V2O5 Thin Films as Nitrogen Dioxide Sensors †
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Film Preparation
2.2. Morphology and Structural Characterization
2.3. Sensing Characterization
3. Results and Discussion
3.1. Structural and Microstructural Characteristics
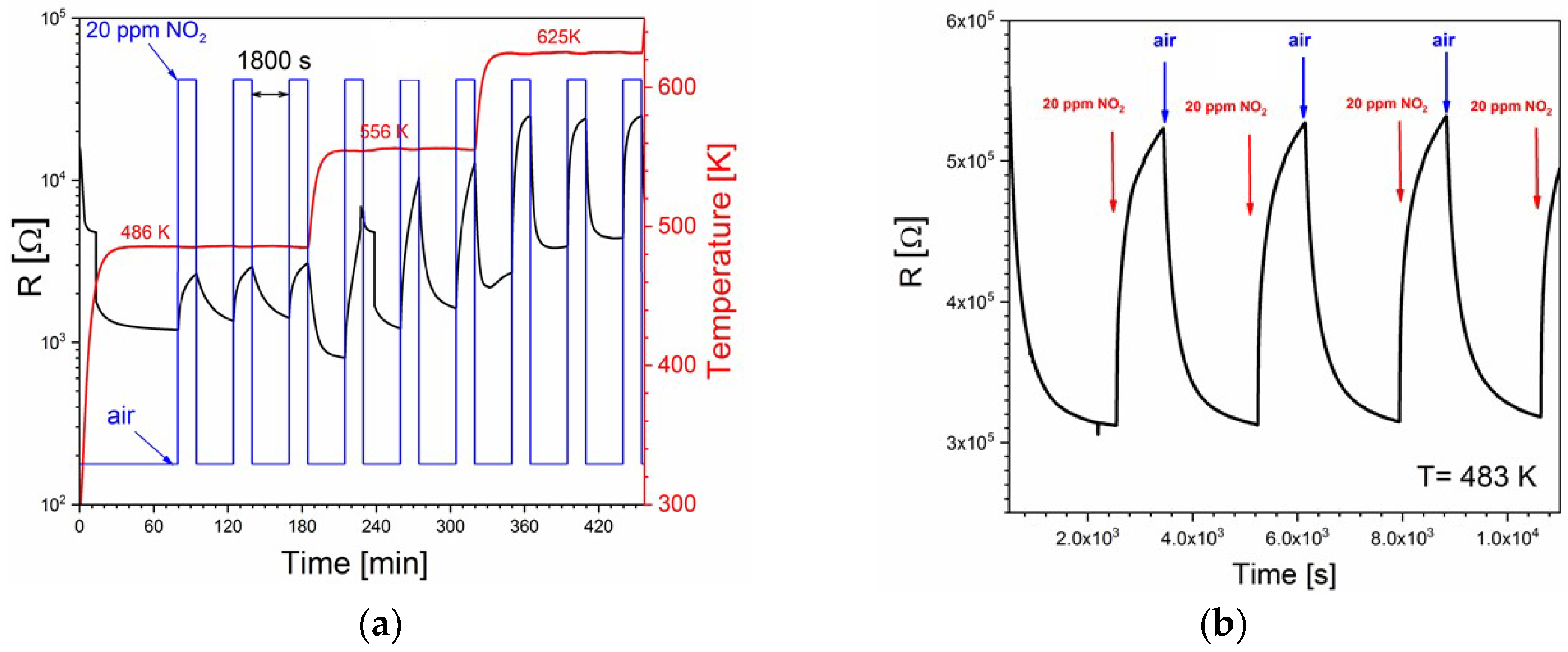
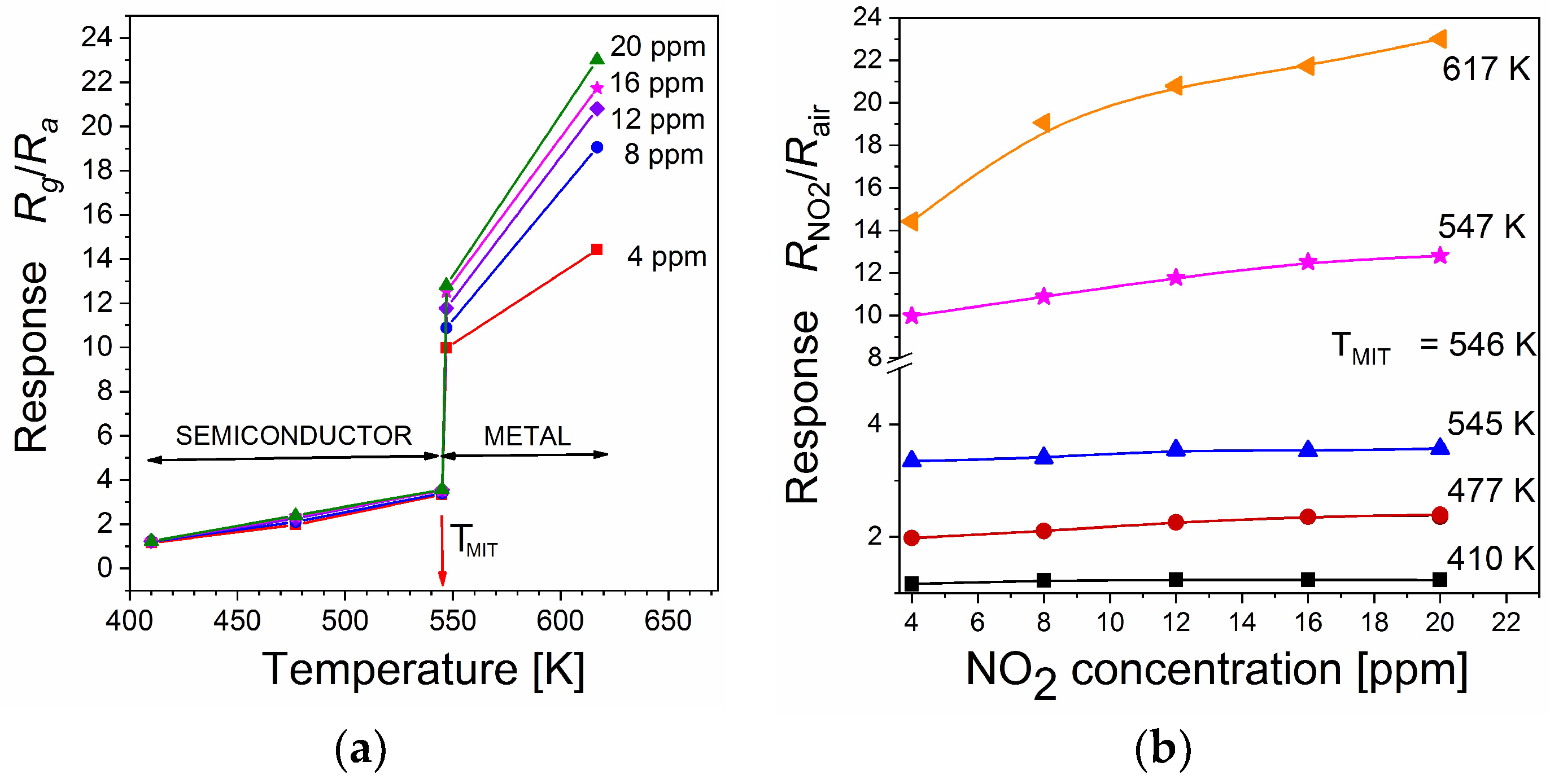
3.2. Sensing Characteristics
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xu, K.; Fu, C.; Gao, Z.; Wei, F.; Yiang, Y.; Xu, C. Nanomaterials-based gas sensor: A review. J. Instr. Sci. Technol. 2018, 46, 115–145. [Google Scholar] [CrossRef]
- Wilson, L.G.; Everett, L.G.; Cullen, S.J. Handbook of Vadose Zone Characterization Monitoring; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Janata, J. Potentiometric microsensors. Chem. Rev. 1990, 5, 691–703. [Google Scholar] [CrossRef]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructured, graphene and 2D metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jiang, C.; Wei, S. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 2, 021304. [Google Scholar] [CrossRef]
- Bishnoi, A.; Kumar, S.; Joshi, N. Wide-Angle X-ray Diffraction (XRD): Technique for Characterization of Nanomaterials and Polymer Nanocomposites. In Microscopy Methods in Nanomaterials Characterization; Sabu, T., Raju, T., Ajesh, Z., Raghvendra, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 313–337. [Google Scholar]
- Szklarski, Z.; Zakrzewska, K.; Rekas, M. Tin oxide films as gas sensors. Thin Solid Films 1989, 174, 269–275. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Teterycz, H.; Kita, J.; Bauer, R.; Golonka, L.J.; Licznerski, B.W.; Nitsch, K.; Wisniewski, K. New design of SnO2 gas sensor on low temperature cofiring ceramics. Sens. Actuators B 1998, 47, 100–103. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Liu, D.; Han, E. Hydrogen sensing and mechanism of doped SnO2 (M = Cr3+, Cu2+ and Pd2+) nanocomposite. Sens. Actuators B 2011, 160, 455–462. [Google Scholar] [CrossRef]
- Gyu, C.N.; Jin, Y.D.; Mi-Jin, J.; Ho-Gi, K.; Tuller, H.L.; Il-Doo, K. Highly sensitive SnO2 hollow nanofiber-based NO2 gas sensor. Sens. Actuators B 2011, 160, 1468–1470. [Google Scholar]
- Jeun, J.H.; Kim, D.H.; Hong, S.H. Synthesis of porous SnO2 foams on SiO2/Si substrate by electrochemical deposition and their gas sensing properties. Sens. Actuators B 2012, 161, 784–790. [Google Scholar] [CrossRef]
- Liu, Y.; Hang, T.; Xie, Y.; Bao, Z.; Song, J.; Zhang, H.; Xie, E. Effect of Mg doping on the hydrogen-sensing characteristics of ZnO thin films. Sens. Actuators B 2011, 160, 266–270. [Google Scholar] [CrossRef]
- Hassan, K.; Chung, G. Catalytically activated quantum size Pt/Pd bimetallic core-shell nanoparticles decorated on ZnO nanorod clusters for accelerated hydrogen gas detection. Sens. Actuators B 2017, 239, 824–833. [Google Scholar] [CrossRef]
- Perfecto, T.M.; Zito, C.A.; Volanti, D.P. Design of nanostructured WO3·0.33H2O via combination of ultrasonic spray nozzle and microwave-assisted hydrothermal methods for enhancing isopropanol gas sensing at room temperature. Cryst. Eng. Comm. 2017, 19, 2733–2738. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Radecka, M.; Rekas, M. Effect of Nb, Cr, Sn additions on gas sensing properties of TiO2 thin films. Thin Solid Films 1997, 310, 161–166. [Google Scholar] [CrossRef]
- Li, Y.; Wlodarski, W.; Galatsis, K.; Moslih, S.H.; Cole, J.; Russo, S.; Rockelman, N. Gas sensing properties of p-type semiconducting Cr-doped TiO2 thin films. Sens. Actuators B 2002, 83, 160–163. [Google Scholar] [CrossRef]
- Lyson-Sypien, B.; Czapla, A.; Lubecka, M.; Gwizdz, P.; Schneider, K.; Zakrzewska, K.; Michalow, K.; Graule, T.; Reszka, A.; Rekas, M.; et al. Nanopowders of chromium doped TiO2 for gas sensors. Sens. Actuators B 2012, 175, 163–172. [Google Scholar] [CrossRef]
- Flak, D.; Braun, A.; Michalow, K.A.; Parlinska-Wojtan, J.W.M.; Graule, T.; Rekas, M. Differences in electrophysical and gas sensing properties of flame spray synthesized Fe2O3 (γ-Fe2O3 and α-Fe2O3). J. Nanosci. Nanotechnol. 2012, 12, 6401–6411. [Google Scholar] [CrossRef] [PubMed]
- Beke, S. A review of the growth of V2O5 films from 1885 to 2010. Thin Solid Films 2011, 519, 1761–1771. [Google Scholar] [CrossRef]
- Han, S.D.; Moon, H.G.; Noh, M.S.; Pyeon, J.J.; Shim, Y.S.; Nahm, S.; Kim, J.S.; Yoo, K.S.; Kang, C.Y. Self-doped nanocolumnar vanadium oxides thin films for highly selective NO2 gas sensing at low temperatures. Sens. Actuators B 2017, 241, 40–47. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Peng, Q.; Li, Y. Vanadium pentoxide nanobelts: Highly selective and stable ethanol sensor material. Adv. Mater. 2005, 17, 764–767. [Google Scholar] [CrossRef]
- Huotari, J.; Bjorklund, R.; Lappalainen, J.; Spetz, L.A. Nanostructured mixed phase vanadium oxide thin films as highly sensitive ammonia sensing material. Procedia Eng. 2014, 87, 1035–1038. [Google Scholar] [CrossRef]
- Schneider, K.; Lubecka, M.; Czapla, A. V2O5 thin films for gas sensor applications. Sens. Actuators B 2016, 206, 970–977. [Google Scholar] [CrossRef]
- Raj, D.; Pazhanieval, T.; Kumar, P.S.; Mangalareaj, D.; Nataraj, D.; Ponpandian, N. Self-assembler V2O5 nanorods for gas sensors. Current Appl. Phys. 2010, 10, 531–537. [Google Scholar]
- Rizzo, G.; Arena, A.; Bonavita, A.; Donato, N.; Neri, G.; Saitt, G. Gasochromic response of nanocrystalline vanadium pentoxide films deposited from ethanol dispersions. Thin Solid Films 2010, 518, 7124–7127. [Google Scholar] [CrossRef]
- Raj, A.D.; Mangalaraj, D.; Ponpandian, N.; Yi, J. Gas sensing properties of chemically synthesized V2O5 thin films. Adv. Mater. Res. 2010, 123, 683–686. [Google Scholar]
- Li, S.C.; Hwang, B.W.; Lee, S.J.; Choi, H.Y.; Kim, S.Y.; Jung, S.Y. A novel tin oxide-based recoverable thick film SO2 gas sensor promoted with magnesium and vanadium oxides. Sens. Actuators B 2011, 160, 1328–1334. [Google Scholar]
- Simo, A.; Kasviyarasu, K.; Mwakilunga, B.; Mokwena, M.; Maaza, M. Room temperature volatile organic compound gas sensor based on vanadium oxide 1-dimension nanoparticles. Ceram. Int. 2017, 43, 1347–1363. [Google Scholar] [CrossRef]
- Izu, N.; Hagen, G.; Schönauer, D.; Röder-Roith, U.; Moos, R. Application of V2O5/WO3/TiO2 for resistive-type SO2 sensors. Sensors 2011, 11, 2982–2991. [Google Scholar]
- Khatibani, A.B.; Abbasi, M.; Rozati, S.M. Pecularities of deposition times on gas sensing behaviour of vanadium oxide thin films. Acta Phys. Pol. A 2016, 129, 1245–1251. [Google Scholar] [CrossRef]
- Vijayakumar, Y.; Mani, G.K.; Reddy, M.R.; Rayappan, J.B.B. Nanostructured flower like V2O5 thin films and its room temperature sensing characteristics. Ceram. Int. 2015, 41, 2221–2227. [Google Scholar] [CrossRef]
- Raible, I.; Burghard, M.; Schlecht, U.; Yasuda, A.; Vossmeyer, T. V2O5 nanofibres: Novel gas sensors with extremely high sensitivity and selectivity to amines. Sens. Actuators B 2005, 106, 730–735. [Google Scholar]
- Modafferi, V.; Trocino, S.; Donato, A.; Panzera, G.; Neri, G. Electrospun V2O5 composite fibers: Synthesis, characterization and ammonia sensing properties. Thin Solid Films 2013, 548, 689–694. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Dong, J.; Qin, N.; Xu, J. Selective BTEX sensor based on SnO2/V2O5 composite. Sens. Actuators B 2013, 186, 126–131. [Google Scholar] [CrossRef]
- Prasad, A.K.; Amirthapandiana, S.; Dharaa, S.; Dasha, S.; Muralib, N.; Tyagia, A.K. Novel single phase vanadium dioxide nanostructured films for methane sensing near room temperature. Sens. Actuators B 2014, 191, 252–256. [Google Scholar] [CrossRef]
- Schönauer-Kamin, D.; Fleischer, M.; Moos, R. Influence of the V2O5 content of the catalyst layer of a non-Nerstian NH3 sensor. Solid State Ion. 2014, 262, 270–273. [Google Scholar]
- Karimov, K.S.; Saleem, M.; Mahroof-Tahir, M.; Akram, R.; Chanee, M.T.S.; Niaz, A.K. Resistive humidity sensor based on vanadium complex films. J. Semicond. 2014, 9, 094001. [Google Scholar] [CrossRef]
- Huotari, J.; Bjorklund, R.; Lappalainen, J.; Spetz, A.L. Pulsed laser deposited nanostructured vanadium oxide thin films characterized as ammonia sensors. Sens. Actuators B 2015, 217, 22–29. [Google Scholar] [CrossRef]
- Abbasi, M.; Rozati, S.M.; Irani, R.; Beke, S. Synthesis and gas sensing behaviour of nanostructured V2O5 thin films prepared by spray pyrolysis. Mater. Sci. Semicond. Process 2015, 29, 132–138. [Google Scholar] [CrossRef]
- Jin, W.; Yan, S.; An, L.; Chenb, W.; Yang, S.; Zhao, C.; Dai, Y. Enhancement of ethanol gas sensing response based on ordered V2O5 nanowire microyarns. Sens. Actuators B 2015, 206, 284–290. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Hu, J.; Cai, P.; Lv, Y. A cataluminescence gas sensor based on nanosized V2O5 for tert-butyl merkaptan. Sens. Actuators B 2015, 206, 284–290. [Google Scholar]
- Tolhurst, T.M.; Leedahl, B.; Andrews, J.L.; Banerjee, S.; Moewes, A. The electronic structure of ε’-V2O5: An expanded band gap in a double-layered polymorph with increased interlayer separation. J. Mater. Chem. A 2017, 45, 23694–23703. [Google Scholar] [CrossRef]
- Huotari, J.; Lappalainen, J.; Puustinen, J.; Spetz, A.L. Gas sensing properties of pulsed laser deposited vanadium oxide thin films with various crystal structures. Sens. Actuators B 2013, 187, 386–394. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Li, W. Magnetron sputtered Au-decorated vanadium oxides composite thin films for methane-sensing properties at room temperature. J. Alloys Compounds 2016, 671, 283–290. [Google Scholar] [CrossRef]
- Chimowa, G.; Tshabalala, Z.P.; Akande, A.A.; Bepete, G.; Mwakikunga, B.; Ray, S.S.; Banecha, E.M. Improving methane gas sensing properties of multi-walled carbon monotubes by vanadium oxide filing. Sens. Actuators B 2017, 247, 11–18. [Google Scholar] [CrossRef]
- Carotta, M.C.; Ferroni, M.; Gherardi, S.; Guidi, V.; Malagu, C.; Martinelli, G.; Sacerdoti, M.; di Vona, M.; Licoccia, S.; Traversa, E. Thick-film gas sensors based on vanadium–titanium oxide powders prepared by sol-gel synthesis. J. Eur. Ceram. Soc. 2004, 24, 1409–1413. [Google Scholar] [CrossRef]
- Yan, W.; Hu, M.; Wang, D.; Li, C. Room temperature gas sensing properties of porous silicon/V2O5nanorods composite. Appl. Surf. Sci. 2015, 346, 216–222. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Chen, Y. Gold/vanadium-tin oxide nanocomposites prepared by coprecipitation method for carbon monoxide gas sensors. Sens. Actuators B 2013, 176, 945–951. [Google Scholar] [CrossRef]
- Jin, A.; Chen, W.; Zhu, Q.; Yang, Y.; Volkov, V.I.; Zakharova, G.S. Structural and electrochromic properties of molybdenum doped vanadium pentoxide thin films by sol-gel and hydrothermal synthesis. Thin Solid Films 2009, 517, 2023–2028. [Google Scholar] [CrossRef]
- Yu, H.Y.; Kang, B.H.; Pi, U.H.; Park, C.W.; Choi, S.; Kim, G.T. V2O5 nanowire based nanoelectronic devices for helium detection. Appl. Phys. Lett. 2005, 86, 253102. [Google Scholar] [CrossRef]
- Maziarz, W.; Kusior, A.; Trenczek-Zajac, A. Nanostructured TiO2-based gas sensors with enhanced sensitivity to reducing gases. Beilstein J. Nanotechnol. 2016, 7, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Hermann, K.; Druzinic, R.; Witko, M.; Wagner, F.; Petersen, M. Geometric and electronic structure of vanadium pentoxide: A density functional bulk and surface study. Phys. Rev. B 1999, 59, 10583–10590. [Google Scholar] [CrossRef]
- Schneider, K. Defect structure of V2O5 thin film gas sensors. In Proceedings of the SPIE 10161, 14th International Conference on Optical and Electronic Sensors, 1016109, Gdansk, Poland, 19–22 June 2016. [Google Scholar]
- Osmolovskaya, O.M.; Murin, I.V.; Smirnov, V.M.; Osmolovsky, M.G. Synthesis of vanadium dioxide thin films and nanoparticles: A brief review. Rev. Adv. Mater. Sci. 2014, 36, 70–74. [Google Scholar]
- Blum, R.P.; Niehus, H.; Hucho, C.; Fortrie, R.; Ganduglia-Pirovano, M.V.; Sauer, J.; Shaikhutdinov, S.; Freund, H.J. Surface metal-insulator transition on a vanadium pentoxide (001) single crystal. Phys. Rev. Lett. 2007, 99, 226103–226104. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, I.; Kim, S.W.; Ryu, J.W.; Park, H.Y. Metal-insulator transition without structural phase transition in V2O5 film. Appl. Phys. Lett. 2011, 98, 131907–131916. [Google Scholar] [CrossRef]
- Strelcov, E.; Lilach, Y.; Kolmakov, A. Gas sensor based on metal-insulator transition in VO2 nanowire thermistor. Nano Lett. 2009, 9, 2322–2326. [Google Scholar] [CrossRef] [PubMed]
- Rella, R.; Siciliano, P.; Cricenti, A.; Generosi, R.; Coluzza, C. A study of physical properties and gas-surface interaction of vanadium oxide thin films. Thin Solid Films 1999, 349, 254–259. [Google Scholar] [CrossRef]
- Mane, A.A.; Suryawanshi, M.P.; Kim, J.H.; Moholkar, A.V. Fast response of sprayed vanadium pentoxide (V2O5) nanorods towards nitrogen dioxide (NO2) gas detection. Appl. Surf. Sci. 2017, 403, 540–550. [Google Scholar] [CrossRef]
- Prasad, A.K.; Dhara, S.; Dash, S. Selective NO2 sensor based on nanostructured vanadium oxide films. Sensor Lett. 2017, 15, 552–556. [Google Scholar] [CrossRef]
- Mane, A.A.; Suryawanshi, M.P.; Kim, J.H.; Moholkar, A.V. Superior selectivity and enhanced response characteristics of palladium sensitized vanadium pentoxide nanorods for detection of nitrogen dioxide gas. J. Colloid Interface Sci. 2017, 495, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kunango, J.; Saha, H.; Basu, S. Pd selenized porous silicon hydrogen sensor-influence of ZnO thin film. Sens. Actuators B 2010, 147, 128–136. [Google Scholar] [CrossRef]



| Gas | Composition | Morphology | Operation Temp (K) | Response | Gas Concentration [ppm] | Sensitive Against | Ref. |
|---|---|---|---|---|---|---|---|
| H2 CH4 C3H8 | V2O5 | Thin films | 420–520 | 1.22 | 5–300 H2 50–3000 CH4, C3H8 | NA | [27] |
| Et1, NH3 | V2O5 | Hollow spheres | NA2 | Et:1.02–1.06 NH3:1.01–1.02 | 100–500 | NA | [28] |
| NH3 | V2O5 | Thin film | RT | Change of color | 100–400 | H2 | [29] |
| Et NH3 | V2O5 | Thin film | NA | 1.04 1.06 | 100–500 100–500 | NA NA | [30] |
| Acetone, CH3OH, HCHO, toluene | VO2 | nanorods | RT3 | 1.015 1.027 1.060 1.055 | 5–100 | NA | [31,32] |
| SO2 | SnO2 + 5 wt% MgO + 2 wt% V2O5 | Thick film | NA | 1.44 | 0.1–1 | NA | [33] |
| Et | V2O5 | Thin film | 508 | NA | 2500 | NA | [34] |
| xylene | V2O5 | Thin film | 300 | 17 27.9 | 800 100 | NH3, Et, toluene, acetone | [35] |
| 1-butyl- amine | V2O5 | Nanofibres | RT | 1.42 | 0.15–9.5 | NH3, propranolol, toluene | [36] |
| NH3 | V2O5 | Composite fibers with polyvinyl acetate and pyrrolidone | 530 | 1.02 1.06 | 0.1–0.8 | [37] | |
| BTEX4 | SnO2/V2O5 | Composite SnO2/V2O5 | 540 | 5.5–6 | 0.5–50 | Et, CH3OH, HCHO | [38] |
| CH4 | VO2 | Thin films | 298–473 | 1.008–1.032 | 50–500 | NO2, H2 | [39] |
| NH3 | VWT: V2O5–WO3–TiO2 | Potentio- metric: Au V2O5–WO3– TiO2 | 820 | 0–150 | 10–300 | NA | [40] |
| H2O | VO2 (3fl) complex | Thick film 20-30μm | RT | NA | RH: 35–70% | NA | [41] |
| NH3 | V2O5–V7O16 | Thin film | 620 | 1.4 | 0.16–0.32 | NO, CO | [42] |
| Et | V2O5 | Thin films | 573–773 | 1.27–1.80 | 500–3000 | NA | [43] |
| Et | V2O5 | Nanowiremicroyarns | 600 | 9.09 | 50–1000 | Higher alcohols | [44] |
| C4H10S Tert-butyl mercaptan | V2O5 | Thick layer (0.2 mm) from nanopowder | 600 | Catalumine- scence | 3600–62,000 | Alcohols, alde-hydes, NH3 | [45] |
| NOx , H2 | V2O5 + VOx | Thin film composed from nanotubes | 448 563 | 2.85 1.075 | 20–80 NO 500–2000 H2 | CO | [46,47] |
| CH4 | Au–VOx | Porous thin film | RT | NA | 1500 | NA | [48] |
| CH4 | C/VOx | C nanotubes filled with VOx | RT | 1.015 | NA | NA | [49] |
| Materials | Operation Temperature [K] | Concentration | Response | Reference |
|---|---|---|---|---|
| VO2 thin film nanocolumnar | 423 | >100 ppm | 5 | [24] |
| V2O5 thin film nanotubes | 563 | 20–80 ppm | 6 | [47] |
| V2O5 thin film nanotubes | 448 | 20–80 ppm | 2.9 | [47] |
| Composite porous Si/ nanorods V2O5 | 298–523 | 0.25–3 | 5–10 | [51] |
| V2O5 thin film | 553–573 | 100 ppm | 1.6 | [62] |
| V2O5 thin films composed from-nanorods | 473 | 100 ppm | 1.24 | [63] |
| V2O5 thin films 450 nm | 323 | 2–20 ppm | 1.8 | [64] |
| V2O5 nanorods | 473 | 100 ppm | 1.75 1.24 | [65] [66] |
| V2O5 thin films | 410–545 | 4 ppm 20 ppm | 1.16 3.35 | This work |
| V2O5 thin films | 546–617 | 4 ppm 20 ppm | 14.4 23.0 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, K.; Maziarz, W.
V2O5 Thin Films as Nitrogen Dioxide Sensors
Schneider K, Maziarz W.
V2O5 Thin Films as Nitrogen Dioxide Sensors
Schneider, Krystyna, and Wojciech Maziarz.
2018. "V2O5 Thin Films as Nitrogen Dioxide Sensors
Schneider, K., & Maziarz, W.
(2018). V2O5 Thin Films as Nitrogen Dioxide Sensors





