Enhanced Sensitivity of a Hydrogen Sulfide Sensor Based on Surface Acoustic Waves at Room Temperature
Abstract
1. Introduction
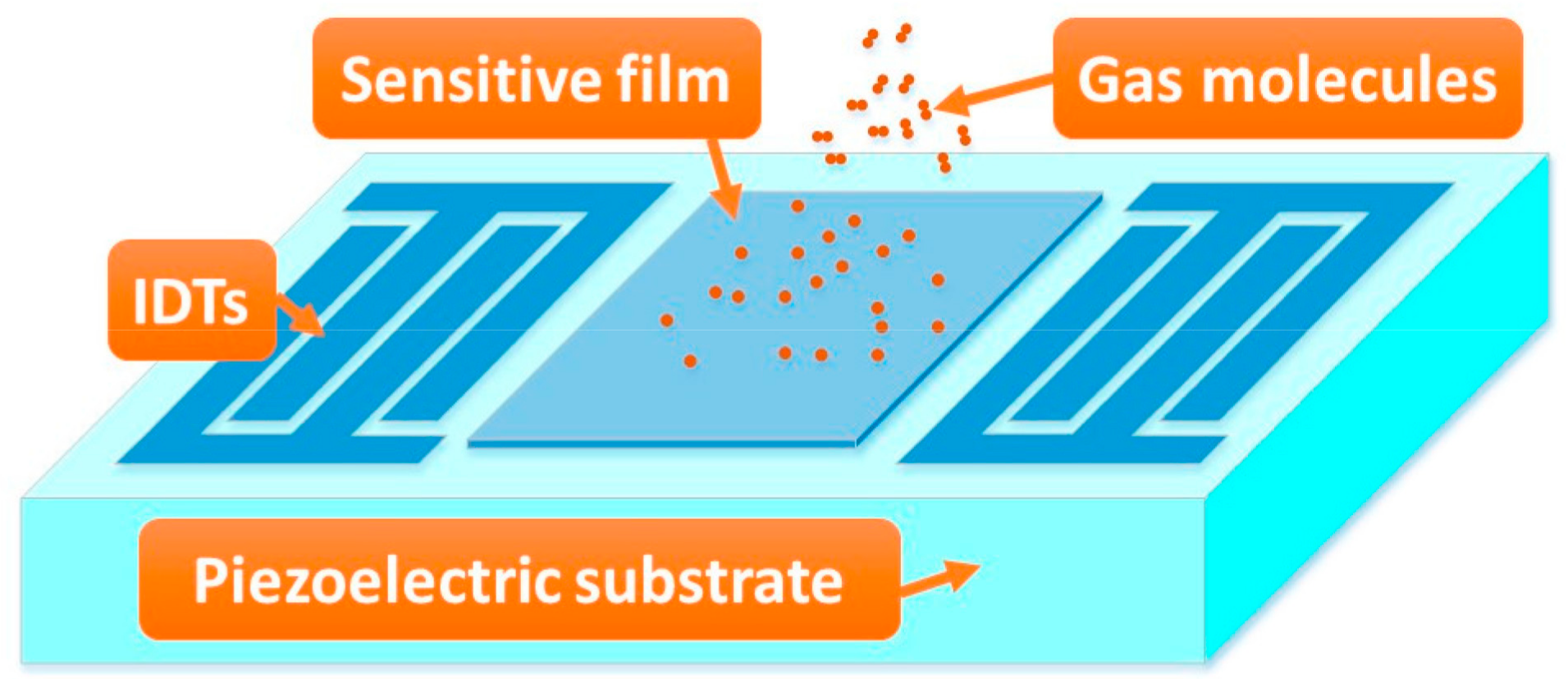
2. Technique Realization
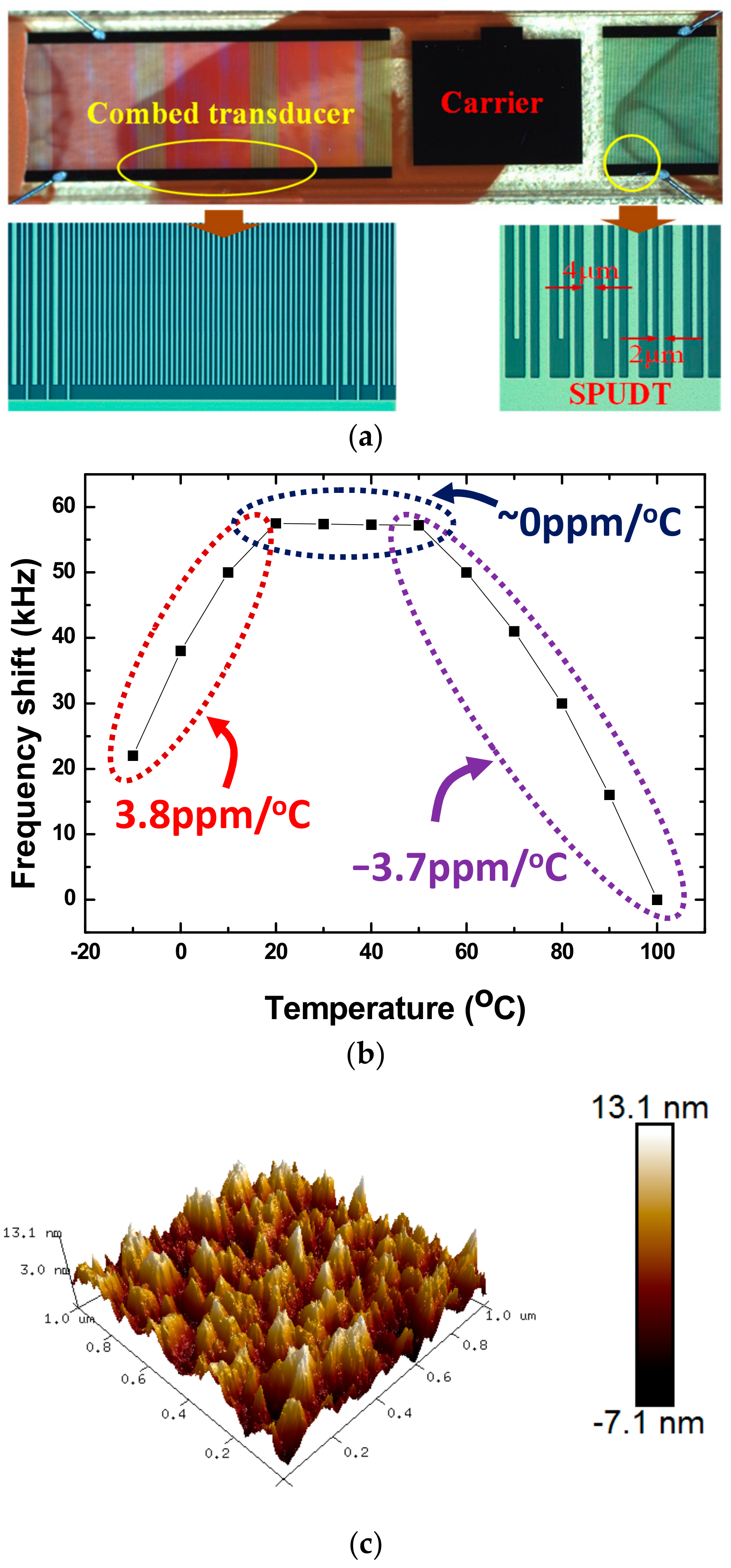
2.1. SAW Devices
2.2. TEA Preparation
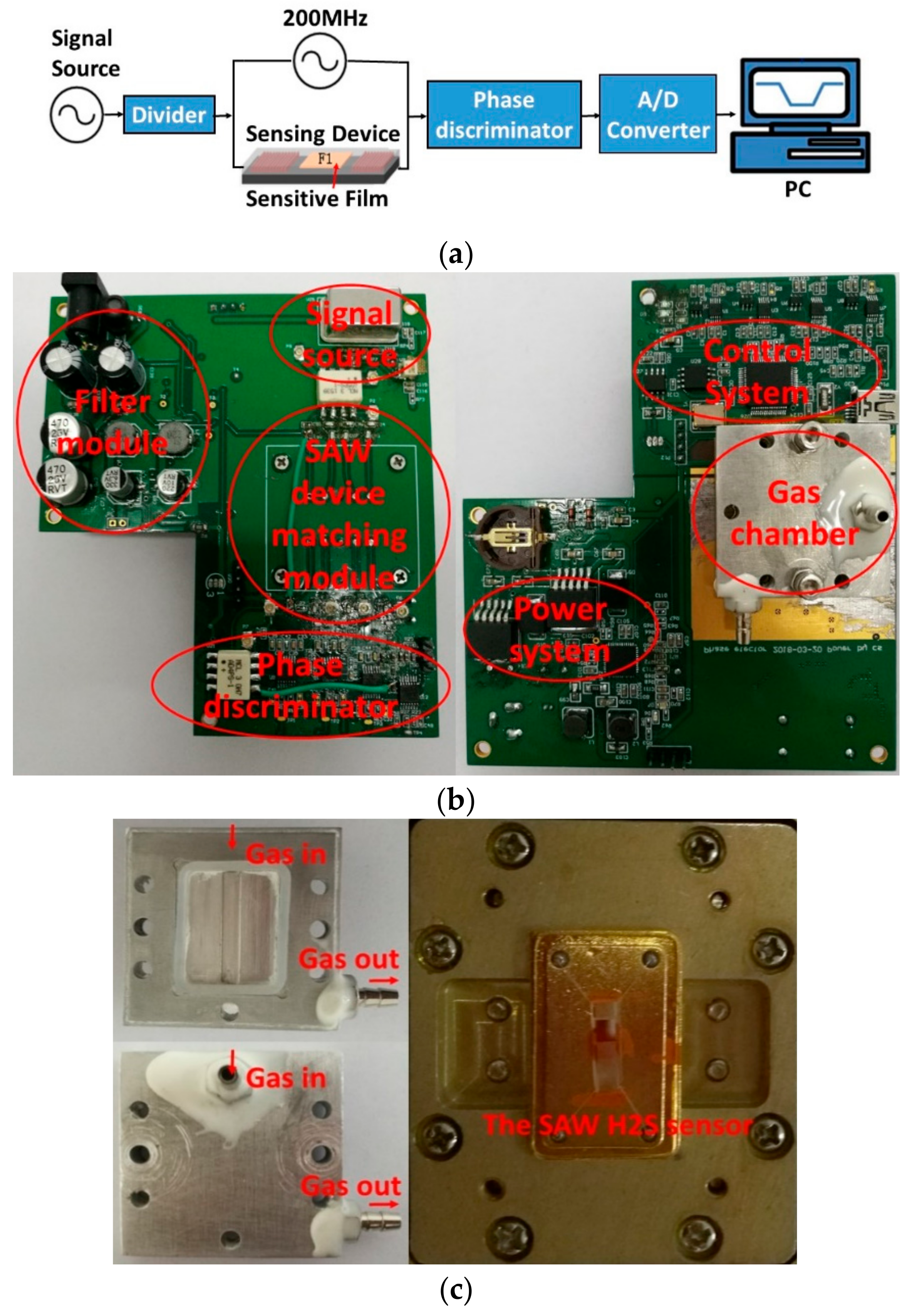
2.3. SAW Sensor System
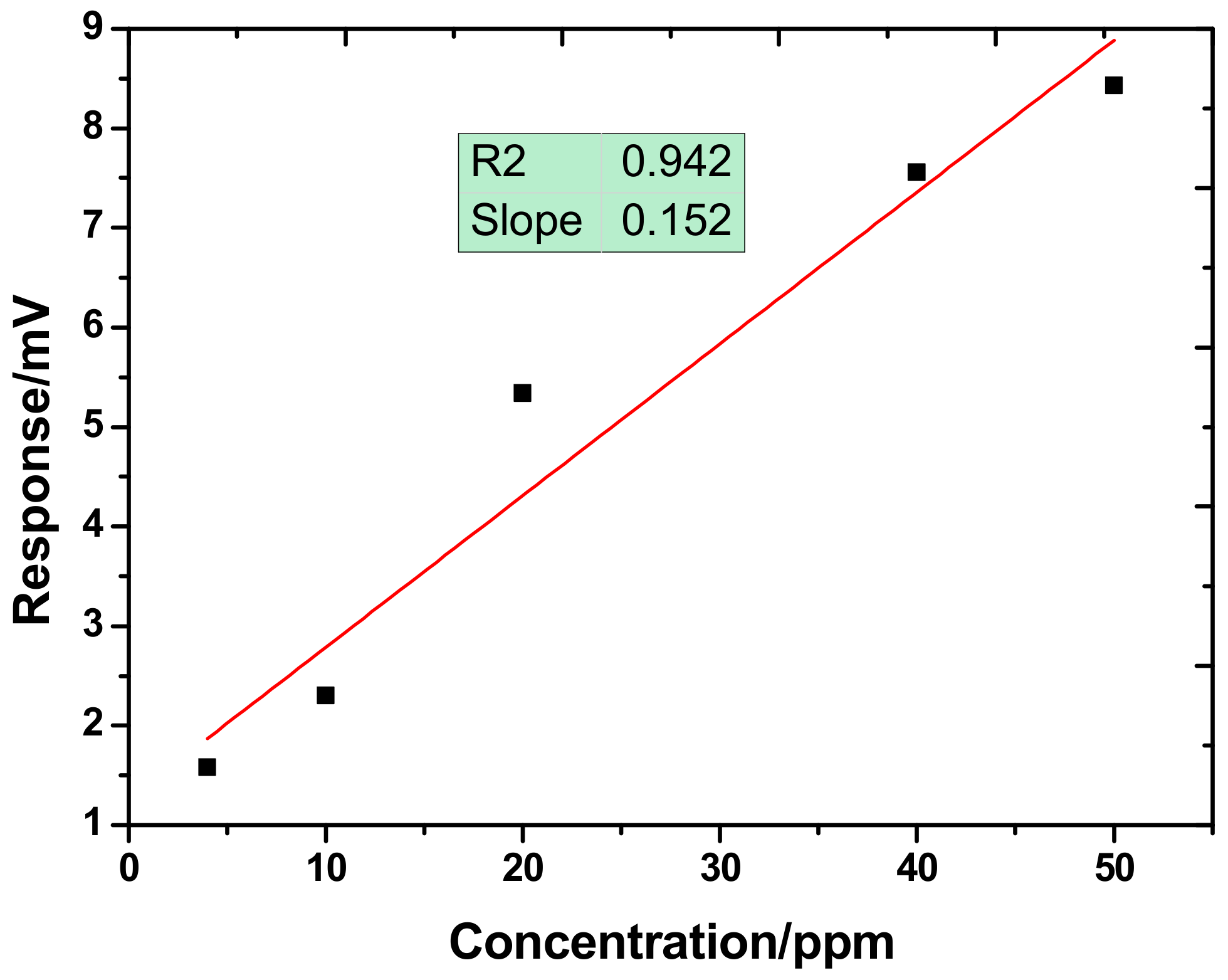
3. Experimental Results and Discussions
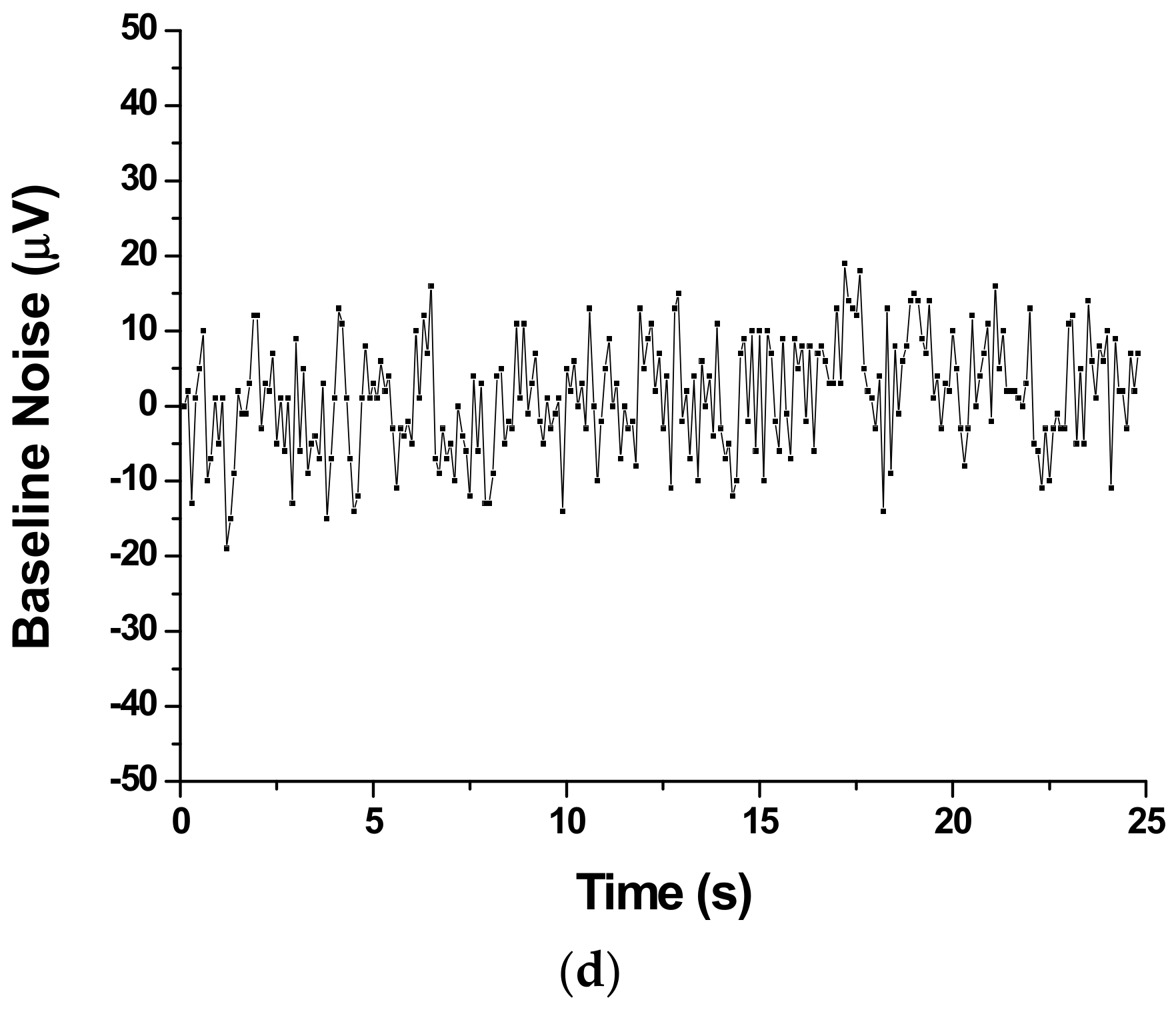
3.1. Experimental Setup
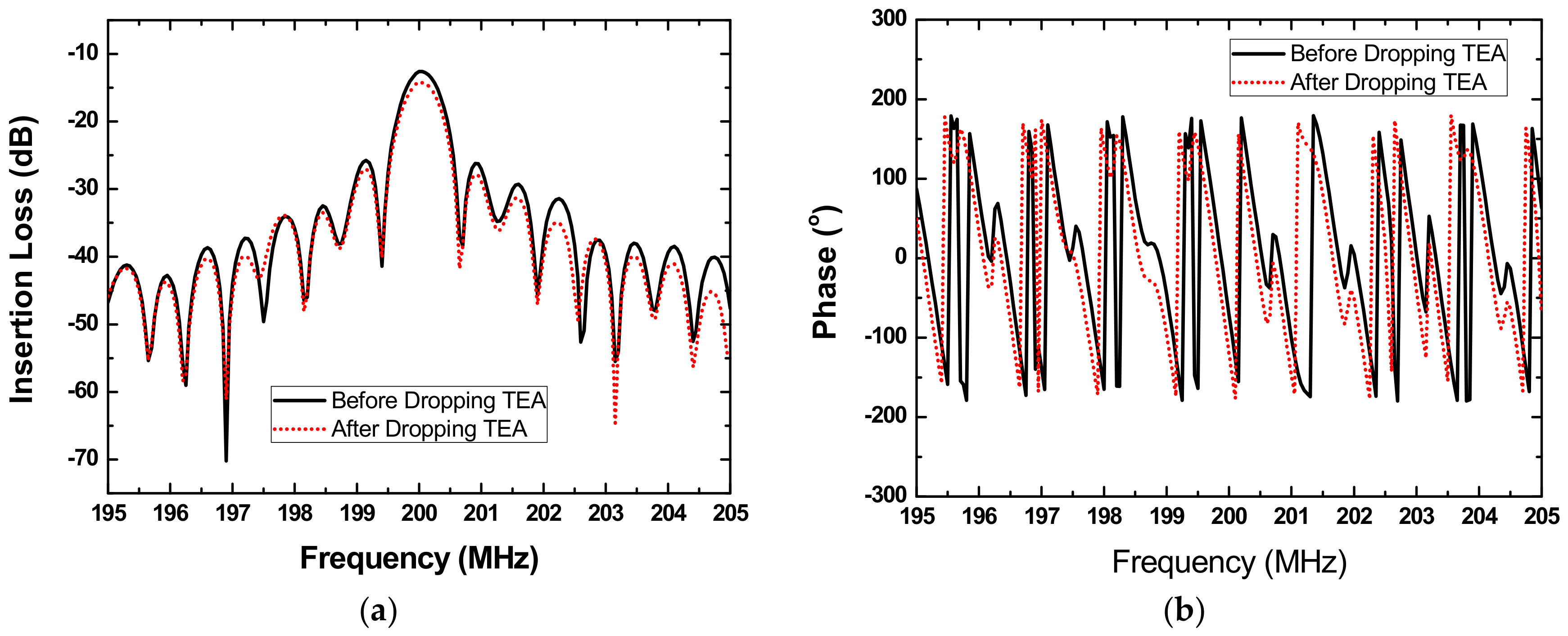
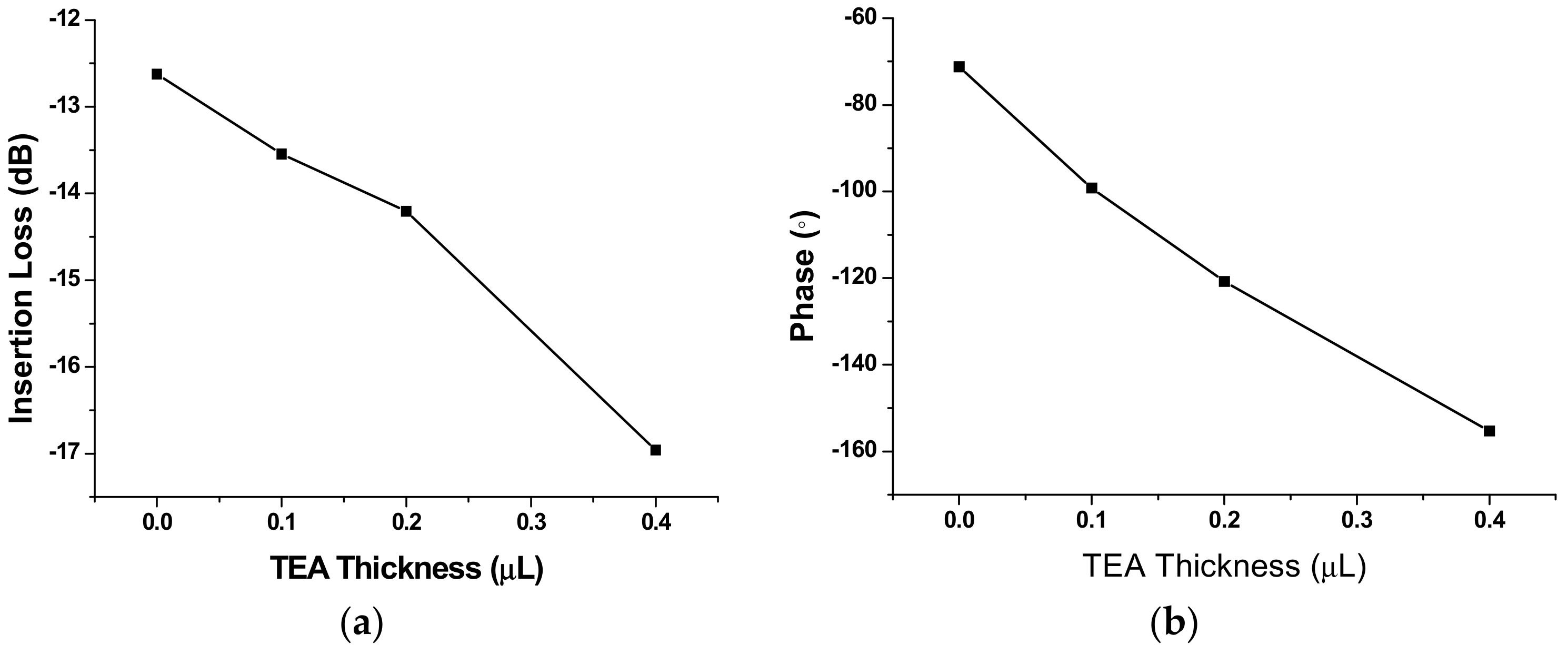
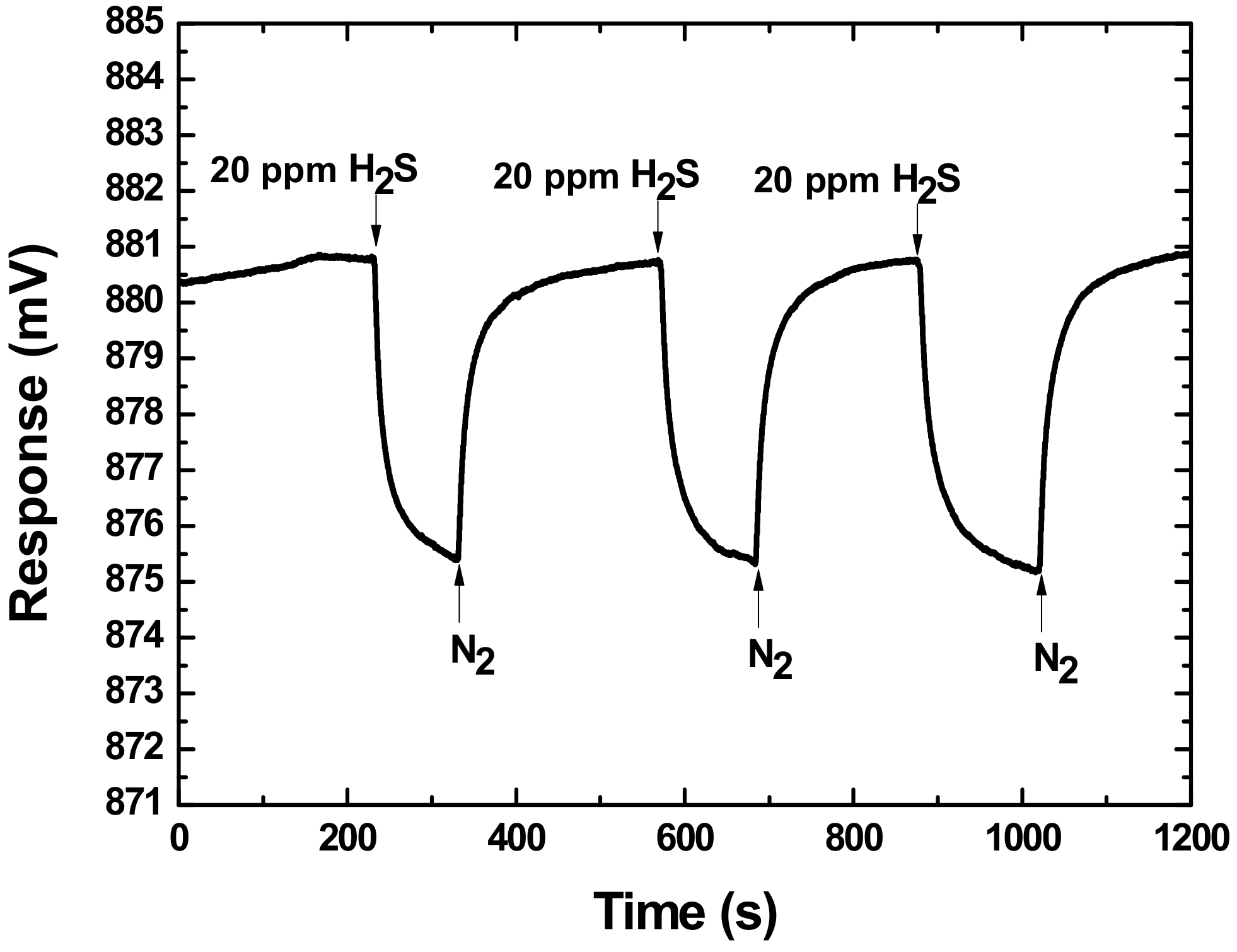
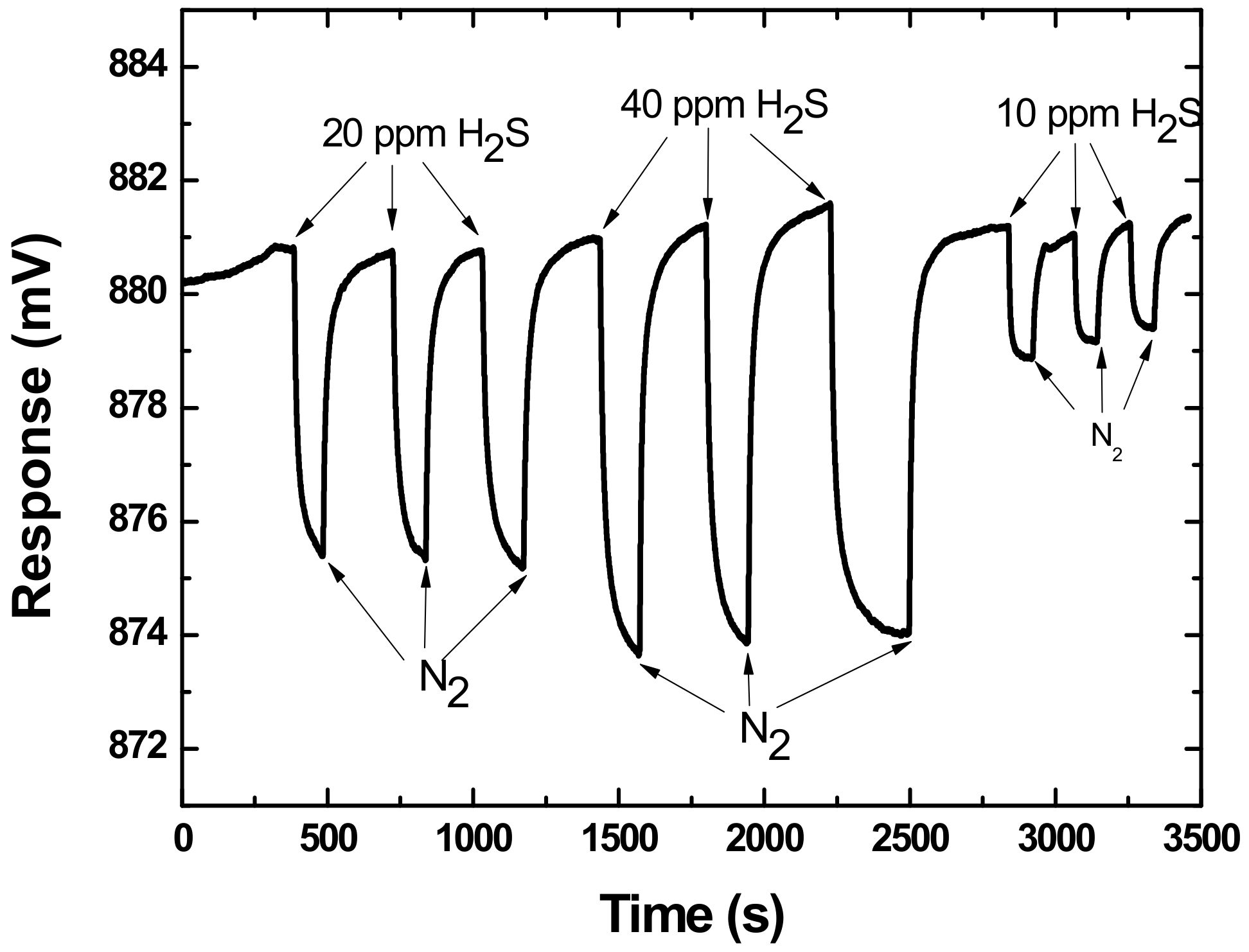
3.2. Gas Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mellor, J.W. A Comprehensive Treatise on Inorganic and Theoretical Chemistry. Nature 2014, 117, 249–250. [Google Scholar] [CrossRef]
- Wohltijon, H.; Ressy, R. Surface Acoustic Wave Probe for Chemical Analysis: Part I—Instruction and Instrument Description, part II-Gas Chromatography Detector. Anal. Chem. 1979, 51, 1458–1469. [Google Scholar] [CrossRef]
- Rana, L.; Gupta, R.; Tomar, M. ZnO/ST-Quartz SAW resonator: An efficient NO2 sensor. Sens. Actuators B Chem. 2017, 252, 252–257. [Google Scholar] [CrossRef]
- Marcu, A.; Viespe, C. Surface acoustic wave sensors for Hydrogen and Deuterium detection. Sensors 2017, 17, 1417. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.B.; Tomar, M.; Nimal, A.T. Nano-crystalline SnO2 thin film based surface acoustic wave sensor for selective and fast detection of NO2 gas. Adv. Sci. Lett. 2014, 20, 1124–1128. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, C.Y.; Shen, C.Y.; Zhi, J.L. Nitric oxide sensing properties of a surface acoustic wave sensor with copper-ion-doped polyaniline/tungsten oxide nanocomposite film. Sens. Actuators B Chem. 2017, 243, 1075–1082. [Google Scholar] [CrossRef]
- Wang, W.; Hu, H.L.; He, S.T.; Pan, Y.; Zhang, C.H.; Dong, C. Development of a room temperature SAW methane gas sensor incorporating a supramolecular cryptophane. A coating. Sensors 2016, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, S.Y.; Liang, Y.; He, S.T.; Pan, Y.; Zhang, C.H.; Dong, C. Enhanced sensitivity of a Love wave based methane gas sensor incorporating Cryptophane—A thin film. Sensors 2018, 18, 3247. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Mu, N.; Shao, S.Y.; Yang, L.; Wang, W.; Xie, X.; He, S.T. Selective Surface Acoustic Wave-Based Organophosphorus Sensor Employing a Host-Guest Self-Assembly Monolayer of β-Cyclodextrin Derivative. Sensors 2015, 15, 17916–17925. [Google Scholar] [CrossRef] [PubMed]
- Sayago, I.; Matatagui, D.; Fernandez, M.J.; Fontecha, J.L.; Jurewicz, J. Graphene oxide as sensitive layer in Love-wave surface acoustic wave sensors for the detection of chemical warfare agent simulants. Talanta 2016, 148, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Cheng, Y.H.; Wang, S.H. A Nitrogen Dioxide Surface Acoustic Wave Sensor Based on Polyaniline/Tungsten Oxide Nanocomposite. In Proceedings of the International Conference on Measuring Technology and Mechatronics Automation, Changsha, China, 13–14 March 2010; pp. 204–207. [Google Scholar]
- Wang, W.; Hu, H.; He, S.; Pan, Y.; Zhang, C.; Dong, C. A room temperature SAW based methane gas sensors. In Proceedings of the IEEE International Ultrasonics Symposium, Prague, Czech, 21–25 July 2013; pp. 2148–2150. [Google Scholar]
- Nikolaou, I.; Hallil, H.; Deligeorgis, G.; Conedera, V.; Garcia, H.; Dejous, C.; Rebière, D. Novel SAW gas sensor based on grapheme. In Proceedings of the Microelectronics Technology and Devices, Salvador, Brazil, 31 August–4 September 2015; pp. 1–4. [Google Scholar]
- Luo, W.; Fu, Q.; Zhou, D.; Deng, J.; Liu, H.; Yan, G. A surface acoustic wave H2S gas sensor employing nanocrystalline SnO2 thin film. Sens. Actuators B Chem. 2013, 176, 746–752. [Google Scholar] [CrossRef]
- Li, H.L.; Wang, X.D.; He, S.T.; Fu, Q.Y.; Luo, W.; Zhou, D.X. A SAW-based H2S sensor coated SnO2 thin film, in: Symposium on Piezoelectricity. Acoust. Waves Device Appl. 2010. [Google Scholar] [CrossRef]
- Luo, W.; Deng, J.; Fu, Q.; Zhou, D.; Hu, Y.; Gong, S.; Zheng, Z. Nanocrystalline SnO2, film prepared by the aqueous sol-gel method and its application as sensing films of the resistance and SAW H2S sensor. Sens. Actuators B Chem. 2015, 217, 119–128. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, H.; Chen, F.; Ke, Y.; He, S. Development of a SnO2/CuO-coated surface acoustic wave-based H2S sensor with switch-like response and recovery. Sens. Actuators B Chem. 2012, 169, 10–16. [Google Scholar] [CrossRef]
- Asad, M.; Sheikhi, M.H. Surface acoustic wave based H2S gas sensors incorporating sensitive layers of single wall carbon nanotubes decorated with Cu nanoparticles. Sens. Actuators B Chem. 2014, 198, 134–141. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, L.; Liu, W.W. A kind of SAW sensor technology for the detection of H2S. Chem. Sens. 2012, 747–752. [Google Scholar] [CrossRef]
- Wang, W.; Lee, K.; Woo, I.; Park, I.; Yang, S. Optimal design on SAW sensor for wireless pressure measurement based on reflective delay line. Sens. Actuators A Phys. 2007, 139, 2–6. [Google Scholar] [CrossRef]
- Wang, W.; He, S. Viscoelastic Analysis of a Surface Acoustic Wave Gas Sensor Coated by a New Deposition Technique. Chin. J. Chem. Phys. 2006, 19, 47–53. [Google Scholar] [CrossRef]

| Response (mV) | Response Time (s) | Recovery Time (s) |
|---|---|---|
| 5.391 | 17.3 | 26.7 |
| 5.344 | 20.5 | 21.6 |
| 5.458 | 23.8 | 24.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, W.; Zhang, Y.; Pan, Y.; Liang, Y.; Li, J. Enhanced Sensitivity of a Hydrogen Sulfide Sensor Based on Surface Acoustic Waves at Room Temperature. Sensors 2018, 18, 3796. https://doi.org/10.3390/s18113796
Liu X, Wang W, Zhang Y, Pan Y, Liang Y, Li J. Enhanced Sensitivity of a Hydrogen Sulfide Sensor Based on Surface Acoustic Waves at Room Temperature. Sensors. 2018; 18(11):3796. https://doi.org/10.3390/s18113796
Chicago/Turabian StyleLiu, Xueli, Wen Wang, Yufeng Zhang, Yong Pan, Yong Liang, and Junhong Li. 2018. "Enhanced Sensitivity of a Hydrogen Sulfide Sensor Based on Surface Acoustic Waves at Room Temperature" Sensors 18, no. 11: 3796. https://doi.org/10.3390/s18113796
APA StyleLiu, X., Wang, W., Zhang, Y., Pan, Y., Liang, Y., & Li, J. (2018). Enhanced Sensitivity of a Hydrogen Sulfide Sensor Based on Surface Acoustic Waves at Room Temperature. Sensors, 18(11), 3796. https://doi.org/10.3390/s18113796





