A Wearable Magneto-Inertial System for Gait Analysis (H-Gait): Validation on Normal Weight and Overweight/Obese Young Healthy Adults
Abstract
1. Background
2. Methods
2.1. Participants
2.2. Procedures
2.2.1. H-Gait
2.2.2. STEP32
2.3. Protocol
- (1)
- Tape measurements between anatomical landmarks were collected from the subject. In particular, the following measurements were taken: pelvis breadth (between the left and right great trochanter), thigh height (between the great trochanter and the lateral epicondyle of the femur), shank height (between the lateral epicondyle of the femur and the lateral malleolus), and sphyrion height (between the lateral malleolus and the floor). Measurements of the lower limbs allow for calculating the body segment coordinate system [36].
- (2)
- Ten reflective markers were placed in correspondence of the volunteer’s greater trochanter, lateral epicondyle of the femur, medial epicondyle of the femur, lateral malleolus and medial malleolus, bilaterally [30].
- (3)
- The volunteer was placed in front of a homogeneous background. Three digital images were taken from the front, left and right sides of the volunteer: the room light was reduced and a flash was used to highlight markers [37]. Subsequently, marker centroids were determined from these images to estimate the inclination of body segments, in order to obtain the rotation matrix to change from the global to the body reference systems (RGlobalToBody) [36].
- (4)
- Reflective markers were removed.
- (5)
- Six STEP32 foot-switches were fixed under both barefoot soles (3 under each foot). In particular, footswitches were positioned beneath the back portion of the heel, the first, and fifth metatarsal heads [38].
- (6)
- Six STEP32 electro-goniometric sensors were fixed in correspondence of ankle, knee and hip joints of each lower limb, with bi-adhesive tape [30].
- (7)
- Elastic bands with Velcro and tape were used to fix the seven H-Gait inertial sensors: two below the medial malleolus [37], two on the shanks in correspondence of the anterior side of the tibia bone, two on the lateral side of the thighs and one on the pelvis, in the posterior center point between the left and right iliac crest. Sensor positions were chosen to minimize motion artifacts and to avoid overlapping among the sensors of the two systems.
3. Signal Processing and Data Analysis
3.1. H-Gait
3.2. STEP32
3.3. Statistical Analysis
3.3.1. Agreement between H-Gait and STEP32
3.3.2. Comparison between NW and OW Groups
4. Results
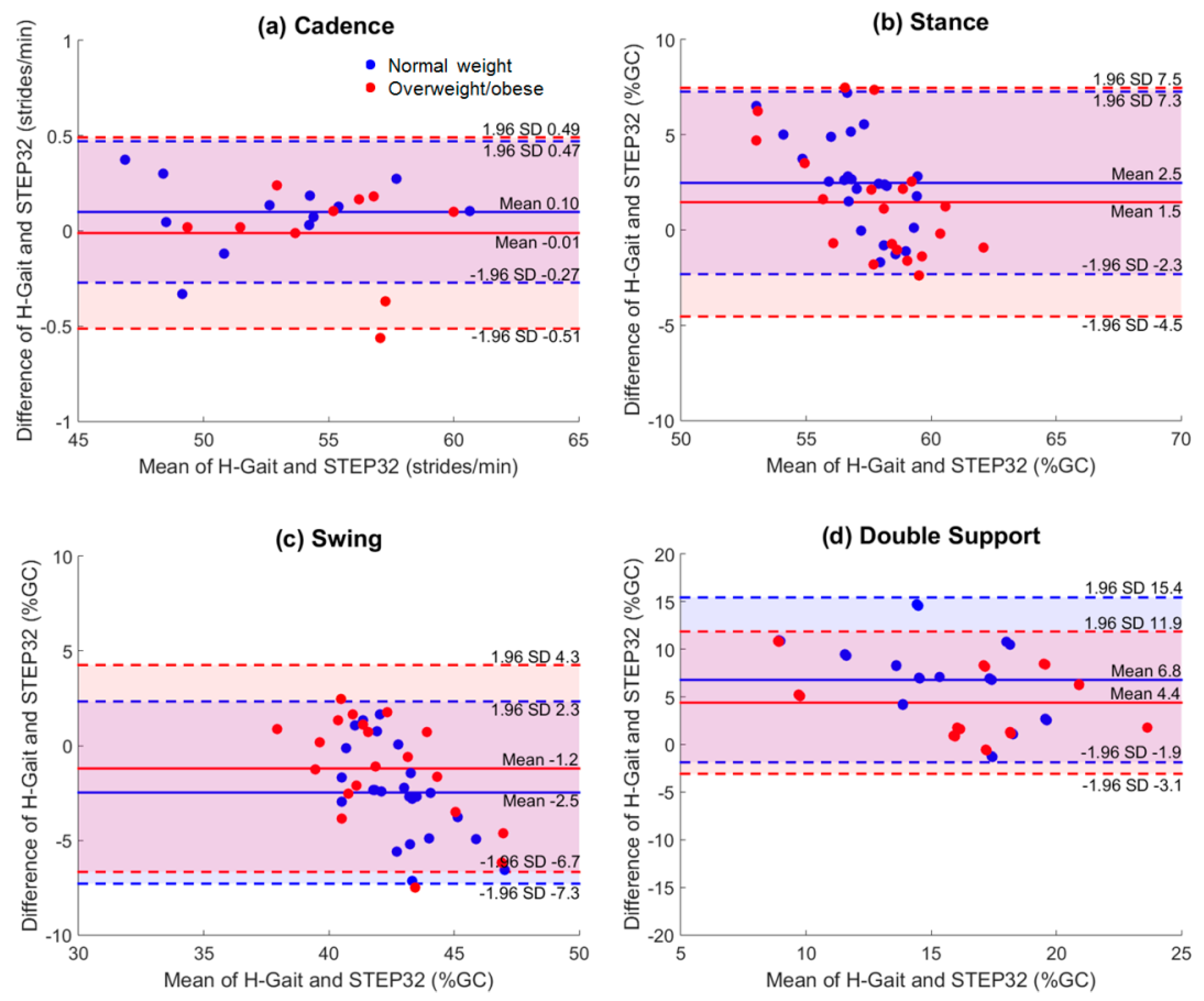
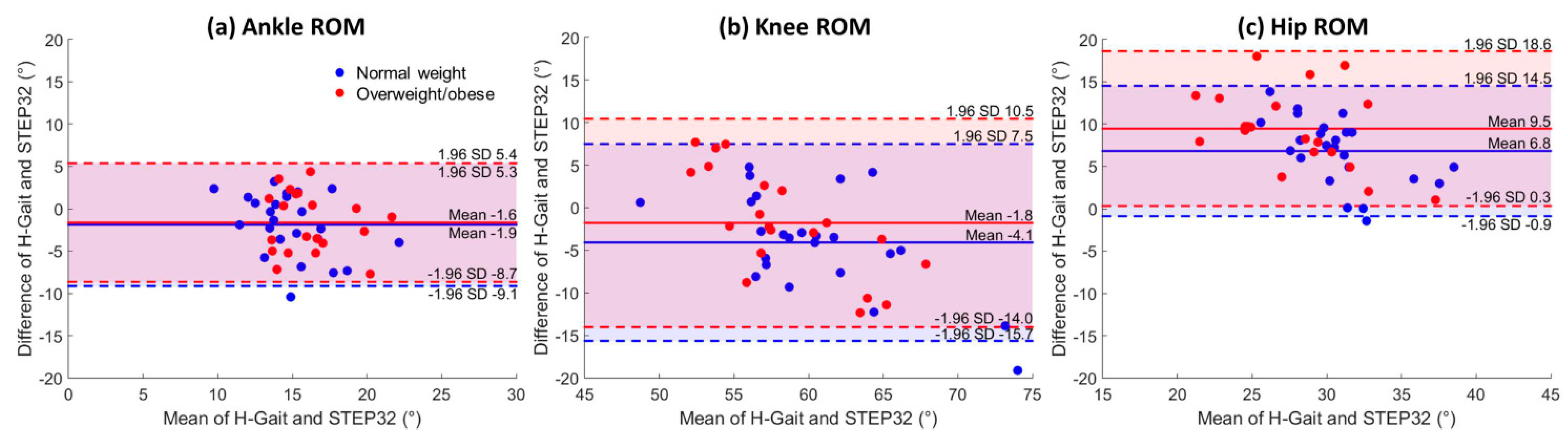
4.1. Agreement between H-Gait and STEP32
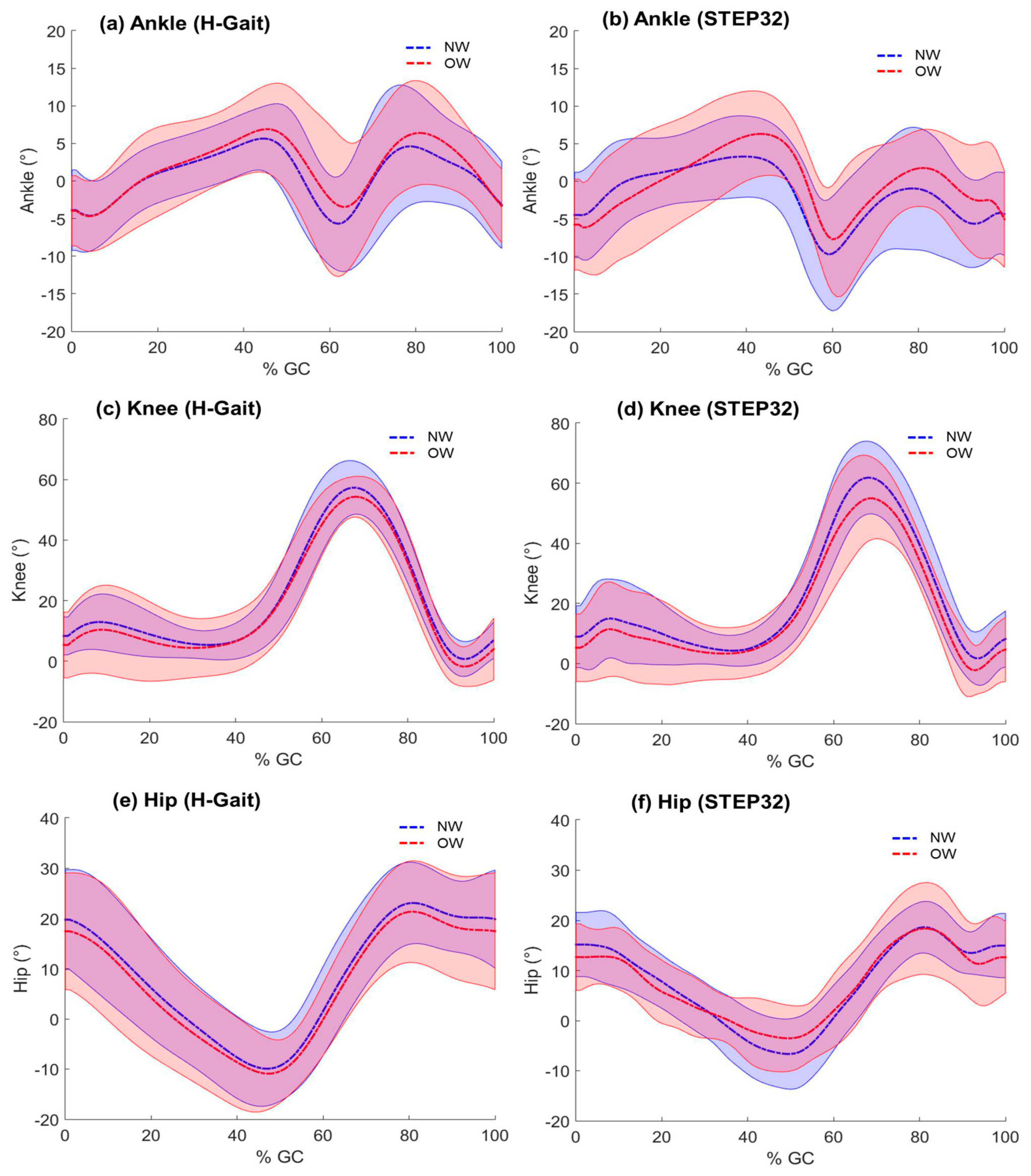
4.2. Comparison between NW and OW Groups
5. Discussion
5.1. Agreement between H-Gait and STEP32
5.1.1. Spatio-Temporal Parameters
5.1.2. Joint Kinematics
5.2. Comparison between NW and OW Groups
5.3. Limitations
6. Conclusions
Acknowledgements
Authors Contributions
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| MIMUs | Magnetic and Inertial Measurement Units |
| NW | Normal Weight |
| OW | Overweight |
| ROM | Range of Motion |
References
- Wren, T.A.L.; Gorton, G.E.; Õunpuu, S.; Tucker, C.A. Efficacy of clinical gait analysis: A systematic review. Gait Posture 2011, 34, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait analysis using wearable sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef] [PubMed]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: an overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef] [PubMed]
- Shull, P.B.; Jirattigalachote, W.; Hunt, M.A.; Cutkosky, M.R.; Delp, S.L. Quantified self and human movement: A review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture 2014, 40, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Picerno, P.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis. Expert Rev. Med. Devices 2016, 13, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Trojaniello, D.; Cereatti, A.; Pelosin, E.; Avanzino, L.; Mirelman, A.; Hausdorff, J.M.; Della Croce, U. Estimation of step-by-step spatio-temporal parameters of normal and impaired gait using shank-mounted magneto-inertial sensors: application to elderly, hemiparetic, parkinsonian and choreic gait. J. Neuroeng. Rehabil. 2014, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, E.; Ligorio, G.; Summa, A.; Vannozzi, G.; Cappozzo, A.; Sabatini, A.M. Estimating orientation using magnetic and inertial sensors and different sensor fusion approaches: accuracy assessment in manual and locomotion tasks. Sensors 2014, 14, 18625–18649. [Google Scholar] [CrossRef] [PubMed]
- Bolink, S.A.A.N.; Naisas, H.; Senden, R.; Essers, H.; Heyligers, I.C.; Meijer, K.; Grimm, B. Validity of an inertial measurement unit to assess pelvic orientation angles during gait, sit-stand transfers and step-up transfers: Comparison with an optoelectronic motion capture system. Med. Eng. Phys. 2016, 38, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Hübsch, T.; Brandt, A.U.; Pfueller, C.; Zange, L.; Seidel, A.; Kühn, A.A.; Paul, F.; Minnerop, M.; Doss, S. Accuracy and repeatability of two methods of gait analysis – GaitRiteTM und Mobility LabTM – in subjects with cerebellar ataxia. Gait Posture 2016, 48, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Donath, L.; Faude, O.; Lichtenstein, E.; Nüesch, C.; Mündermann, A. Validity and reliability of a portable gait analysis system for measuring spatiotemporal gait characteristics: comparison to an instrumented treadmill. J. Neuroeng. Rehabil. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Storm, F.A.; Buckley, C.J.; Mazzà, C. Gait event detection in laboratory and real life settings: Accuracy of ankle and waist sensor based methods. Gait Posture 2016, 50, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Tadano, S.; Natorigawa, A.; Todoh, M.; Yoshinari, S. Gait posture estimation using wearable acceleration and gyro sensors. J. Biomech. 2009, 42, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Inoue, Y.; Shibata, K. Development of a wearable sensor system for quantitative gait analysis. Measurement 2009, 42, 978–988. [Google Scholar] [CrossRef]
- Takeda, R.; Lisco, G.; Fujisawa, T.; Gastaldi, L.; Tohyama, H.; Tadano, S. Drift Removal for Improving the Accuracy of Gait Parameters Using Wearable Sensor Systems. Sensors 2014, 14, 23230–23247. [Google Scholar] [CrossRef] [PubMed]
- Picerno, P.; Cereatti, A.; Cappozzo, A. Joint kinematics estimate using wearable inertial and magnetic sensing modules. Gait Posture 2008, 28, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, A.M.; Martelloni, C.; Scapellato, S.; Cavallo, F. Assessment of walking features from foot inertial sensing. IEEE Trans. Biomed. Eng. 2005, 52, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Robert-Lachaine, X.; Mecheri, H.; Larue, C.; Plamondon, A. Effect of local magnetic field disturbances on inertial measurement units accuracy. Appl. Ergon. 2017, 63, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.; Rossi, S.; Patanè, F.; Cappa, P. Experimental evaluation of indoor magnetic distortion effects on gait analysis performed with wearable inertial sensors. Physiol. Meas. 2014, 35, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.; Rossi, S.; Marini, F.; Patanè, F.; Cappa, P. Experimental evaluation of accuracy and repeatability of a novel body-to-sensor calibration procedure for inertial sensor-based gait analysis. Meas. J. Int. Meas. Confed. 2014, 52, 145–155. [Google Scholar] [CrossRef]
- Favre, J.; Aissaoui, R.; Jolles, B.M.; de Guise, J.A.; Aminian, K. Functional calibration procedure for 3D knee joint angle description using inertial sensors. J. Biomech. 2009. [Google Scholar] [CrossRef] [PubMed]
- Leardini, A.; Chiari, A.; Della Croce, U.; Cappozzo, A. Human movement analysis using stereophotogrammetry Part 3. Soft tissue artifact assessment and compensation. Gait Posture 2005, 21, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Della Croce, U.; Leardini, A.; Chiari, L.; Cappozzo, A. Human movement analysis using stereophotogrammetry Part 4: Assessment of anatomical landmark misplacement and its effects on joint kinematics. Gait Posture 2005, 21, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Galna, B.; Sangeux, M.; Morris, M.; Baker, R. Quantification of soft tissue artifact in lower limb human motion analysis: a systematic review. Gait Posture 2010, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Forner-Cordero, A.; Mateu-Arce, M.; Forner-Cordero, I.; Alcántara, E.; Moreno, J.C.; Pons, J.L. Study of the motion artefacts of skin-mounted inertial sensors under different attachment conditions. Physiol. Meas. 2008, 29, N21–N31. [Google Scholar] [CrossRef] [PubMed]
- Browning, R.C.; Kram, R. Effects of obesity on the biomechanics of walking at different speeds. Med. Sci. Sports Exerc. 2007, 39, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Roan, M.; Lee, M. The effect of evenly distributed load carrying on lower body gait dynamics for normal weight and overweight subjects. Gait Posture 2010, 32, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Runhaar, J.; Koes, B.W.; Clockaerts, S.; Bierma-Zeinstra, S.M. A. A systematic review on changed biomechanics of lower extremities in obese individuals: A possible role in development of osteoarthritis. Obes. Rev. 2011, 12, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Freedman Silvernail, J.; Milner, C.E.; Thompson, D.; Zhang, S.; Zhao, X. The influence of body mass index and velocity on knee biomechanics during walking. Gait Posture 2013, 37, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Tadano, S.; Takeda, R.; Sasaki, K.; Fujisawa, T.; Tohyama, H. Gait characterization for osteoarthritis patients using wearable gait sensors (H-Gait systems). J. Biomech. 2016, 49, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Agostini, V.; Knaflitz, M.; Antenucci, L.; Lisco, G.; Gastaldi, L.; Tadano, S. Wearable sensors for gait analysis. In 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings; IEEE: Torino, Italy, 2015; pp. 146–150. [Google Scholar]
- Agostini, V.; Nascimbeni, A.; Gaffuri, A.; Knaflitz, M. Multiple gait patterns within the same Winters class in children with hemiplegic cerebral palsy. Clin. Biomech. 2015, 30, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, F.; Maranesi, E.; Mengarelli, A.; Ghetti, G.; Burattini, L.; Fioretti, S. Assessment of the variability of vastii myoelectric activity in young healthy females during walking: A statistical gait analysis. J. Electromyogr. Kinesiol. 2015, 25, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Agostini, V.; Lanotte, M.; Carlone, M.; Campagnoli, M.; Azzolin, I.; Scarafia, R.; Massazza, G.; Knaflitz, M. Instrumented gait analysis for an objective pre-/postassessment of tap test in normal pressure hydrocephalus. Arch. Phys. Med. Rehabil. 2015, 96, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Cutti, A.G.; Ferrari, A.; Garofalo, P.; Raggi, M.; Cappello, A.; Ferrari, A. “Outwalk”: A protocol for clinical gait analysis based on inertial and magnetic sensors. Med. Biol. Eng. Comput. 2010, 48, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Bachmann, E.R. Design, Implementation, and Experimental Results of a Quaternion-Based Kalman Filter for Human Body Motion Tracking. IEEE Trans. Robot. 2006, 22, 1216–1227. [Google Scholar] [CrossRef]
- Tadano, S.; Takeda, R.; Miyagawa, H. Three dimensional gait analysis using wearable acceleration and gyro sensors based on quaternion calculations. Sensors 2013, 13, 9321–9343. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, L.; Rosso, V.; Gabola, V.; Agostini, V.; Frutos, M.M.L.; Knaflitz, M.; Takeda, R.; Tadano, S. Technical challenges using magneto-inertial sensors for gait analysis. In 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA); IEEE: Benevento, Italy, 2016; pp. 1–6. [Google Scholar]
- Agostini, V.; Balestra, G.; Knaflitz, M. Segmentation and classification of gait cycles. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J.L. The Design and Analysis of Clinical Experiments; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999; ISBN 9781118032923. [Google Scholar]
- Bland, J.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Laroche, D.; Duval, A.; Morisset, C.; Beis, J.-N.; d’Athis, P.; Maillefert, J.-F.; Ornetti, P. Test–retest reliability of 3D kinematic gait variables in hip osteoarthritis patients. Osteoarthr. Cartil. 2011, 19, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Agostini, V.; Nascimbeni, A.; Gaffuri, A.; Imazio, P.; Benedetti, M.G.; Knaflitz, M. Normative EMG activation patterns of school-age children during gait. Gait Posture 2010, 32, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.D.; Morrison, S.C.; Drechsler, W.I. Foot loading patterns in normal weight, overweight and obese children aged 7 to 11 years. J. Foot Ankle Res. 2013, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Hennig, E.M.; McDonald, M.; Bar-Or, O. Plantar pressure differences between obese and non-obese adults: a biomechanical analysis. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Gates, D.H.; Darter, B. J.; Dingwell, J.B.; Wilken, J.M. Comparison of walking overground and in a Computer Assisted Rehabilitation Environment (CAREN) in individuals with and without transtibial amputation. J. Neuroeng. Rehabil. 2012, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.J.; Cavanagh, P.R. Measurement of the screw-home motion of the knee is sensitive to errors in axis alignment. J. Biomech. 2000, 33, 1029–1034. [Google Scholar] [CrossRef]
- de Oliveira Sato, T.; Hansson, G.Å.; Coury, H.J.C.G. Goniometer crosstalk compensation for knee joint applications. Sensors 2010, 10, 9994–10005. [Google Scholar] [CrossRef] [PubMed]
- Wearing, S.C.; Hennig, E.M.; Byrne, N.M.; Steele, J.R.; Hills, A.P. The biomechanics of restricted movement in adult obesity. Obes. Rev. 2006, 7, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kirtley, C.; Whittle, M.W.; Jefferson, R.J. Influence of walking speed on gait parameters. J. Biomed. Eng. 1985, 7, 282–288. [Google Scholar] [CrossRef]
- DeVita, P.; Hortobágyi, T. Obesity is not associated with increased knee joint torque and power during level walking. J. Biomech. 2003, 36, 1355–1362. [Google Scholar] [CrossRef]
- Spyropoulos, P.; Pisciotta, J.C.; Pavlou, K.N.; Cairns, M.A.; Simon, S.R. Biomechanical gait analysis in obese men. Arch. Phys. Med. Rehabil. 1991, 72, 1065–1070. [Google Scholar] [PubMed]
- Iosa, M.; Cereatti, A.; Merlo, A.; Campanini, I.; Paolucci, S.; Cappozzo, A. Assessment of waveform similarity in clinical gait data: The linear fit method. Biomed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Grazia, M.; Pavan, E.; Frigo, C.; Bettinelli, D.; Rabuffetti, M.; Crenna, P.; Leardini, A.; Benedetti, M.G.; Pavan, E.; et al. Quantitative comparison of five current protocols in gait analysis. Gait Posture 2008, 28, 207–216. [Google Scholar] [CrossRef] [PubMed]
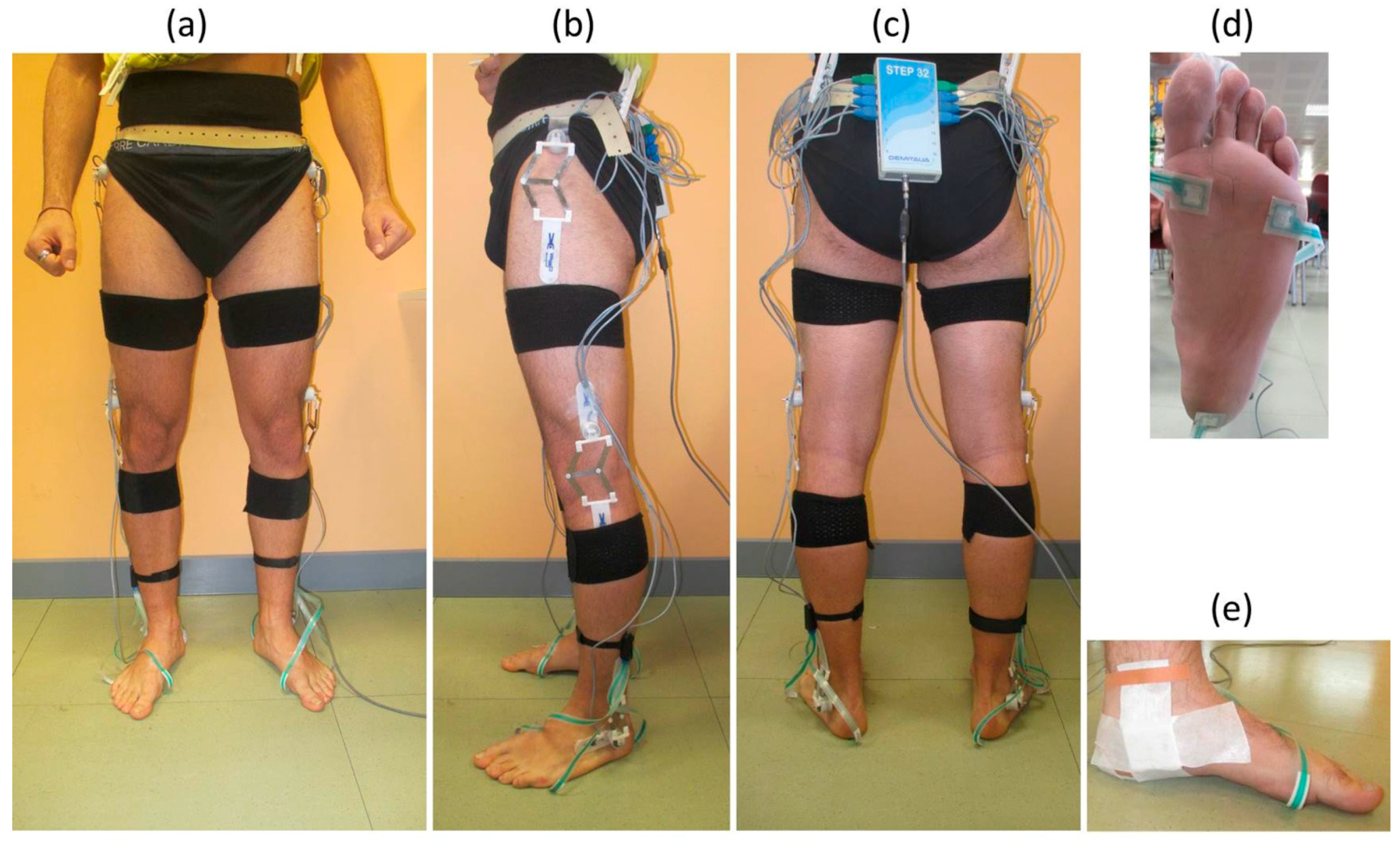
| Age (years) | Height (cm) | Body Mass (kg) | BMI | |
|---|---|---|---|---|
| NW (N = 12) | 26.2 ± 1.5 | 180.4 ± 8.8 | 74.3 ± 8.2 * | 22.8 ± 1.1 * |
| OW (N = 10) | 25.6 ± 2.2 | 175.3 ± 7.6 | 95.5 ± 12.4 * | 31.1 ± 3.3 * |
| NW | OW | |||||
|---|---|---|---|---|---|---|
| Difference a (Mean ± SD) | ICC b | ICC 95%-CI | Difference a (Mean ± SD) | ICC b | ICC 95%-CI | |
| Spatio-Temporal Parameters | ||||||
| Cadence (strides/min) | 0.1 ± 0.2 | 0.99 | [0.97, 1.0] | −0.01 ± 0.3 | 1.00 | [1.00, 1.00] |
| Stance (%GC) | 2.5 ± 2.5 | 0.23 | [−0.78, 0.67] | 1.5 ± 3.0 | 0.66 | [0.14, 0.87] |
| Swing (%GC) | −2.5 ± 2.5 | 0.36 | [−0.47, 0.73] | −1.2 ± 2.8 | 0.67 | [0.16, 0.87] |
| Double support (%GC) | 6.8 ± 4.4 | 0.44 | [−0.30, 0.76] | 4.4 ± 3.8 | 0.82 | [0.53, 0.93] |
| Joint Kinematic Parameters | ||||||
| Ankle ROM (°) | −1.9 ± 3.7 | 0.49 | [−0.18, 0.78] | −1.6 ± 3.6 | 0.43 | [−0.44, 0.77] |
| Knee ROM (°) | −4.1 ± 5.9 | 0.72 | [0.36, 0.88] | −1.8 ± 6.2 | 0.56 | [−0.13, 0.82] |
| Hip ROM (°) | 6.8 ± 3.9 | 0.61 | [0.11, 0.83] | 9.5 ± 4.7 | 0.69 | [0.21, 0.88] |
| H-Gait | STEP32 | |||||
|---|---|---|---|---|---|---|
| NW | OW | p-value | NW | OW | p-value | |
| Spatio-Temporal Parameters | ||||||
| Cadence (strides/min) | 52.9 ± 4.0 | 55.0 ± 3.0 | - | 52.6 ± 4.1* | 55.0 ± 3.1* | 0.04 |
| Stance (%GC) | 58.0 ± 2.2 | 58.7 ± 2.0 | - | 55.9 ± 2.7 | 57.2 ± 3.6 | 0.18 |
| Swing (%GC) | 41.8 ± 1.5 | 41.5 ± 2.0 | - | 44.2 ± 2.7 | 42.7 ± 3.4 | 0.12 |
| Double support (%GC) | 18.6 ± 2.6 | 19.0 ± 4.2 | - | 11.8 ± 4.6 | 14.5 ± 5.4 | 0.08 |
| Joint Kinematic Parameters | ||||||
| Ankle ROM (°) | 13.9 ±2.5 | 15.3 ± 2.8 | - | 15.8 ± 3.7 | 16.9 ± 3.2 | 0.30 |
| Knee ROM (°) | 58.4 ± 4.4 | 57.5 ± 3.1 | - | 62.5 ± 7.8 | 59.2 ± 7.3 | 0.17 |
| Hip ROM (°) | 34.1 ± 2.6 | 32.5 ± 3.8 | - | 27.3 ± 4.6 * | 23.0 ± 5.6 * | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostini, V.; Gastaldi, L.; Rosso, V.; Knaflitz, M.; Tadano, S. A Wearable Magneto-Inertial System for Gait Analysis (H-Gait): Validation on Normal Weight and Overweight/Obese Young Healthy Adults. Sensors 2017, 17, 2406. https://doi.org/10.3390/s17102406
Agostini V, Gastaldi L, Rosso V, Knaflitz M, Tadano S. A Wearable Magneto-Inertial System for Gait Analysis (H-Gait): Validation on Normal Weight and Overweight/Obese Young Healthy Adults. Sensors. 2017; 17(10):2406. https://doi.org/10.3390/s17102406
Chicago/Turabian StyleAgostini, Valentina, Laura Gastaldi, Valeria Rosso, Marco Knaflitz, and Shigeru Tadano. 2017. "A Wearable Magneto-Inertial System for Gait Analysis (H-Gait): Validation on Normal Weight and Overweight/Obese Young Healthy Adults" Sensors 17, no. 10: 2406. https://doi.org/10.3390/s17102406
APA StyleAgostini, V., Gastaldi, L., Rosso, V., Knaflitz, M., & Tadano, S. (2017). A Wearable Magneto-Inertial System for Gait Analysis (H-Gait): Validation on Normal Weight and Overweight/Obese Young Healthy Adults. Sensors, 17(10), 2406. https://doi.org/10.3390/s17102406






