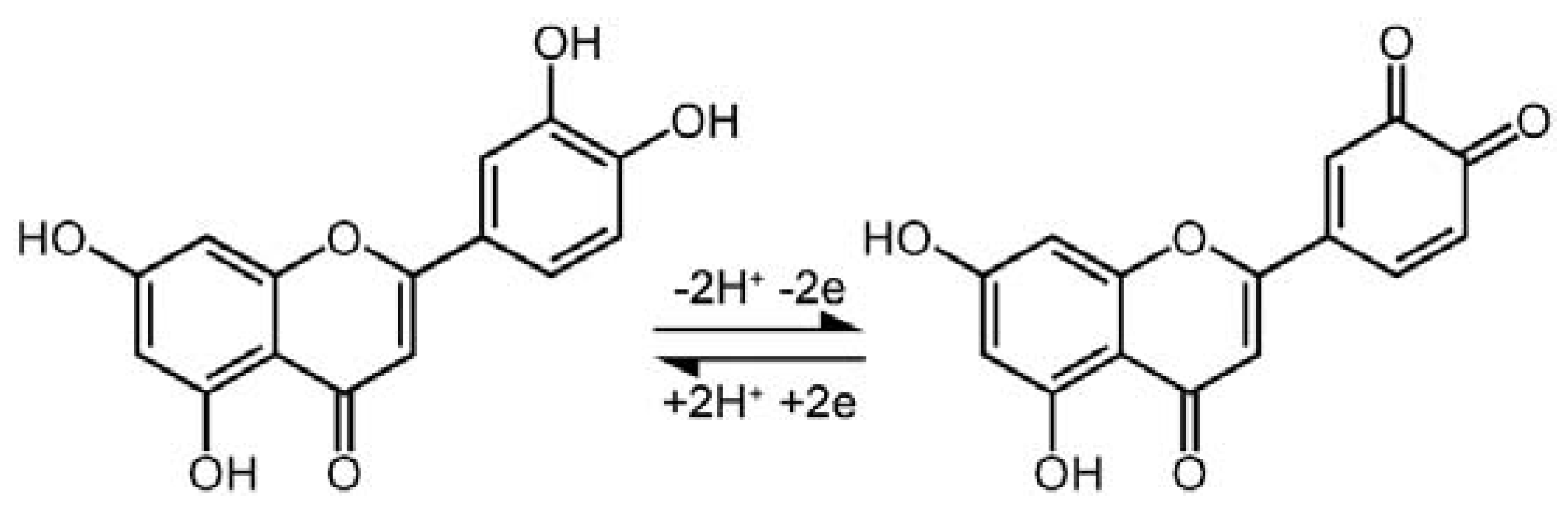
3.2. Direct Electrochemical Behavior of Luteolinon the Modified Electrode
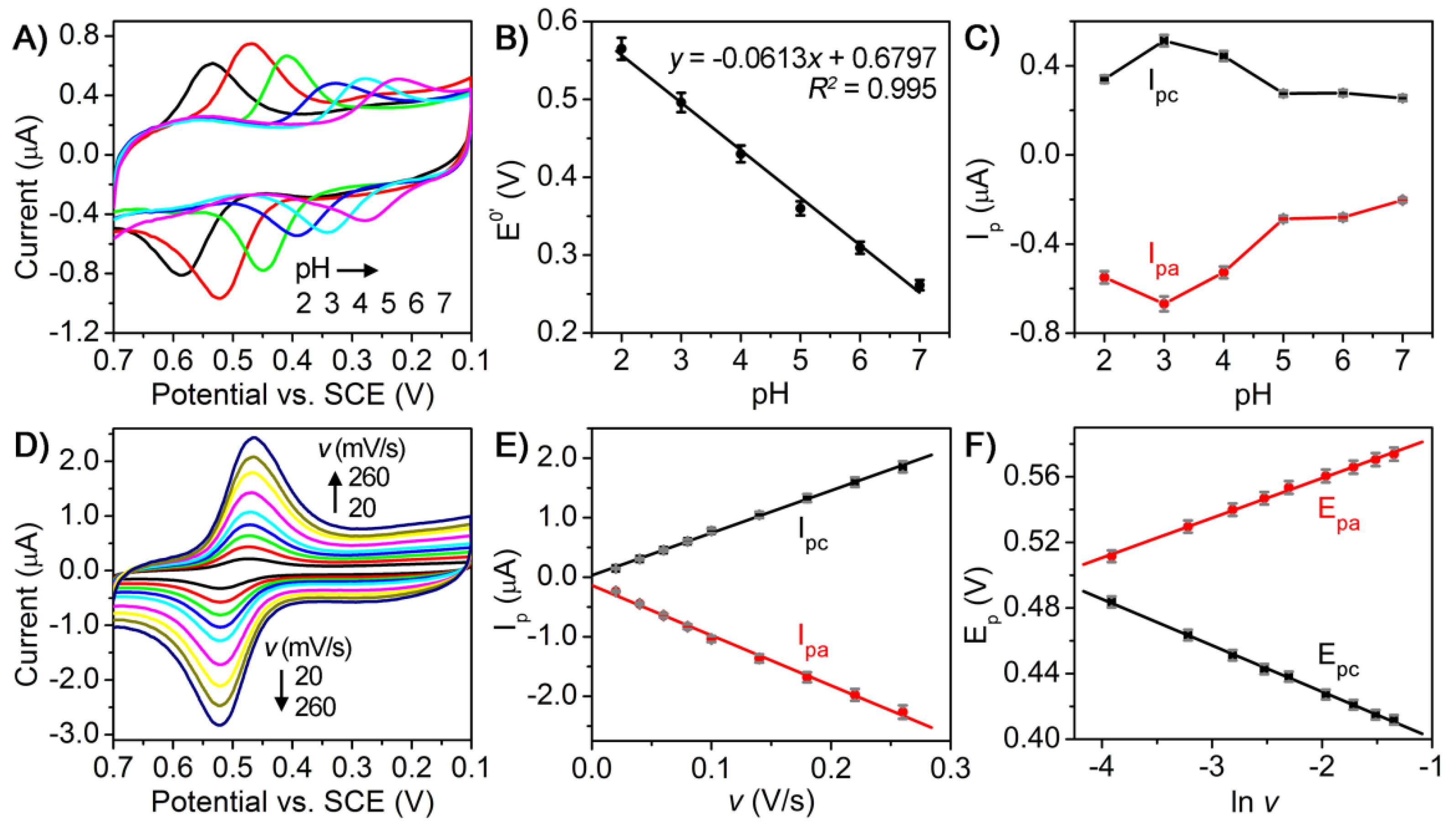
3.3. Electrochemical Investigations
3.4. Analytical Performance
3.5. Monitoring of Real Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GCE | glassy carbon electrode |
| GNs | Graphenenano sheets |
| PDDA-RGO | Poly (diallyldimethylammonium chloride) functionalized reduced graphene oxide |
| MWNTs | multi-walled carbon nanotubes |
| MPC | macroporous carbon |
| HA | hydroxyapatite |
| Nbim | nitro-substituted3,3′-bis (indolyl) methane |
| PBS | phosphate buffer solutions |
| CNT | carbon nanotube |
| HPPF6 | 1-butylpyridinium hexafluorophosphate |
References
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- Neuhouser, M.L. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, A.C.; Vieira, I.C.; Dupont, J.; Scheeren, C.W.; de Oliveira, L.F. Biosensor for luteolin based on silver or gold nanoparticles in ionic liquid and laccase immobilized in chitosan modified with cyanuric chloride. Analyst 2009, 134, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, S.; Huang, L.; Cao, Y.; Yao, H.; Chen, W.; Lin, X. Electrochemical oxidation of luteolin at a glassy carbon electrode and its application in pharmaceutical analysis. Chem. Pharm. Bull. 2008, 56, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Wang, A. Poly (diallyldimethylammonium chloride) functionalized reduced graphene oxide based electrochemical sensing platform for luteolin determination. Int. J. Electrochem. Sci. 2015, 10, 3518–3529. [Google Scholar]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; He, X.S.; Liu, C. Effect of luteolin on blood lipid level and antioxidant capability in hyperlipidemic rats. Food Sci. 2009, 30, 348–350. [Google Scholar]
- Cheng, W.; Li, J.; You, T.; Hu, C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linné. J. Ethnopharmacol. 2005, 101, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Uehara, M.; Yanagitani, H.; Hashimoto, T. Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Arch. Biochem. Biophys. 2006, 455, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Robards, K.; Antolovich, M. Analytical chemistry of fruit bioflavonoids: A review. Analyst 1997, 122, 11R–34R. [Google Scholar] [CrossRef]
- Merken, H.M.; Beecher, G.R. Measurement of Food Flavonoids by high-Performance liquid chromatography: A review. J. Agric. Food Chem. 2000, 48, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Song, Y.S.; Zhang, K.J.; Ryu, J.C.; Kim, M.; Zhou, T.H. Gas chromatographic/mass spectrometric profiling of luteolin and its metabolites in rat urine and bile. J. Pharm. Biomed. Anal. 1995, 13, 1409–1414. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Huang, K.J. Determination of flavonoids by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A. 2004, 1032, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, I.; Rarog, D. Application of derivative spectrophotometry to determination of flavonoid mixtures. Talanta 2001, 55, 209–212. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Feng, L.; Qi, Q.; Wang, S. Sensitive electrochemical determination of luteolin in peanut hulls using multi-walled carbon nanotubes modified electrode. Food Chem. 2011, 127, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, Y.; Wang, H.; Guo, L. Electrochemical behavior of luteolin and its detection based on macroporous carbon modified glassy carbon electrode. Anal. Methods 2013, 5, 3365–3370. [Google Scholar] [CrossRef]
- Pang, P.; Liu, Y.; Zhang, Y.; Gao, Y.; Hu, Q. Electrochemical determination of luteolin in peanut hulls using graphene and hydroxyapatite nanocomposite modified electrode. Sens. Actuators B 2014, 194, 397–403. [Google Scholar] [CrossRef]
- Ibrahim, H.; Temerk, Y. Novel sensor for sensitive electrochemical determination of luteolin based on In2O3 nanoparticles modified glassy carbon paste electrode. Sens. Actuators B 2015, 206, 744–752. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, N.; Ni, Y.; Xu, J.; Shao, S. Electrochemical sensor based on Nbim/CNT composite for selective determination of luteolin in the flavonoids. J. Electroanal. Chem. 2015, 754, 94–99. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold nanocages: From synthesis to theranostic applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, Q.; Chen, J.; Xia, Y. A Comparison Study of the Catalytic Properties of Au-Based Nanocages, Nanoboxes, and Nanoparticles. Nano Lett. 2010, 10, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, J.B. Comparative investigation on electrochemical behavior of hydroquinone at carbon ionic liquid electrode, ionic liquid modified carbon paste electrode and carbon paste electrode. Electrochim. Acta 2007, 52, 7210–7216. [Google Scholar] [CrossRef]
- Sun, W.; Li, Y.; Yang, M.; Liu, S.; Jiao, K. Direct electrochemistry of single-stranded DNA on an ionic liquid modified carbon paste electrode. Electrochem. Commun. 2008, 10, 298–301. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Tillekeratne, L.M.V.; Kirchhoff, J.R.; Hudson, R.A. High performance liquid chromatographic separation and pH-dependent electrochemical properties of pyrroloquinoline quinone and three closely related isomeric analogues. Biochem. Biophys. Res. Commun. 1995, 22, 41–47. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Wu, S.H.; Zhu, B.J.; Huang, Z.X.; Sun, J.J. A heated pencil lead disk electrode with direct current and its preliminary application for highly sensitive detection of luteolin. Electrochem. Commun. 2013, 28, 47–50. [Google Scholar] [CrossRef]




| Electrodes | Linear Range (nmol/L) | LOD (nmol/L) | Refs |
|---|---|---|---|
| GCE | 10~1.0 × 103 | 5 | [4] |
| PDDA-RGO-modified GCE | 1~1.0 × 104 | 1 | [5] |
| MWNTs-modified GCE | 0.2~3 | 0.06 | [16] |
| MPC-modified GCE | 3.0 × 102~3.0 × 104 | 1.3 | [17] |
| GNs-HA-modified GCE | 20~1.0 × 104 | 10 | [18] |
| In2O3-nanoparticle-modified GCE | 9.98~88.4 | 0.199 | [19] |
| Nbim/CNT-modified GCE | 5~320 | 0.6 | [20] |
| heated pencil lead disk electrode | 4~1.0 × 104 | 1 | [31] |
| AuNCs/CILE | 1~1.0 × 103 | 0.4 | This work |
| Interfering Substances | Concentration (µM) | Relative Deviation (%) |
|---|---|---|
| Na+ | 50 | 0.74 |
| K+ | 50 | 3.32 |
| Ba2+ | 50 | −2.31 |
| Cd2+ | 50 | 4.90 |
| Mn2+ | 50 | 3.79 |
| Co2+ | 50 | 3.55 |
| Lysine | 50 | 4.39 |
| Threonine | 50 | 2.30 |
| Glycine | 50 | 0.98 |
| Alanine | 50 | 3.18 |
| Leucine | 50 | 0.56 |
| Baicalein | 50 | 2.97% |
| Quercetin | 50 | 4.24% |
| Sample No. | Spiked (µM) | Found (µM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| 1 | 0 | 0.99 | 1.44 | No application |
| 2 | 0.2 | 1.18 | 1.32 | 95.0 |
| 3 | 0.4 | 1.37 | 1.97 | 95.0 |
| 4 | 0.6 | 1.57 | 1.62 | 96.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zou, R.; Niu, Y.; Sun, W.; Shao, T.; Chen, X. Gold Nanocage-Based Electrochemical Sensing Platform for Sensitive Detection of Luteolin. Sensors 2018, 18, 2309. https://doi.org/10.3390/s18072309
Li X, Zou R, Niu Y, Sun W, Shao T, Chen X. Gold Nanocage-Based Electrochemical Sensing Platform for Sensitive Detection of Luteolin. Sensors. 2018; 18(7):2309. https://doi.org/10.3390/s18072309
Chicago/Turabian StyleLi, Xiaobao, Ruyi Zou, Yanyan Niu, Wei Sun, Taiming Shao, and Xiaoqin Chen. 2018. "Gold Nanocage-Based Electrochemical Sensing Platform for Sensitive Detection of Luteolin" Sensors 18, no. 7: 2309. https://doi.org/10.3390/s18072309
APA StyleLi, X., Zou, R., Niu, Y., Sun, W., Shao, T., & Chen, X. (2018). Gold Nanocage-Based Electrochemical Sensing Platform for Sensitive Detection of Luteolin. Sensors, 18(7), 2309. https://doi.org/10.3390/s18072309





