Abstract
Electroencephalography (EEG) is an objective diagnostic tool in the evaluation of cerebral functionality, both in human and veterinary medicine. For EEG acquisition, different types of electrodes are used, as long as they have no impact on the recorded background activity. However, to date, the influence of electrode type on quantitative EEG and cerebral coherence has not been investigated. Twenty EEG traces (ten with needle electrodes and ten with disk electrodes) were recorded from ten mesocephalic vigil dogs in a monopolar montage. Values for interhemispheric coherence for each frequency band were compared between stainless needle and silver disk electrodes traces. Our results show that the values of interhemispheric coherence in vigil dogs are depending of the type of electrodes used in EEG recordings. In the frontal (FP) channel, for delta and theta frequency bands, the registered coherence is significantly higher when stainless needle electrodes are used. Our results might have important consequences in the field of canine neurology and applied neuroscience, as the frontal channel analysis is preferred in aging and behavior studies.
1. Introduction
Electroencephalography (EEG) is an essential diagnostic tool in the evaluation of cerebral disorders [1,2,3,4]. It is widely used in establishing a diagnosis of epilepsy [5,6,7], but also in assessing the efficacy of anticonvulsivants or other drugs. In a clinical environment, EEG traces are analyzed visually [8]. In addition, quantitative EEG (qEEG) may offer an in-depth look at different frequency bands, making it possible to further appreciate brain coherence (the normalized linear correlation which exists between two pairs of EEG channels and informs the frequency components common to them). From a mathematical point of view, coherence is calculated with the following formula:
where Coh(f) is the coherence function, f is frequency, N is the number of EEG realizations involved in averaging, F1(f) and F2(f) are Fourier transforms of EEG signals in two different channels, and * denotes a complex conjugation.
In humans, intra- and interhemispheric coherence is extensively studied in normal and pathological situations [9,10,11,12,13,14,15]. In veterinary medicine, due to the lack of an optimal protocol for EEG recordings [7], there are only few reports about cerebral coherence in healthy dogs or individuals with behavior problems, qEEG being mainly used in conditioning studies [14,16,17].
To record EEG traces, different types of electrodes are used [18,19,20]. Their usage is directly related to the type of investigation [21]. In human medicine, disk or cup electrodes are classically used, and those with a central hole allow periodic refilling with electrode conductive gel [22]. Needles are inserted just beneath the skin, parallel to the surface of the scalp and should be secured with an adhesive paste. Usually, they are not recommended for vigil patients or for prolonged recording, but have the advantage of being easy to place and may be used for short-term EEG in some comatose patients [22]. In veterinary medicine, the most used electrodes are the subdermal needle and disk electrodes. Subdermal needle electrodes have the advantages that they are easy to place, are minimally invasive, and are more suitable for short-term EEG (less than 30–40 min). Their disadvantages are related to a higher occurrence of muscle artifact registration in comparison with the disk electrodes, as well as the risk of their displacement from the area of interest, especially in non-sedated patients. Disk electrodes can be made from tin, pure silver, pure silver with a gold coating, silver-silver chloride, or gold. They are not invasive and are attached to the skin with a paste that has good electrical conductivity [23]. Disk electrodes allow a prolonged EEG recording (even in vigil dogs), but the time of acquisition is directly influenced by the properties of the conductive paste. In sedated dogs, the EEG traces are characterized by a significant reduction in artifacts, but the differences among the electrodes are not considered clinically relevant [23]. However, in this study, the authors focused on the impacts of electrodes over the visual background activity and the presence of artifacts; however, no qEEG assessment was performed. The disk electrodes are able to acquire the biopotentials from a wider cortical area compared to the needle electrodes. When the brainstem auditory evoked potentials were assessed using disk electrodes, Musteata (2013) observed that the brainstem latencies were shorter in comparison with those obtained when needle electrodes were used [24]. Because disk electrodes record electrical impulses of a higher number of neurons than the needle electrodes, it is possible to have a different distribution of waves in the different frequency bands and different values for cerebral coherence. To our knowledge, there is no previous study in which the value of dog cerebral coherences are evaluated in relation to the type of electrode used.
The goal of this study is to analyze the interhemispheric cerebral coherence values in vigil dogs when needle and disk electrodes are used.
2. Materials and Methods
The study was conducted at the Neurology Department of the Veterinary Teaching Hospital (VTH) of Faculty of Veterinary Medicine of Iasi, Romania. Ethical approval for the study was obtained from the Ethics Committee of the Faculty of Veterinary Medicine, University of Agricultural Sciences and Veterinary Medicine “Ion Ionescu de la Brad” of Iași (No. 424/05.05.2018).
2.1. Animals
The study was performed on ten mesocephalic dogs (6 females and 4 males) referred to VTH Iași. For all patients, a general physical examination, followed by a neurological one, was performed. All dogs were free of any intracranial pathology (based on the absence of any cognitive impairment, deficits in cranial nerves and no changes in behavior). The dogs were aged between six months and four years.
2.2. EEG System and Electrodes
Electroencephalographic examinations were performed with an 8-channel digital system, “Neuron-spectrum-1” (Neuron-Spectrum.net, Neurosoft Ltd., Ivanovo, Russia) and the registered traces were analyzed using the corresponding software.
For EEG trace acquisition, two types of electrodes (disk and needle) were used: Disk electrodes (pure silver) with a 10-mm diameter and 1.5-mm touch proof connector (NE134A, Nihon Kohden, Tokyo, Japan), and 0.22 × 22.5 mm and 1.5 mm proof connector subdermal electrodes (stainless steel) (NE-224S EEG, Nihon Kohden, Tokyo, Japan).
The parameters used for each electroencephalographic recording were: sensitivity, 10 μV; time constant, 0.3 s; filter pass-down of 35 Hz; filter pass-up 0.5 Hz; and electrode impedance <10 Ω, notch filter active.
2.3. Experiment Protocol
Before EEG acquisition, each dog was left for 30 min in the investigation room to accommodate to the environment and operators. To avoid the dog becoming distracted during EEG acquisition, the experiment was performed in a quiet room and no other individuals other than designated operators were allowed inside.
For each patient, a ten minute EEG with an 8-channel examination was recorded for each type of electrode. The electrodes were placed according to previously described protocols [8,25] as follows: FP1, FP2, C3, C4, T3, T4, O1 and O2 in monopolar montage with reference electrodes (A1, A2) placed on the corresponding ear. The needle electrodes were positioned subcutaneously. The disk electrodes were placed in the same position and fixed with a conductive paste (Elefix paste for EEG). All EEG examinations were performed in vigil status (no chemical constraints were used).
To exclude the influence over EEG traces of the same sequence of electrode placements, the order of examination (first needle electrode then disk/first disk then needle) was randomized. The positioning of the electrodes was made each time by the same operator and the correctness was verified by a second operator.
2.4. EEG Processing
After a visual assessment of the background, 3 artifact-free epochs of 20 s were selected for each dog and each type of electrode for further processing. In total, 60 datasets (30 for needle electrodes and 30 for disk electrodes) were analyzed. Interhemispheric coherence and correlations were assessed by applying cross scheme 8 (Fp1–Fp2, C3–C4, T3–T4, O1–O2) for each epoch. The dominant frequency (DF) and mean of coherence (MIC) were automatically generated by the software for each frequency band (delta: 0–4 Hz, theta: 4–7 Hz, alpha: 7–13 Hz, low frequency beta: 13–20 Hz and high frequency beta: >20 Hz).
A 0.4 value of coherence (which range between 0—no coherence up to 1—maximal coherence) was established as a threshold.
2.5. Statistical Analysis
Data are expressed as mean and standard deviations with maximum and minimum values. After Kurtosis and Skewness test of normality, Mann–Whitney U test and Kruskal Wallis analysis were chosen to compare variables inside and between groups. Values of p lower than 0.05 were considered statistically significant. All statistics were performed with the SPSS IBM vs. 17 package program.
3. Results
Twenty EEG traces (ten with needle electrodes and ten with disk electrodes for each dog) were recorded and analyzed.
When DF was analyzed in each frequency band, no differences were noted between individuals (p > 0.05). In addition, when the DF of each frequency band was compared in relation to the acquisition protocol for each dog, no significant differences were noticed (p > 0.05) (Figure 1).
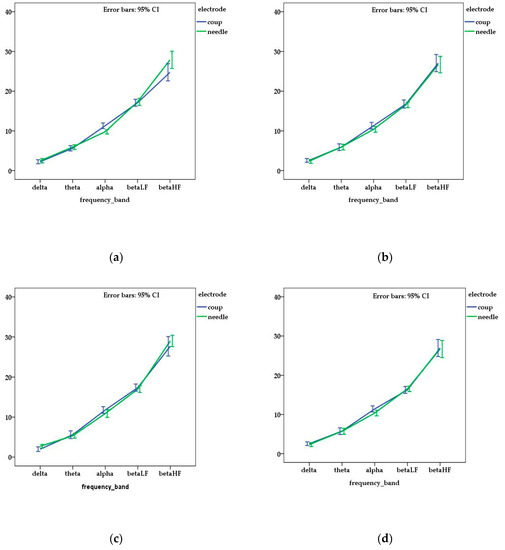
Figure 1.
Mean and standard error of dominant frequency for each channel ((a) frontal—FP; (b) central—C, (c) temporal—T; (d) occipital—O) and frequency bands related with the type of electrode used in the electroencephalography (EEG) acquisition. No statistical differences (p > 0.05) were observed between the obtained values of DF (dominant frequency).
However, when the means of DF of each frequency band were compared between the type of electrodes, statistical differences were observed for FP channel for alpha (p = 0.001) and beta HF (p = 0.011) waves and for T channel for delta waves (p = 0.046). The mean values of DF are presented in Table 1.

Table 1.
Mean of dominant frequency in all frequency bands relative to the electrode.
Regarding the mean interhemispheric coherence (MIC), the highest value observed was 0.78, with only a few others that ranged between 0.4 and 0.6. The mean and standard deviation of MIC values obtained for all individuals are presented in Table 2.

Table 2.
Mean interhemispheric coherence in all frequency bands relative to the electrode.
Statistical differences (p < 0.05) were noticed when MIC values were compared for each dog in relation to the type of electrode used. Furthermore, when MIC values were compared for each type of electrode for the entire dog group, significant differences were observed (p < 0.05).
When mean values of MIC were compared in relation to the electrode type, a higher coherence was observed for needle electrode traces in FP channel for delta (p = 0.006) and theta (p = 0.001) frequency bands. In the alpha and beta frequency bands, the coherence values were higher in traces acquired with disk electrodes and, when compared with needle electrodes (Figure 2), significant differences were obtained for C channel in the alpha and beta LF frequency bands (p = 0.039 and 0.020 respectively).
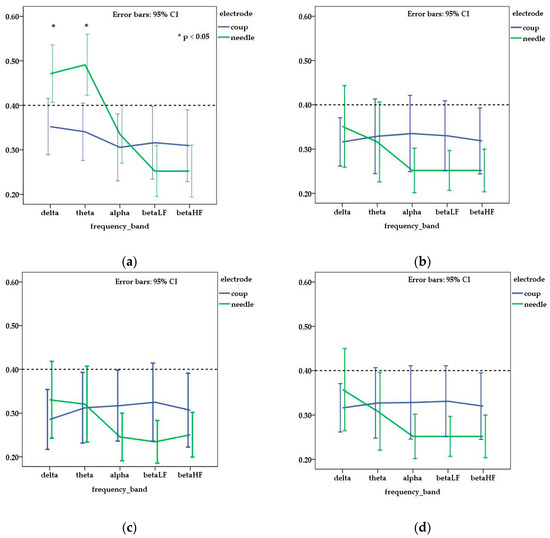
Figure 2.
Mean and standard error of interhemispheric coherence observed for needle and disk electrodes in each channel ((a) frontal—FP; (b) central—C, (c) temporal—T; (d) occipital—O) and frequency bands. Dotted line (threshold for medium coherence) is fixed at 0.4 corresponding to a medium coherence. The value of obtained coherence was higher than 0.4 in frontal channel for delta and theta frequency bands only on EEG traces acquired with needle electrodes.
4. Discussion
The goal of our study was to characterize interhemispheric coherence in vigil dogs when needle and disk electrodes are used for recording EEG traces. To our knowledge, there are no studies in which cerebral coherence is assessed in vigil dogs relative to the type of electrode used during EEG acquisition. Our study shows that the type of electrode influences the registered values of interhemispheric coherence.
Previous research has described data obtained for EEGs in vigil dogs [26] and sedated individuals [8,25]. Brain frequency and coherence were studied in anesthetized dogs [15], and in both healthy and neurological patients [27]. In the present study, we did not find any differences in the background activity and DF between the EEG traces acquired either with needle or disk electrodes. Our results are similar to those previously described by James (2011).
Regarding cerebral coherence in chemically restrained dogs, when needle electrodes were used, a higher interhemispheric coherence (>0.6) was observed in the dorsal channel (FP, P, O) for theta and alpha frequency bands [15]. In our study, the mean of coherence was lower than 0.6 in all the analyzed channels. These results might be a consequence of the fact that we recorded EEG traces only on vigil patients. In the vigil status, coherence is not necessarily predominant in one single frequency band, but different grades of coherence in multiple frequency bands may coexist [14]. In dogs, a coherence value superior to 0.4 was observed only in the FP channel, mainly in the delta and theta frequency bands, when the EEG traces were recorded using needle electrodes. A statistically different value was obtained also for the C channel in the alpha and beta LF frequency bands, but, in those cases, the mean of coherence was inferior to the threshold value (0.4) of the study. This observation is surprising and suggests that, for lower frequency bands, in the FP channel, registered values of the coherence might be dependent on the type of electrode used in the acquisition process.
Our results might have important consequences in the field of canine neurology and applied neuroscience, as frontal channel analysis is preferred in aging and behavior studies. In humans, it is considered that delta oscillations are involved in many cognitive processes [28] and theta waves in the frontal lobes are an indicator of an attentive status. In consequence, cognitive dysfunctions will change the cerebral coherence when assessed using the EEG technique [29]. In dogs, a decrease in total brain volume occurs only in geriatric individuals (12 years and older), but frontal lobe atrophy appears two to four years earlier (8–11 years old). In consequence, the functions related of this region are compromised early in aging [30]. Canine cognitive dysfunction syndrome is usually diagnosed based on imaging appearance, clinical signs, and detection of some metabolites in the cerebrospinal fluid. The clinical signs displayed by affected dogs include disorientation, a decrease in social interactions, changes in sleep–wake cycles, a loss of prior housetraining, increased anxiety, and changes in level of activity [31]. Similar to humans, qEEG may be used as a noninvasive way to determine in a more objective manner, in vigil dogs, the onset and progression of this condition through the assessment of cerebral coherence (especially in the frontal lobes). Therefore, in the light of this study, stainless needle electrodes may be more suitable than the silver disk electrodes in the evaluation of frontal lobe interhemispheric coherence.
Although in our study we observed a net segregation of the obtained coherence values between the frequency bands in the FP channel in relation to the electrode types, we acknowledge some limitations. The study was performed on a limited number of subjects. It is possible that by increasing the number of animals, a higher level of coherence might appear in some frequency bands, different to those presented in our study. There is also a lack of reference values for coherence mapping in vigil dogs, which means that intra- or interbreed brain activity coherence variations must be considered. Finally, our EEG study was performed only on mesocephalic dogs. Our results are not necessarily applicable for dogs with different skull shapes (brahicephalic or dolicocephalic ones).
5. Conclusions
In this paper we compared the registered values of interhemispheric cerebral coherence when needle and disk electrodes were used in mesocephalic vigil dogs. Sixty datasets (30 for needle electrodes and 30 for disk ones) were analyzed for dominant frequency and interhemispheric coherence in cross scheme 8 at a 0.4 threshold, established as medium coherence. First, the type of electrode used during EEG acquisition had no influence over the DF values of each frequency band in each individual (p > 0.05). Second, the registered values of coherence might be dependent on the type of electrode used in the acquisition process. Thus, the MIC was significantly higher (p > 0.05) in the FP channel for the delta and theta frequency bands when stainless needle electrodes were used. Therefore, our results may influence the field of applied neuroscience and canine neurology as the frontal channel analysis is preferred in aging and behavior studies.
Although the results show that the type of electrode influences the registered value of interhemispheric coherence, this paper focuses only on mesocephalic dogs. Thus, the impact of the electrode type on interhemispheric coherence in dogs with different skull shapes (brachicephalic or dolicocephalic ones) must be considered.
Author Contributions
Conceptualization, M.M. and R.L.; formal analysis, M.M. and G.S.; investigation, M.M., D.G.B. and R.S.; methodology, M.M., D.G.B. and R.L.; supervision, M.M. and G.S.; writing—original draft, M.M., D.G.B., G.S. and R.L.; writing—review & editing, M.M.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sutter, R.; Kaplan, P.W.; Cervenka, M.C.; Thakur, K.T.; Asemota, A.O.; Venkatesan, A.; Geocadin, R.G. Electroencephalography for diagnosis and prognosis of acute encephalitis. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015, 126, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Naim-Feil, J.; Rubinson, M.; Freche, D.; Grinshpoon, A.; Peled, A.; Moses, E.; Levit-Binnun, N. Altered brain network dynamics in schizophrenia: A cognitive electroencephalography study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, M.; Murphy, N.; Peraza, L.R.; Graziadio, S.; Cromarty, R.; Killen, A.; O’Brien, J.T.; Thomas, A.J.; LeBeau, F.E.N.; Taylor, J.P. Quantitative electroencephalography as a marker of cognitive fluctuations in dementia with lewy bodies and an aid to differential diagnosis. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2018, 129, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, C.; Cavanaugh, J.M.; Kallakuri, S.; Desai, A.; Zhang, L.; King, A.I. Quantitative electroencephalography in a swine model of blast-induced brain injury. Brain Inj. 2017, 31, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Debicki, D.B. Electroencephalography after a single unprovoked seizure. Seizure 2017, 49, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Berendt, M.; Hogenhaven, H.; Flagstad, A.; Dam, M. Electroencephalography in dogs with epilepsy: Similarities between human and canine findings. Acta Neurol. Scand. 1999, 99, 276–283. [Google Scholar] [CrossRef] [PubMed]
- De Risio, L.; Bhatti, S.; Munana, K.; Penderis, J.; Stein, V.; Tipold, A.; Berendt, M.; Farqhuar, R.; Fischer, A.; Long, S.; et al. International veterinary epilepsy task force consensus proposal: Diagnostic approach to epilepsy in dogs. BMC Vet. Res. 2015, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.; Ives, J.R.; Karczewski, M.; Dziadkowiak, E.; Gruszka, E. The relationship between epileptiform discharges and background activity in the visual analysis of electroencephalographic examinations in dogs with seizures of different etiologies. Vet. J. 2017, 222, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Youh, J.; Hong, J.S.; Han, D.H.; Chung, U.S.; Min, K.J.; Lee, Y.S.; Kim, S.M. Comparison of electroencephalography (eeg) coherence between major depressive disorder (mdd) without comorbidity and mdd comorbid with internet gaming disorder. J. Korean Med. Sci. 2017, 32, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Lin, C.T.; Chuang, C.H.; Lai, K.L.; Yang, A.C.; Fuh, J.L.; Wang, S.J. Resting-state eeg power and coherence vary between migraine phases. J. Headache Pain 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Rubega, M.; Sparacino, G.; Sejling, A.S.; Juhl, C.B.; Cobelli, C. Hypoglycemia-induced decrease of eeg coherence in patients with type 1 diabetes. Diabetes Technol. Ther. 2016, 18, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Yu, H.; Wei, X.; Yang, C.; Deng, B. Power spectral density and coherence analysis of alzheimer’s eeg. Cogn. Neurodyn. 2015, 9, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Shida-Tokeshi, J.; Vanderbilt, D.L.; Smith, B.A. Electroencephalography power and coherence changes with age and motor skill development across the first half year of life. PLoS ONE 2018, 13, e0190276. [Google Scholar] [CrossRef] [PubMed]
- Preobrazhenskaya, L.A. Functional regional asymmetry in intrahemisphere coherencein the eeg during conditioning in dogs. Neurosci. Behav. Physiol. 2007, 37, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Vidal Figueredo, R.J.; Pellegrino, F.C. Ritmos cerebrales: Componente frecuencial theta dominante asociado a perros con epilepsia idiopática. Anal. Vet. Murcia 2014, 30, 85–93. [Google Scholar]
- Preobrazhenskaia, L.A. Eeg dynamics during conditioning in symmetrical neocortical regions in dogs. Zhurnal Vysshei Nervnoi Deiatelnosti Imeni i p Pavlova 2002, 52, 441–449. [Google Scholar] [PubMed]
- Preobrazhenskaia, L.A. Functional asymmetry of the neocortex electrical activity during food conditioning in dogs. Zhurnal Vysshei Nervnoi Deiatelnosti IMENI i p Pavlova 2000, 50, 434–446. [Google Scholar] [PubMed]
- Fiedler, P.; Pedrosa, P.; Griebel, S.; Fonseca, C.; Vaz, F.; Supriyanto, E.; Zanow, F.; Haueisen, J. Novel multipin electrode cap system for dry electroencephalography. Brain Topogr. 2015, 28, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kappel, S.L.; Rank, M.L.; Toft, H.O.; Andersen, M.; Kidmose, P. Dry-contact electrode ear-eeg. IEEE Trans. Bio-Med. Eng. 2018. [Google Scholar] [CrossRef]
- Pasion, R.; Paiva, T.O.; Pedrosa, P.; Gaspar, H.; Vasconcelos, B.; Martins, A.C.; Amaral, M.H.; Nobrega, J.M.; Pascoa, R.; Fonseca, C.; et al. Assessing a novel polymer-wick based electrode for eeg neurophysiological research. J. Neurosci. Methods 2016, 267, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Nakamoto, H.; Egawa, S.; Kawamata, T. Continuous eeg monitoring in icu. J. Intensive Care 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Caricato, A.; Melchionda, I.; Antonelli, M. Continuous electroencephalography monitoring in adults in the intensive care unit. Crit. Care 2018, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- James, F.M.; Allen, D.G.; Bersenas, A.M.; Grovum, W.L.; Kerr, C.L.; Monteith, G.; Parent, J.M.; Poma, R. Investigation of the use of three electroencephalographic electrodes for long-term electroencephalographic recording in awake and sedated dogs. Am. J. Vet. Res. 2011, 72, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Musteata, M.; Neculae, I.; Armasu, M.; Balan, C.B.; Solcan, G. Brainstem auditory evoked potentials in healthy cats recorded with surface electrodes. Acta Vet. Brno 2013, 82, 97–101. [Google Scholar] [CrossRef]
- Pellegrino, F.C.; Sica, R.E. Canine electroencephalographic recording technique: Findings in normal and epileptic dogs. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2004, 115, 477–487. [Google Scholar] [CrossRef]
- James, F.M.K.; Cortez, M.A.; Monteith, G.; Jokinen, T.S.; Sanders, S.; Wielaender, F.; Fischer, A.; Lohi, H. Diagnostic utility of wireless video-electroencephalography in unsedated dogs. J. Vet. Intern. Med. 2017, 31, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Bochenska, A.; Kwiatkowska, M.; Pomianowski, A.; Monowid, T.; Adamiak, Z. Electroencephalography recording analysis in monitoring of canine idiopathic epilepsy treated with phenobarbital. Pilot study. Pol. J. Vet. Sci. 2014, 17, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Basar, E.; Basar-Eroglu, C.; Karakas, S.; Schurmann, M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2001, 39, 241–248. [Google Scholar]
- Murias, M.; Swanson, J.M.; Srinivasan, R. Functional connectivity of frontal cortex in healthy and adhd children reflected in eeg coherence. Cereb. Cortex 2007, 17, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Tapp, P.D.; Siwak, C.T.; Gao, F.Q.; Chiou, J.Y.; Black, S.E.; Head, E.; Muggenburg, B.A.; Cotman, C.W.; Milgram, N.W.; Su, M.Y. Frontal lobe volume, function, and beta-amyloid pathology in a canine model of aging. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 8205–8213. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Munshi, S.; Banerjee, K.; Thakurta, I.G.; Sinha, M.; Bagh, M.B. Mitochondrial dysfunction during brain aging: Role of oxidative stress and modulation by antioxidant supplementation. Aging Dis. 2011, 2, 242–256. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).