Fast and Reliable Determination of Virgin Olive Oil Quality by Fruit Inspection Using Computer Vision
Abstract
1. Introduction
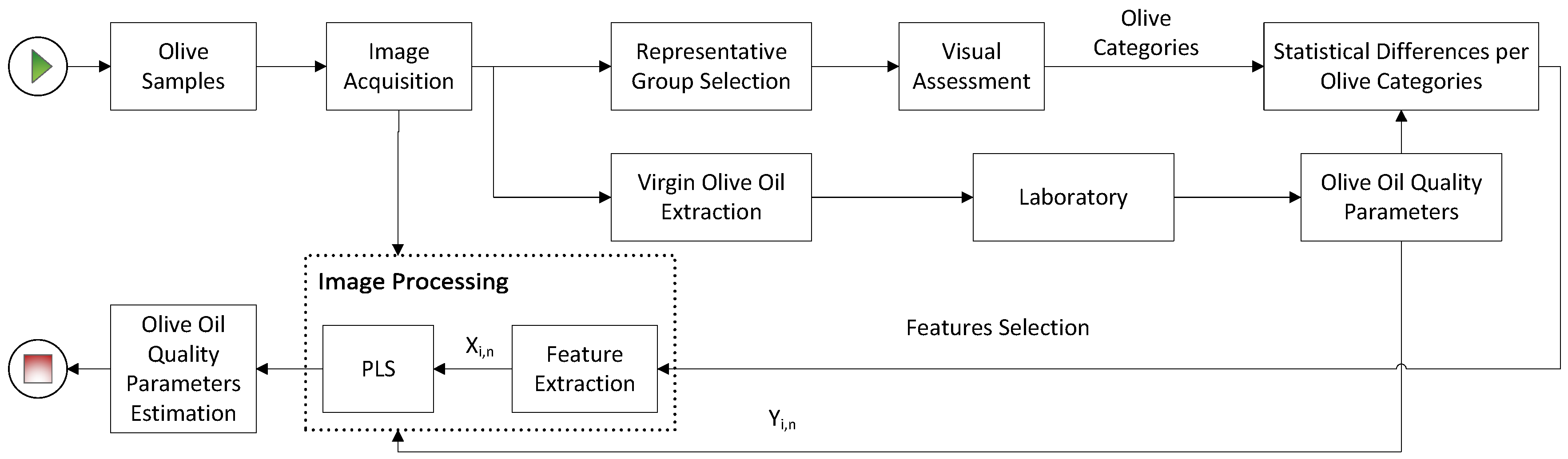
2. Material and Methods
2.1. Olive Batches Preparation and Oil Extraction
2.2. Visual Assessment
2.3. Image Processing
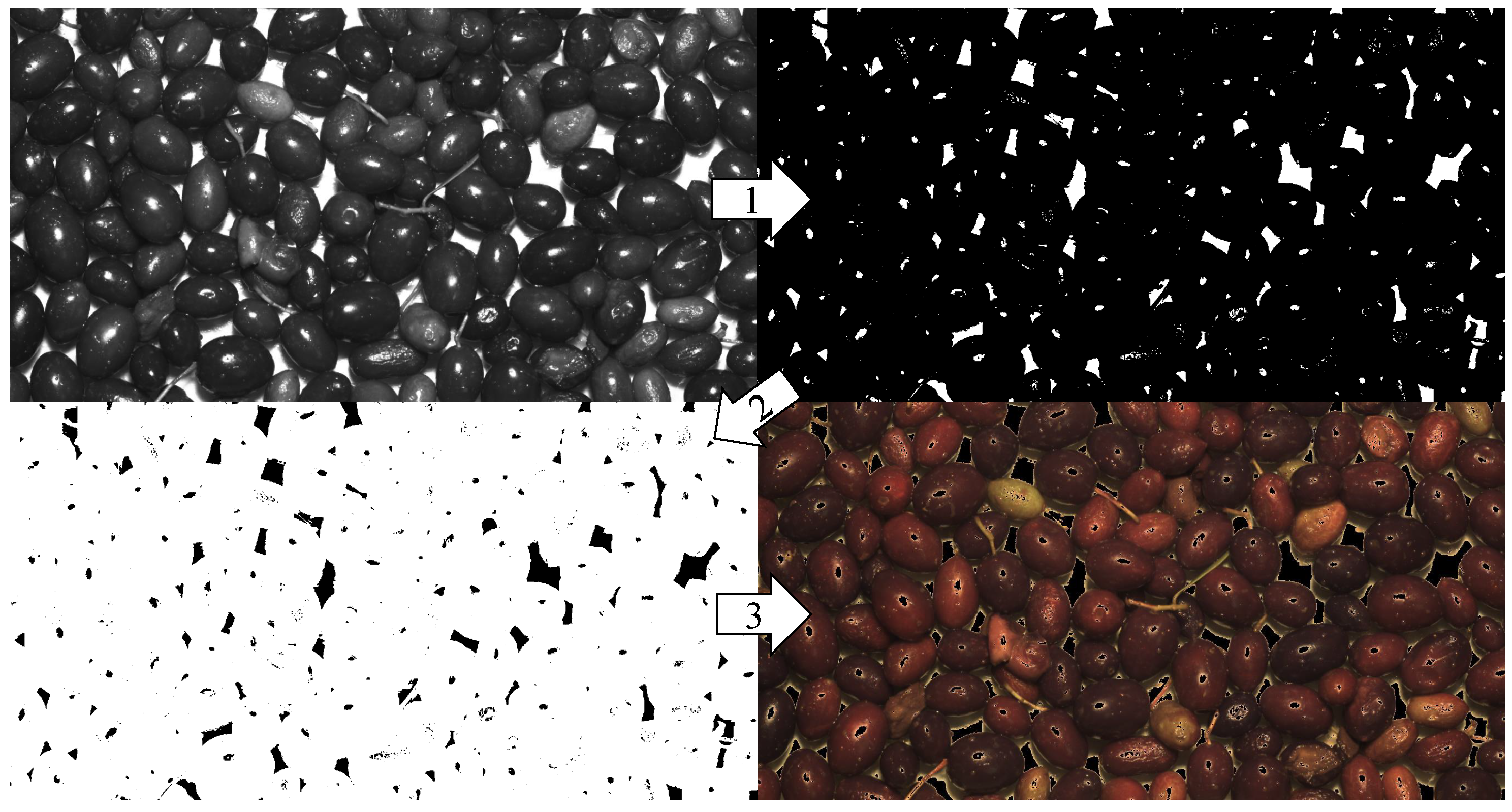
2.3.1. Features Extraction
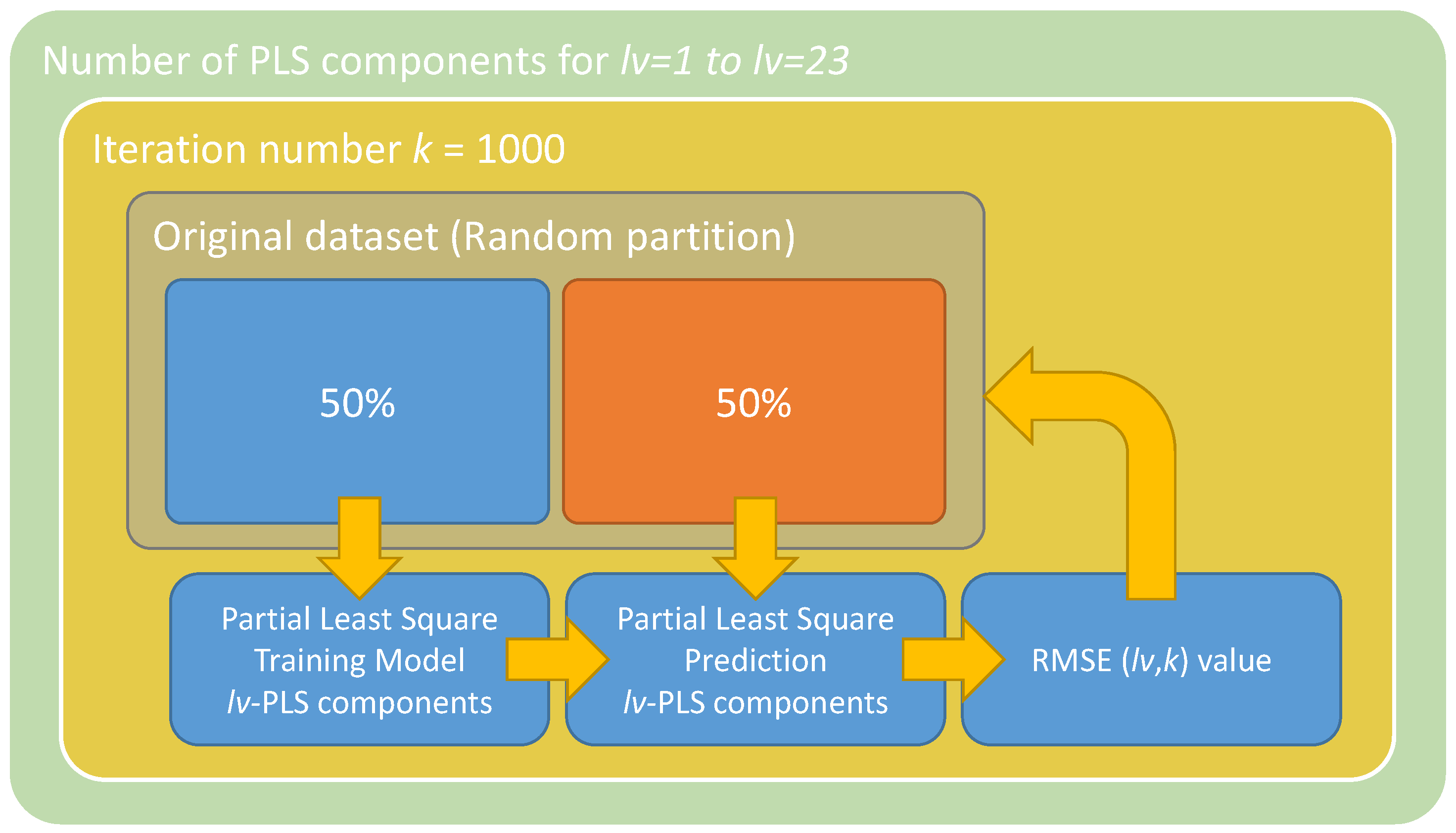
2.3.2. Regression Model
3. Results and Discussion
3.1. Laboratory Results for the Analytical Parameters
3.2. Image Processing Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VOO | Virgin olive oil |
| VOOs | Virgin olive oils |
| EVOO | Extra virgin olive oil |
| LVOO | Lampante virgin olive oil |
| PLS | Partial least squares |
| RMSE | Root mean square error |
| R | Regression coefficient |
| RPD | Ratio of performance to deviation |
References
- International Olive Council. MARKET NEWSLETTER No 125–MARCH 2018, 18, 1–6. Available online: http://webcache.googleusercontent.com/search?q=cache:jlu1QppfQEcJ:www.internationaloliveoil.org/documents/viewfile/13363-market-newsletter-march-2018+&cd=1&hl=en&ct=clnk&gl=hk (accessed on 7 November 2018).
- Roselli, L.; Clodoveo, M.L.; Corbo, F.; De Gennaro, B. Are health claims a useful tool to segment the category of extra-virgin olive oil? Threats and opportunities for the Italian olive oil supply chain. Trends Food Sci. Technol. 2017, 68, 176–181. [Google Scholar] [CrossRef]
- International Olive Council. Trade Standard Applying to Olive Oils and Olive Pomace Oils. COI/T.15/NC No 3/Rev. 12. June 2018. Available online: http://webcache.googleusercontent.com/search?q=cache:2kCpZ3swD_AJ:www.internationaloliveoil.org/documents/viewfile/9708-norma-english+&cd=1&hl=en&ct=clnk&gl=hk (accessed on 7 November 2018).
- Salazar-Ordóñez, M.; Rodríguez-Entrena, M.; Cabrera, E.R.; Henseler, J. Understanding product differentiation failures: The role of product knowledge and brand credence in olive oil markets. Food Qual. Preference 2018, 68, 146–155. [Google Scholar] [CrossRef]
- Fregapane, G.; Gómez-Rico, A.; Inarejos, A.M.; Salvador, M.D. Relevance of minor components stability in commercial olive oil quality during the market period. Eur. J. Lipid Sci. Technol. 2013, 115, 541–548. [Google Scholar] [CrossRef]
- Fuentes, E.; Paucar, F.; Tapia, F.; Ortiz, J.; Jimenez, P.; Romero, N. Effect of the composition of extra virgin olive oils on the differentiation and antioxidant capacities of twelve monovarietals. Food Chem. 2018, 243, 285–294. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EC Regulation 432/2012; Technical Report; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Tsimidou, M.Z.; Nenadis, N.; Servili, M.; Luis, D.; Gonzáles, G. Why Tyrosol Derivatives Have to Be Quantified in the Calculation of “Olive Oil Polyphenols” Content to Support the Health Claim Provisioned in the EC Reg. 432/2012. Eur. J. Agron. 2018. [Google Scholar] [CrossRef]
- Lazzerini, C.; Domenici, V.; Lazzerini, C.; Domenici, V. Pigments in Extra-Virgin Olive Oils Produced in Tuscany (Italy) in Different Years. Foods 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.N.; Romero, M.P.; Casanovas, M.; Motilva, M.J. Pigment profile and colour of monovarietal virgin olive oils from Arbequina cultivar obtained during two consecutive crop seasons. Food Chem. 2008, 110, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Ruiz, R.; Gandul-Rojas, B. Decoloration kinetics of chlorophylls and carotenoids in virgin olive oil by autoxidation. Food Res. Int. 2014, 65, 199–206. [Google Scholar] [CrossRef]
- Jolayemi, O.S.; Tokatli, F.; Ozen, B. Effects of malaxation temperature and harvest time on the chemical characteristics of olive oils. Food Chem. 2016, 211, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, G.; Bejaoui, M.A.; Jimenez, A.; Sanchez-Ortiz, A. Ethanol in Olive Fruit. Changes during Ripening. J. Agric. Food Chem. 2015, 63, 5309–5312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, W.; Li, J.; Zhao, C.; Fan, S.; Wu, J.; Liu, C. Principles, developments and applications of computer vision for external quality inspection of fruits and vegetables: A review. Food Res. Int. 2014, 62, 326–343. [Google Scholar] [CrossRef]
- Riquelme, M.T.; Barreiro, P.; Ruiz-Altisent, M.; Valero, C. Olive classification according to external damage using image analysis. J. Food Eng. 2008, 87, 371–379. [Google Scholar] [CrossRef]
- Ram, T.; Wiesman, Z.; Parmet, I.; Edan, Y. Olive oil content prediction models based on image processing. Biosyst. Eng. 2010, 105, 221–232. [Google Scholar] [CrossRef]
- Furferi, R.; Governi, L.; Volpe, Y. ANN-based method for olive Ripening Index automatic prediction. J. Food Eng. 2010, 101, 318–328. [Google Scholar] [CrossRef]
- Guzmán, E.; Baeten, V.; Pierna, J.A.F.; García-Mesa, J.A. Infrared machine vision system for the automatic detection of olive fruit quality. Talanta 2013, 116, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, O.M.; Martínez, D.M.G.; Aguilera, D.P.; Gámez, J.G.; Gómez, J.O. Automatic determination of peroxides and acidity of olive oil using machine vision in olive fruits before milling process. In Proceedings of the IST 2015—2015 IEEE International Conference on Imaging Systems and Techniques, Macau, China, 16–18 September 2015. [Google Scholar] [CrossRef]
- Aguilera, D.; Martínez, D.M.G.; Gamez, J.G.; Gómez, J.O. Sorting Olive Batches for the Milling Process Using Image Processing. Sensors 2015, 15, 15738–15754. [Google Scholar] [CrossRef]
- Ponce, J.M.; Aquino, A.; Millán, B.; Andújar, J.M.; Ponce, J.M.; Aquino, A.; Millán, B.; Andújar, J.M. Olive-Fruit Mass and Size Estimation Using Image Analysis and Feature Modeling. Sensors 2018, 18, 2930. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gȩbicki, J.; Namieśnik, J.; Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gȩbicki, J.; Namieśnik, J. Portable Electronic Nose Based on Electrochemical Sensors for Food Quality Assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, L.; Ngadi, M.; Huang, H.; Liu, L.; Ngadi, M.O. Recent Developments in Hyperspectral Imaging for Assessment of Food Quality and Safety. Sensors 2014, 14, 7248–7276. [Google Scholar] [CrossRef] [PubMed]
- Melesse, A.; Weng, Q.; Thenkabail, P.; Senay, G.; Melesse, A.M.; Weng, Q.; Thenkabail, P.S.; Senay, G.B. Remote Sensing Sensors and Applications in Environmental Resources Mapping and Modelling. Sensors 2007, 7, 3209–3241. [Google Scholar] [CrossRef] [PubMed]
- Makantasis, K.; Doulamis, A.D.; Doulamis, N.D.; Nikitakis, A. Tensor-Based Classification Models for Hyperspectral Data Analysis. IEEE Trans. Geosci. Remote Sens. 2018, 1–15. [Google Scholar] [CrossRef]
- Díaz-Varela, R.; de la Rosa, R.; León, L.; Zarco-Tejada, P.; Díaz-Varela, R.A.; de la Rosa, R.; León, L.; Zarco-Tejada, P.J. High-Resolution Airborne UAV Imagery to Assess Olive Tree Crown Parameters Using 3D Photo Reconstruction: Application in Breeding Trials. Remote Sens. 2015, 7, 4213–4232. [Google Scholar] [CrossRef]
- Jha, M.; Levy, J.; Gao, Y. Advances in Remote Sensing for Oil Spill Disaster Management: State-of-the-Art Sensors Technology for Oil Spill Surveillance. Sensors 2008, 8, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Benalia, S.; Bernardi, B.; Blasco, J.; Fazari, A.; Zimbalatti, G. Assessment of the Ripening of Olives Using Computer Vision. Chem. Eng. Trans. 2017, 58, 355–360. [Google Scholar] [CrossRef]
- International Olive Council. Guide for the Determination of the Characteristics of Oil-Olives; International Olive Council: Madrid, Spain, 2011. [Google Scholar]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Dag, A.; Boim, S.; Sobotin, Y.; Zipori, I. Effect of Mechanically Harvested Olive Storage Temperature and Duration on Oil Quality. HortTechnology 2012, 22, 528–533. [Google Scholar]
- Jabeur, H.; Zribi, A.; Abdelhedi, R.; Bouaziz, M. Effect of olive storage conditions on Chemlali olive oil quality and the effective role of fatty acids alkyl esters in checking olive oils authenticity. Food Chem. 2015, 169, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.; Aranda, F.; Fregapane, G. Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality a study of four successive crop seasons. Food Chem. 2001, 73, 45–53. [Google Scholar] [CrossRef]
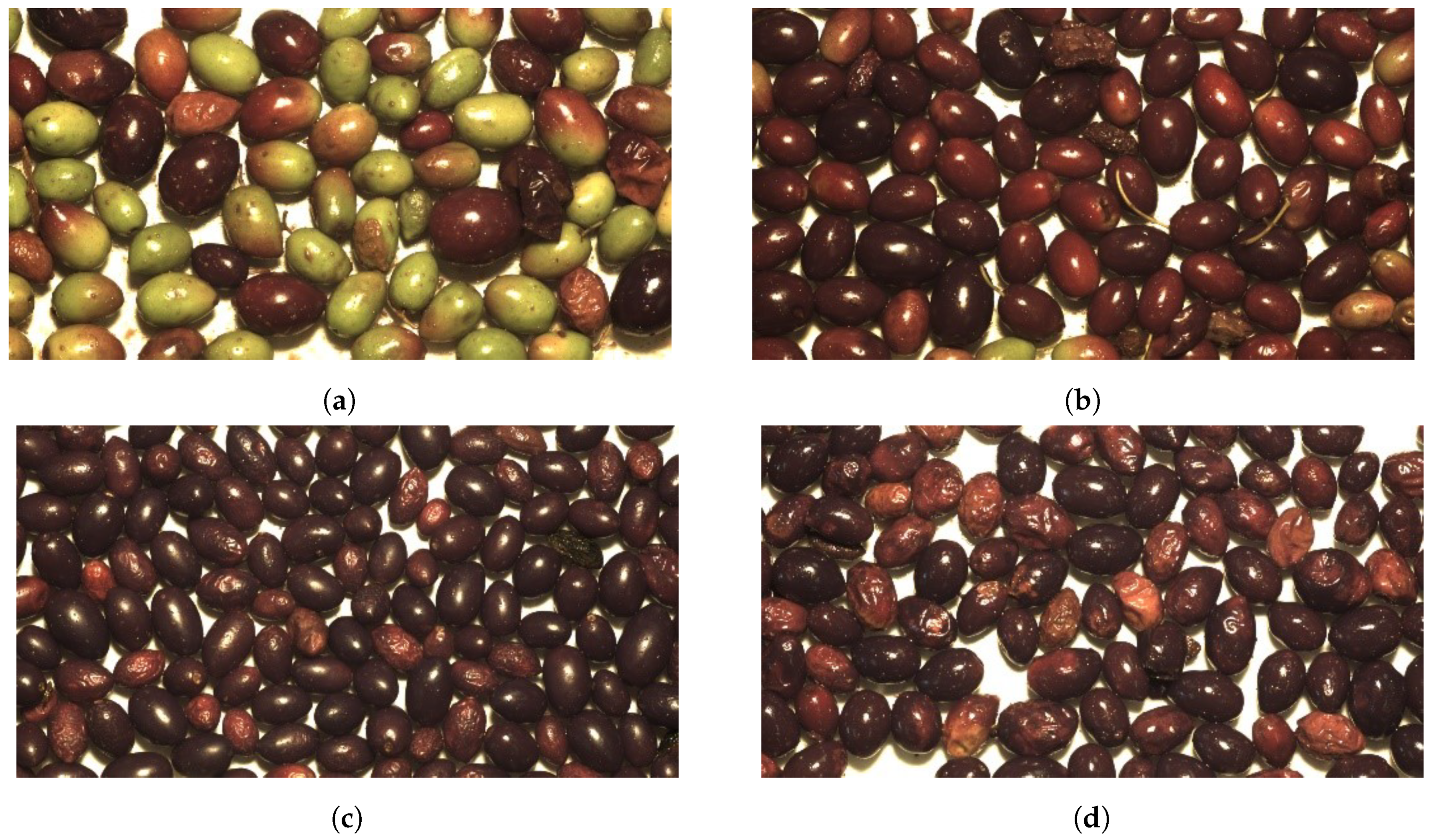
| Acidity Index (%) | Peroxide Index (meq/Kg) | K270 | K232 | Ethyl Ester (mg/Kg) | Ethyl Palmitate (mg/Kg) | Ethyl Oleate (mg/Kg) | Polyphenols | Chlorophyll | Carotenoids | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat-1 | Min. | 0.17 | 2.70 | 0.09 | 1.26 | 3.00 | 2.00 | 1.00 | 241.00 | 9.80 | 8.83 |
| Max. | 0.25 | 4.80 | 0.17 | 1.51 | 10.00 | 4.00 | 6.00 | 841.00 | 34.73 | 16.53 | |
| Mean | 0.20 a | 3.87 a | 0.13 a | 1.38 a | 5.77 a | 2.92 a | 2.85 a | 538.31 a | 21.26 c | 13.20 b | |
| Cat-2 | Min. | 0.12 | 2.10 | 0.12 | 1.34 | 4.00 | 2.00 | 1.00 | 452.00 | 6.24 | 7.49 |
| Max. | 0.31 | 5.80 | 0.28 | 1.92 | 9.00 | 4.00 | 5.00 | 1275.00 | 31.88 | 20.46 | |
| Mean | 0.22 a | 3.75 a | 0.17 a | 1.53 b | 5.77 a | 2.62 a | 3.15 a | 765.92 b | 17.42 bc | 12.20 ab | |
| Cat-3 | Min. | 0.17 | 2.60 | 0.11 | 1.39 | 5.00 | 2.00 | 3.00 | 182.00 | 6.30 | 4.96 |
| Max. | 0.59 | 15.40 | 0.21 | 1.70 | 64.00 | 15.00 | 50.00 | 864.00 | 20.30 | 16.91 | |
| Mean | 0.35 b | 6.76 ab | 0.15 a | 1.52 b | 17.00 ab | 4.92 ab | 12.00 ab | 585.54 ab | 12.89 ab | 10.50 ab | |
| Cat-4 | Min. | 0.15 | 2.70 | 0.10 | 1.31 | 5.00 | 2.00 | 2.00 | 171.00 | 2.42 | 3.04 |
| Max. | 0.79 | 22.50 | 0.20 | 1.77 | 104.00 | 21.00 | 83.00 | 846.00 | 22.90 | 14.78 | |
| Mean | 0.41 b | 8.98 b | 0.15 a | 1.50 b | 31.00 b | 7.38 b | 23.31 b | 457.62 a | 10.42 a | 9.39 a | |
| Parameters | Sampling Dates | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 21/11/2017 | 28/11/2017 | 4/12/2017 | 12/12/2017 | 18/12/2017 | 10/1/2018 | 17/1/2018 | 23/1/2018 | ||
| min | 0.12 | 0.17 | 0.20 | 0.15 | 0.19 | 0.27 | 0.29 | 0.15 | |
| max | 0.25 | 0.24 | 0.31 | 0.30 | 0.48 | 0.79 | 0.50 | 0.66 | |
| mean | 0.19 | 0.20 | 0.25 | 0.22 | 0.28 | 0.45 | 0.39 | 0.40 | |
| SD | 0.03 | 0.02 | 0.04 | 0.04 | 0.08 | 0.18 | 0.08 | 0.14 | |
| min | 2.10 | 2.80 | 2.70 | 2.40 | 5.10 | 3.00 | 2.80 | 2.60 | |
| max | 5.80 | 4.60 | 4.30 | 5.40 | 11.30 | 17.20 | 10.90 | 22.50 | |
| mean | 4.20 | 3.58 | 3.62 | 3.55 | 7.43 | 8.26 | 4.89 | 7.55 | |
| SD | 0.93 | 0.55 | 0.56 | 1.01 | 1.97 | 5.50 | 2.99 | 6.06 | |
| min | 0.12 | 0.11 | 0.09 | 0.10 | 0.11 | 0.12 | 0.10 | 0.13 | |
| max | 0.19 | 0.28 | 0.22 | 0.24 | 0.23 | 0.19 | 0.21 | 0.21 | |
| mean | 0.14 | 0.17 | 0.16 | 0.17 | 0.15 | 0.15 | 0.16 | 0.16 | |
| SD | 0.02 | 0.04 | 0.04 | 0.05 | 0.04 | 0.03 | 0.04 | 0.02 | |
| min | 1.29 | 1.26 | 1.27 | 1.31 | 1.43 | 1.28 | 1.31 | 1.41 | |
| max | 1.67 | 1.92 | 1.76 | 1.97 | 1.84 | 1.68 | 1.70 | 1.76 | |
| mean | 1.41 | 1.50 | 1.49 | 1.63 | 1.57 | 1.47 | 1.56 | 1.53 | |
| SD | 0.11 | 0.17 | 0.14 | 0.23 | 0.14 | 0.12 | 0.13 | 0.11 | |
| min | 3.00 | 5.00 | 4.00 | 11.00 | 5.00 | 11.00 | 5.00 | 8.00 | |
| max | 7.00 | 10.00 | 6.00 | 34.00 | 15.00 | 104.00 | 57.00 | 70.00 | |
| mean | 5.40 | 7.10 | 4.90 | 19.00 | 9.09 | 31.77 | 14.57 | 24.46 | |
| SD | 1.51 | 1.60 | 0.88 | 7.42 | 3.21 | 27.75 | 18.82 | 15.89 | |
| min | 2.00 | 2.00 | 2.00 | 3.00 | 2.00 | 3.00 | 2.00 | 3.00 | |
| max | 4.00 | 4.00 | 3.00 | 10.00 | 5.00 | 21.00 | 13.00 | 15.00 | |
| mean | 2.07 | 3.40 | 2.10 | 6.30 | 3.36 | 7.54 | 4.14 | 6.23 | |
| SD | 0.82 | 0.70 | 0.32 | 2.00 | 0.92 | 5.46 | 3.98 | 3.14 | |
| min | 1.00 | 3.00 | 2.00 | 6.00 | 3.00 | 8.00 | 2.00 | 5.00 | |
| max | 5.00 | 6.00 | 4.00 | 24.00 | 10.00 | 83.00 | 44.00 | 55.00 | |
| mean | 2.60 | 3.80 | 2.80 | 12.20 | 5.82 | 24.23 | 10.43 | 18.15 | |
| SD | 1.35 | 1.14 | 0.79 | 5.98 | 2.23 | 22.45 | 14.93 | 12.78 | |
| min | 321.00 | 329.00 | 241.00 | 290.00 | 358.00 | 182.00 | 171.00 | 221.00 | |
| max | 782.00 | 1275.00 | 1089.00 | 1176.00 | 916.00 | 864.00 | 861.00 | 757.00 | |
| mean | 532.60 | 739.20 | 744.60 | 719.20 | 662.45 | 516.54 | 597.00 | 535.69 | |
| SD | 136.45 | 246.97 | 232.71 | 315.84 | 197.70 | 239.18 | 233.79 | 148.25 | |
| min | 9.84 | 8.83 | 7.49 | 6.88 | 9.40 | 4.96 | 3.04 | 4.49 | |
| max | 17.22 | 20.46 | 14.69 | 22.26 | 19.41 | 15.74 | 7.02 | 14.78 | |
| mean | 13.92 | 13.38 | 10.54 | 11.73 | 13.09 | 10.47 | 5.74 | 10.11 | |
| SD | 2.33 | 3.20 | 2.45 | 4.58 | 2.91 | 3.47 | 1.56 | 3.07 | |
| min | 13.51 | 12.13 | 6.24 | 5.40 | 5.60 | 2.40 | 3.20 | 2.42 | |
| max | 34.73 | 31.88 | 19.58 | 39.50 | 19.20 | 28.60 | 12.10 | 14.01 | |
| mean | 23.80 | 20.89 | 11.76 | 12.80 | 11.78 | 15.71 | 7.50 | 8.63 | |
| SD | 6.57 | 5.25 | 4.21 | 10.06 | 4.69 | 8.31 | 3.25 | 3.20 | |
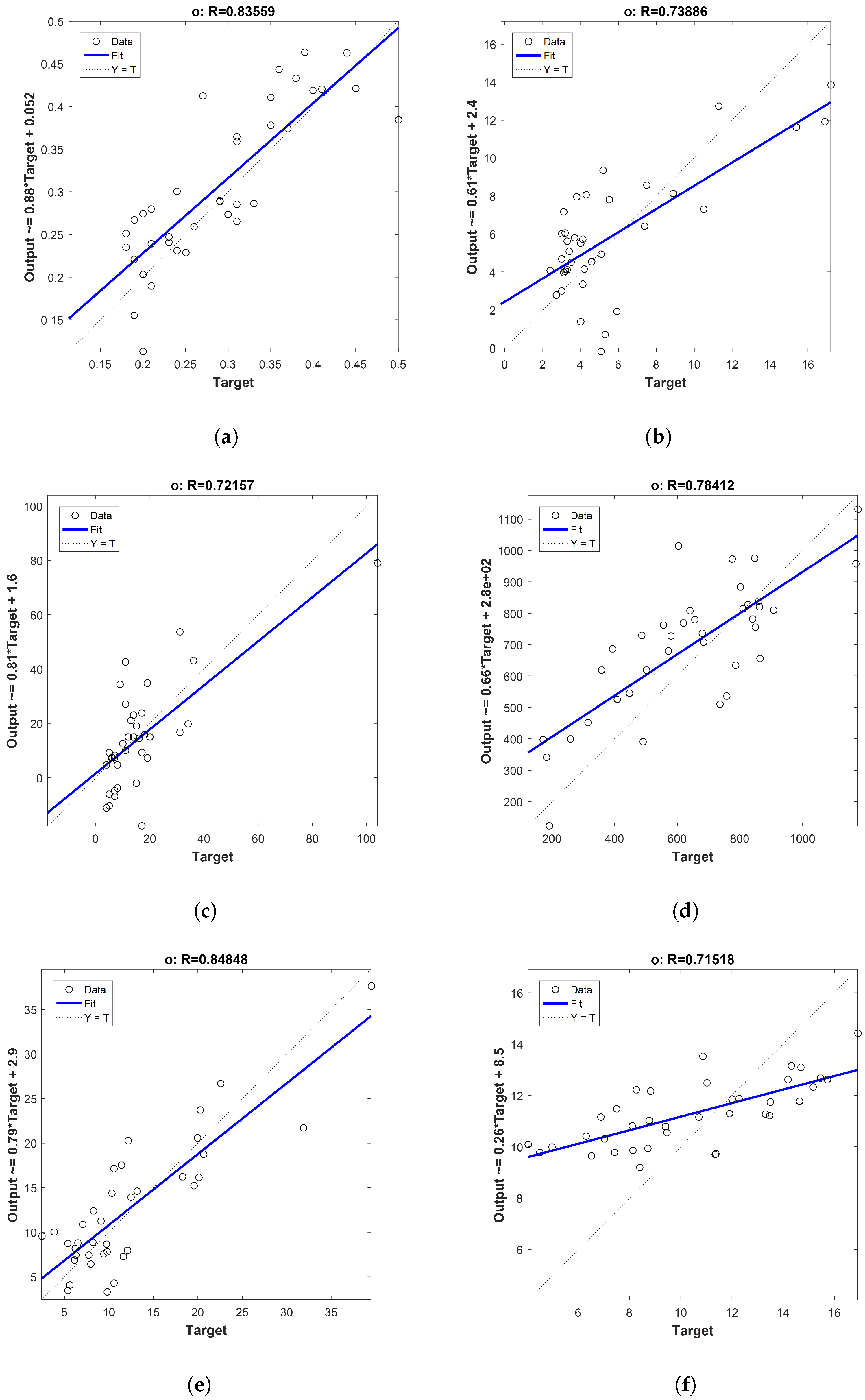
| Parameter | lv | RPD | ||||
|---|---|---|---|---|---|---|
| Acidity Index | 0.89 | 0.84 | 0.11 | 0.12 | 2 | 2.16 |
| Peroxide value | 0.87 | 0.74 | 3.97 | 4.12 | 1 | 1.01 |
| K270 | 0.83 | 0.64 | 0.03 | 0.04 | 3 | 1.28 |
| K232 | 0.83 | 0.69 | 0.13 | 0.15 | 4 | 1.29 |
| Ethyl Ester | 0.88 | 0.72 | 15.74 | 16.89 | 2 | 1.07 |
| Ethyl Palmitate | 0.85 | 0.68 | 3.21 | 3.42 | 2 | 1.17 |
| Ethyl Oleate | 0.88 | 0.74 | 12.67 | 13.52 | 2 | 1.07 |
| Polyphenols | 0.88 | 0.78 | 200.6 | 243.50 | 5 | 1.32 |
| Chlorophyll | 0.91 | 0.85 | 5.96 | 6.71 | 2 | 1.08 |
| Carotenoids | 0.88 | 0.72 | 3.02 | 3.40 | 2 | 1.44 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro Soto, J.; Satorres Martínez, S.; Martínez Gila, D.; Gómez Ortega, J.; Gámez García, J. Fast and Reliable Determination of Virgin Olive Oil Quality by Fruit Inspection Using Computer Vision. Sensors 2018, 18, 3826. https://doi.org/10.3390/s18113826
Navarro Soto J, Satorres Martínez S, Martínez Gila D, Gómez Ortega J, Gámez García J. Fast and Reliable Determination of Virgin Olive Oil Quality by Fruit Inspection Using Computer Vision. Sensors. 2018; 18(11):3826. https://doi.org/10.3390/s18113826
Chicago/Turabian StyleNavarro Soto, Javiera, Silvia Satorres Martínez, Diego Martínez Gila, Juan Gómez Ortega, and Javier Gámez García. 2018. "Fast and Reliable Determination of Virgin Olive Oil Quality by Fruit Inspection Using Computer Vision" Sensors 18, no. 11: 3826. https://doi.org/10.3390/s18113826
APA StyleNavarro Soto, J., Satorres Martínez, S., Martínez Gila, D., Gómez Ortega, J., & Gámez García, J. (2018). Fast and Reliable Determination of Virgin Olive Oil Quality by Fruit Inspection Using Computer Vision. Sensors, 18(11), 3826. https://doi.org/10.3390/s18113826





