Internal and External Temperature Monitoring of a Li-Ion Battery with Fiber Bragg Grating Sensors
Abstract
:1. Introduction
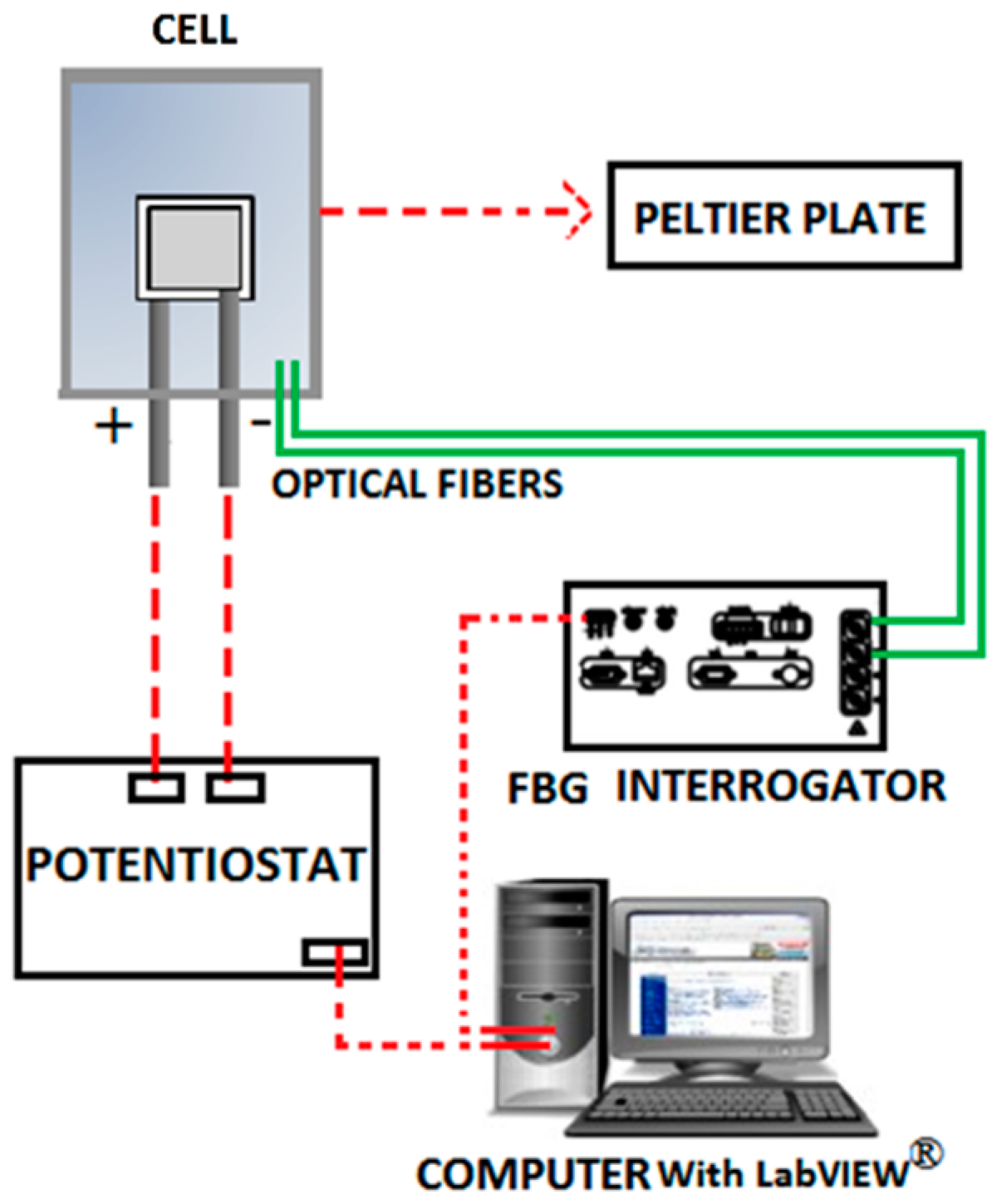
2. Experimental Setup and Testing
2.1. Silica Fiber Stability Test
2.2. Fiber Bragg Grating Sensors
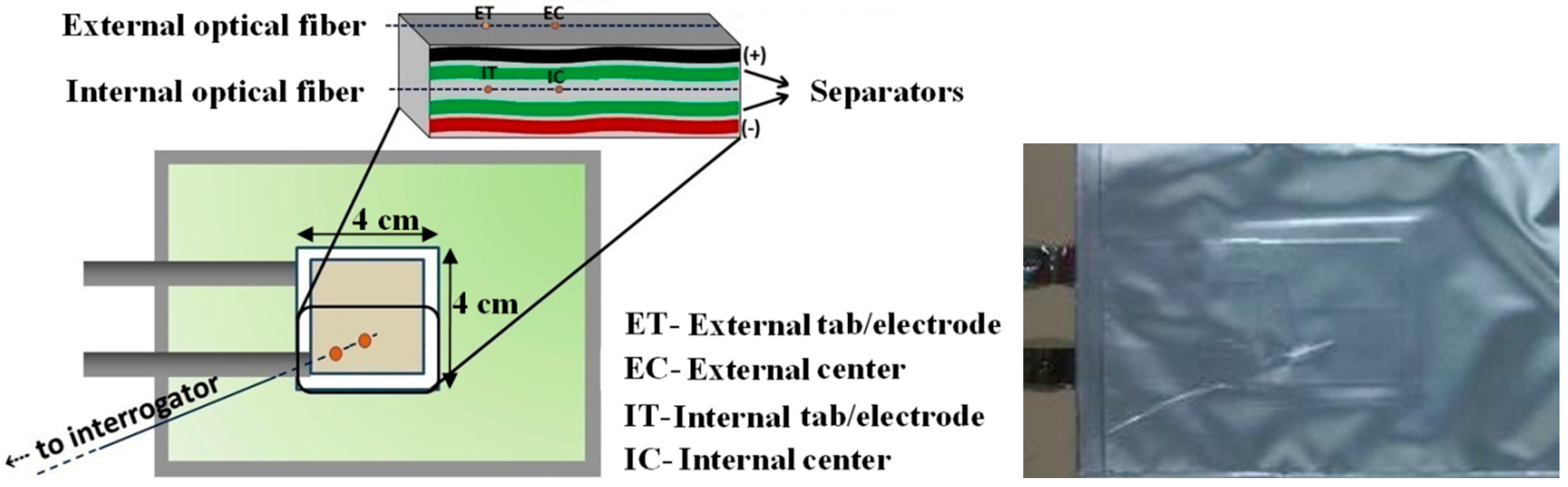
2.3. Li-Ion Cell Assembly and Microsensor Integration
2.4. Thermal Calibration of FBG Sensors
2.5. Electrochemical Testing
3. Results and Discussion
3.1. Silica Fiber Chemical Inertness Study
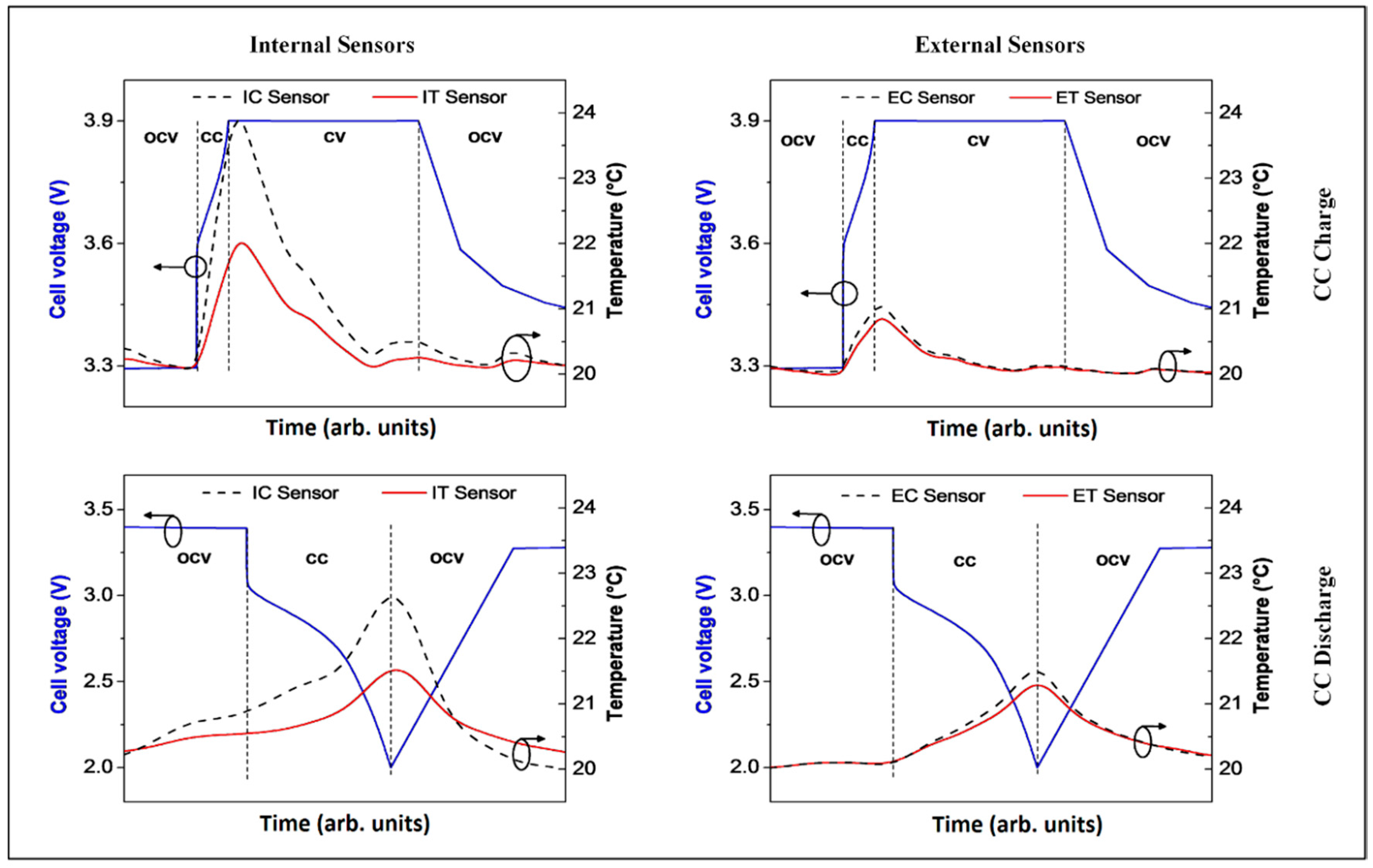
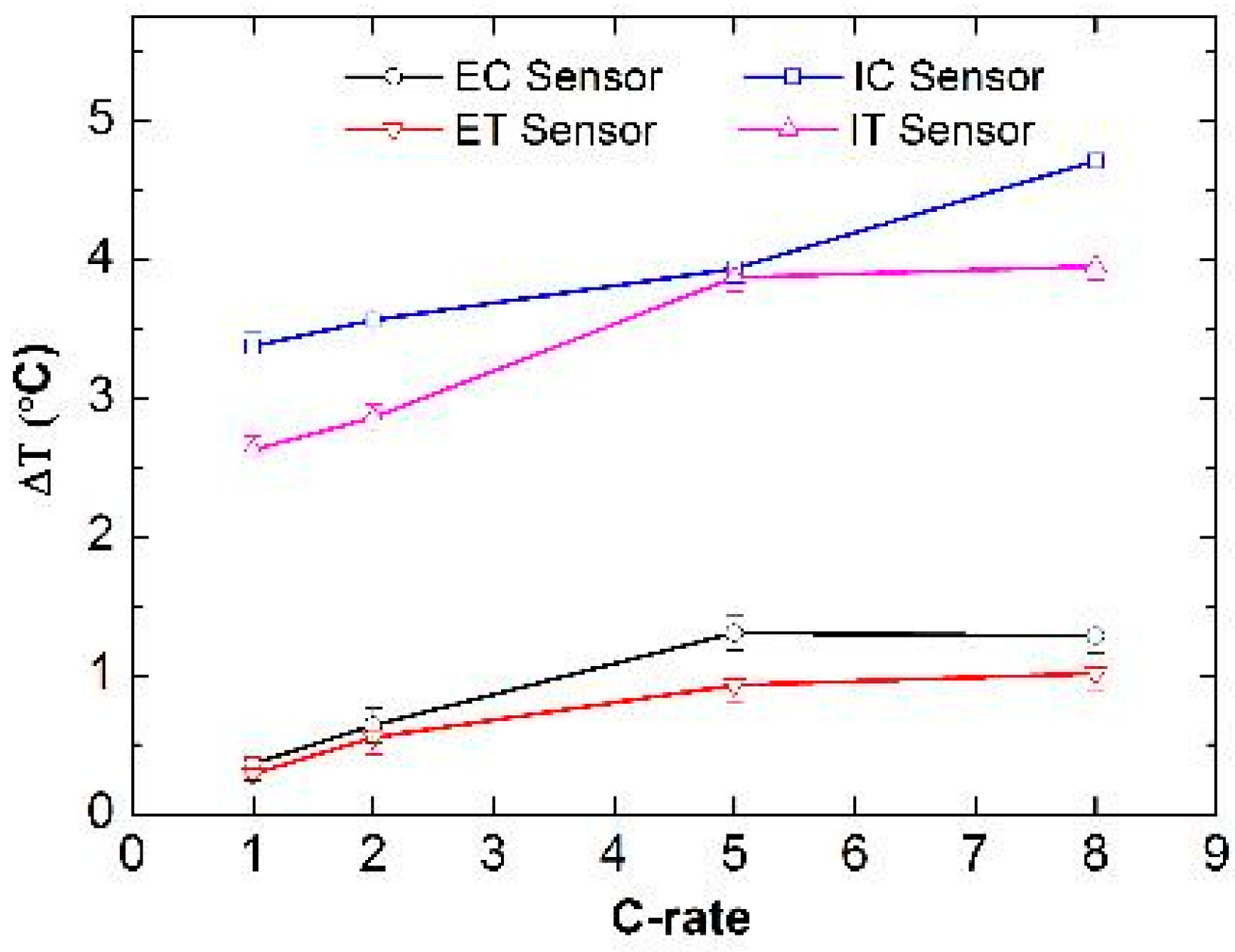
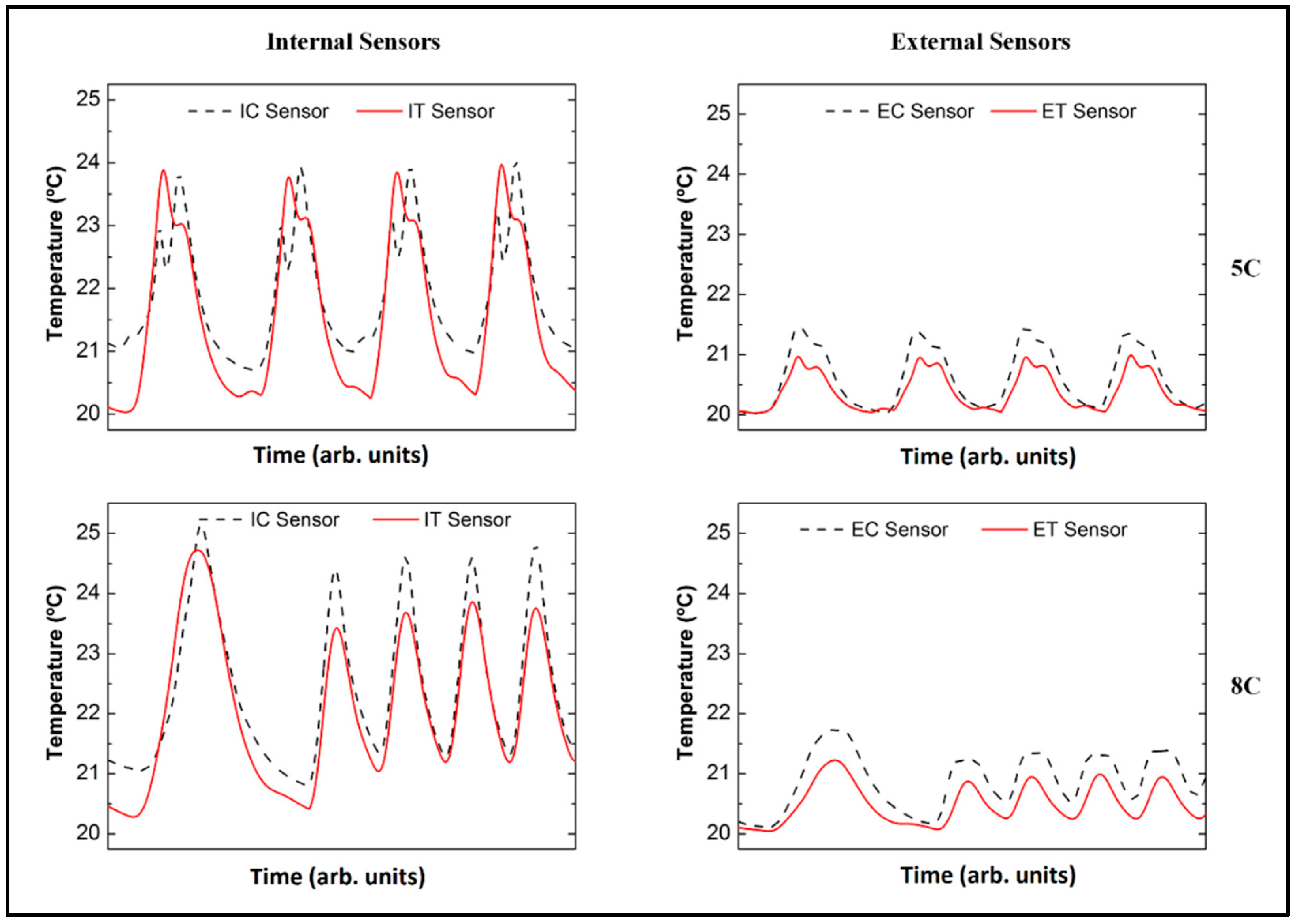
3.2. Analysis of the FBG Sensors Response under Different Operating Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, J.; Cheng, F. Combination of lightweight elements and nanostructured materials for batteries. Acc. Chem. Res. 2009, 42, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Kizilel, R.; Sabbah, R.; Selman, J.R.; Al-Hallaj, S. An alternative cooling system to enhance the safety of Li-ion battery packs. J. Power Sour. 2009, 194, 1105–1112. [Google Scholar] [CrossRef]
- Sethuramana, V.A.; Van Winklea, N.; Abrahamb, D.P.; Bowera, A.F.; Guduru, P.R. Real-time stress measurements in lithium-ion battery negative-electrodes. J. Power Sour. 2012, 206, 334–342. [Google Scholar] [CrossRef]
- Kima, U.S.; Yia, J.; Shin, C.B. Modelling the thermal behavior of a lithium-ion battery during charge. J. Power Sour. 2011, 196, 5115–5121. [Google Scholar] [CrossRef]
- Yang, G.; Leitão, C.; Lib, Y.; Pinto, J.; Jiang, X. Real-time temperature measurement with fiber Bragg sensors in lithium batteries for safety usage. Measurement 2013, 46, 3166–3172. [Google Scholar] [CrossRef]
- Spotnitza, R.M.; Weavera, J.; Yeduvaka, G.; Doughty, D.H.; Roth, E.P. Simulation of abuse tolerance of lithium-ion battery packs. J. Power Sour. 2007, 163, 1080–1086. [Google Scholar] [CrossRef]
- Roder, P.; Stiaszny, B.; Ziegler, J.C.; Wiemhofer, H.-D. The impact of calendar aging on the thermal stability of an Mn2O4-Li (Ni1/3Mn1/3Co1/3) O2/graphite lithium-ion cell. J. Power Sour. 2014, 268, 315–325. [Google Scholar] [CrossRef]
- Fan, J.; Tan, S. Studies on charging lithium-ion cells at low temperatures. J. Electrochem. Soc. 2006, 153, A1081–A1092. [Google Scholar] [CrossRef]
- Huang, C.-K.; Sakamoto, J.S.; Wolfenstine, J.; Surampudi, S. The limits of low-temperature performance of Li-ion cells. J. Electrochem. Soc. 2000, 147, 2893–2896. [Google Scholar] [CrossRef]
- Kima, U.S.; Shina, C.B.; Kim, C.-S. Effect of electrode configuration on the thermal behavior of a lithium-polymer battery. J. Power Sour. 2008, 180, 909–916. [Google Scholar] [CrossRef]
- Richardson, R.R.; Ireland, P.T.; Howey, D.A. Battery internal temperature estimation by combined impedance and surface temperature measurement. J. Power Sour. 2014, 265, 254–261. [Google Scholar] [CrossRef]
- Antunes, P.; Lima, H.; Alberto, N.; Bilro, L.; Pinto, P.; Costa, A.; Rodrigues, H.; Pinto, J.L.; Nogueira, R.; Varum, H.; et al. Optical sensors based on FBG for structural health monitoring. In New Developments in Sensing Technology for Structural Health Monitoring; Mukhopadhyay, S.C., Ed.; Springer-Verlag: Berlin, Germany, 2011. [Google Scholar]
- David, N.A.; Wild, P.M.; Hu, J.; Djilali, N. In-fibre Bragg grating sensors for distributed temperature measurement in a polymer electrolyte membrane fuel cell. J Power Sour. 2009, 192, 376–380. [Google Scholar] [CrossRef]
- Nascimento, M.; Novais, S.; Leitão, C.; Domingues, M.F.; Alberto, N.; Antunes, P.; Pinto, J.L. Lithium batteries temperature and strain fiber monitoring. In Proceedings of the SPIE 9634, 24th International Conference on Optical Fibre Sensors, Curitiba, Brazil, 28 September–2 October 2015; Volume 9634, pp. 9634V-1–9634V-4.
- Sommer, L.W.; Raghavan, A.; Kiesel, P.; Saha, B.; Schwartz, J.; Lochbaum, A.; Ganguli, A.; Bae, C.-J.; Alamgir, M. Monitoring of Intercalation Stages in Lithium-Ion Cells over Charge-Discharge Cycles with Fiber Optics Sensors. J. Electrochem. Soc. 2015, 162, A2664–A2669. [Google Scholar] [CrossRef]
- Sommer, L.W.; Kiesel, P.; Ganguli, A.; Lochbaum, A.; Saha, B.; Schwartz, J.; Bae, C.-J.; Alamgir, M.; Raghavan, A. Fast and slow ion diffusion processes in lithium ion pouch cells during cycling observed with fiber optic strain sensors. J. Power Sour. 2015, 296, 46–52. [Google Scholar] [CrossRef]
- Bae, C.; Manandhar, A.; Kiesel, P.; Raghavan, A. Monitoring the Strain Evolution of Lithium-Ion Battery Electrodes using an Optical Fiber Bragg Grating Sensor. Energy Technol. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- Loeffler, N.; Zamory, J.; Laszczynski, N.; Doberdo, I.; Kim, G.-T.; Passerini, S. Performance of LiNi1/3Mn1/3Co1/3O2/graphite batteries based on aqueous binder. J. Power Sour. 2014, 248, 915–922. [Google Scholar] [CrossRef]
- Srinivasan, R.; Baisden, A.C.; Carkhuff, B.G.; Butler, M.H. The five modes of heat generation in a Li-ion cell under discharge. J. Power Sour. 2014, 262, 93–103. [Google Scholar] [CrossRef]

| (1) | Constant Current Constant Voltage (CCCV) charge |
| (2) | Two cycles each composed of Constant Current (CC) discharge followed by CCCV charge |
| (3) | Open Circuit Voltage (OCV) |
| (4) | CC discharge |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novais, S.; Nascimento, M.; Grande, L.; Domingues, M.F.; Antunes, P.; Alberto, N.; Leitão, C.; Oliveira, R.; Koch, S.; Kim, G.T.; et al. Internal and External Temperature Monitoring of a Li-Ion Battery with Fiber Bragg Grating Sensors. Sensors 2016, 16, 1394. https://doi.org/10.3390/s16091394
Novais S, Nascimento M, Grande L, Domingues MF, Antunes P, Alberto N, Leitão C, Oliveira R, Koch S, Kim GT, et al. Internal and External Temperature Monitoring of a Li-Ion Battery with Fiber Bragg Grating Sensors. Sensors. 2016; 16(9):1394. https://doi.org/10.3390/s16091394
Chicago/Turabian StyleNovais, Susana, Micael Nascimento, Lorenzo Grande, Maria Fátima Domingues, Paulo Antunes, Nélia Alberto, Cátia Leitão, Ricardo Oliveira, Stephan Koch, Guk Tae Kim, and et al. 2016. "Internal and External Temperature Monitoring of a Li-Ion Battery with Fiber Bragg Grating Sensors" Sensors 16, no. 9: 1394. https://doi.org/10.3390/s16091394
APA StyleNovais, S., Nascimento, M., Grande, L., Domingues, M. F., Antunes, P., Alberto, N., Leitão, C., Oliveira, R., Koch, S., Kim, G. T., Passerini, S., & Pinto, J. (2016). Internal and External Temperature Monitoring of a Li-Ion Battery with Fiber Bragg Grating Sensors. Sensors, 16(9), 1394. https://doi.org/10.3390/s16091394











