PlaIMoS: A Remote Mobile Healthcare Platform to Monitor Cardiovascular and Respiratory Variables
Abstract
:1. Introduction
1.1. Research Background and Motivation
1.2. Research Objective
- A framework to manage and analyze information collected by PlaIMoS platform;
- A wireless sensor network (WSN) infrastructure that includes a scalable network algorithm;
- A mobile application to provide real-time monitoring and information access for patients, doctors and caregivers.
2. Related Work
2.1. Importance of e-Health Platforms
2.2. e-Health Platforms
- Discover and naming: communication pathways between sensors and receiving devices are established;
- Robust routing: a sensor node reports its data to multiple receiving nodes, thus providing system redundancy;
- Prioritization of critical data: critical and time sensitive data have priority over other control traffic;
- Tracking device location: radio frequency (RF) signals are used to track patient and rescue device locations.
- Health sensors: the types of measurements the platform can perform using a wearable device.
- Communication system: the communication architecture that transmits data from the wearable device to the collector system.
- Data security: the security mechanism used to protect the information generated on the platform.
- Emergency detection: the capacity to launch a push notification if an emergency event occurs.
- Display devices: the type of devices that display data generated by the platform.
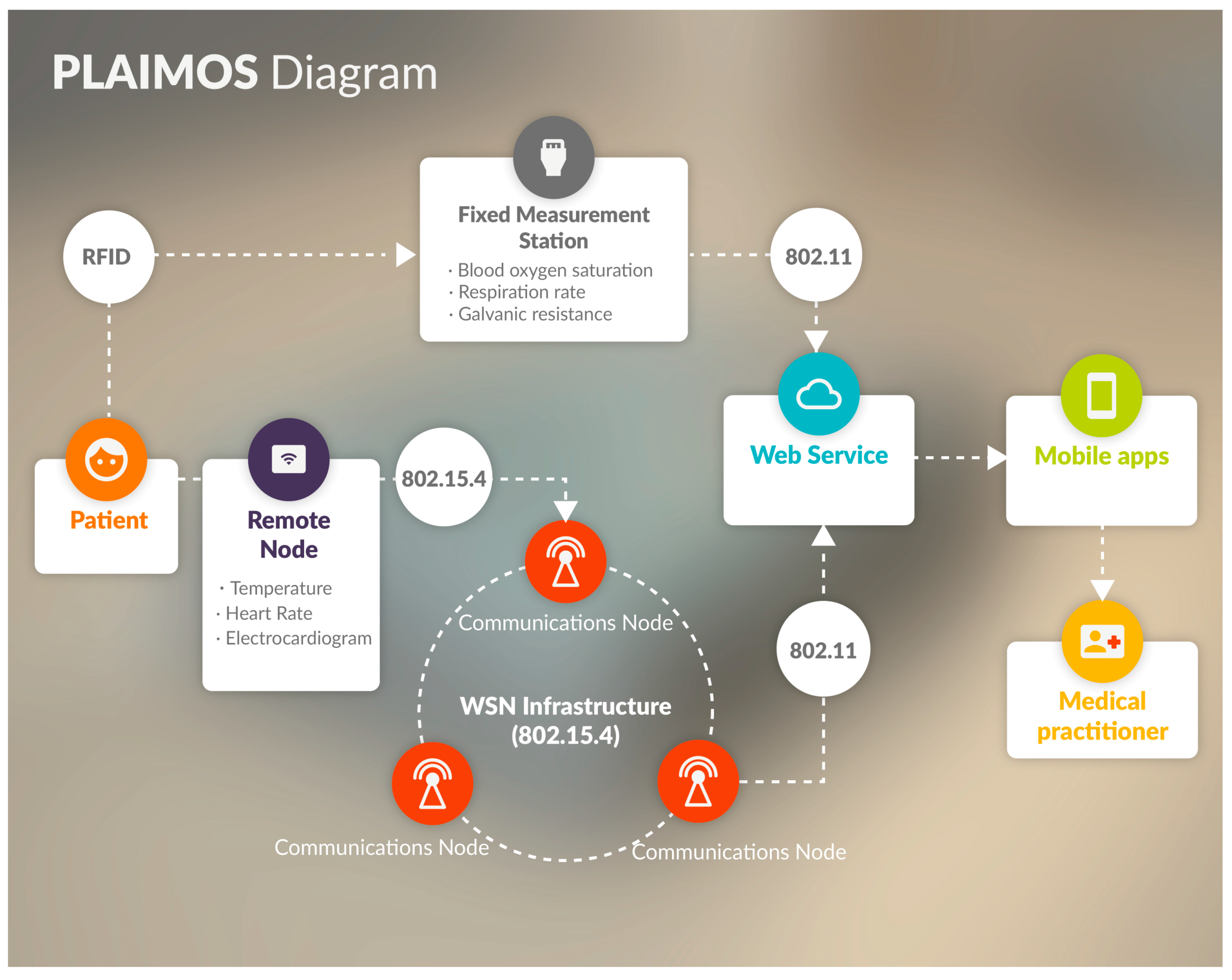
3. Proposal Architecture
3.1. Architecture
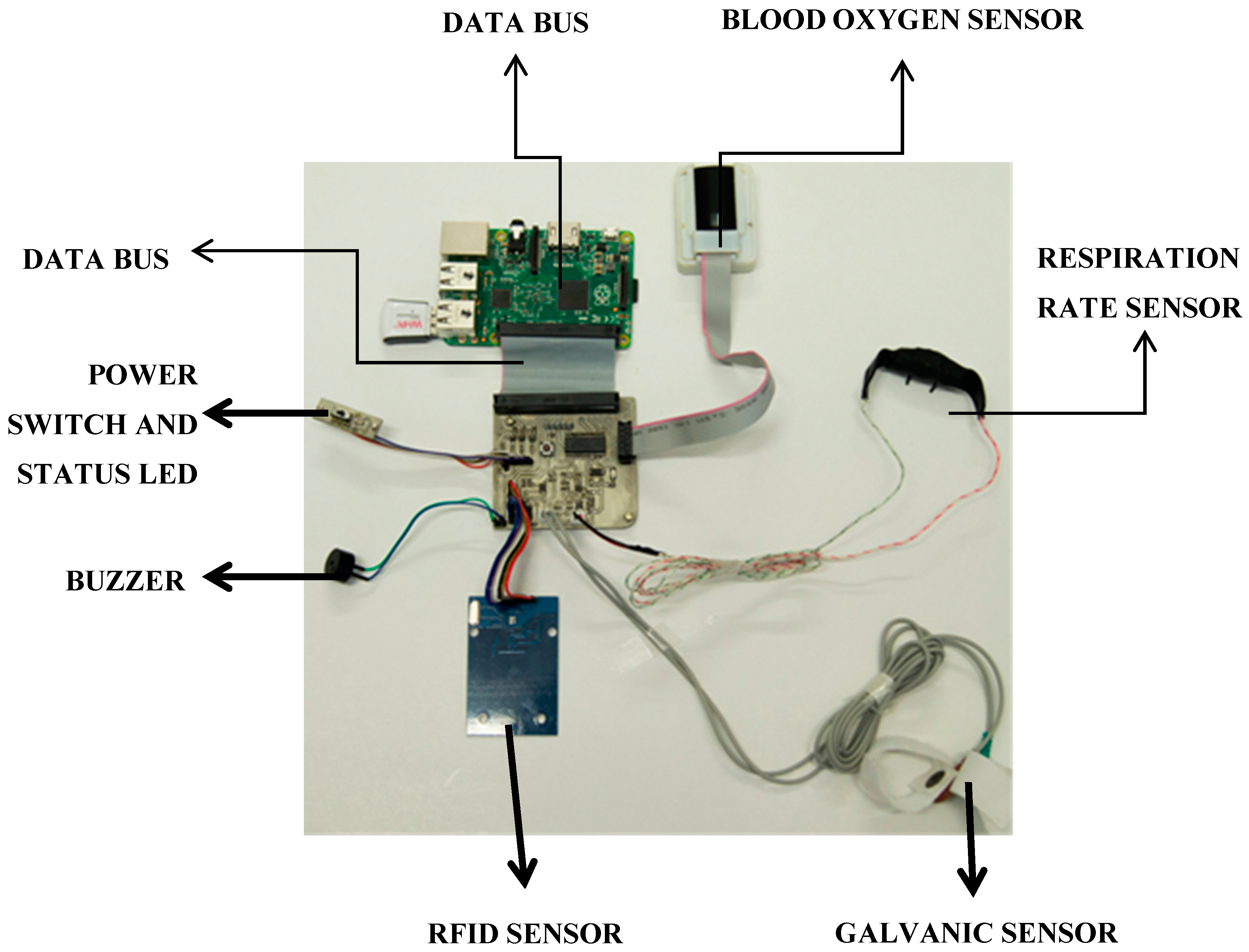
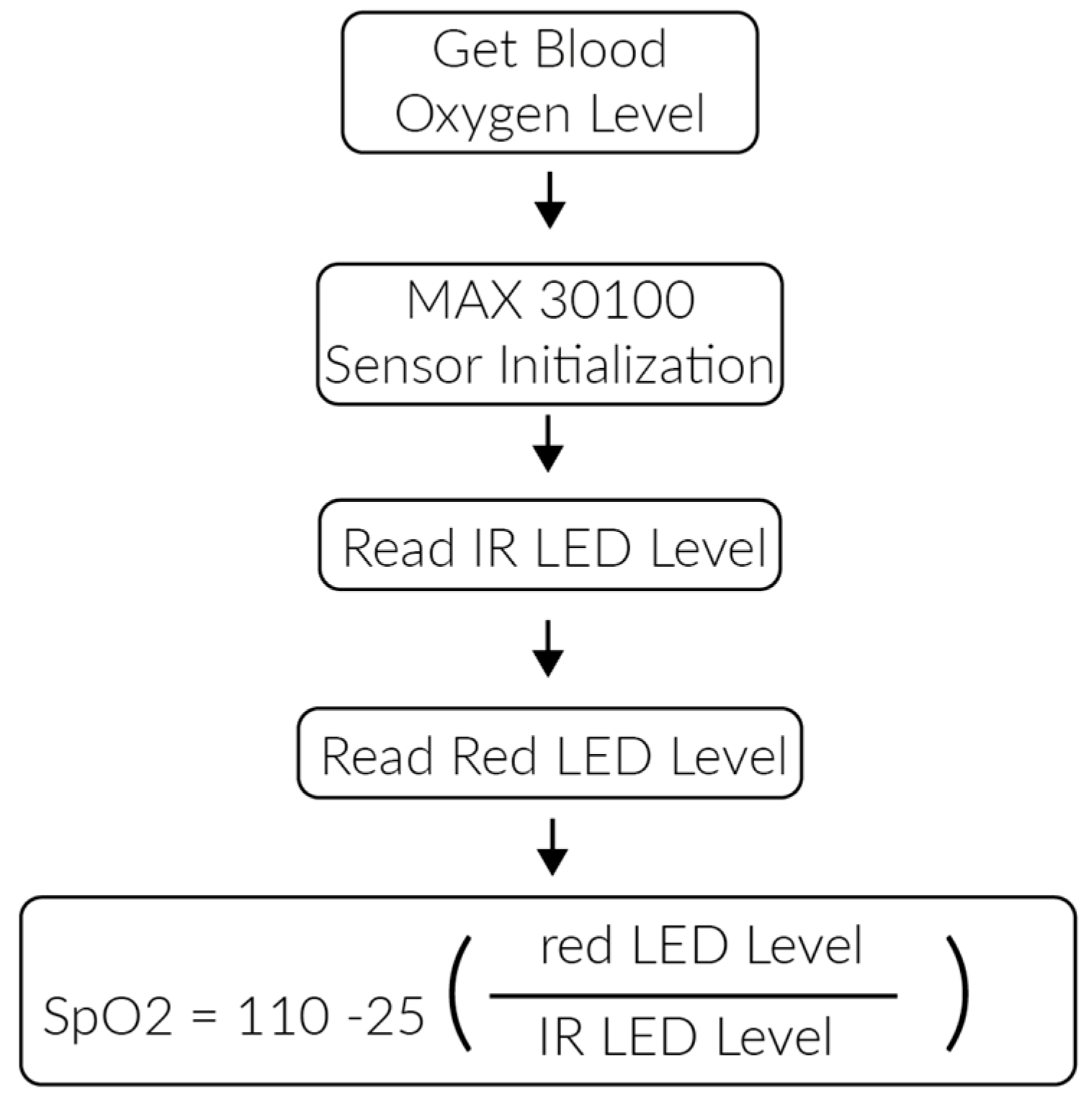
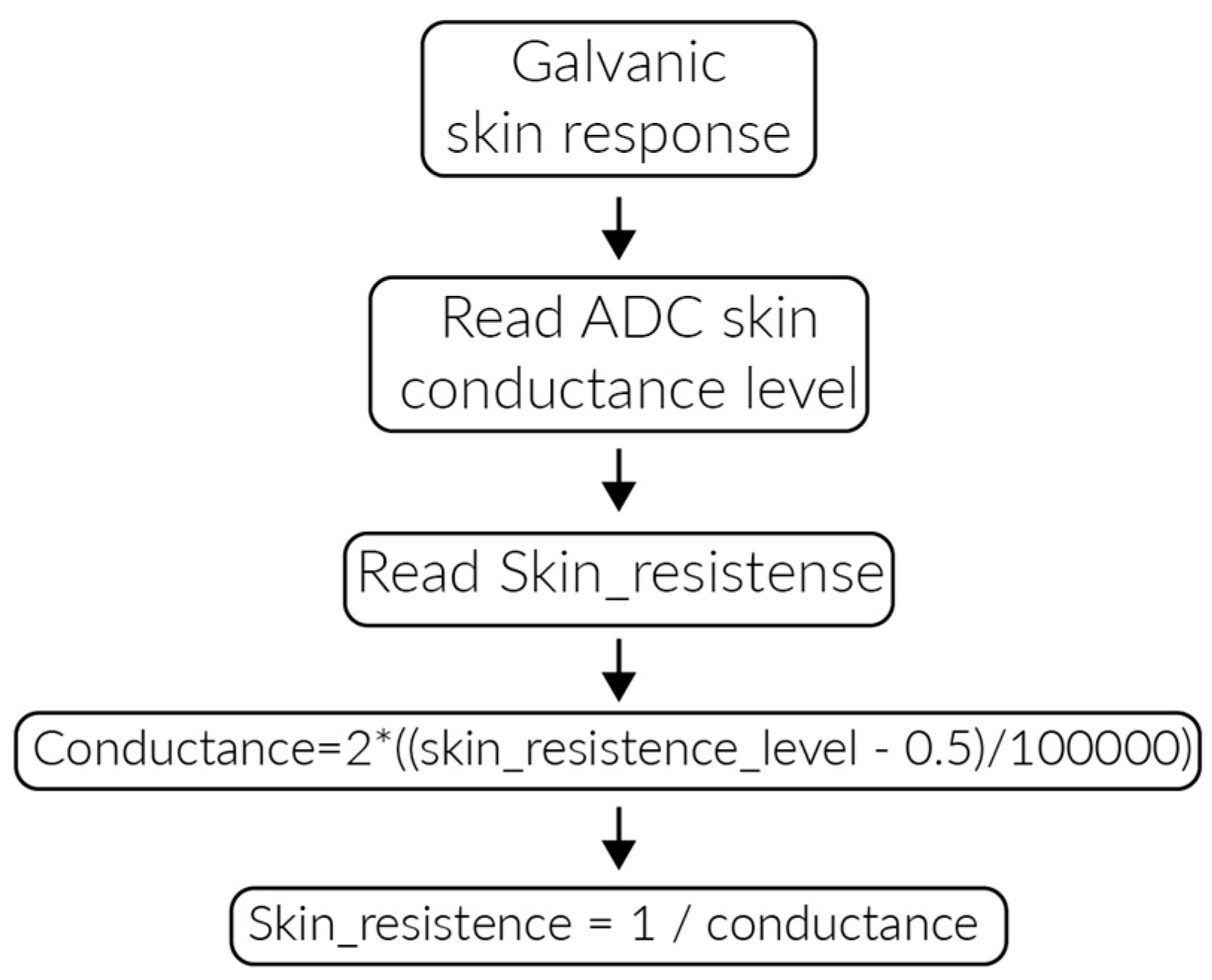
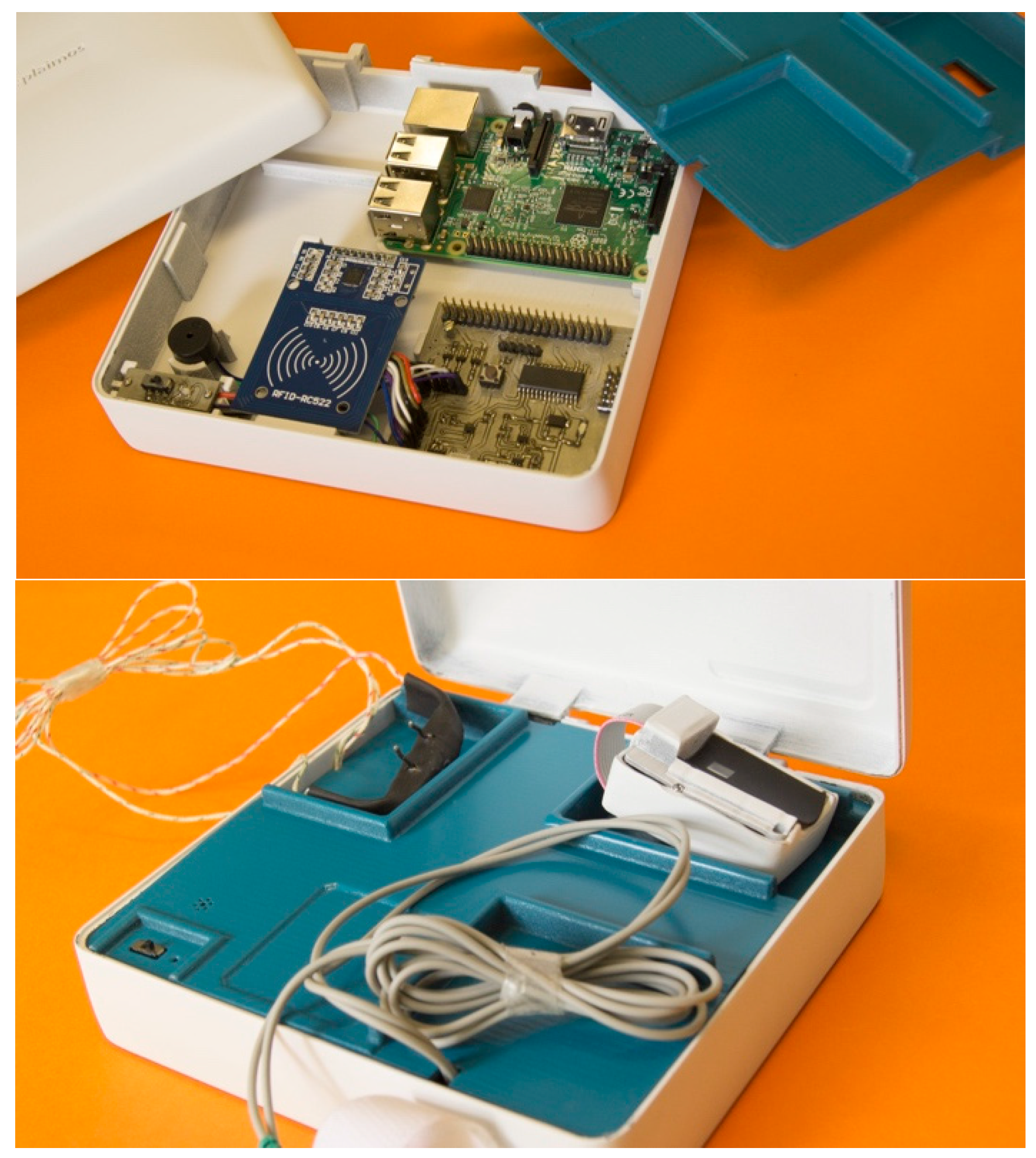
3.2. PlaIMoS Fixed Measurement Station
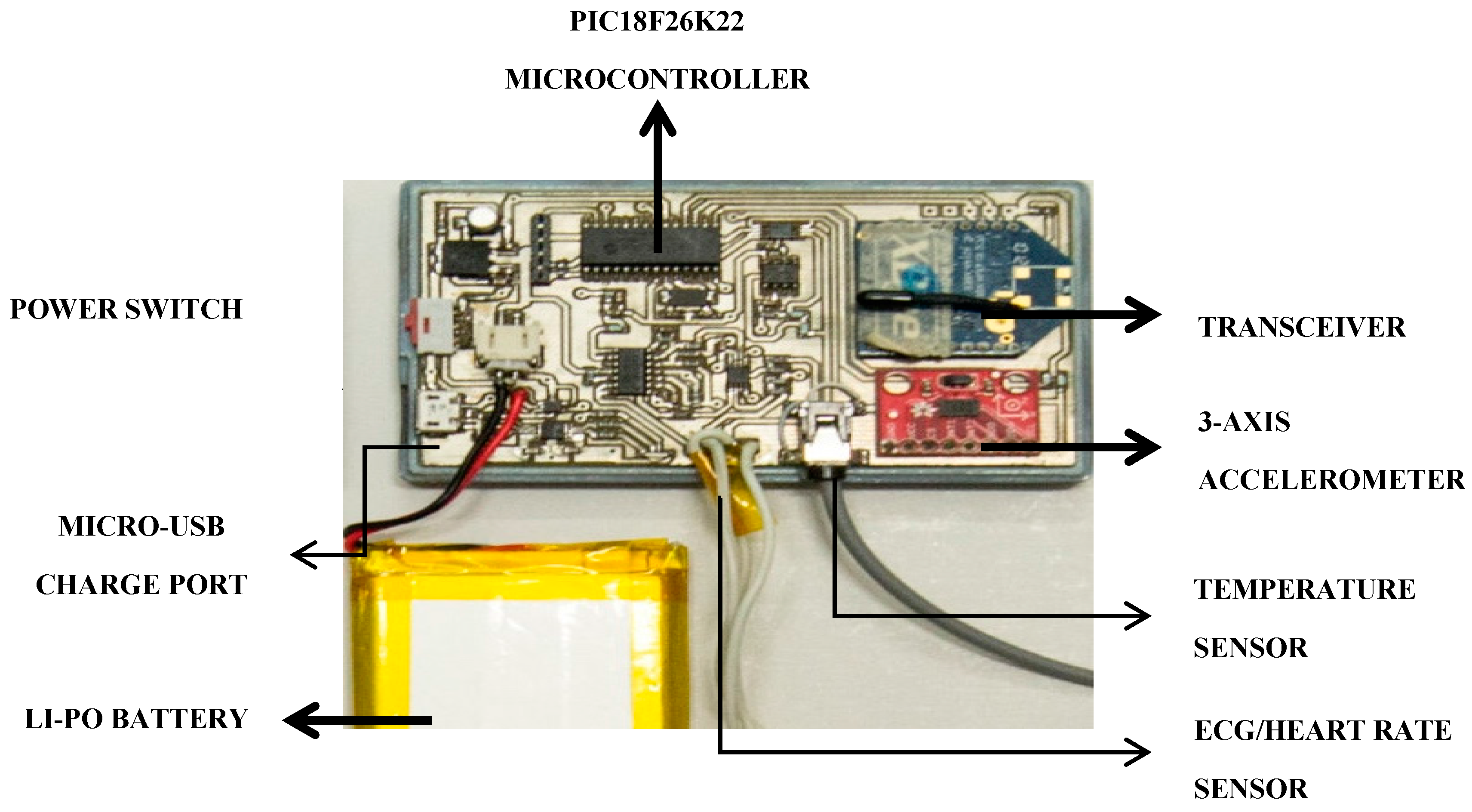
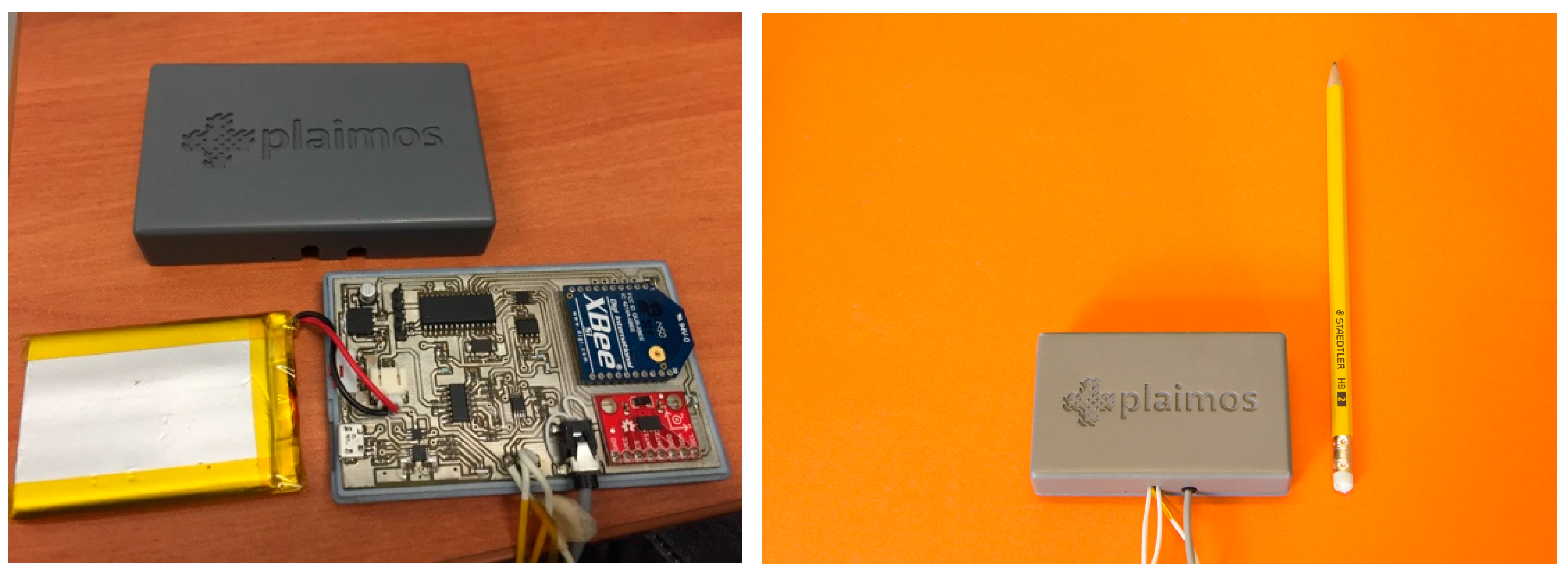
3.3. PlaIMoS Remote Node
3.4. Wireless Sensor Network Infrastructure
- It is scalable; because it is hierarchical, the networks created under this protocol have the ability to incorporate other devices without losing their efficiency. This feature is achieved in large part because each network device possesses a specific role (PlaIMoS remote node, cluster, gateway and sink);
- It provides accurate user location; because of its distributed scheme and its centroid localization algorithm, the PlaIMoS remote node can be located with an accuracy that depends on the number of nodes in the WSN infrastructure;
- It permits user mobility; PlaIMoS-Routing combines a routing protocol developed to operate in static networks where the components are motionless, along with a scheme in which the network nodes can be in constant motion;
- It employs redundant links; during network formation, PlaIMoS-Routing stores each node’s link in a routing table, thus ensuring alternate routes if the data cannot be transmitted via the main link. PlaIMoS-Routing determines the main links through the creation of a metric (determined as weight), which is formed by the intensity of the received signal (RSSI), the number of hops and the lifetime of each node (TTL);
- It is fault tolerant; because of possible redundant links, PlaIMoS-Routing allows the network to continue operating should any link or node fail. If a node has the ability to re-establish its operation, the protocol allows it to rejoin the network and adopt the best suited role at that moment;
- It implements security schemes; PlaIMoS-Routing encrypts data using AES 128, which authorizes the persons or devices authorized to access and use the information generated in the network;
- It utilizes a reactive/ proactive scheme; PlaIMoS-Routing can operate in two different schemes, meaning it can be programmed to operate exclusively to detect any device possessing information that needs to be transmitted, thereby reducing the energy resources required by the network devices, or it can be scheduled to continuously transmit information.
- Remote node; the device that patients use to continuously send their vital signs to the WSN using IEEE 802.15.4;
- Cluster device; the device responsible for group conformation within the network which manages and receives information from remote sensing node using IEEE 802.15.4;
- Gateway device; the device that acts as the communication link between cluster devices to forward information using IEEE 802.15.4;
- Sink device; the device that receives and forwards all information from WSN infrastructure to the PlaIMoS server using IEEE 802.15.4, 802.11 and 802.3.
3.5. Software Application
- Name of the measurement performed
- Date and time of the measurement performed
- Value of the measurement performed
- Minimum measurement value
- Maximum measurement value
- The Web service detects if a measurement is outside the normal preset range.
- The Web service solicits the notification server (Apple, Google and Microsoft Server) to send a push notification to a particular device.
- The notification server receives the request and forwards the notification push to the indicated device.
- The device (smartphone) emits a visual and audible alert with the incidence reported by the Web service regardless of whether the device is locked or the application is running.
4. Results
- relaying information through the WSN Infrastructure using the PlaIMoS-Routing protocol
- locating the indoor location of PlaIMoS remote nodes
- transmitting clinical parameters through the PlaIMoS fixed measurement station
- generating alarms when patient clinical parameters exceeded the present ranges (temperature and patient falls)
- viewing clinical patient information
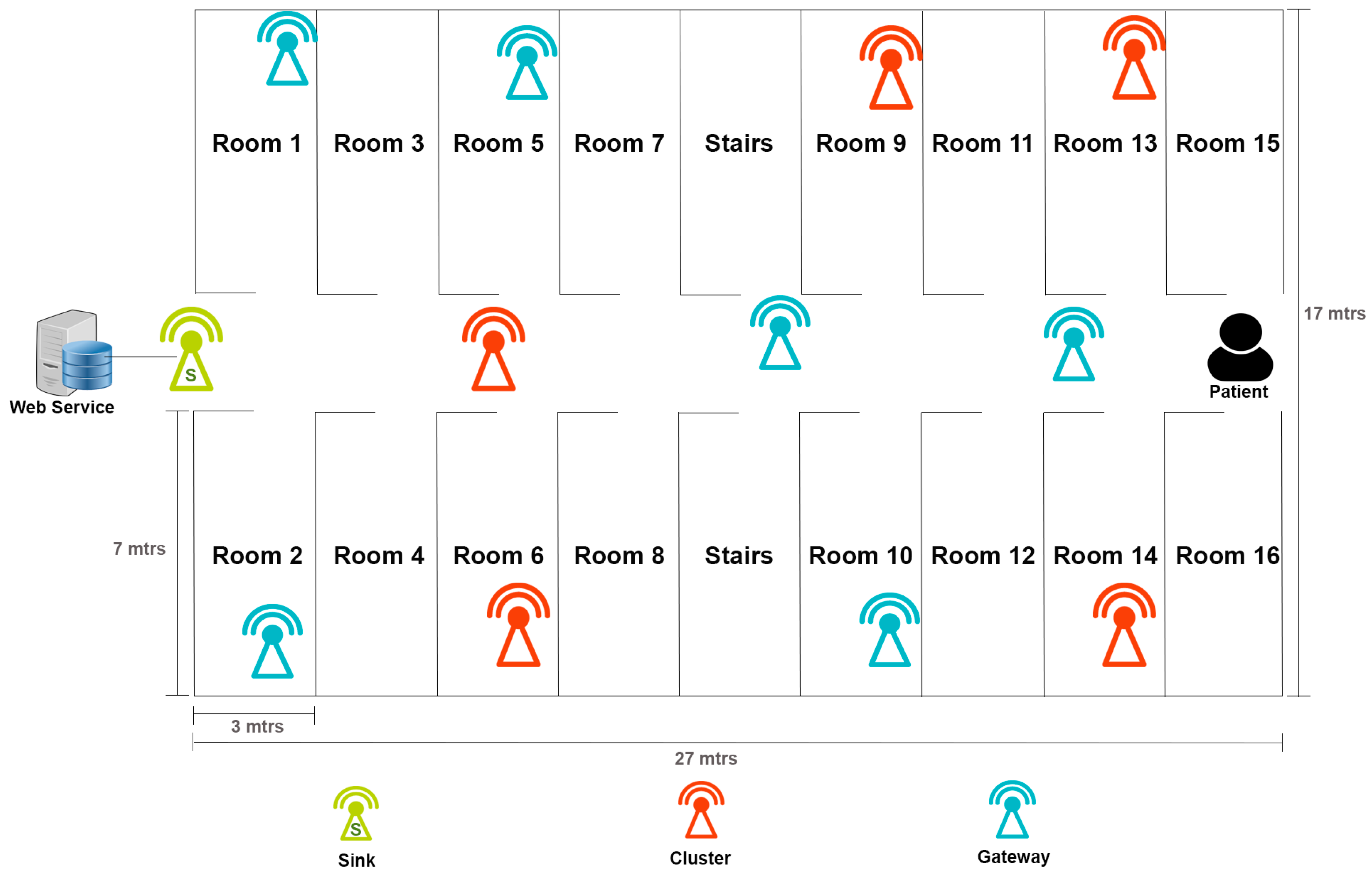
4.1. Pilot Study
4.2. Results of the WSN Infrastructure Using the PlaIMoS-Routing Protocol
4.3. Results of Indoor Location for PlaIMoS Remote Node
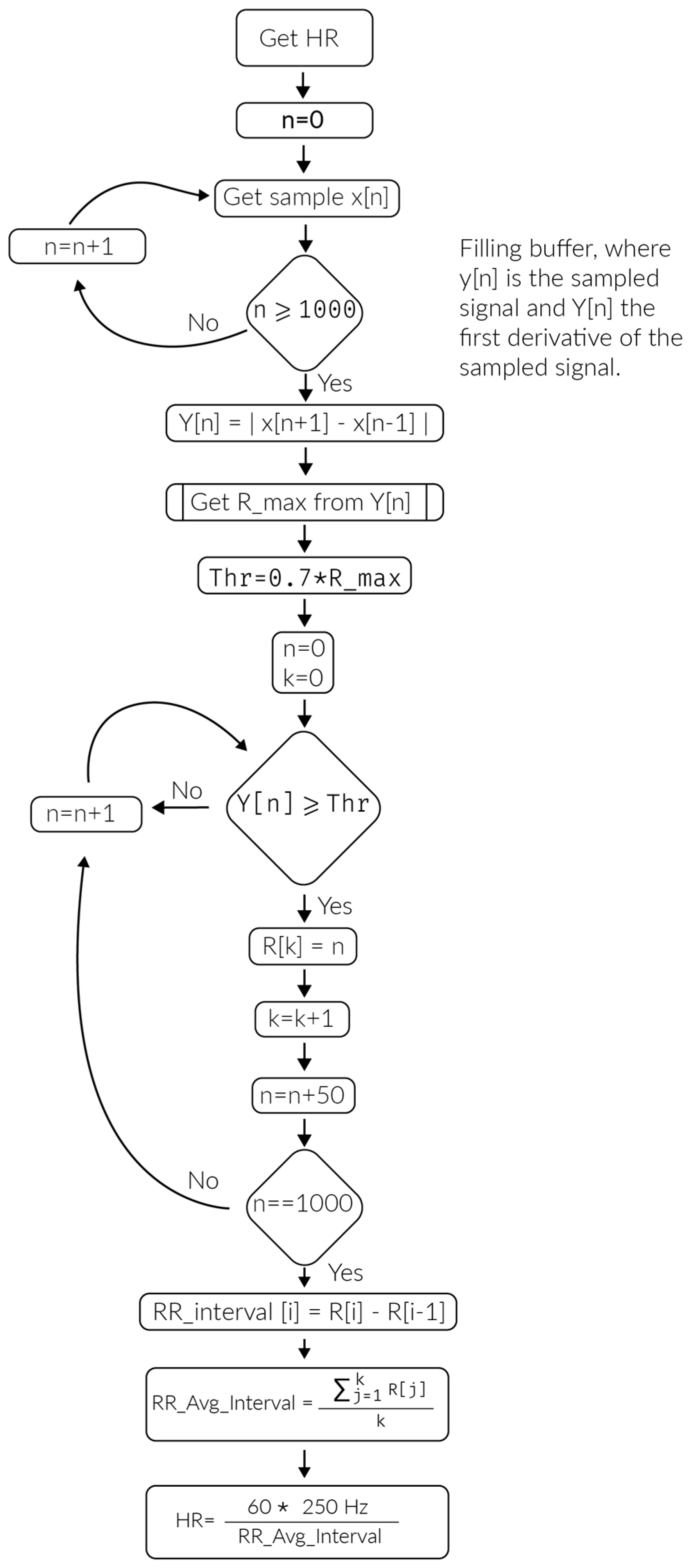
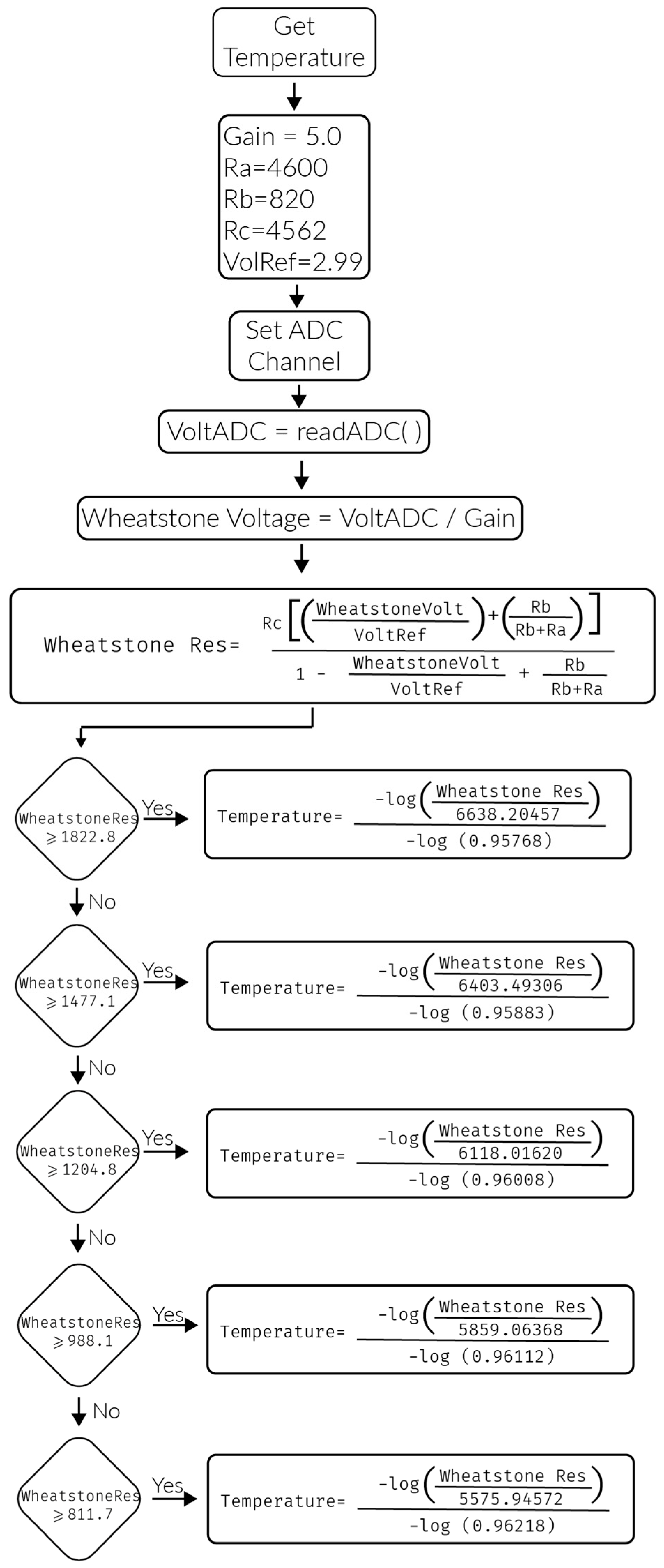
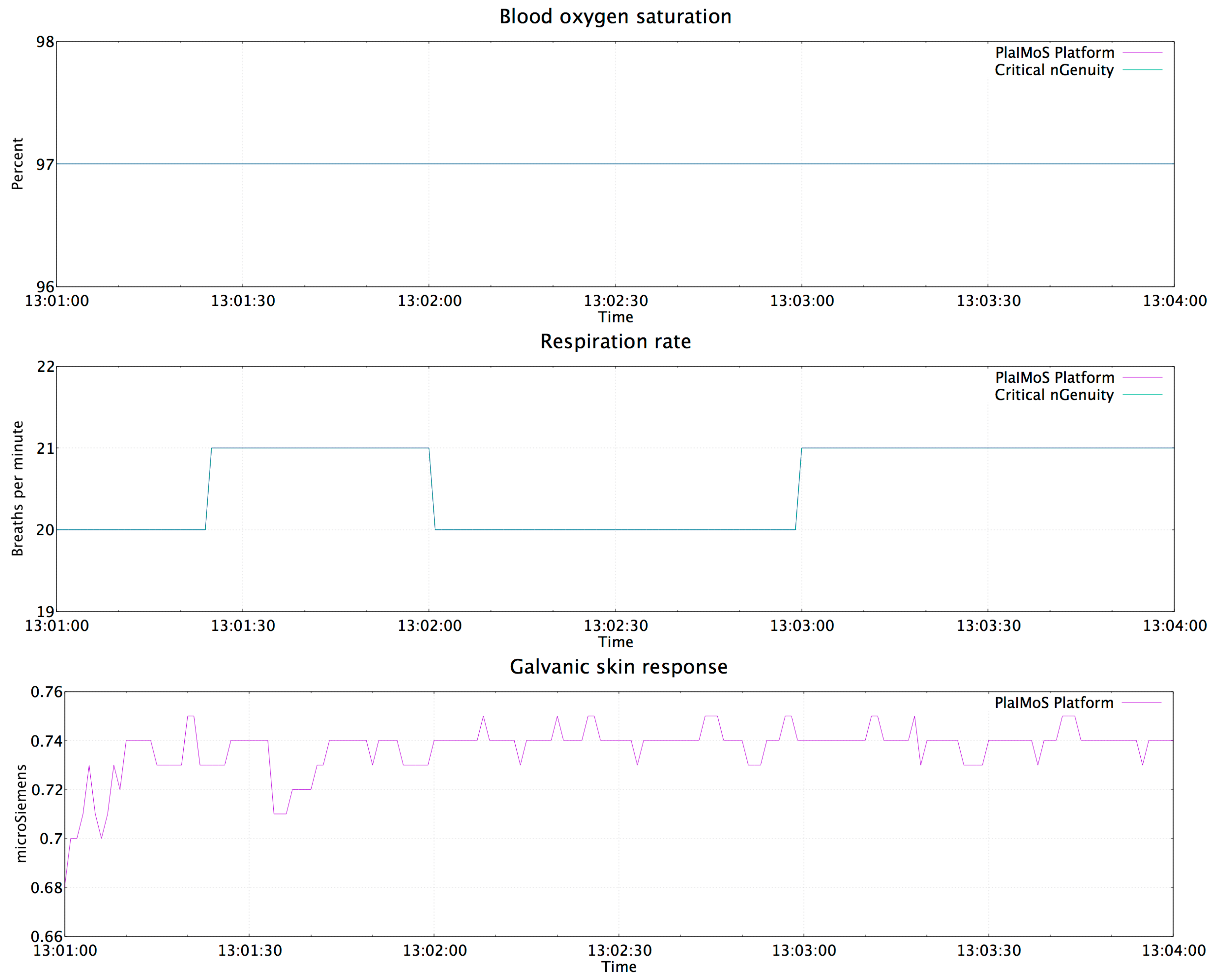
4.4. Results of the PlaIMoS Fixed Measurement Station
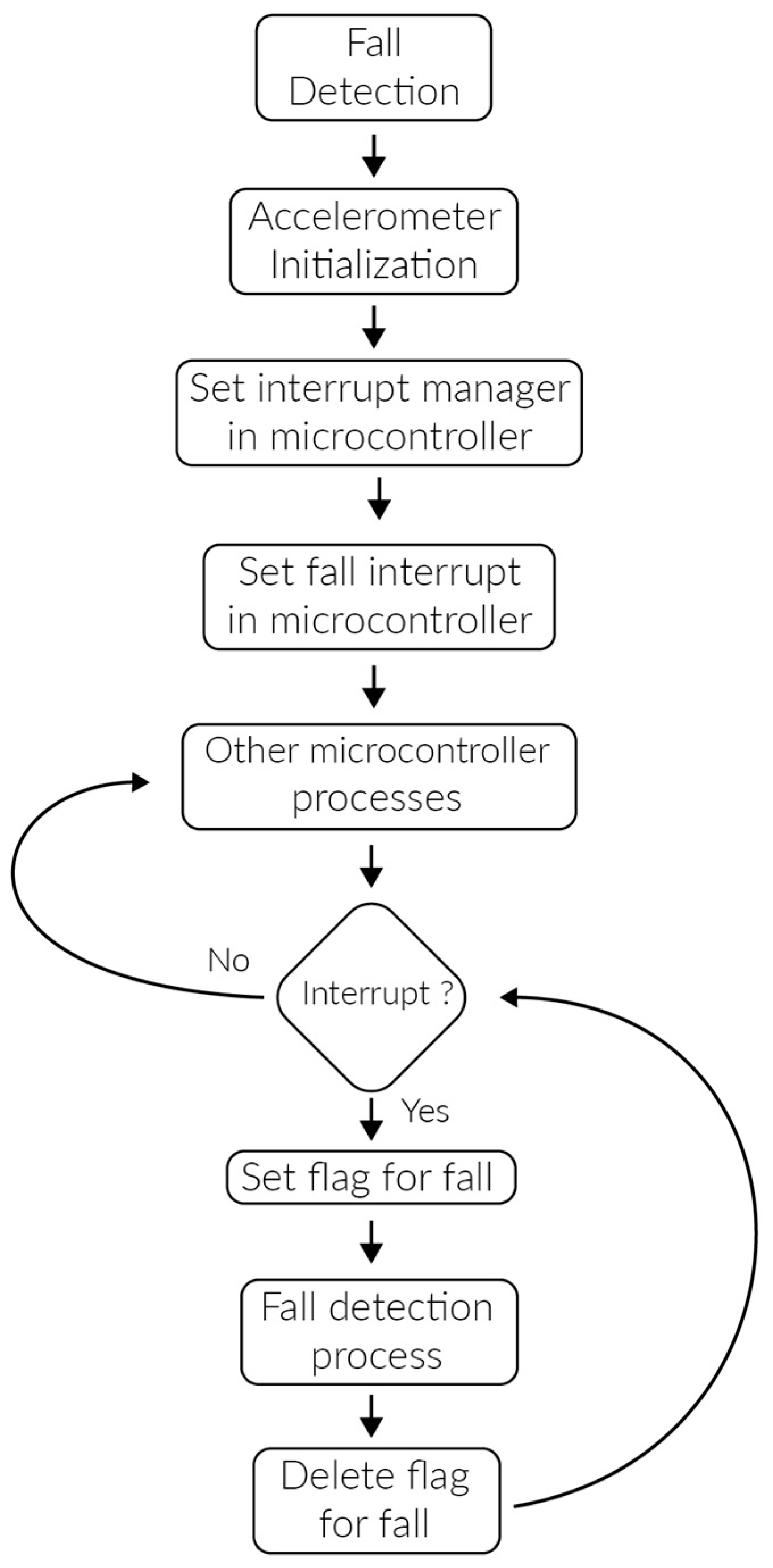
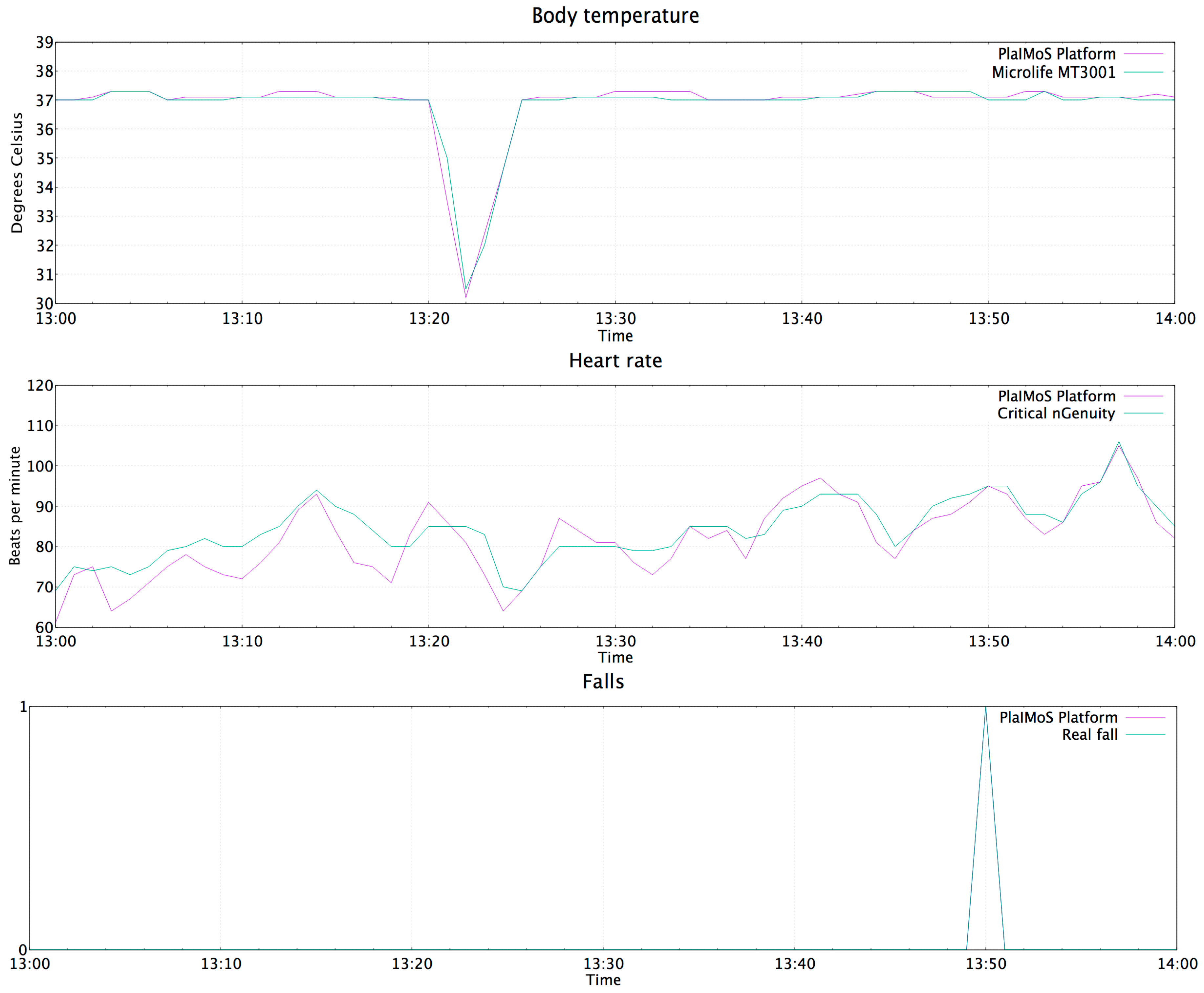
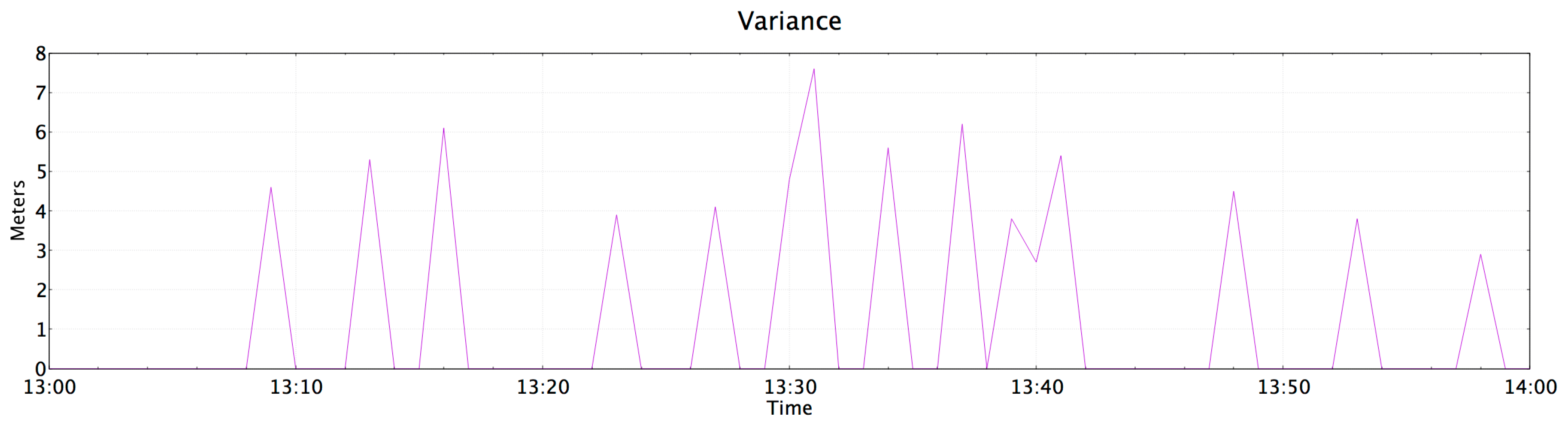
4.5. Push Notification in Case of Abnormalities
- Temperature of 30 °C. (a cold object was placed over the temperature sensor)
- The patient let himself fall to represent a faint
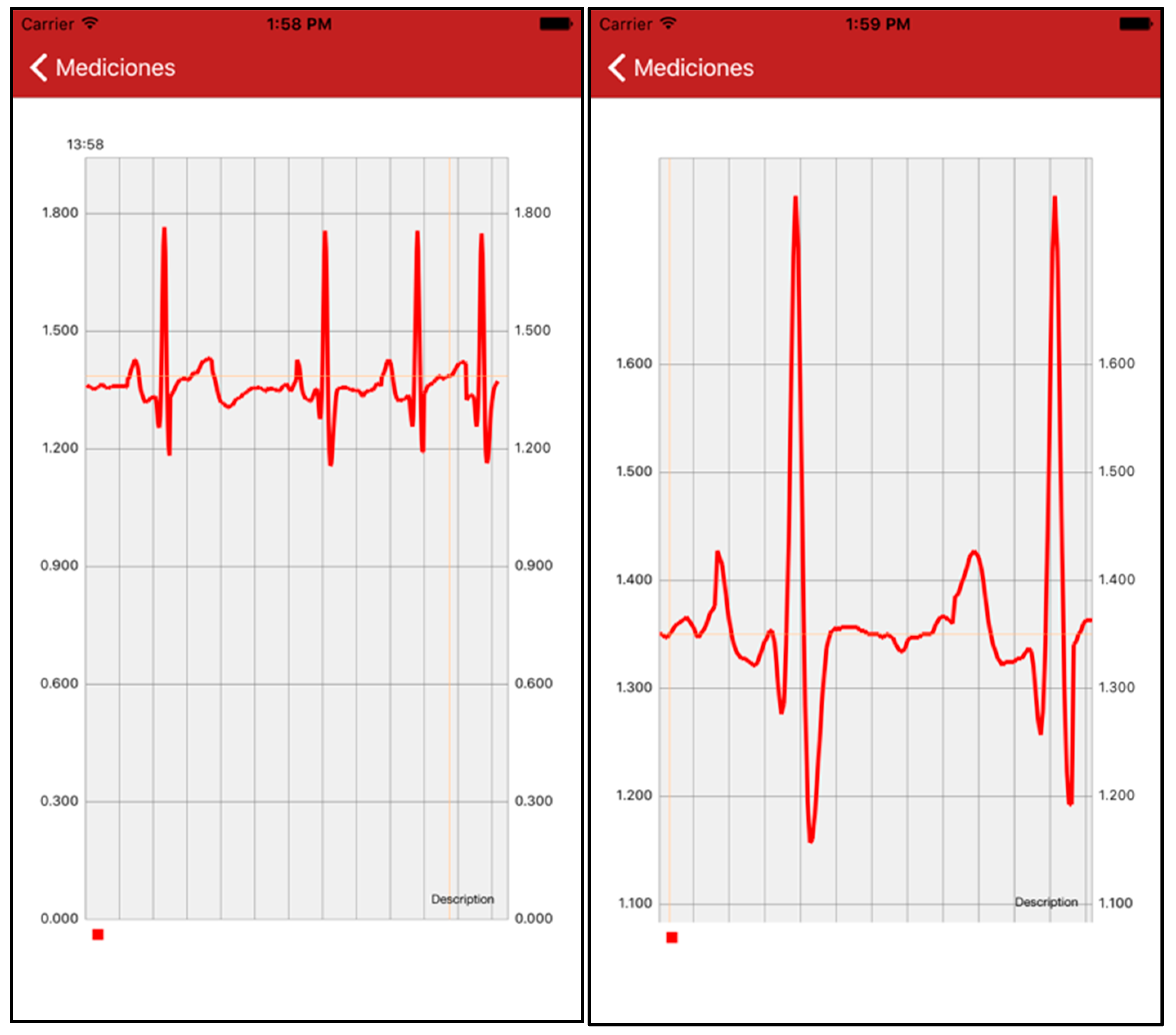
4.6. App to Display Patient Information
5. Conclusions and Future Work
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Chronic Diseases and Health Promotion. Available online: http://www.who.int/chp/en/ (accessed on 18 May 2016).
- World Health Organization. Cardiovascular Diseases. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 20 May 2016).
- British Heart Foundation. Cardiovascular Disease Statistics 2015. Available online: https://www.bhf.org.uk/publications/statistics/cvd-stats-2015 (accessed on 17 November 2016).
- Antman, E.; Bassand, J.P.; Klein, W.; Ohman, M.; Sendon, J.L.L.; Rydén, L.; Simoons, M.; Tendera, M. Myocardial infarction redefined—A consensus document of the joint european society of cardiology/american college of cardiology committee for the redefinition of myocardial infarction: The joint european society of cardiology/american college of cardiology committee. J. Am. Coll. Cardiol. 2000, 36, 959–969. [Google Scholar]
- World Health Organization. Chronic Respiratory Diseases. Available online: http://www.who.int/respiratory/en/ (accessed on 20 May 2016).
- MedlinePlus. Dificultad Respiratoria. Available online: https://medlineplus.gov/spanish/ency/article/003075.htm (accessed on 10 July 2016).
- Sanitas. Complicaciones de Enfermos que Pasan Mucho Tiempo en la Cama. Available online: http://www.sanitas.es/sanitas/seguros/es/particulares/biblioteca-de-salud/tercera-edad/control-patologias-cronicas/complicaciones-enfermos-cama.html (accessed on 15 July 2016).
- Avilés, J.M.; Gil-García, J.R.; Ramirez-Hernández, F. E-salud en mexico: Antecedentes, objetivos, logros y retos. Espac. Públicos 2012, 15, 65–94. [Google Scholar]
- Mundt, C.W.; Montgomery, K.N.; Udoh, U.E.; Barker, V.N.; Thonier, G.C.; Tellier, A.M.; Ricks, R.D.; Darling, R.B.; Cagle, Y.D.; Cabrol, N.A.; et al. A Multiparameter Wearable Physiological Monitoring System for Space and Terrestrial Applications. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, V.; Chen, B.; Lorincz, K.; Fulford-Jones, T.R.F.; Welsh, M. Sensor Networks for Medical Care; Technical Report TR-08-05; Division of Engineering and Applied Sciences, Harvard University: Cambridge, MA, USA, 2005. [Google Scholar]
- Ko, J.; Lu, C.; Srivastava, M.B.; Stankovic, J.A.; Terzis, A.; Welsh, M. Wireless Sensor Networks for Healthcare. Proc. IEEE 2010, 98, 1947–1960. [Google Scholar] [CrossRef]
- Lorincz, K.; Malan, D.J.; Fulford-Jones, T.R.F.; Nawoj, A.; Clavel, A.; Shnayder, V.; Mainland, G.; Welsh, M.; Moulton, S. Sensor networks for emergency response: Challenges and opportunities. IEEE Pervasive Comput. 2004, 3, 16–23. [Google Scholar] [CrossRef]
- Pandian, P.S.; Mohanavelu, K.; Safeer, K.P.; Kotresh, T.M.; Shakunthala, D.T.; Gopal, P.; Padaki, V.C. Smart Vest: Wearable Multi-Parameter Remote Physiological Monitoring System. Med. Eng. Phys. 2007, 30, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Gyselinckx, B.; Penders, J.; Vullers, R. Potential and Challenges of Body Area Networks for Cardiac Monitoring. J. Electr. 2007, 40, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Lim, J.H.; Chen, Y.; Musaloiu-E, R.; Terzis, A.; Masson, G.M.; Gao, T.; Destler, W.; Selavo, L.; Dutton, R.P. MEDiSN: Medical Emergency Detection in Sensor Networks. ACM Trans. Embed. Comput. Syst. (TECS) 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Iyengar, S.; Bonda, F.T.; Gravina, R.; Guerrieri, A.; Fortino, G.; Sangiovanni-Vincentelli, A. A framework for creating healthcare monitoring applications using wireless body sensor networks. In Proceedings of the ICST 3rd International Conference on Body Area Networks (BodyNets ‘08), ICST (Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering), Tempe, AZ, USA, 13–17 March 2008.
- Fortino, G.; Giannantonio, R.; Gravina, R.; Kuryloski, P.; Jafari, R. Enabling Effective Programming and Flexible Management of Efficient Body Sensor Network Applications. IEEE Trans. Hum.-Mach. Syst. 2013, 43, 115–133. [Google Scholar] [CrossRef]
- Gravina, R.; Fortino, G. Automatic Methods for the Detection of Accelerative Cardiac Defense Response. IEEE Trans. Affect. Comput. 2016, 7, 286–298. [Google Scholar] [CrossRef]
- Fortino, G.; Guerrieri, A.; Bellifemine, F.; Giannantonio, R. Platform-independent development of collaborative wireless body sensor network applications: SPINE2. In Proceedings of the IEEE International Conference on Systems, Man and Cybernetics, San Antonio, TX, USA, 11–14 October 2009; pp. 3144–3150.
- Fortino, G.; Guerrieri, A.; Bellifemine, F.L.; Giannantonio, R. SPINE2: Developing BSN applications on heterogeneous sensor nodes. In Proceedings of the IEEE International Symposium on Industrial Embedded Systems, Lausanne, Switzerland, 8–10 July 2009; pp. 128–131.
- Fortino, G.; Giampà, V. PPG-based methods for non invasive and continuous blood pressure measurement: an overview and development issues in body sensor networks. In Proceedings of the IEEE International Workshop on Medical Measurements and Applications, Ottawa, ON, Canada, 30 April–1 May 2010; pp. 10–13.
- Fortino, G.; Gravina, R. Fall-MobileGuard: A smart real-time fall detection system. In Proceedings of the 10th EAI International Conference on Body Area Networks (BodyNets ‘15), ICST (Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering), Sydney, Australia, 28–30 September 2015; pp. 44–50.
- Custodio, V.; Herrera, F.J.; López, G.; Moreno, J.I. A Review on Architectures and Communications Technologies for Wearable Health-Monitoring Systems. Sensors 2012, 12, 13907–13946. [Google Scholar] [CrossRef] [PubMed]
- Cooking-Hacks. E-Health Sensor Platform v2.0 for Arduino and Raspberry pi [Biometric/Medical Applications]. Available online: https://www.cooking-hacks.com/documentation/tutorials/ehealth-biometric-sensor-platform-arduino-raspberry-pi-medical/ (accessed on 10 February 2016).
- Analog Dialogue. A forum for the exchange of circuits, systems, and software for real-world signal processing. Detecting Human Falls with a 3-Axis Digital Accelerometer. Available online: http://www.analog.com/media/en/analog-dialogue/volume-43/number-3/articles/volume43-number3.pdf (accessed on 19 July 2016).
- Markandey, V. Ecg Implementation on the tms320c5515 dsp Medical Development Kit (mdk). Available online: http://www.ti.com/lit/an/sprab36b/sprab36b.pdf (accessed on 16 January 2017).
- Bianchi, G.; Fratta, L.; Oliveri, M. Performance evaluation and enhancement of the CSMA/CA MAC protocol for 802.11 wireless LANs. In Proceedings of the Seventh IEEE International Symposium on Personal, Indoor and Mobile Radio Communications, PIMRC’96, Taipei, Taiwan, 15–18 October 1996; Volume 2, pp. 392–396.
- Apple. Notifications. Available online: https://developer.apple.com/notifications/ (accessed on 14 August 2016).
- Criticare Technologies, Inc. 8100E nGenuity. Available online: http://www.criticareusa.com/ (accessed on 28 July 2016).
- Microlife. Termometros. Available online: http://www.microlife.es/products/fever/ (accessed on 28 July 2016).
- Joorabchi, M.E.; Ali, M.; Mesbah, A. Detecting inconsistencies in multi-platform mobile apps. In Proceedings of the IEEE 26th International Symposium on Software Reliability Engineering (ISSRE), Gaithersbury, MD, USA, 2–5 November 2015; pp. 450–460.






| Platforms/Characteristics | LifeGuard [9] | Code Blue [10] | Smartvest [13] | Human++ [14] | MEDiSN [15] | SPINE2 [19] | PlaIMoS |
|---|---|---|---|---|---|---|---|
| Health sensors | Blood pressure ECG heart rate respiration rate oxygen saturation temperature body position | Blood oxygen heart rate ECG EMG | Blood pressure ECG EMG EEG temperature respiration rate skin response oxygen saturation heart rate | ECG EEG EMG | ECG heart rate blood oxygen | ECG heart rate temperature respiration rate skin response fall detection | ECG heart rate temperature blood oxygen respiration rate skin response fall detection |
| Communication system | Bluetooth | Wireless sensor network | Wireless sensor network | Ultra-wide band communication | Wireless sensor network | Wireless body sensor network | Wireless sensor network |
| Data security | Not provided | Not provided | Not provided | Not provided | 128-bit AES encryption into the WSN | ZigBee security layer | 128-bit AES encryption into the WSN and database |
| Patient localization | Not provided | RF-based localization system for indoor location | GPS | Not provided | Not provided | Not provide | centroid localization algorithm for indoor location |
| Emergency detection | Buzzer is used to alert the user | Not provide | Automatic alarms at the remote monitoring | Not provided | Alarm through back-end server | Alarm through wireless body sensor network | Push notification from server to display device |
| Display devices | Base station computer | Computer | Remote monitoring station | Computer PDA | Not provided | Computer | Computer tablets smartphones |
| Package_id | Patient_id | Blood_oxygen | Respiration_rate | Galvanic_resistance |
|---|---|---|---|---|
| Int | Int | Float | Smallint | Float |
| Package_id | Patient_id | Temperature | Heart_rate | Fall | Location |
|---|---|---|---|---|---|
| Int | Int | Int | Float | Int | Int |
| Package_id | Patient_id | Sample 1 | … | Sample 80 |
|---|---|---|---|---|
| Int | Int | Float | … | Float |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miramontes, R.; Aquino, R.; Flores, A.; Rodríguez, G.; Anguiano, R.; Ríos, A.; Edwards, A. PlaIMoS: A Remote Mobile Healthcare Platform to Monitor Cardiovascular and Respiratory Variables. Sensors 2017, 17, 176. https://doi.org/10.3390/s17010176
Miramontes R, Aquino R, Flores A, Rodríguez G, Anguiano R, Ríos A, Edwards A. PlaIMoS: A Remote Mobile Healthcare Platform to Monitor Cardiovascular and Respiratory Variables. Sensors. 2017; 17(1):176. https://doi.org/10.3390/s17010176
Chicago/Turabian StyleMiramontes, Ramses, Raúl Aquino, Arturo Flores, Guillermo Rodríguez, Rafael Anguiano, Arturo Ríos, and Arthur Edwards. 2017. "PlaIMoS: A Remote Mobile Healthcare Platform to Monitor Cardiovascular and Respiratory Variables" Sensors 17, no. 1: 176. https://doi.org/10.3390/s17010176
APA StyleMiramontes, R., Aquino, R., Flores, A., Rodríguez, G., Anguiano, R., Ríos, A., & Edwards, A. (2017). PlaIMoS: A Remote Mobile Healthcare Platform to Monitor Cardiovascular and Respiratory Variables. Sensors, 17(1), 176. https://doi.org/10.3390/s17010176







