The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties
Abstract
:1. Introduction
2. Gas-Sensing Mechanism
2.1. Adsorption Oxygen on the Surface of Semiconductor Oxides
2.2. Band Gap
2.3. Schottky Barrier Contact
2.4. Catalysis-Based Sensing Mechanism
2.5. Heterojunction-Based Sensing Mechanism
3. Different Morphologies of Nanomaterials for Gas-Sensing Performances
3.1. Zero-Dimensional Sensing Materials
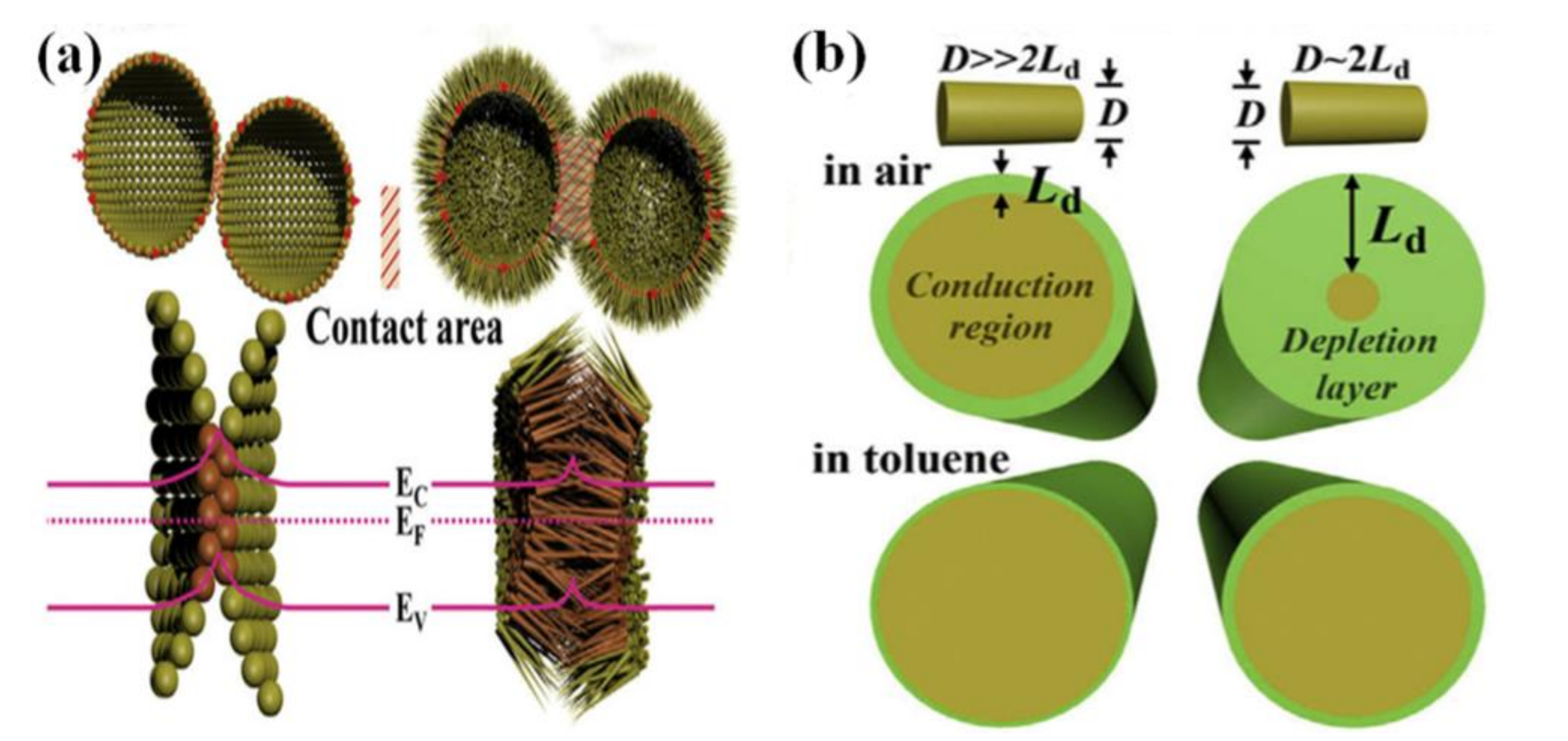
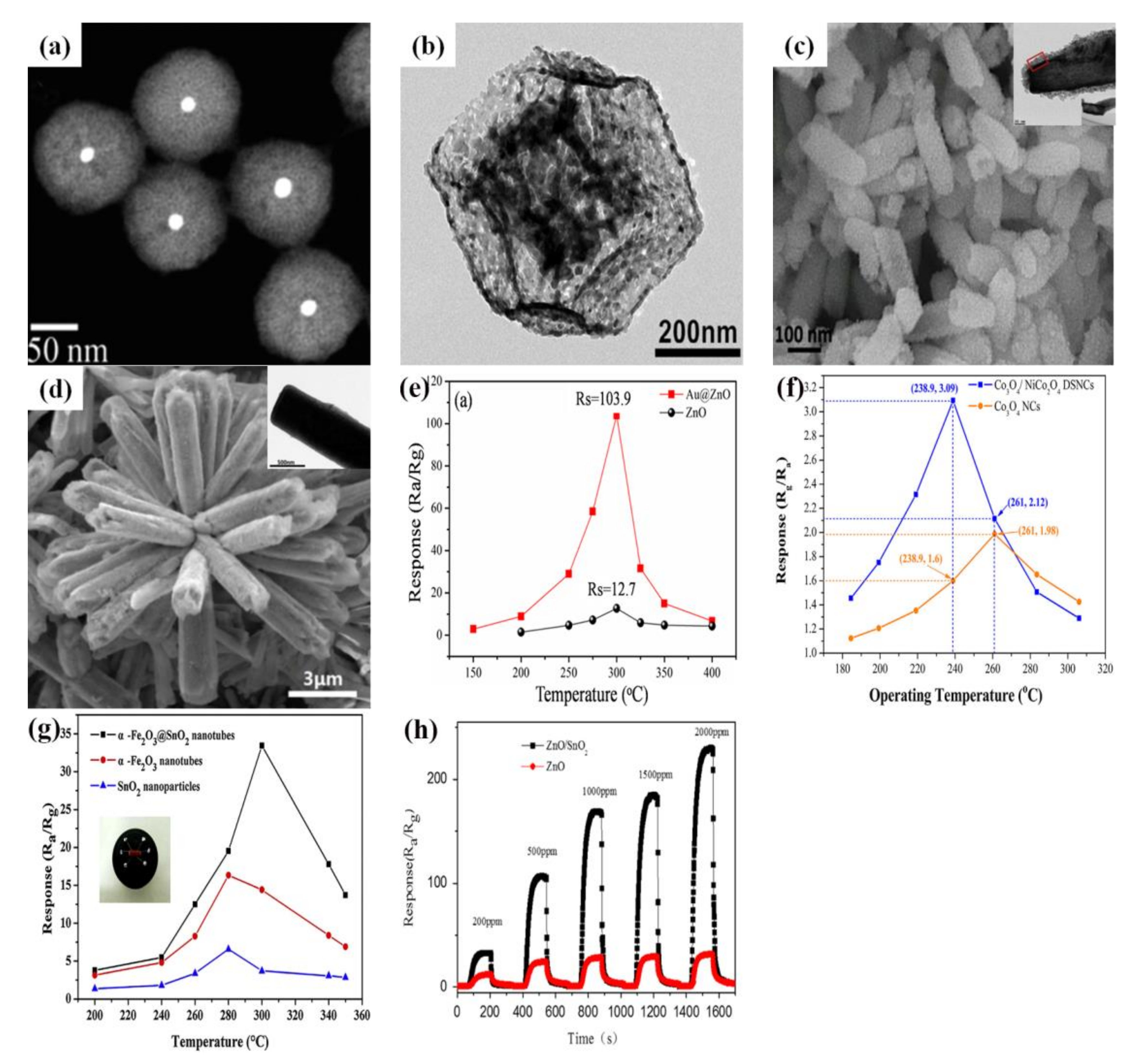
3.2. One-Dimensional Sensing Materials
3.3. Two-Dimensional Sensing Materials
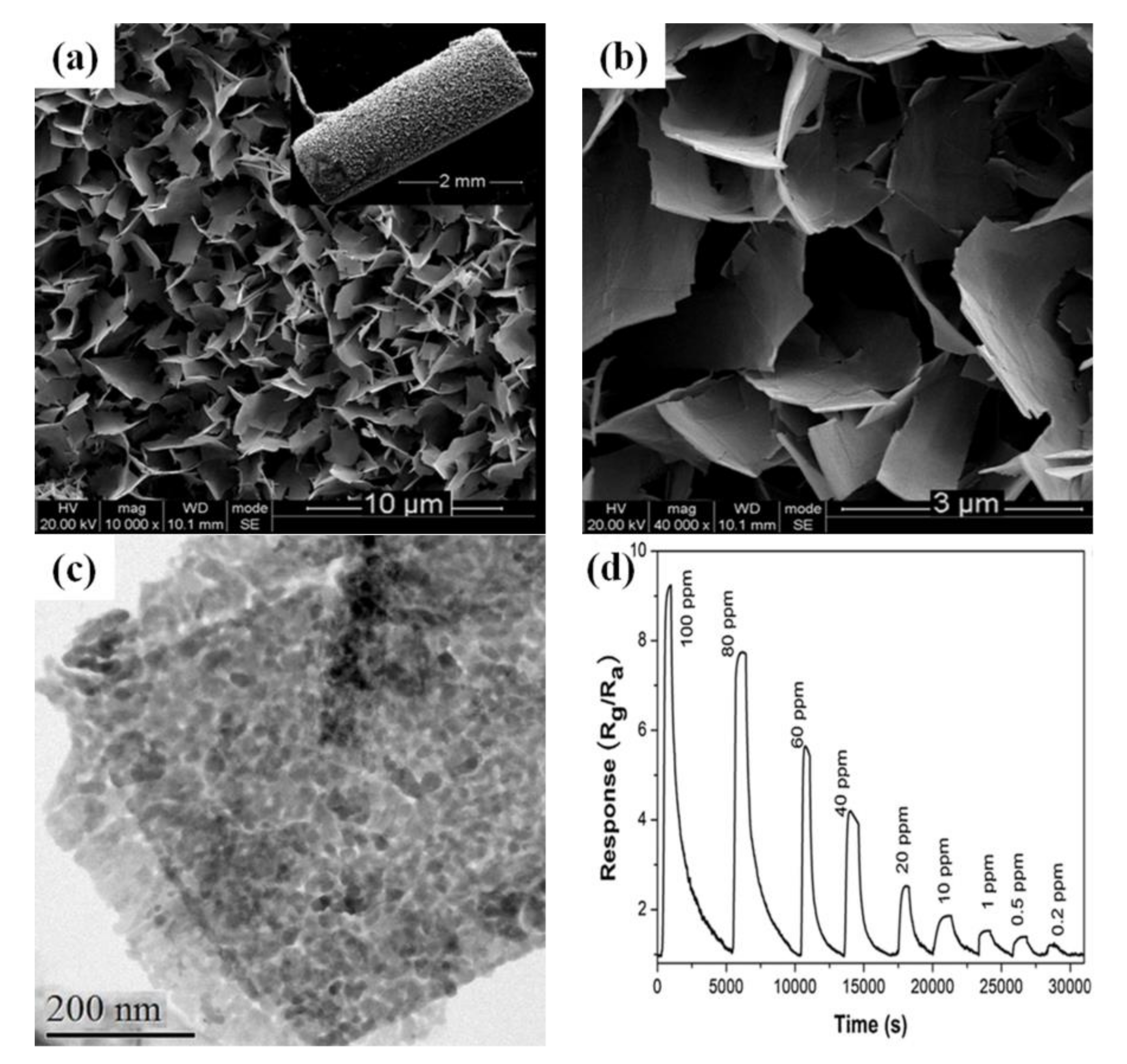
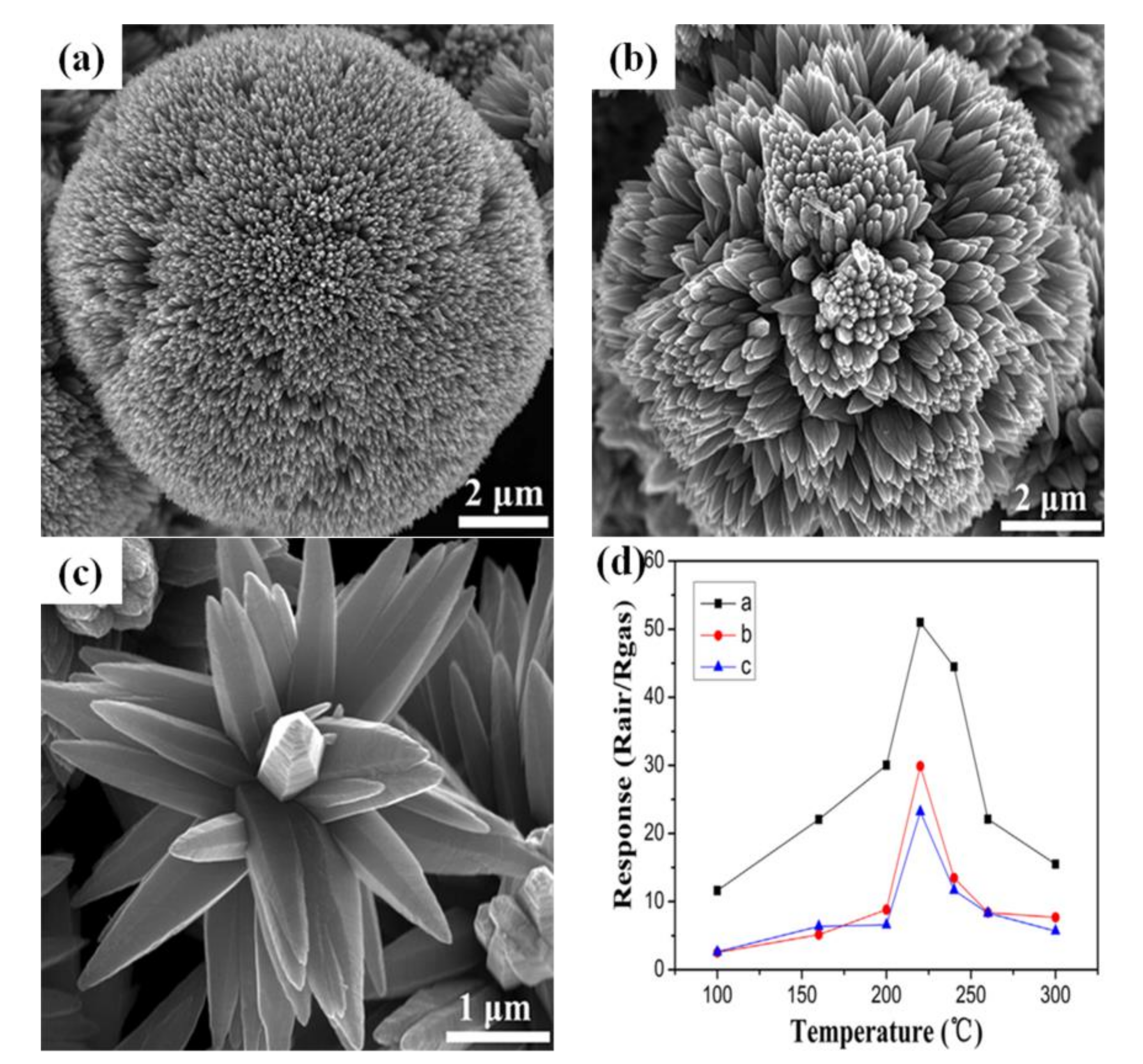
3.4. Three-Dimensional Sensing Materials
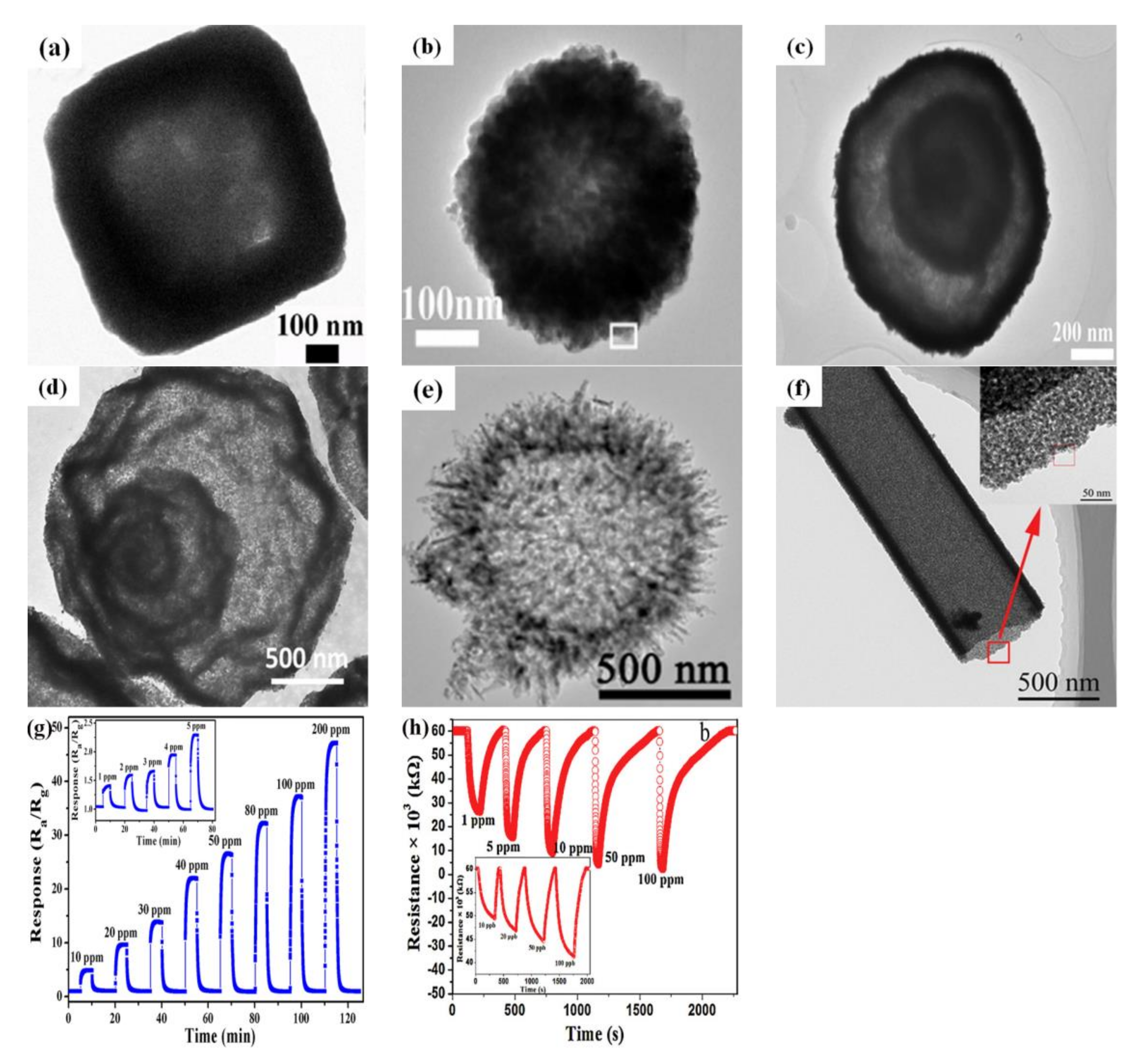
4. Hollow Semiconductor Oxide Structures for Gas-Sensing Performance
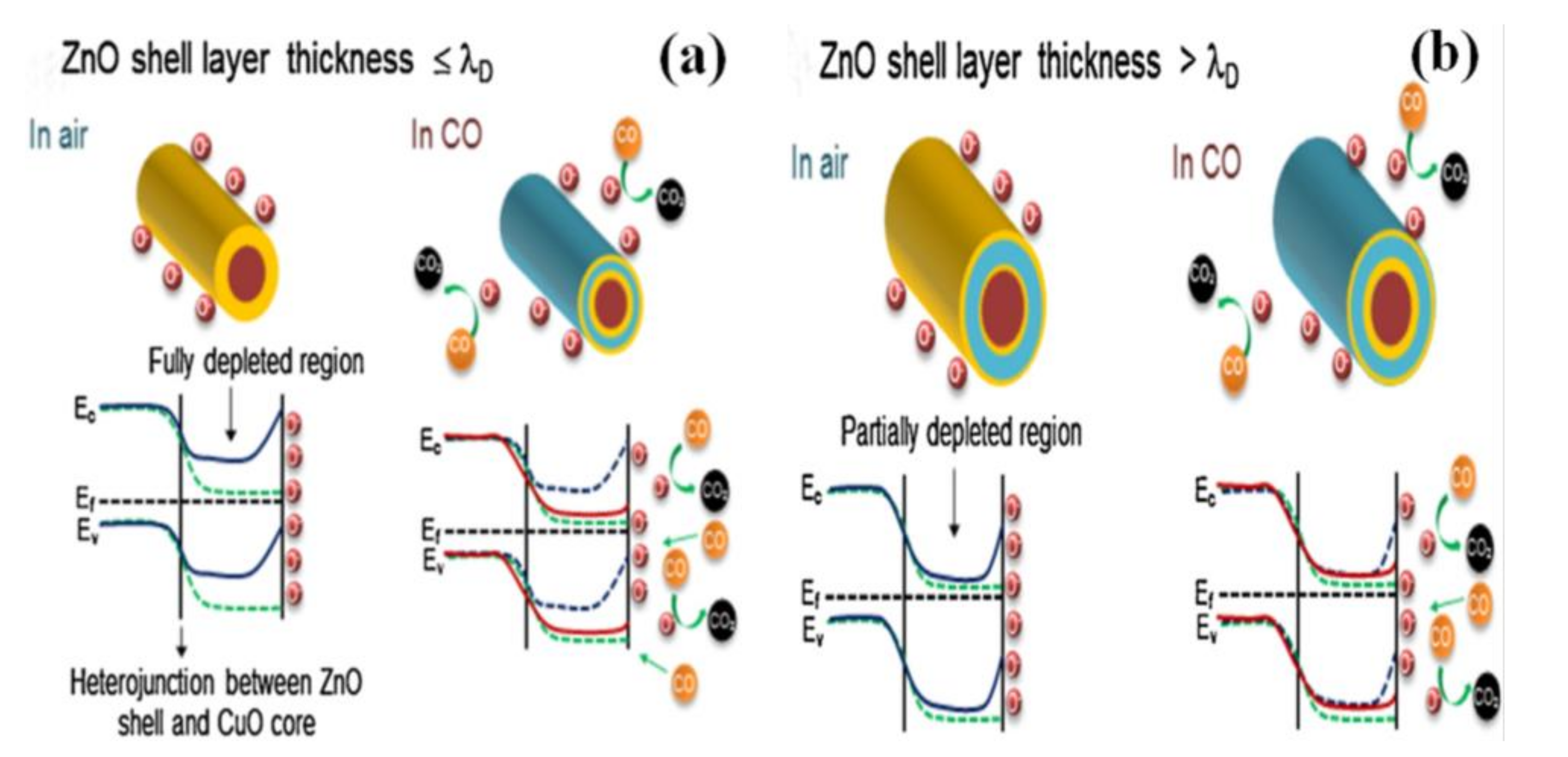
5. Core-Shell Structure of Semiconductor Oxide for Gas-Sensing Performance
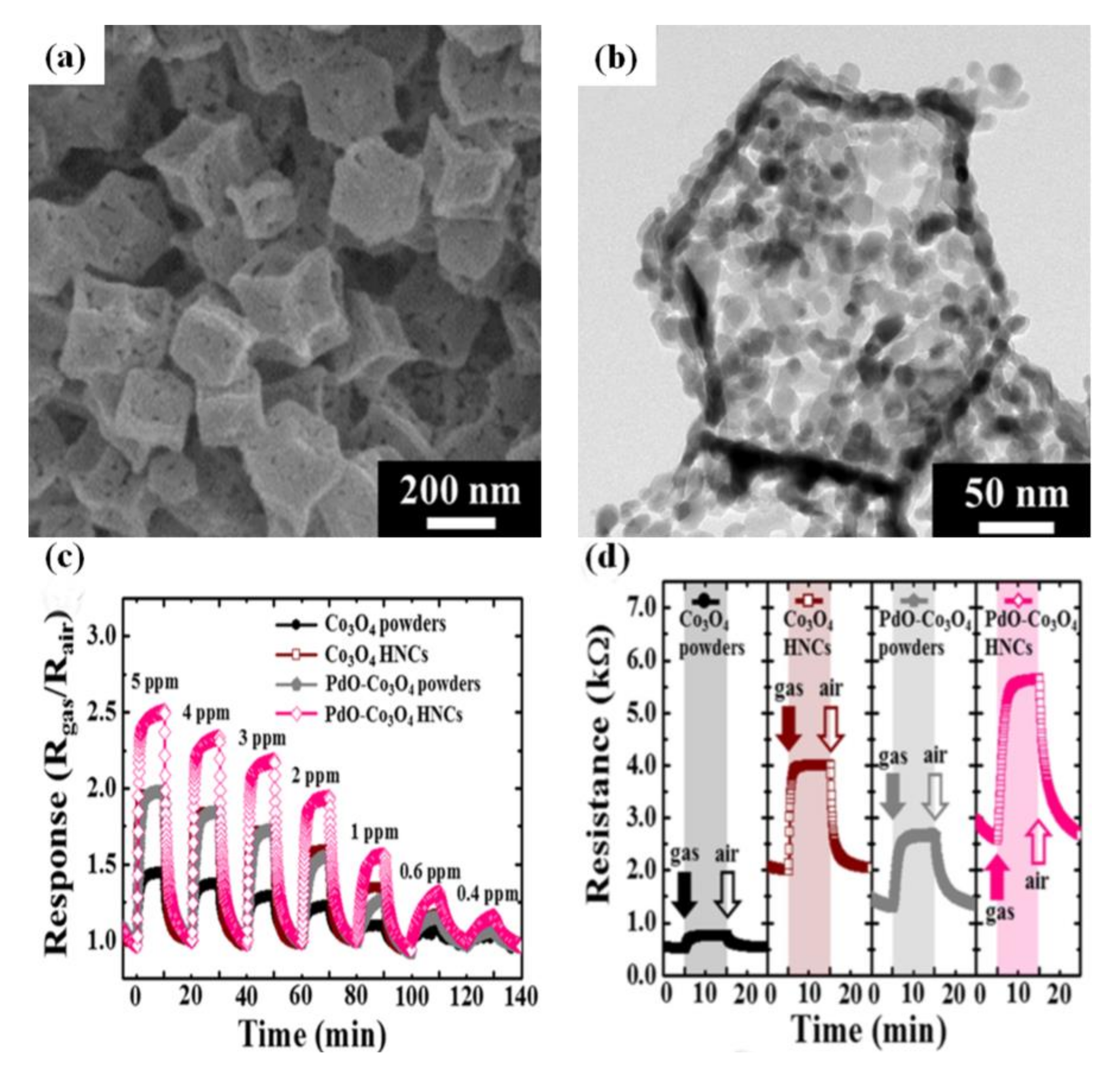
6. The Effect of Catalyst on Gas-Sensing Performance
7. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, H.L.; Nie, L.; Li, J.; Wang, Y.F.; Wang, G.; Wang, J.H.; Hao, Z.P. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Llobet, E.; Brunet, J.; Pauly, A.; Ndiaye, A.; Varenne, C. Nanomaterials for the Selective Detection of Hydrogen Sulfide in Air. Sensors 2017, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Li, X.G.; Zhao, Y.Y.; Wang, J. Enhanced NO2 sensing of SnO2/SnS2 heterojunction based sensor. Sens. Actuators B Chem. 2017, 244, 67–76. [Google Scholar] [CrossRef]
- Wang, Q.J.; Wang, C.; Sun, H.B.; Sun, P.; Wang, Y.Z.; Lin, J.; Lu, G.Y. Microwave assisted synthesis of hierarchical Pd/SnO2 nanostructures for CO gas sensor. Sens. Actuators B Chem. 2016, 222, 257–263. [Google Scholar] [CrossRef]
- Ma, L.T.; Fan, H.Q.; Tian, H.L.; Fang, J.W.; Qian, X.Z. The n-ZnO/n-In2O3 heterojunction formed by a surface-modification and their potential barrier-control in methanal gas sensing. Sens. Actuators B Chem. 2016, 222, 508–516. [Google Scholar] [CrossRef]
- Kheel, H.; Sun, G.-J.; Lee, J.K.; Lee, S.; Dwivedi, R.P.; Lee, C. Enhanced H2S sensing performance of TiO2-decorated α-Fe2O3 nanorod sensors. Ceram. Int. 2016, 42, 18597–18604. [Google Scholar] [CrossRef]
- Perrozzi, F.; Emamjomeh, S.M.; Paolucci, V.; Taglieri, G.; Ottaviano, L.; Cantalini, C. Thermal stability of WS2 flakes and gas sensing properties of WS2/WO3 composite to H2, NH3 and NO2. Sens. Actuators B Chem. 2017, 243, 812–822. [Google Scholar] [CrossRef]
- Dhahri, R.; Leonardi, S.G.; Hjiri, M.; Mir, L.E.; Bonavita, A.; Donato, N.; Iannazzo, D.; Neri, G. Enhanced performance of novel calcium/aluminum co-doped zinc oxide for CO2 sensors. Sens. Actuators B Chem. 2017, 239, 36–44. [Google Scholar] [CrossRef]
- Wang, B.; Jin, H.T.; Zheng, Z.Q.; Zhou, Y.H.; Gao, C. Low-temperature and highly sensitive C2H2 sensor based on Au decorated ZnO/In2O3 belt-tooth shape nano-heterostructures. Sens. Actuators B Chem. 2017, 244, 344–356. [Google Scholar] [CrossRef]
- Wang, Q.J.; Li, X.; Liu, F.M.; Sun, Y.F.; Wang, C.; Li, X.W.; Sun, P.; Lin, J.; Lu, G.Y. Three-dimensional flake-flower Co/Sn oxide composite and its excellent ethanol sensing properties. Sens. Actuators B Chem. 2016, 230, 17–24. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yang, W.; Wang, M.X.; Xiao, L.; Liu, S.T. Fabrication of lotus-like Au@TiO2 nanocomposites with enhanced gas-sensing properties. Sens. Actuators B Chem. 2016, 236, 490–498. [Google Scholar] [CrossRef]
- Miller, D.R.; Williams, R.E.; Akbar, S.A.; Morris, P.A.; McComb, D.W. STEM-Cathodoluminescence of SnO2 nanowires and powders. Sens. Actuators B Chem. 2017, 240, 193–203. [Google Scholar] [CrossRef]
- Karthik, T.V.; Olvera Mde, L.; Maldonado, A.; Gomez Pozos, H. CO Gas Sensing Properties of Pure and Cu-Incorporated SnO2 Nanoparticles: A Study of Cu-Induced Modifications. Sensors 2016, 16, 1283. [Google Scholar] [CrossRef] [PubMed]
- Huber, F.; Riegert, S.; Madel, M.; Thonke, K. H2S sensing in the ppb regime with zinc oxide nanowires. Sens. Actuators B Chem. 2017, 239, 358–363. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, S.J.; Kim, S.J.; Cho, H.J.; Jang, J.S.; Koo, W.T.; Kim, M.; Kim, I.D. Highly sensitive and selective acetone sensing performance of WO3 nanofibers functionalized by Rh2O3 nanoparticles. Sens. Actuators B Chem. 2016, 224, 185–192. [Google Scholar] [CrossRef]
- Yan, H.H.; Song, P.; Zhang, S.; Zhang, J.; Yang, Z.X.; Wang, Q. Au nanoparticles modified MoO3 nanosheets with their enhanced properties for gas sensing. Sens. Actuators B Chem. 2016, 236, 201–207. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, C.; Yang, Q.Y.; Gao, Y.; Zhou, X.; Liang, X.S.; Sun, P.; Lu, G.Y. Hydrothermal synthesis and gas-sensing properties of flower-like Sn3O4. Sens. Actuators B Chem. 2016, 224, 128–133. [Google Scholar] [CrossRef]
- Wang, Q.J.; Li, X.; Liu, F.M.; Liu, C.; Su, T.; Lin, J.; Sun, P.; Sun, Y.F.; Liu, F.M.; Lu, G.Y. The enhanced CO gas sensing performance of Pd/SnO2 hollow sphere sensors under hydrothermal conditions. RSC Adv. 2016, 6, 80455–80461. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.W.; Kim, S.S. Ultra-sensitive benzene detection by a novel approach: Core-shell nanowires combined with the Pd-functionalization. Sens. Actuators B Chem. 2017, 239, 578–585. [Google Scholar] [CrossRef]
- Shankar, P.; Rayappan, J.B.B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases—A review. Sci. Lett. J. 2015, 4, 126. [Google Scholar]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Yin, L.W.; Zhang, L.Y.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Mane, A.A.; Moholkar, A.V. Effect of film thickness on NO2 gas sensing properties of sprayed orthorhombic nanocrystalline V2O5 thin films. Appl. Surf. Sci. 2017, 416, 511–520. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Makeeva, E.A.; Badalyan, S.M.; Zhukova, A.A.; Gaskov, A.M. Nanocrystalline SnO2 and In2O3 as materials for gas sensors: The relationship between microstructure and oxygen chemisorption. Thin Solid Films 2009, 518, 1283–1288. [Google Scholar] [CrossRef]
- Qiao, P.Y.; Zhang, L.X.; Zhu, M.Y.; Yin, Y.Y.; Zhao, Z.W.; Sun, H.N.; Dong, J.Y.; Bie, L.J. Acetylene sensing enhancement of mesoporous ZnO nanosheets with morphology and defect induced structural sensitization. Sens. Actuators B Chem. 2017, 250, 189–197. [Google Scholar] [CrossRef]
- Sui, L.; Zhang, X.; Cheng, X.; Wang, P.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Au-Loaded Hierarchical MoO3 Hollow Spheres with Enhanced Gas-Sensing Performance for the Detection of BTX (Benzene, Toluene, And Xylene) and the Sensing Mechanism. ACS Appl. Mater. Interfaces 2017, 9, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Bing, Y.; Zeng, Y.; Feng, S.; Qiao, L.; Wang, Y.; Zheng, W. Multistep assembly of Au-loaded SnO2 hollow multilayered nanosheets for high-performance CO detection. Sens. Actuators B Chem. 2016, 227, 362–372. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Yan, H.; Wang, Q. Self-assembled hierarchical Au-loaded In2O3 hollow microspheres with superior ethanol sensing properties. Sens. Actuators B Chem 2016, 231, 245–255. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, X.; Wang, Y.; Bing, Y.; Qiao, L.; Liang, Z.; Yu, S.; Zeng, Y.; Zheng, W. Pd-loaded SnO2 ultrathin nanorod-assembled hollow microspheres with the significant improvement for toluene detection. Sens. Actuators B Chem. 2017, 243, 465–474. [Google Scholar] [CrossRef]
- Shao, F.; Hoffmann, M.W.G.; Prades, J.D.; Zamani, R.; Arbiol, J.; Morante, J.R.; Varechkina, E.; Rumyantseva, M.; Gaskov, A.; Giebelhaus, I.; et al. Heterostructured p-CuO (nanoparticle)/n-SnO2 (nanowire) devices for selective H2S detection. Sens. Actuators B Chem. 2013, 181, 130–135. [Google Scholar] [CrossRef]
- Kim, J.H.; Katoch, A.; Kim, S.S. Optimum shell thickness and underlying sensing mechanism in p-n CuO-ZnO core-shell nanowires. Sens. Actuators B Chem. 2016, 222, 249–256. [Google Scholar] [CrossRef]
- Tan, W.; Ruan, X.; Yu, Q.; Yu, Z.; Huang, X. Fabrication of a SnO2-based acetone gas sensor enhanced by molecular imprinting. Sensors 2014, 15, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pang, W.; Feng, Z.H.; Chen, X.J.; Chen, Y.; Zhang, Q.; Zhang, H.; Sun, C.L.; Yang, J.J.; Zhang, D.H. Enabling selectivity and fast recovery of ZnO nanowire gas sensors through resistive switching. Sens. Actuators B Chem. 2017, 238, 357–363. [Google Scholar] [CrossRef]
- Yin, M.L.; Yu, L.M.; Liu, S.Z. Synthesis of thickness-controlled cuboid WO3 nanosheets and their exposed facets-dependent acetone sensing properties. J. Alloys Compd. 2017, 696, 490–497. [Google Scholar] [CrossRef]
- Li, F.R.; Jian, J.K.; Wu, R.; Li, J.; Sun, Y.F. Synthesis, electrochemical and gas sensing properties of In2O3 nanostructures with different morphologies. J. Alloys Compd. 2015, 645, 178–183. [Google Scholar] [CrossRef]
- Tian, K.; Wang, X.X.; Li, H.Y.; Nadimicherla, R.; Guo, X. Lotus pollen derived 3-dimensional hierarchically porous NiO microspheres for NO2 gas sensing. Sens. Actuators B Chem. 2016, 227, 554–560. [Google Scholar] [CrossRef]
- Peng, X.Y.; Wang, Z.M.; Huang, P.; Chen, X.; Fu, X.Z.; Dai, W.X. Comparative Study of Two Different TiO2 Film Sensors on Response to H2 under UV Light and Room Temperature. Sensors 2016, 16, 1249. [Google Scholar] [CrossRef] [PubMed]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Pokropivny, V.V.; Skorokhod, V.V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. NO and NO2 sensing properties of WO3 and Co3O4 based gas sensors. Sensors 2013, 13, 12467–12481. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.K.; Bhardwaj, N.; Kukkar, M.; Sharma, A.L.; Kim, K.H.; Deep, A. Formation of High-Purity Indium Oxide Nanoparticles and Their Application to Sensitive Detection of Ammonia. Sensors 2015, 15, 31930–31938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Yang, B.; Liu, J.Q.; Chen, X.; Wang, X.L.; Yang, C.S. Highly sensitive and low operating temperature SnO2 gas sensor doped by Cu and Zn two elements. Sens. Actuators B Chem. 2017, 243, 982–989. [Google Scholar] [CrossRef]
- Wang, Q.J.; Liu, F.M.; Lin, J.; Lu, G.Y. Gas-sensing properties of In-Sn oxides composites synthesized by hydrothermal method. Sens. Actuators B Chem. 2016, 234, 130–136. [Google Scholar] [CrossRef]
- Gao, H.T.; Jia, H.; Bierer, B.; Wöllenstein, J.; Lu, Y.; Palzer, S. Scalable gas sensors fabrication to integrate metal oxide nanoparticles with well-defined shape and size. Sens. Actuators B Chem. 2017, 249, 639–646. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, R.; Zhou, T.T.; Lou, Z.; Deng, J.N.; Zhang, T. Concave Cu2O octahedral nanoparticles as an advanced sensing material for benzene (C6H6) and nitrogen dioxide (NO2) detection. Sens. Actuators B Chem. 2016, 223, 311–317. [Google Scholar] [CrossRef]
- Yu, H.X.; Song, Z.L.; Liu, Q.; Ji, X.; Liu, J.Q.; Xu, S.M.; Kan, H.; Zhang, B.H.; Liu, J.Y.; Jiang, J.J.; et al. Colloidal synthesis of tungsten oxide quantum dots for sensitive and selective H2S gas detection. Sens. Actuators B Chem. 2017, 248, 1029–1036. [Google Scholar] [CrossRef]
- Xiao, B.X.; Wang, F.; Zhai, C.B.; Wang, P.; Xiao, C.H.; Zhang, M.Z. Facile synthesis of In2O3 nanoparticles for sensing properties at low detection temperature. Sens. Actuators B Chem. 2016, 235, 251–257. [Google Scholar] [CrossRef]
- Xu, C.N.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain-size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B Chem. 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Liang, S.; Li, J.P.; Wang, F.; Qin, J.L.; Lai, X.Y.; Jiang, X.M. Highly sensitive acetone gas sensor based on ultrafine α-Fe2O3 nanoparticles. Sens. Actuators B Chem. 2017, 238, 923–927. [Google Scholar] [CrossRef]
- Devan, R.S.; Patil, R.A.; Lin, J.H.; Ma, Y.R. One-Dimensional Metal-Oxide Nanostructures: Recent Developments in Synthesis, Characterization, and Applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Qurashi, A.; Yamazaki, T.; El-Maghraby, E.M.; Kikuta, T. Fabrication and gas sensing properties of In2O3 nanopushpins. Appl. Phys. Lett. 2009, 95, 153109. [Google Scholar] [CrossRef]
- Kong, X.H.; Li, Y.D. High sensitivity of CuO modified SnO2 nanoribbons to H2S at room temperature. Sens. Actuators B Chem. 2005, 105, 449–453. [Google Scholar] [CrossRef]
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S. Gas sensors based on one dimensional nanostructured metal-oxides: A review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Wan, Q.; Song, Z.T.; Feng, S.L. SnO2 nanowhiskers and their ethanol sensing characteristics. Nanotechnology 2004, 15, 1682–1684. [Google Scholar] [CrossRef]
- Hu, P.Q.; Du, G.J.; Zhou, W.J.; Cui, J.J.; Lin, J.J.; Liu, H.; Liu, D.; Wang, J.Y.; Chen, S.W. Enhancement of ethanol vapor sensing of TiO2 nanobelts by surface engineering. ACS Appl. Mater. Interfaces 2010, 2, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Tian, J.; Sang, Y.H.; Cabot, A.; Liu, H. Structure, synthesis, and applications of TiO2 nanobelts. Adv. Mater. 2015, 27, 2557–2582. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Bai, L.; Song, D.S.; Yang, H.P.; Sun, X.M.; Sun, H.Y.; Zhu, J. Ag-Modified In2O3/ZnO Nanobundles with High Formaldehyde Gas-Sensing Performance. Sensors 2015, 15, 20086–20096. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Aldalbahi, A.; Feng, P.X. Nanostructured Tungsten Oxide Composite for High-Performance Gas Sensors. Sensors 2015, 15, 27035–27046. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.S.; Na, C.W.; Lee, J.H. Design of Highly Selective Gas Sensors via Physicochemical Modification of Oxide Nanowires: Overview. Sensors 2016, 16, 1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Abideen, Z.U.; Zheng, Y.; Kim, S.S. Improvement of Toluene-Sensing Performance of SnO2 Nanofibers by Pt Functionalization. Sensors 2016, 16, 1857. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cheung, K.W.; Yan, W.H.A.; Li, Y.X.B.; Ho, D. High-sensitivity low-power tungsten doped niobium oxide nanorods sensor for nitrogen dioxide air pollution monitoring. Sens. Actuators B Chem. 2017, 238, 204–213. [Google Scholar] [CrossRef]
- Zhao, C.H.; Bai, J.L.; Huang, B.Y.; Wang, Y.L.; Zhou, J.Y.; Xie, E.Q. Grain refining effect of calcium dopants on gas-sensing properties of electrospun alpha-Fe2O3 nanotubes. Sens. Actuators B Chem. 2016, 231, 552–560. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Ababii, N.; Hoppe, M.; Cretu, V.; Tiginyanu, I.; Sontea, V.; Pauporté, T.; Viana, B.; Adelung, R. Influence of CuO nanostructures morphology on hydrogen gas sensing performances. Microelectron. Eng. 2016, 164, 63–70. [Google Scholar] [CrossRef]
- Sun, J.B.; Sun, P.; Zhang, D.L.; Xu, J.; Liang, X.S.; Liu, F.M.; Lu, G.Y. Growth of SnO2 nanowire arrays by ultrasonic spray pyrolysis and their gas sensing performance. RSC Adv. 2014, 4, 43429–43435. [Google Scholar] [CrossRef]
- Nakla, W.; Wisitsora-at, A.; Tuantranont, A.; Singjai, P.; Phanichphant, S.; Liewhiran, C. H2S sensor based on SnO2 nanostructured film prepared by high current heating. Sens. Actuators B Chem. 2014, 203, 565–578. [Google Scholar] [CrossRef]
- Wang, H.R.; Sun, Q.; Yao, Y.Q.; Li, Y.X.; Wang, J.X.; Chen, L. A micro sensor based on TiO2 nanorod arrays for the detection of oxygen at room temperature. Ceram. Int. 2016, 42, 8565–8571. [Google Scholar] [CrossRef]
- Oros, C.; Horprathum, M.; Wisitsoraat, A.; Srichaiyaperk, T.; Samransuksamer, B.; Limwichean, S.; Eiamchai, P.; Phokharatkul, D.; Nuntawong, N.; Chananonnawathorn, C.; et al. Ultra-sensitive NO2 sensor based on vertically aligned SnO2 nanorods deposited by DC reactive magnetron sputtering with glancing angle deposition technique. Sens. Actuators B Chem. 2016, 223, 936–945. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Shingange, K.; Dhonge, B.P.; Ntwaeaborwa, O.M.; Mhlongo, G.H.; Motaung, D.E. Fabrication of ultra-high sensitive and selective CH4 room temperature gas sensing of TiO2 nanorods: Detailed study on the annealing temperature. Sens. Actuators B Chem. 2017, 238, 402–419. [Google Scholar] [CrossRef]
- Cao, S.X.; Zhao, C.; Han, T.; Peng, L.L. Hydrothermal synthesis, characterization and gas sensing properties of the WO3 nanofibers. Mater. Lett. 2016, 169, 17–20. [Google Scholar] [CrossRef]
- Acharyya, D.; Bhattacharyya, P. Alcohol sensing performance of ZnO hexagonal nanotubes at low temperatures: A qualitative understanding. Sens. Actuators B Chem. 2016, 228, 373–386. [Google Scholar] [CrossRef]
- Kaur, M.; Kailasaganapathi, S.; Ramgir, N.; Datta, N.; Kumar, S.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Gas dependent sensing mechanism in ZnO nanobelt sensor. Appl. Surf. Sci. 2017, 394, 258–266. [Google Scholar] [CrossRef]
- Cai, Z.X.; Li, H.Y.; Yang, X.N.; Guo, X. NO sensing by single crystalline WO3 nanowires. Sens. Actuators B Chem. 2015, 219, 346–353. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, P.; Xu, T.; Zheng, D.; Li, X. ZnO-nanowire size effect induced ultra-high sensing response to ppb -level H2S. Sens. Actuators B Chem. 2017, 240, 264–272. [Google Scholar] [CrossRef]
- Lupan, O.; Ursaki, V.V.; Chai, G.; Chow, L.; Emelchenko, G.A.; Tiginyanu, I.M.; Gruzintsev, A.N.; Redkin, A.N. Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuators B Chem. 2010, 144, 56–66. [Google Scholar] [CrossRef]
- Qin, Y.X.; Xie, W.W.; Liu, Y.; Ye, Z.H. Thermal-oxidative growth of aligned W18O49 nanowire arrays for high performance gas sensor. Sens. Actuators B Chem. 2016, 223, 487–495. [Google Scholar] [CrossRef]
- Wang, L.H.; Cao, J.; Qian, X.M.; Zhang, H.M. Facile synthesis and enhanced gas sensing properties of grain size-adjustable In2O3 micro/nanotubes. Mater. Lett. 2016, 171, 30–33. [Google Scholar] [CrossRef]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.L.; Chen, W.; Jin, W.; Zhou, J.; Zhang, H.; Zakharova, G.S. High sensitivity and good selectivity of ultralong MoO3 nanobelts for trimethylamine gas. Sens. Actuators B Chem. 2016, 226, 478–485. [Google Scholar] [CrossRef]
- Khalil, A.; Kim, J.J.; Tuller, H.L.; Rutledge, G.C.; Hashaikeh, R. Gas sensing behavior of electrospun nickel oxide nanofibers: Effect of morphology and microstructure. Sens. Actuators B Chem. 2016, 227, 54–64. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, H.J.; Jeong, H.M.; Lee, J.H. Gas sensing characteristics of p-type Cr2O3 and Co3O4 nanofibers depending on inter-particle connectivity. Sens. Actuators B Chem. 2014, 202, 263–271. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Meher, S.R.; Sivacoumar, R.; Alex, Z.C. Influence of surface functionalization on the gas sensing characteristics of ZnO nanorhombuses. J. Alloys Compd. 2017, 706, 186–197. [Google Scholar] [CrossRef]
- Dou, Y.H.; Xu, J.T.; Ruan, B.Y.; Liu, Q.N.; Pan, Y.D.; Sun, Z.Q.; Dou, S.X. Atomic Layer-by-Layer Co3O4/Graphene Composite for High Performance Lithium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501835. [Google Scholar] [CrossRef]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Zhang, G.W.; Hu, X.Y.; Mu, J.L.; Han, T.X.; Fan, J.; Zhu, C.J.; Song, L.X.; Bai, J.T.; Hou, X. A novel strategy to prepare 2D g-C3N4 nanosheets and their photoelectrochemical properties. J. Alloys Compd. 2017, 690, 669–676. [Google Scholar] [CrossRef]
- Nijamudheen, A.; Akimov, A.V. Excited-State Dynamics in Two-Dimensional Heterostructures: SiR/TiO2 and GeR/TiO2 (R = H, Me) as Promising Photocatalysts. J. Phys. Chem. C 2017, 121, 6520–6532. [Google Scholar] [CrossRef]
- Ji, F.X.; Ren, X.P.; Zheng, X.Y.; Liu, Y.C.; Pang, L.Q.; Jiang, J.X.; Liu, S.Z. 2D-MoO3 nanosheets for superior gas sensors. Nanoscale 2016, 8, 8696–8703. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Q.; Xue, Z.G.; Qin, N.; Cheng, Z.X.; Xiang, Q. The crystal facet-dependent gas sensing properties of ZnO nanosheets: Experimental and computational study. Sens. Actuators B Chem. 2017, 242, 148–157. [Google Scholar] [CrossRef]
- Liu, C.; Lu, H.B.; Zhang, J.N.; Yang, Z.B.; Zhu, G.Q.; Yin, F.; Gao, J.Z.; Chen, C.J.; Xin, X. Abnormal p-type sensing response of TiO2 nanosheets with exposed {001} facets. J. Alloys Compd. 2017, 705, 112–117. [Google Scholar] [CrossRef]
- Godbole, R.; Godbole, V.P.; Bhagwat, S. Surface morphology dependent tungsten oxide thin films as toxic gas sensor. Mater. Sci. Semicond. Process. 2017, 63, 212–219. [Google Scholar] [CrossRef]
- Cho, Y.H.; Ko, Y.N.; Kang, Y.C.; Kim, I.-D.; Lee, J.-H. Ultraselective and ultrasensitive detection of trimethylamine using MoO3 nanoplates prepared by ultrasonic spray pyrolysis. Sens. Actuators B Chem. 2014, 195, 189–196. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chen, H.; Li, G.D.; Shi, Z.F.; Zou, H.F.; Zou, X.X. Ultrathin In2O3 Nanosheets with Uniform Mesopores for Highly Sensitive Nitric Oxide Detection. ACS Appl. Mater. Interfaces 2017, 9, 16335–16342. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Lin, Z.J.; Wang, N.N.; Wang, J.Q.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z.G. High precision NH3 sensing using network nano-sheet Co3O4 arrays based sensor at room temperature. Sens. Actuators B Chem. 2016, 235, 222–231. [Google Scholar] [CrossRef]
- Escalante, G.; Juárez, H.; Fernández, P. Characterization and sensing properties of ZnO film prepared by single source chemical vapor deposition. Adv. Powder Technol. 2017, 28, 23–29. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Wen, Z.; Ye, Z.Z.; Zhu, L.P. Gas sensors based on ultrathin porous Co3O4 nanosheets to detect acetone at low temperature. RSC Adv. 2015, 5, 59976–59982. [Google Scholar] [CrossRef]
- Xiao, C.H.; Yang, T.Y.; Chuai, M.Y.; Xiao, B.X.; Zhang, M.Z. Synthesis of ZnO nanosheet arrays with exposed (100) facets for gas sensing applications. Phys. Chem. Chem. Phys. 2016, 18, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Feng, Q.Q.; Tao, Y.F.; Zheng, L.J.; Han, Z.Y.; Ma, J.M. Systematic investigation on the gas-sensing performance of TiO2 nanoplate sensors for enhanced detection on toxic gases. Mater. Res. Bull. 2016, 73, 302–307. [Google Scholar] [CrossRef]
- Shendage, S.S.; Patil, V.L.; Vanalakar, S.A.; Patil, S.P.; Harale, N.S.; Bhosale, J.L.; Kim, J.H.; Patil, P.S. Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens. Actuators B Chem. 2017, 240, 426–433. [Google Scholar] [CrossRef]
- Qin, H.Y.; Cao, Y.L.; Xie, J.; Xu, H.; Jia, D.Z. Solid-state chemical synthesis and xylene-sensing properties of α-MoO3 arrays assembled by nanoplates. Sens. Actuators B Chem. 2017, 242, 769–776. [Google Scholar] [CrossRef]
- Maity, A.; Majumder, S.B. NO2 sensing and selectivity characteristics of tungsten oxide thin films. Sens. Actuators B Chem. 2015, 206, 423–429. [Google Scholar] [CrossRef]
- Teimoori, F.; Khojier, K.; Dehnavi, N.Z. Investigation of sensitivity and selectivity of ZnO thin film to volatile organic compounds. J. Theor. Appl. Phys. 2017, 11, 157–163. [Google Scholar] [CrossRef]
- Karthikeyan, P.S.; Dhivya, P.; Deepak Raj, P.; Sridharan, M. V2O5 thin film for 2-Propanol vapor sensing. Mater. Today Proc. 2016, 3, 1510–1516. [Google Scholar] [CrossRef]
- Yu, L.M.; Guo, F.; Liu, S.; Yang, B.; Jiang, Y.X.; Qi, L.J.; Fan, X.H. Both oxygen vacancies defects and porosity facilitated NO2 gas sensing response in 2D ZnO nanowalls at room temperature. J. Alloys Compd. 2016, 682, 352–356. [Google Scholar] [CrossRef]
- Wu, B.F.; Wang, L.L.; Wu, H.Y.; Kan, K.; Zhang, G.; Xie, Y.; Tian, Y.; Li, L.; Shi, K.Y. Templated synthesis of 3D hierarchical porous Co3O4 materials and their NH3 sensor at room temperature. Microporous Mesoporous Mater. 2016, 225, 154–163. [Google Scholar] [CrossRef]
- Wang, S.R.; Yang, J.D.; Zhang, H.X.; Wang, Y.S.; Gao, X.L.; Wang, L.W.; Zhu, Z.Y. One-pot synthesis of 3D hierarchical SnO2 nanostructures and their application for gas sensor. Sens. Actuators B Chem. 2015, 207, 83–89. [Google Scholar] [CrossRef]
- Li, T.M.; Zeng, W. New insight into the gas sensing performance of SnO2 Nanorod-assembled urchins based on their assembly density. Ceram. Int. 2017, 43, 728–735. [Google Scholar] [CrossRef]
- Xu, K.; Yang, L.; Zou, J.P.; Yang, Y.; Li, Q.L.; Qu, Y.H.; Ye, J.R.; Yuan, C.L. Fabrication of novel flower-like Co3O4 structures assembled by single-crystalline porous nanosheets for enhanced xylene sensing properties. J. Alloys Compd. 2017, 706, 116–125. [Google Scholar] [CrossRef]
- Diao, K.D.; Zhou, M.J.; Zhang, J.C.; Tang, Y.J.; Wang, S.X.; Cui, X.D. High response to H2S gas with facile synthesized hierarchical ZnO microstructures. Sens. Actuators B Chem. 2015, 219, 30–37. [Google Scholar] [CrossRef]
- Liu, J.; Huang, H.W.; Zhao, H.; Yan, X.T.; Wu, S.J.; Li, Y.; Wu, M.; Chen, L.H.; Yang, X.Y.; Su, B.L. Enhanced Gas Sensitivity and Selectivity on Aperture-Controllable 3D Interconnected Macro-Mesoporous ZnO Nanostructures. ACS Appl. Mater. Interfaces 2016, 8, 8583–8590. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Zhang, H.J.; Hu, X.L.; Sun, P.; Zhu, Y.S.; He, C.Z.; Hou, S.J.; Sun, Y.F.; Lu, G.Y. Hierarchical nanorod-flowers indium oxide microspheres and their gas sensing properties. Sens. Actuators B Chem. 2016, 227, 547–553. [Google Scholar] [CrossRef]
- Li, Y.X.; Chen, N.; Deng, D.Y.; Xing, X.X.; Xiao, X.C.; Wang, Y.D. Formaldehyde detection: SnO2 microspheres for formaldehyde gas sensor with high sensitivity, fast response/recovery and good selectivity. Sens. Actuators B Chem. 2017, 238, 264–273. [Google Scholar] [CrossRef]
- Sui, L.L.; Xu, Y.M.; Zhang, X.F.; Cheng, X.L.; Gao, S.; Zhao, H.; Cai, Z.; Huo, L.H. Construction of three-dimensional flower-like α-MoO3 with hierarchical structure for highly selective triethylamine sensor. Sens. Actuators B Chem. 2015, 208, 406–414. [Google Scholar] [CrossRef]
- Jiang, X.H.; Ma, S.Y.; Sun, A.M.; Zhang, Z.M.; Jin, W.X.; Wang, T.T.; Li, W.Q.; Xu, X.L.; Luo, J.; Cheng, L.; et al. Hydrothermal self-assembly of novel porous flower-like SnO2 architecture and its application in ethanol sensor. Appl. Surf. Sci. 2015, 355, 1192–1200. [Google Scholar] [CrossRef]
- Miao, R.Y.; Zeng, W. Hydrothermal synthesis of flake-flower NiO architectures: Structure, growth and gas-sensing properties. Mater. Lett. 2016, 171, 200–203. [Google Scholar] [CrossRef]
- Song, L.M.; Li, Y.; Li, S.C.; Liu, L.; Wang, L.Y.; Guo, X.X.; Lian, H.W. Porous ZnO microflowers with ultrahigh sensitive and selective properties to ethanol. J. Mater. Sci. Mater. Electron. 2016, 28, 652–656. [Google Scholar] [CrossRef]
- Jin, W.X.; Ma, S.Y.; Tie, Z.Z.; Li, W.Q.; Luo, J.; Cheng, L.; Xu, X.L.; Wang, T.T.; Jiang, X.H.; Mao, Y.Z. Synthesis of hierarchical SnO2 nanoflowers with enhanced acetic acid gas sensing properties. Appl. Surf. Sci. 2015, 353, 71–78. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Feng, C.H.; Sun, Y.F.; Lu, G.Y. Nanosheets assembled hierarchical flower-like WO3 nanostructures: Synthesis, characterization, and their gas sensing properties. Sens. Actuators B Chem. 2015, 210, 75–81. [Google Scholar] [CrossRef]
- Cao, S.X.; Chen, H.; Han, T.; Zhao, C.; Peng, L.L. Rose-like Cu2O nanoflowers via hydrothermal synthesis and their gas sensing properties. Mater. Lett. 2016, 180, 135–139. [Google Scholar] [CrossRef]
- Qiang, Z.; Ma, S.Y.; Jiao, H.Y.; Jin, W.X.; Wang, T.T.; Jiang, X.H.; Zhang, Z.Y. Solvothermal synthesis of 3D leaf-like α-Fe2O3 and its gas-sensing properties research. Mater. Lett. 2016, 181, 29–33. [Google Scholar] [CrossRef]
- Cao, S.X.; Chen, H. Nanorods assembled hierarchical urchin-like WO3 nanostructures: Hydrothermal synthesis, characterization, and their gas sensing properties. J. Alloys Compd. 2017, 702, 644–648. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.H.; Ma, S.Y.; Jin, W.X.; Yan, S.H.; Xu, X.L.; Jiang, X.H.; Wang, T.T.; Yang, H.M.; Chen, H.; et al. Synthesis of cactus-like NiO nanostructure and their gas-sensing properties. Mater. Lett. 2016, 164, 48–51. [Google Scholar] [CrossRef]
- Wang, S.M.; Cao, J.; Cui, W.; Li, X.F.; Li, D.J. Facile synthesis and excellent formaldehyde gas sensing properties of novel spindle-like In2O3 porous polyhedra. Sens. Actuators B Chem. 2016, 237, 944–952. [Google Scholar] [CrossRef]
- Sun, P.; Wang, C.; Liu, J.Y.; Zhou, X.; Li, X.W.; Hu, X.L.; Lu, G.Y. Hierarchical Assembly of alpha-Fe2O3 Nanosheets on SnO2 Hollow Nanospheres with Enhanced Ethanol Sensing Properties. ACS Appl. Mater. Interfaces 2015, 7, 19119–19125. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Lai, X.Y.; Wang, J.Y.; Tang, H.J.; Ren, H.; Yang, Y.; Jin, Q.; Zhang, L.J.; Yu, R.B.; Ma, G.H.; et al. Multi-shelled hollow micro-/nanostructures. Chem. Soc. Rev. 2015, 44, 6749–6773. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.G.; Li, X.X.; Wang, J.; Tang, Z.A. UV activated hollow ZnO microspheres for selective ethanol sensors at low temperatures. Sens. Actuators B Chem. 2016, 232, 158–164. [Google Scholar] [CrossRef]
- Shen, J.Y.; Zhang, L.; Ren, J.; Wang, J.C.; Yao, H.C.; Li, Z.J. Highly enhanced acetone sensing performance of porous C-doped WO3 hollow spheres by carbon spheres as templates. Sens. Actuators B Chem. 2017, 239, 597–607. [Google Scholar] [CrossRef]
- Li, W.H.; Wu, X.F.; Han, N.; Chen, J.Y.; Qian, X.H.; Deng, Y.Z.; Tang, W.X.; Chen, Y.F. MOF-derived hierarchical hollow ZnO nanocages with enhanced low-concentration VOCs gas-sensing performance. Sens. Actuators B Chem. 2016, 225, 158–166. [Google Scholar] [CrossRef]
- Zhou, T.T.; Zhang, T.; Zhang, R.; Deng, J.N.; Lou, Z.; Lu, G.Y.; Wang, L.L. Highly sensitive sensing platform based on ZnSnO3 hollow cubes for detection of ethanol. Appl. Surf. Sci. 2017, 400, 262–268. [Google Scholar] [CrossRef]
- Liu, J.Y.; Dai, M.J.; Wang, T.S.; Sun, P.; Liang, X.S.; Lu, G.Y.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Ma, S.Y.; Jiao, H.Y.; Chen, Q.; Lu, Y.; Jin, W.X.; Li, W.Q.; Wang, T.T.; Jiang, X.H.; Qiang, Z.; et al. Synthesis of Zn2SnO4 hollow spheres by a template route for high-performance acetone gas sensor. Sens. Actuators B Chem. 2017, 245, 493–506. [Google Scholar] [CrossRef]
- Sun, H.M.; Wang, L.M.; Chu, D.Q.; Ma, Z.C.; Wang, A.X. Facile fabrication of multishelled Cr2O3 hollow microspheres with enhanced gas sensitivity. Mater. Lett. 2015, 140, 158–161. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.W.; Sun, H.B.; Sun, P.; Liang, X.S.; Liu, F.M.; Hu, X.L.; Lu, G.Y. Nanosheet-assembled ZnFe2O4 hollow microspheres for high-sensitive acetone sensor. ACS Appl. Mater. Interfaces 2015, 7, 15414–15421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, X.L.; Zhang, X.F.; Xu, Y.M.; Gao, S.; Zhao, H.; Huo, L.H. High selectivity to ppb-level HCHO sensor based on mesoporous tubular SnO2 at low temperature. Sens. Actuators B Chem. 2017, 247, 664–672. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Wang, G.Z.; Liu, L.L.; Yuan, C.L.; Yu, T.; Li, Q.L.; Zeng, F.Y.; Gu, G. Enhanced Gas-Sensing Properties of the Hierarchical TiO2 Hollow Microspheres with Exposed High-Energy {001} Crystal Facets. ACS Appl. Mater. Interfaces 2015, 7, 24902–24908. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Ma, S.Y.; Yang, G.J.; Jin, W.X.; Wang, T.T.; Jiang, X.H.; Li, W.Q. High sensitive and low concentration detection of methanol by a gas sensor based on one-step synthesis α-Fe2O3 hollow spheres. Mater. Lett. 2016, 169, 73–76. [Google Scholar] [CrossRef]
- Li, J.W.; Liu, X.; Cui, J.S.; Sun, J.B. Hydrothermal synthesis of self-assembled hierarchical tungsten oxides hollow spheres and their gas sensing properties. ACS Appl. Mater. Interfaces 2015, 7, 10108–10114. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, C.H.; Wang, M.; Li, X.; Cheng, P.F.; Zhang, H.; Sun, Y.F.; Sun, P.; Lu, G.Y. One-pot synthesis of hierarchical WO3 hollow nanospheres and their gas sensing properties. RSC Adv. 2015, 5, 29698–29703. [Google Scholar] [CrossRef]
- Yang, J.D.; Wang, S.R.; Dong, R.; Zhang, L.P.; Zhu, Z.Y.; Gao, X.L. One-pot synthesis of SnO2 hollow microspheres and their formaldehyde sensor application. Mater. Lett. 2016, 184, 9–12. [Google Scholar] [CrossRef]
- Jia, X.H.; Tian, M.G.; Dai, R.R.; Lian, D.D.; Han, S.; Wu, X.Y.; Song, H.J. One-pot template-free synthesis and highly ethanol sensing properties of ZnSnO3 hollow microspheres. Sens. Actuators B Chem. 2017, 240, 376–385. [Google Scholar] [CrossRef]
- Qiang, Z.; Ma, S.Y.; Jiao, H.Y.; Wang, T.T.; Jiang, X.H.; Jin, W.X.; Yang, H.M.; Chen, H. Highly sensitive and selective ethanol sensors using porous SnO2 hollow spheres. Ceram. Int. 2016, 42, 18983–18990. [Google Scholar] [CrossRef]
- Wang, T.T.; Ma, S.Y.; Cheng, L.; Jiang, X.H.; Zhang, M.; Li, W.Q.; Jin, W.X. Facile fabrication of multishelled SnO2 hollow microspheres for gas sensing application. Mater. Lett. 2016, 164, 56–59. [Google Scholar] [CrossRef]
- Dong, C.J.; Xing, X.X.; Chen, N.; Liu, X.; Wang, Y.D. Biomorphic synthesis of hollow CuO fibers for low-ppm-level n-propanol detection via a facile solution combustion method. Sens. Actuators B Chem. 2016, 230, 1–8. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.Z.; Qiao, L.; Bing, Y.F.; Zou, B.; Zheng, W.T. Synthesis and the improved sensing properties of hierarchical SnO2 hollow nanosheets with mesoporous and multilayered interiors. Sens. Actuators B Chem. 2016, 222, 354–361. [Google Scholar] [CrossRef]
- Wei, S.H.; Han, L.X.; Wang, M.Y.; Zhang, H.H.; Du, W.M.; Zhou, M.H. Hollow cauliflower-like WO3 nanostructures: Hydrothermal synthesis and their CO sensing properties. Mater. Lett. 2017, 186, 259–262. [Google Scholar] [CrossRef]
- Jin, W.X.; Ma, S.Y.; Tie, Z.Z.; Xu, X.L.; Jiang, X.H.; Li, W.Q.; Wang, T.T.; Lu, Y.; Yan, S.H. Synthesis of monodisperse ZnO hollow six-sided pyramids and their high gas-sensing properties. Mater. Lett. 2015, 159, 102–105. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, S.Y.; Jiao, H.Y.; Zhang, G.H.; Chen, H.; Xu, X.L.; Yang, H.M.; Qiang, Z. Synthesis of novel ZnSnO3 hollow polyhedrons with open nanoholes: Enhanced acetone-sensing performance. Ceram. Int. 2017, 43, 1617–1621. [Google Scholar] [CrossRef]
- Xu, W.; Li, J.W.; Sun, J.B. Fabrication of monodispersed hollow flower-like porous In2O3 nanostructures and their application as gas sensors. RSC Adv. 2015, 5, 81407–81414. [Google Scholar] [CrossRef]
- Tan, J.F.; Dun, M.H.; Li, L.; Zhao, J.Y.; Tan, W.H.; Lin, Z.D.; Huang, X.T. Synthesis of hollow and hollowed-out Co3O4 microspheres assembled by porous ultrathin nanosheets for ethanol gas sensors: Responding and recovering in one second. Sens. Actuators B Chem. 2017, 249, 44–52. [Google Scholar] [CrossRef]
- Zhang, J.; Song, P.; Li, J.; Yang, Z.X.; Wang, Q. Template-assisted synthesis of hierarchical MoO3 microboxes and their high gas-sensing performance. Sens. Actuators B Chem. 2017, 249, 458–466. [Google Scholar] [CrossRef]
- Li, J.; Tang, P.G.; Zhang, J.J.; Feng, Y.J.; Luo, R.X.; Chen, A.F.; Li, D.Q. Facile Synthesis and Acetone Sensing Performance of Hierarchical SnO2 Hollow Microspheres with Controllable Size and Shell Thickness. Ind. Eng. Chem. Res. 2016, 55, 3588–3595. [Google Scholar] [CrossRef]
- Katoch, A.; Abideen, Z.U.; Kim, J.H.; Kim, S.S. Influence of hollowness variation on the gas-sensing properties of ZnO hollow nanofibers. Sens. Actuators B Chem. 2016, 232, 698–704. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. Metal-core@metal oxide-shell nanomaterials for gas-sensing applications: A review. J. Nanopart. Res. 2015, 17, 371. [Google Scholar] [CrossRef]
- Majhi, S.M.; Rai, P.; Yu, Y.T. Facile Approach to Synthesize Au@ZnO Core-Shell Nanoparticles and Their Application for Highly Sensitive and Selective Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 9462–9468. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.D.; Jiang, H.F.; Yang, M.H. MOF-derived Co3O4/NiCo2O4 double-shelled nanocages with excellent gas sensing properties. Mater. Lett. 2017, 190, 75–78. [Google Scholar] [CrossRef]
- Yu, Q.X.; Zhu, J.H.; Xu, Z.Y.; Huang, X.T. Facile synthesis of α-Fe2O3@SnO2 core–shell heterostructure nanotubes for high performance gas sensors. Sens. Actuators B Chem. 2015, 213, 27–34. [Google Scholar] [CrossRef]
- Zhang, B.W.; Fu, W.Y.; Li, H.Y.; Fu, X.L.; Wang, Y.; Bala, H.; Sun, G.; Wang, X.D.; Wang, Y.; Cao, J.L.; et al. Actinomorphic ZnO/SnO2 core–shell nanorods: Two-step synthesis and enhanced ethanol sensing propertied. Mater. Lett. 2015, 160, 227–230. [Google Scholar] [CrossRef]
- Song, H.M.; Chon, B.S.; Jeon, S.H.; Rai, P.; Yu, Y.T.; Dutta, P.K. Synthesis of Au@SnO2 core–shell nanoparticles with controllable shell thickness and their CO sensing properties. Mater. Chem. Phys. 2015, 166, 87–94. [Google Scholar] [CrossRef]
- Zhu, Z.; Chang, J.L.; Wu, R.J. Fast ozone detection by using a core–shell Au@TiO2 sensor at room temperature. Sens. Actuators B Chem. 2015, 214, 56–62. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G.Y.; Barsan, N. Design of Core-Shell Heterostructure Nanofibers with Different Work Function and Their Sensing Properties to Trimethylamine. ACS Appl. Mater. Interfaces 2016, 8, 19799–19806. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Z.Y.; Wang, R.; Liu, S.; Fei, T.; Wang, L.J.; Zhang, T. Synthesis of core–shell alpha-Fe2O3@NiO nanofibers with hollow structures and their enhanced HCHO sensing properties. J. Mater. Chem. A 2015, 3, 5635–5641. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yang, P.; Li, J.; Matras-Postolek, K.; Yue, Y.L.; Huang, B.B. Formation of SiO2@SnO2 core–shell nanofibers and their gas sensing properties. RSC Adv. 2016, 6, 13371–13376. [Google Scholar] [CrossRef]
- Li, W.Q.; Ma, S.Y.; Li, Y.F.; Yang, G.J.; Mao, Y.Z.; Luo, J.; Gengzang, D.; Xu, X.L.; Yan, S.H. Enhanced ethanol sensing performance of hollow ZnO–SnO2 core–shell nanofibers. Sens. Actuators B Chem. 2015, 211, 392–402. [Google Scholar] [CrossRef]
- Qin, Y.X.; Zhang, X.J.; Wang, Y.Y.; Liu, Y. Remarkable improvement of W18O49/TiO2 heteronanowires in ambient temperature-responsive NO2-sensing abilities and its unexpected n-p transition phenomenon. Sens. Actuators B Chem. 2017, 240, 477–486. [Google Scholar] [CrossRef]
- Wang, Y.F.; Qu, F.D.; Liu, J.; Wang, Y.; Zhou, J.R.; Ruan, S.P. Enhanced H2S sensing characteristics of CuO-NiO core-shell microspheres sensors. Sens. Actuators B Chem. 2015, 209, 515–523. [Google Scholar] [CrossRef]
- Wang, L.L.; Lou, Z.; Zhang, R.; Zhou, T.T.; Deng, J.N.; Zhang, T. Hybrid Co3O4/SnO2 Core–Shell Nanospheres as Real-Time Rapid-Response Sensors for Ammonia Gas. ACS Appl. Mater. Interfaces 2016, 8, 6539–6545. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Yoon, J.W.; Kwak, C.H.; Lee, J.H. Role of Pd nanoparticles in gas sensing behaviour of Pd@In2O3 yolk–shell nanoreactors. J. Mater. Chem. A 2016, 4, 264–269. [Google Scholar] [CrossRef]
- Gu, F.B.; Chen, H.H.; Han, D.M.; Wang, Z.H. Metal–organic framework derived Au@ZnO yolk–shell nanostructures and their highly sensitive detection of acetone. RSC Adv. 2016, 6, 29727–29733. [Google Scholar] [CrossRef]
- Gao, X.M.; Li, C.Y.; Yin, Z.X.; Chen, Y.J. Synthesis and H2S sensing performance of MoO3/Fe2(MoO4)3 yolk/shell nanostructures. RSC Adv. 2015, 5, 37703–37709. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, B.Q.; Sun, H.B.; Wang, C.; Sun, P.; Li, X.W.; Hu, X.L.; Lu, G.Y. Template-free synthesis of hierarchical ZnFe2O4 yolk-shell microspheres for high-sensitivity acetone sensors. Nanoscale 2016, 8, 5446–5453. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Lou, Z.; Deng, J.N.; Zhang, R.; Zhang, T. Ethanol Gas Detection Using a Yolk-Shell (Core-Shell) alpha-Fe2O3 Nanospheres as Sensing Material. ACS Appl. Mater. Interfaces 2015, 7, 13098–13104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, J.W.; Hong, Y.J.; Kang, Y.C.; Abdel-Hady, F.; Wazzan, A.A.; Lee, J.H. Highly sensitive and selective detection of ppb-level NO2 using multi-shelled WO3 yolk–shell spheres. Sens. Actuators B Chem. 2016, 229, 561–569. [Google Scholar] [CrossRef]
- Bing, Y.F.; Liu, C.; Qiao, L.; Zeng, Y.; Yu, S.S.; Liang, Z.Z.; Liu, J.P.; Luo, J.Z.; Zheng, W.T. Multistep synthesis of non-spherical SnO2@SnO2 yolk-shell cuboctahedra with nanoparticle-assembled porous structure for toluene detection. Sens. Actuators B Chem. 2016, 231, 365–375. [Google Scholar] [CrossRef]
- Li, X.W.; Liu, J.Y.; Guo, H.; Zhou, X.; Wang, C.; Sun, P.; Hu, X.L.; Lu, G.Y. Au@In2O3 core–shell composites: A metal–semiconductor heterostructure for gas sensing applications. RSC Adv. 2015, 5, 545–551. [Google Scholar] [CrossRef]
- Qu, F.D.; Jiang, H.F.; Yang, M.H. Designed formation through a metal organic framework route of ZnO/ZnCo2O4 hollow core-shell nanocages with enhanced gas sensing properties. Nanoscale 2016, 8, 16349–16356. [Google Scholar] [CrossRef] [PubMed]
- Dankeaw, A.; Poungchan, G.; Panapoy, M.; Ksapabutr, B. In-situ one-step method for fabricating three-dimensional grass-like carbon-doped ZrO2 films for room temperature alcohol and acetone sensors. Sens. Actuators B Chem. 2017, 242, 202–214. [Google Scholar] [CrossRef]
- Vallejos, S.; Grácia, I.; Chmela, O.; Figueras, E.; Hubálek, J.; Cané, C. Chemoresistive micromachined gas sensors based on functionalized metal oxide nanowires: Performance and reliability. Sens. Actuators B Chem. 2016, 235, 525–534. [Google Scholar] [CrossRef]
- Han, D.; Song, P.; Zhang, S.; Zhang, H.H.; Xu, Q.; Wang, Q. Enhanced methanol gas-sensing performance of Ce-doped In2O3 porous nanospheres prepared by hydrothermal method. Sens. Actuators B Chem. 2015, 216, 488–496. [Google Scholar] [CrossRef]
- NaderiNasrabadi, M.; Mortazavi, Y.; Khodadadi, A.A. Highly sensitive and selective Gd2O3-doped SnO2 ethanol sensors synthesized by a high temperature and pressure solvothermal method in a microreactor. Sens. Actuators B Chem. 2016, 230, 130–139. [Google Scholar] [CrossRef]
- Wang, S.C.; Shaikh, M.O. A Room Temperature H2 Sensor Fabricated Using High Performance Pt-Loaded SnO2 Nanoparticles. Sensors 2015, 15, 14286–14297. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Bing, Y.F.; Wang, Y.Z.; Yu, S.S.; Liang, Z.Z.; Zeng, Y. Enhanced toluene sensing performances of Pd-loaded SnO2 cubic nanocages with porous nanoparticle-assembled shells. Sens. Actuators B Chem. 2017, 241, 1121–1129. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liu, J.; Cui, X.B.; Gao, Y.; Ma, J.; Sun, Y.F.; Sun, P.; Liu, F.M.; Liang, X.S.; Zhang, T.; et al. NH3 gas sensing performance enhanced by Pt-loaded on mesoporous WO3. Sens. Actuators B Chem. 2017, 238, 473–481. [Google Scholar] [CrossRef]
- Fan, F.Y.; Zhang, J.J.; Li, J.; Zhang, N.; Hong, R.R.; Deng, X.C.; Tang, P.G.; Li, D.Q. Hydrogen sensing properties of Pt-Au bimetallic nanoparticles loaded on ZnO nanorods. Sens. Actuators B Chem. 2017, 241, 895–903. [Google Scholar] [CrossRef]
- Koo, W.T.; Yu, S.; Choi, S.J.; Jang, J.S.; Cheong, J.Y.; Kim, I.D. Nanoscale PdO Catalyst Functionalized Co3O4 Hollow Nanocages Using MOF Templates for Selective Detection of Acetone Molecules in Exhaled Breath. ACS Appl. Mater. Interfaces 2017, 9, 8201–8210. [Google Scholar] [CrossRef] [PubMed]
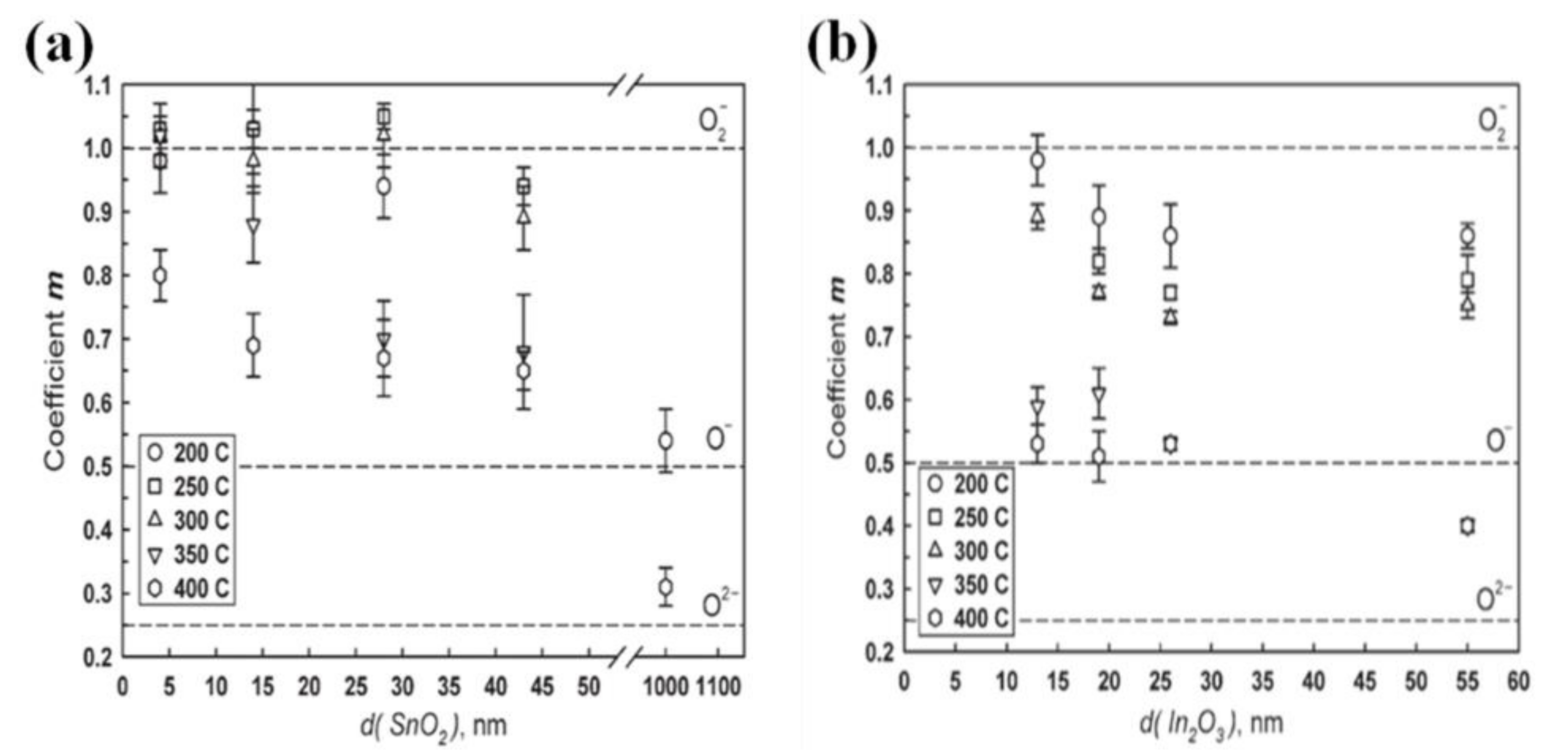
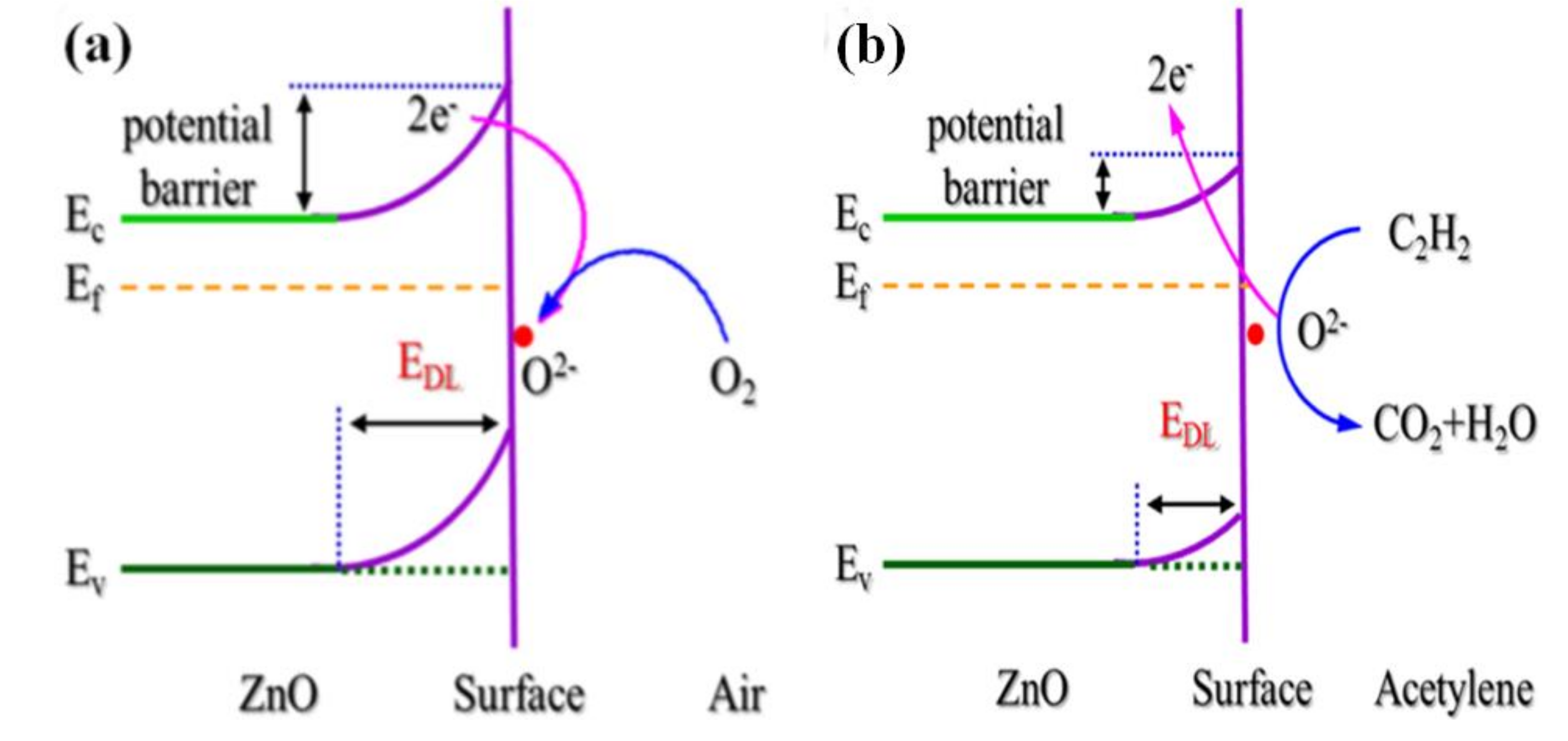
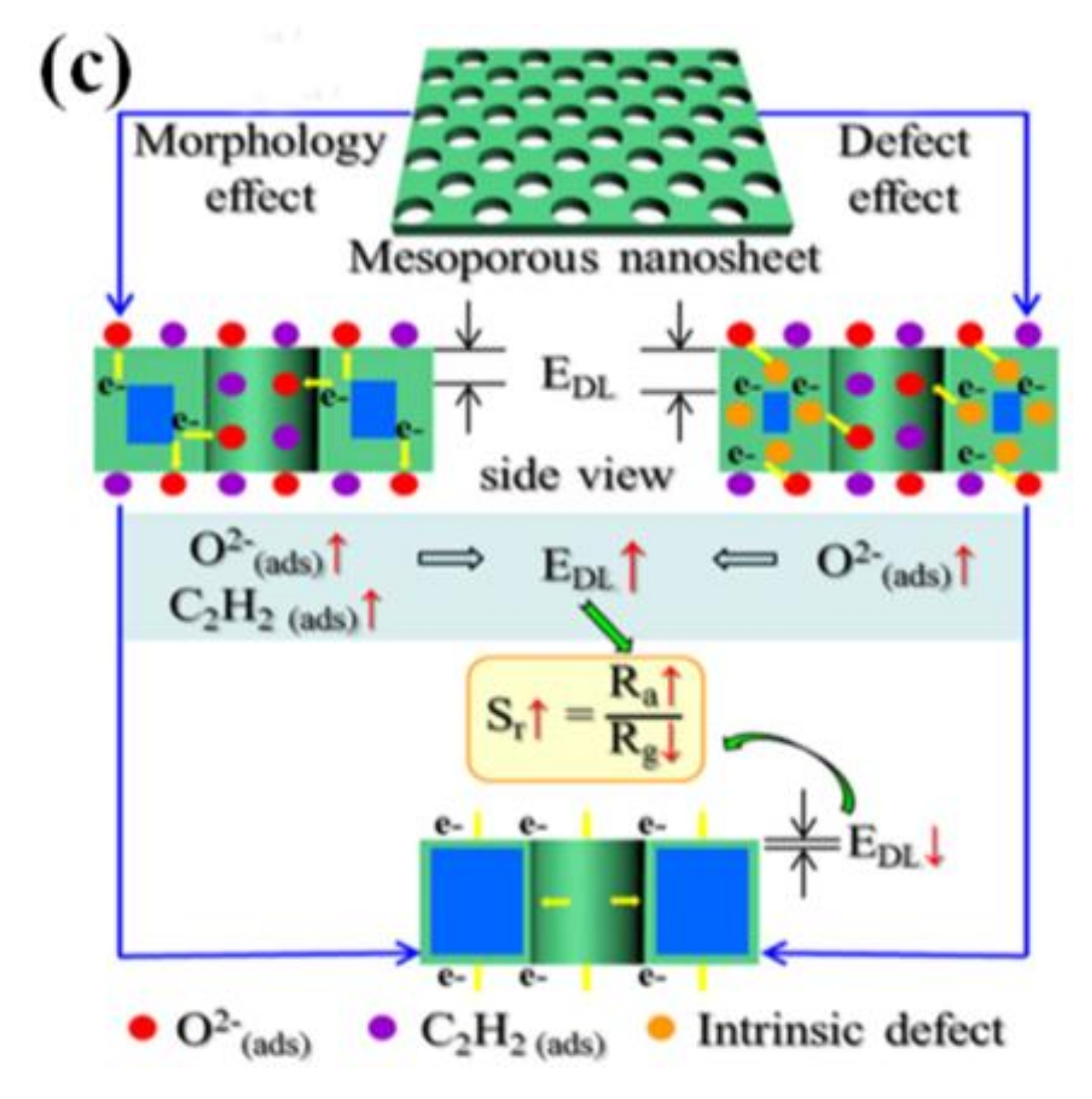
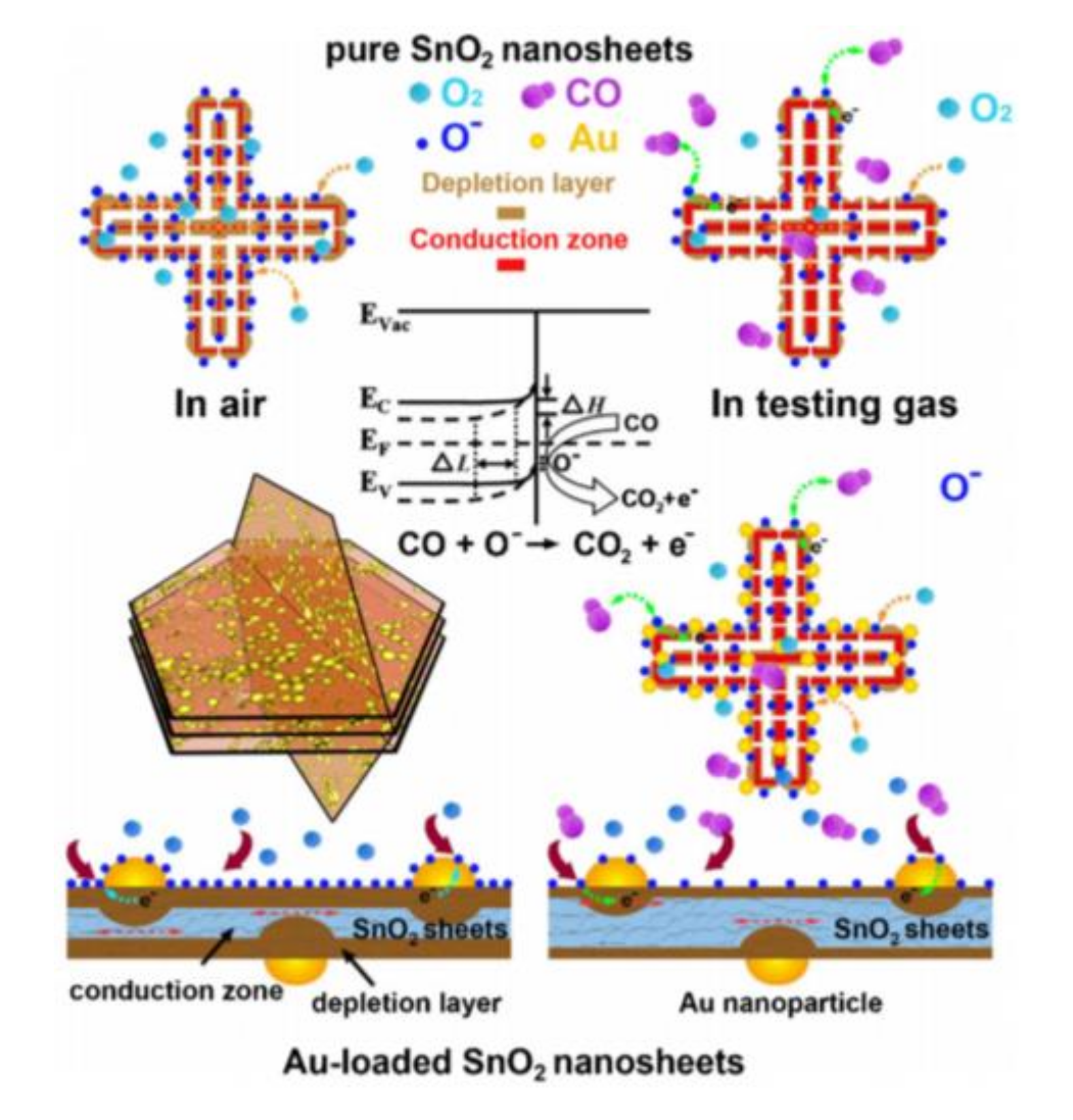


| Morphology | Gases | Materials | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| nanowire | H2 | CuO | 100 | NA | 300 | 60 | 2 | 340 b | [68] |
| nanowire | NO2 | SnO2 | 2 | NA | 150 | 25 min | 45 min | 6.9 b | [69] |
| nanowire | H2S | SnO2 | 10 | NA | 250 | 2.3 | 5 min | 380 a | [70] |
| nanorod array | O2 | TiO2 | 4% | NA | RT | 40 | 75 | 1.68 b | [71] |
| nanorod | NO2 | SnO2 | 5 | 0.03 | 150 | 5.9 min | 2.6 min | 5310 b | [72] |
| nanorod | CH4 | TiO2 | 60 | NA | RT | 45 | 33 | 6028 a | [73] |
| nanofiber | ethanol | WO3 | 100 | NA | 350 | NA | NA | 62 a | [74] |
| nanotube | methanol | ZnO | 700 | NA | NA | 2.24 min | 1.03 min | 51.23% c | [75] |
| nanobelt | NO | ZnO | 50 | 0.5 | RT | NA | NA | 6.5 b | [76] |
| Morphology | Gases | Materials | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| nanosheet | acetone | Co3O4 | 100 | 1.8 | 150 | NA | NA | 11.4 b | [100] |
| nanosheet arrays | NO2 | ZnO | 10 | NA | 180 | 3 | 12 | 20 b | [101] |
| nanoplate | H2S | TiO2 | 100 | NA | 300 | NA | NA | 5.5 a | [102] |
| nanoplate | NO2 | WO3 | 100 | 1 | 100 | NA | NA | 131.75 b | [103] |
| Nanoplate arrays | xylene | α-MoO3 | 100 | NA | 370 | 1 | 15 | 19.2 a | [104] |
| thin film | NO2 | WO3 | 1 | NA | 300 | 7 | 8 | 4.1 b | [105] |
| thin film | acetone | ZnO | 100 | NA | 280 | 6 | 18 | 30 a | [106] |
| thin film | 2-proponal | V2O5 | 5 | NA | NA | 3 | 10 | 176 a | [107] |
| nanowall | NO2 | ZnO | 50 | NA | 450 | 23 | 11 | 6.4 b | [108] |
| Morphology | Gases | Materials | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| microsphere | NO2 | In2O3 | 1 | NA | 145 | 60 | 30 | 132 b | [115] |
| microsphere | HCHO | SnO2 | 100 | NA | 200 | 17 | 25 | 38.3 a | [116] |
| flower-like | TEA | α-MoO3 | 100 | 0.5 | 250 | 3 | 1283 | 416 a | [117] |
| flower-like | ethanol | SnO2 | 500 | NA | 300 | 8 | 7 | 208 a | [118] |
| flower-like | ethanol | NiO | 400 | NA | 300 | 4 | 8 | 32 b | [119] |
| flower-like | ethanol | ZnO | 100 | 0.2 | 260 | NA | NA | 123 a | [120] |
| flower-like | acetic acid | SnO2 | 100 | NA | 260 | 18 | 11 | 47.7 a | [121] |
| flower-like | NO2 | WO3 | 0.4 | 0.04 | 120 | NA | NA | 103 b | [122] |
| rose-like | NO2 | Cu2O | 200 | NA | 340 | NA | NA | 6.8 b | [123] |
| leaf-like | acetone | α-Fe2O3 | 200 | NA | 260 | 8 | 9 | 95.4 a | [124] |
| urchin-like | ethanol | WO3 | 100 | NA | 350 | 28 | 12 | 68.56 a | [125] |
| cactus-like | acetone | NiO | 100 | NA | 260 | 24 | 39 | 13.51 b | [126] |
| spindle-like polyhedra | HCHO | In2O3 | 20 | NA | 240 | 1 | 2 | 8.2 a | [127] |
| Morphology | Gases | Materials | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| hollow sphere | acetone | TiO2 | 100 | NA | 320 | NA | NA | 6.9 a | [139] |
| hollow sphere | methanol | α-Fe2O3 | 10 | 1 | 280 | 8 | 9 | 25 a | [140] |
| hollow sphere | NO2 | WO3 | 0.1 | NA | 140 | 90 | 400 | 18 b | [141] |
| hollow sphere | NO2 | WO3 | 1 | 0.04 | 100 | 237 | 88 | 89 b | [142] |
| hollow sphere | HCHO | SnO2 | 200 | NA | 300 | NA | NA | 9 a | [143] |
| hollow sphere | ethanol | ZnSnO3 | 5 | NA | 280 | NA | NA | 4.5 a | [144] |
| hollow sphere | ethanol | SnO2 | 200 | NA | 260 | 10 | 8 | 274.5 a | [145] |
| multi-shelled hollow sphere | acetone | SnO2 | 200 | NA | 200 | 10 | 12 | 153 a | [146] |
| hollow nanofiber | n-propanol | CuO | 100 | 1 | 200 | 19.18 | 63 | 4.66 b | [147] |
| hollow nanosheet | acetone | SnO2 | 50 | NA | 300 | 0.9 | 5.8 | 18.3 a | [148] |
| hollow cauliflower-like | CO | WO3 | 300 | NA | 270 | NA | NA | 41.9 a | [149] |
| hollow six-sided pyramids | ethanol | ZnO | 200 | NA | 240 | 11 | 9 | 187 a | [150] |
| hollow polyhedrons | acetone | ZnSnO3 | 50 | NA | 240 | 17 | 10 | 12.48 a | [151] |
| Morphology | Gases | Materials | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| core-shell nanoparticle | CO | Au/SnO2 | 200 | NA | 200 | NA | NA | 1.3 a | [162] |
| core-shell nanoparticle | O3 | Au/TiO2 | 2.5 | 0.36 | RT | 5 | 24 | 3.27 a | [163] |
| core-shell nanofiber | TMA | In2O3/SnO2 | 10 | NA | 280 | 3 | 32 | 7.11 a | [164] |
| core-shell nanofiber | HCHO | NiO/α-Fe2O3 | 50 | 1 | 240 | 2 | 9 | 12.8 a | [165] |
| core-shell nanofiber | ethanol | SiO2/SnO2 | 200 | NA | NA | 13 | 16 | 37 a | [166] |
| core-shell nanofiber | ethanol | ZnO/SnO2 | 100 | NA | 200 | 75 | 12 | 392.29 a | [167] |
| core-shell nanowire | NO2 | W18O49/TiO2 | 5 | NA | RT | NA | NA | 36.5 a | [168] |
| core-shell nanosphere | H2S | CuO/NiO | 100 | NA | 260 | 18 | 29 | 47.6 b | [169] |
| core-shell nanosphere | NH3 | Co3O4/SnO2 | 50 | NA | 200 | 4 | 17 | 13.6 a | [170] |
| yolk-shell nanoparticle | ethanol | Pd/In2O3 | 5 | NA | 350 | NA | NA | 159.02 a | [171] |
| yolk-shell nanoparticle | acetone | Au/ZnO | 10 | 0.05 | 280 | 15 | 12 | 43 a | [172] |
| yolk-shellnanostructure | H2S | MoO3/Fe2(MoO4)3 | 1 | NA | 70 | 20 | 70 | 1.7 a | [173] |
| yolk-shellnanosphere | acetone | ZnFe2O4 | 50 | 0.5 | 200 | NA | NA | 28.5 a | [174] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Lv, X.; Li, S.; Wang, Q. The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties. Sensors 2017, 17, 2779. https://doi.org/10.3390/s17122779
Lin T, Lv X, Li S, Wang Q. The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties. Sensors. 2017; 17(12):2779. https://doi.org/10.3390/s17122779
Chicago/Turabian StyleLin, Tingting, Xin Lv, Shuang Li, and Qingji Wang. 2017. "The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties" Sensors 17, no. 12: 2779. https://doi.org/10.3390/s17122779
APA StyleLin, T., Lv, X., Li, S., & Wang, Q. (2017). The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties. Sensors, 17(12), 2779. https://doi.org/10.3390/s17122779





