Smart Sensing System for the Prognostic Monitoring of Bone Health
Abstract
:1. Introduction
2. Materials and Methods
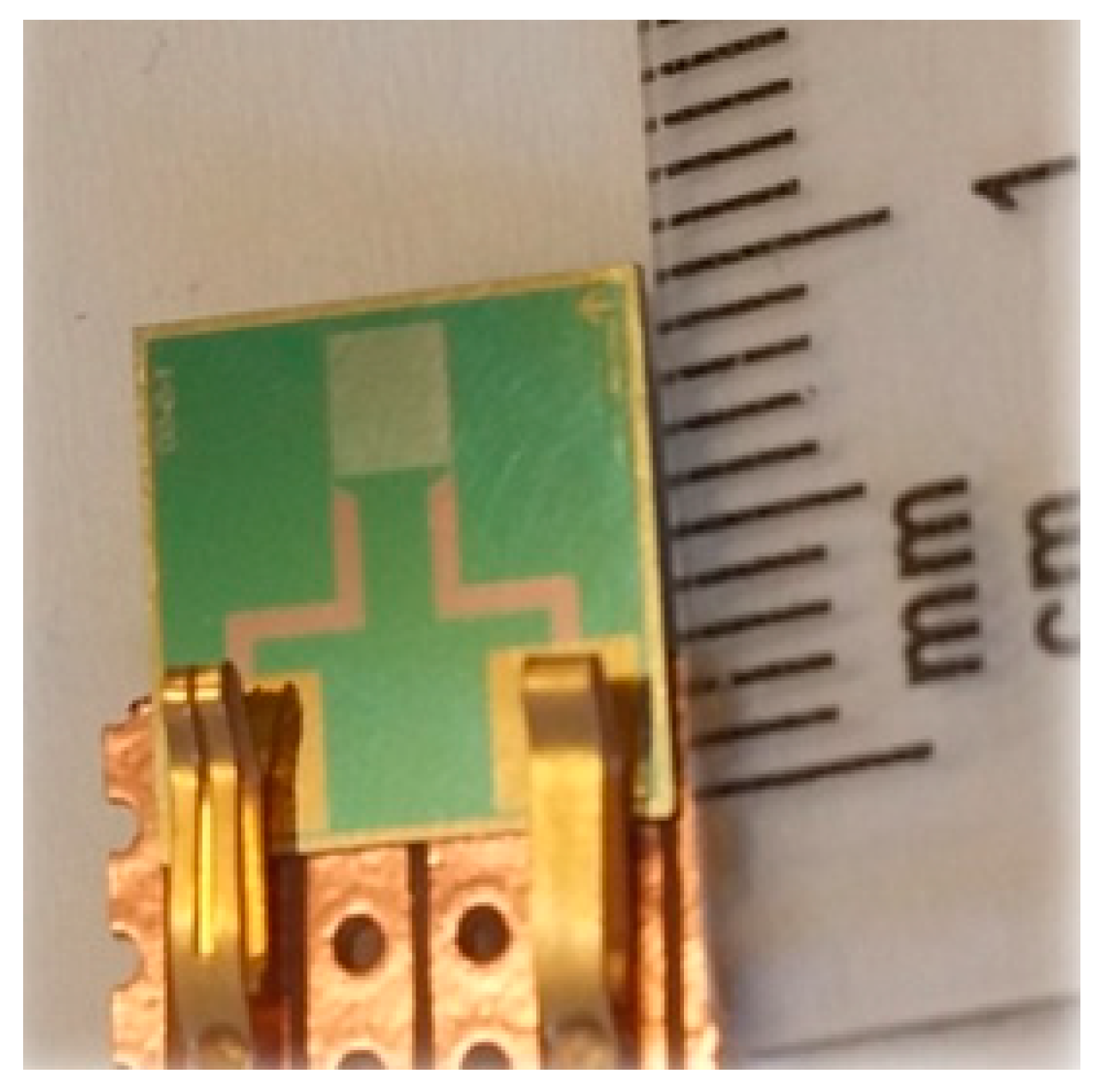
2.1. High Penetration Depth Planar Interdigital Sensor
2.2. Materials and Chemicals
2.3. Experiments Using the Serum CrossLaps® ELISA Kit
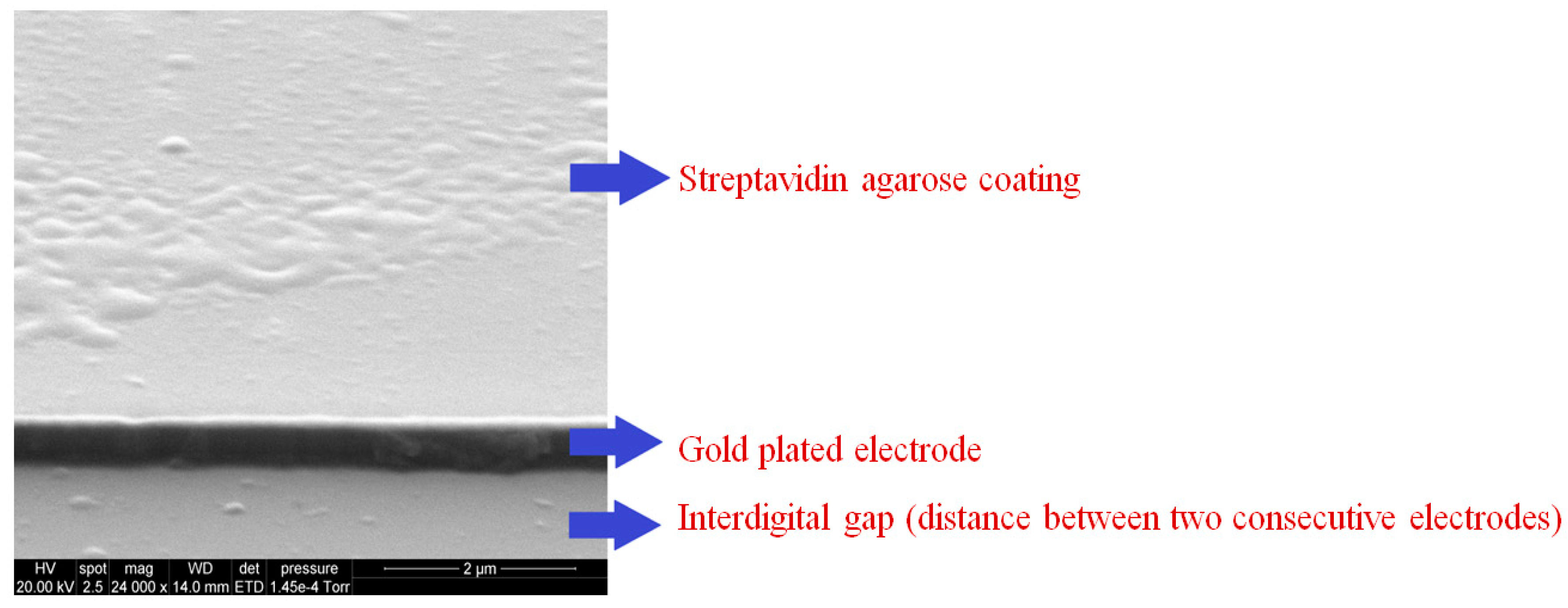
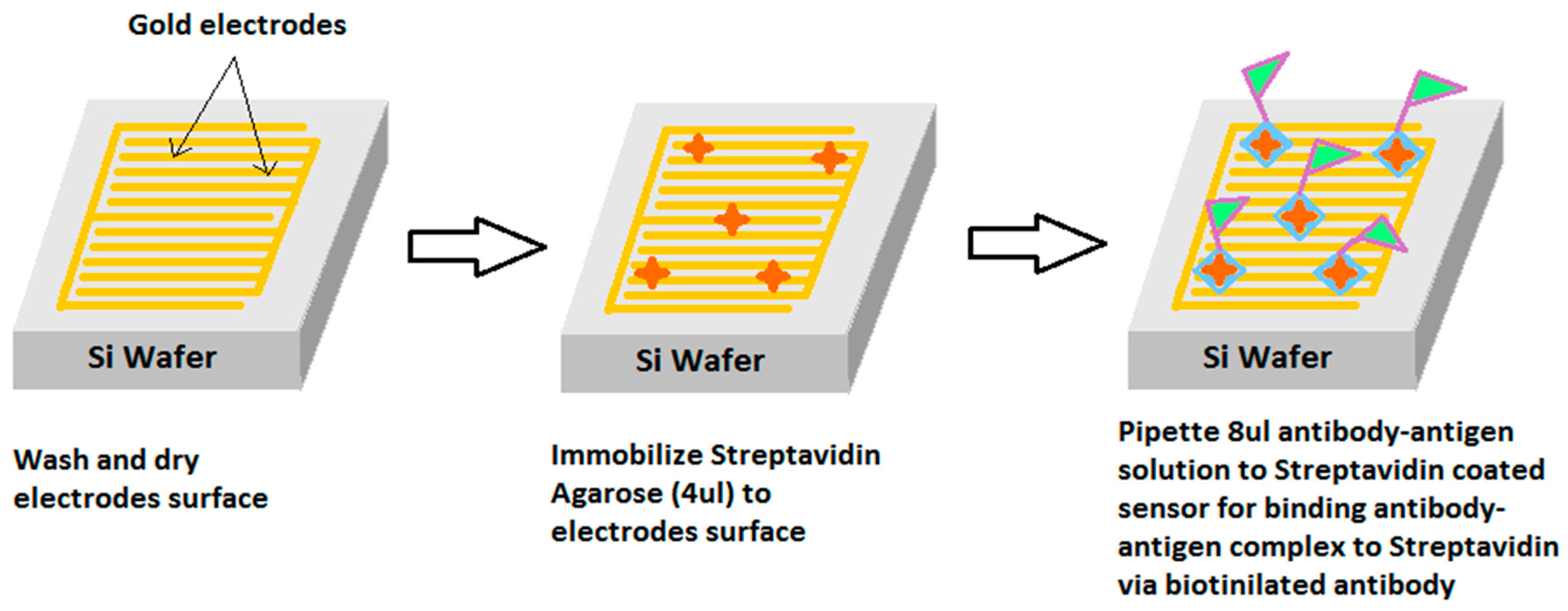
2.4. Coating of Sensor for Inducing Selectivity
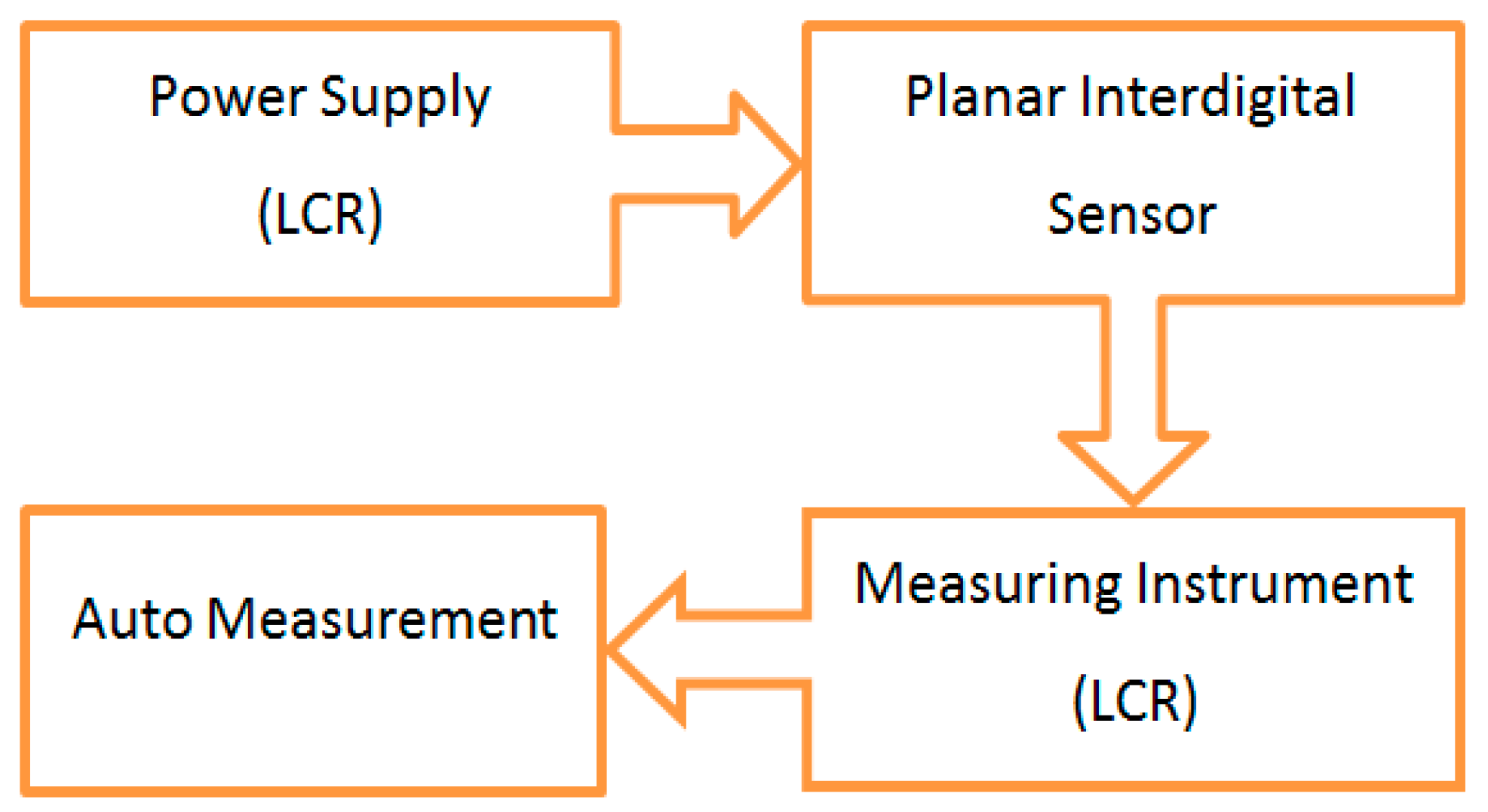
2.5. Experimental Setup and Electrochemical Impedance Spectroscopy
3. Results and Discussions
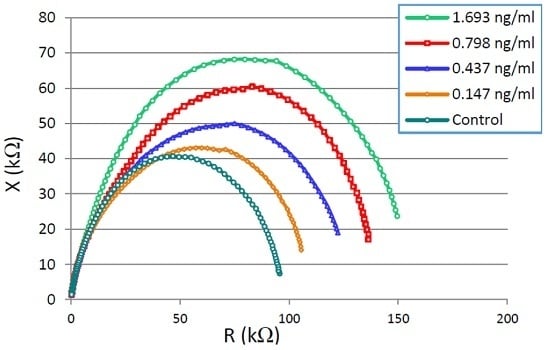
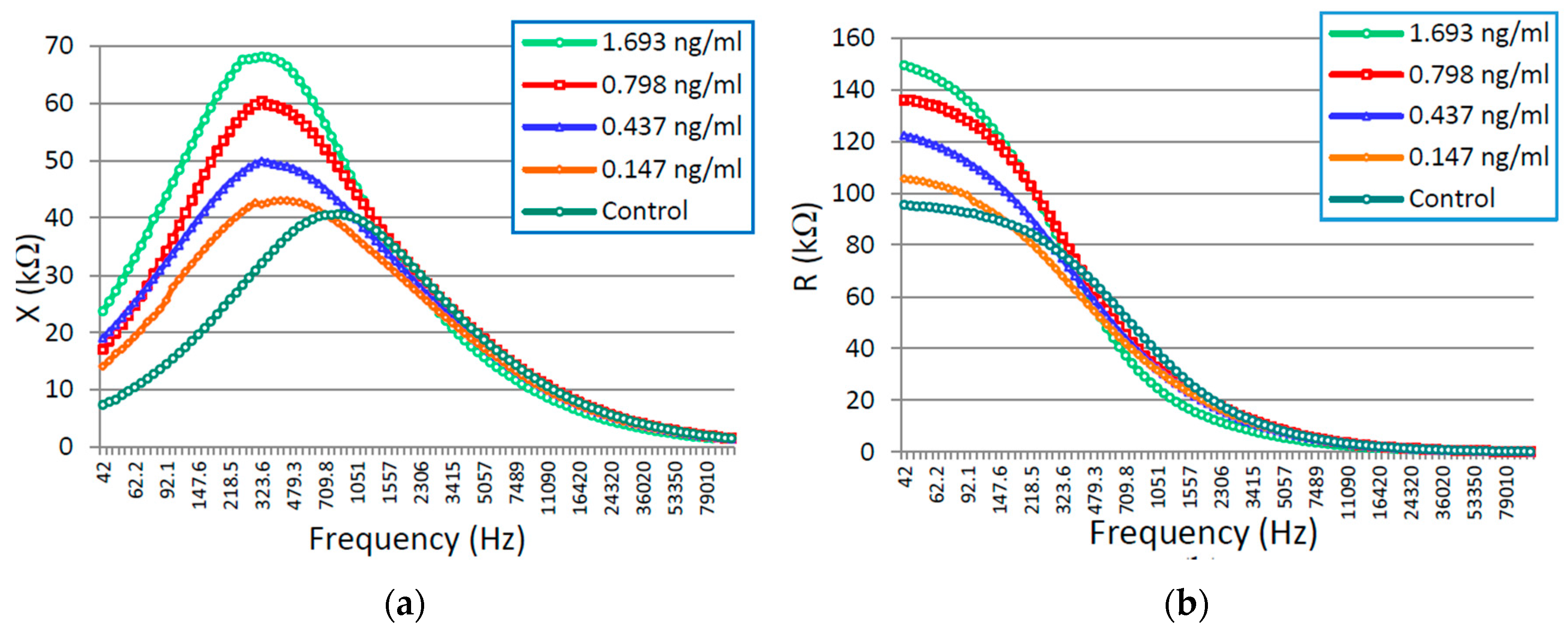
3.1. CTx-I Measurement in Known Samples Using the Proposed Sensing System
3.2. Data Analyses Using Non-Linear Least Square Curve Fitting
3.3. Multivariate Chemometric Analyses
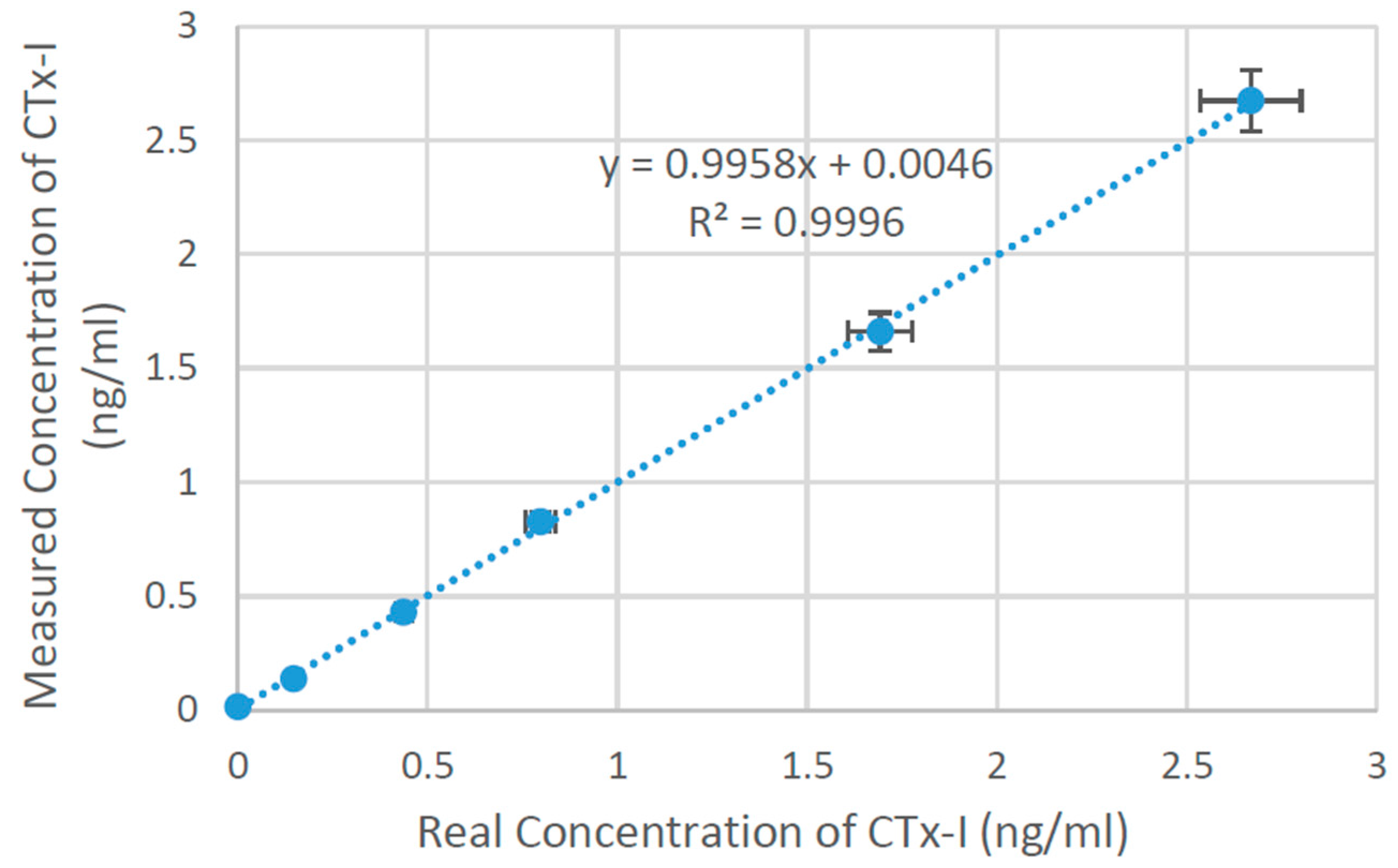
3.4. CTx-I Measurement in Unknown Samples Using the Proposed Sensing System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seibel, M.J.; Robins, S.P.; Bilezikian, J.P. Dynamics of Bone and Cartilage Metabolism: Principles and Clinical Applications; Academic Press: Sydney, Australia, 2006. [Google Scholar]
- Seeman, E. Osteoporosis in Men: The “Silent Epidemic” Strikes Men Too; International Osteoporosis Foundation: Melbourne, Australia, 2004. [Google Scholar]
- Snyman, L. Menopause-related osteoporosis. S. Afr. Fam. Pract. 2014, 56, 174–177. [Google Scholar] [CrossRef]
- Khan, A.; Fortier, M. Osteoporosis in menopause. J. Obstet. Gynaecol. Can. 2014, 36, S1–S15. [Google Scholar] [CrossRef]
- Melton, L.J.; Chrischilles, E.A.; Cooper, C.; Lane, A.W.; Riggs, B.L. How many women have osteoporosis? J. Bone Min. Res. 2005, 20, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.; Johnell, O.; Oden, A.; Sernbo, I.; Redlund-Johnell, I.; Dawson, A.; de Laet, C.; Jonsson, B. Long-term risk of osteoporotic fracture in malmö. Osteoporos. Int. 2000, 11, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; MacFarlane, G.; Srivastava, V.; Mohan, S.; Baylink, D. A new monoclonal antibody elisa for detection and characterization of C-telopeptide fragments of type I collagen in urine. Calcif. Tissue Int. 2001, 69, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.; Morin, S.; Cheung, A.M.; Atkinson, S.; Brown, J.P.; Feldman, S.; Hanley, D.A.; Hodsman, A.; Jamal, S.A.; Kaiser, S.M. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. Can. Med. Assoc. J. 2010, 182, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Delmas, P. Biochemical markers of bone turnover: Potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos. Int. 2008, 19, 1683–1704. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Vacic, A.; Li, C.; Ishikawa, F.N.; Zhou, C.; Reed, M.A.; Fahmy, T.M. A nanoelectronic enzyme-linked immunosorbent assay for detection of proteins in physiological solutions. Small 2010, 6, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Paulie, S.; Perlmann, H. Enzyme-Linked Immunosorbent Assay. In eLS; John Wiley & Sons Ltd.: Chichester, UK, 2016. [Google Scholar]
- Gan, S.D.; Patel, K.R. Enzyme immunoassay and enzyme-linked immunosorbent assay. J. Investig. Dermatol. 2013, 133, e12. [Google Scholar] [CrossRef] [PubMed]
- Christenson, R.H. Biochemical markers of bone metabolism: An overview. Clin. Biochem. 1997, 30, 573–593. [Google Scholar] [CrossRef]
- Abu-Abed, A.S.; Lindquist, R.G. Capacitive interdigital sensor with inhomogeneous nematic liquid crystal film. Prog. Electromagn. Res. B 2008, 7, 75–87. [Google Scholar] [CrossRef]
- Mamishev, A.V.; Sundara-Rajan, K.; Yang, F.; Du, Y.; Zahn, M. Interdigital sensors and transducers. Proc. IEEE 2004, 92, 808–845. [Google Scholar] [CrossRef]
- Syaifudin, A.M.; Jayasundera, K.; Mukhopadhyay, S. A low cost novel sensing system for detection of dangerous marine biotoxins in seafood. Sens. Actuators B Chem. 2009, 137, 67–75. [Google Scholar] [CrossRef]
- Syaifudin, A.M.; Mukhopadhyay, S.; Yu, P. Electromagnetic field computation using comsol multiphysics to evaluate the performance of novel interdigital sensors. In Proceedings of the Applied Electromagnetics Conference (AEMC), Kolkata, India, 14–16 December 2009; pp. 1–4.
- Syaifudin, A.M.; Yunus, M.; Mukhopadhyay, S.; Jayasundera, K. A novel planar interdigital sensor for environmental monitoring. In Proceedings of the 2009 IEEE Sensors, Christchurch, New Zealand, 25–28 October 2009; pp. 105–110.
- Ramulu, T.; Venu, R.; Sinha, B.; Lim, B.; Jeon, S.; Yoon, S.; Kim, C. Nanowires array modified electrode for enhanced electrochemical detection of nucleic acid. Biosens. Bioelectron. 2013, 40, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.I.; Mukhopadhyay, S.C.; Yu, P.-L.; Al-Bahadly, I.; Gooneratne, C.P.; Kosel, J. Rapid and molecular selective electrochemical sensing of phthalates in aqueous solution. Biosens. Bioelectron. 2015, 67, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; He, F.; Wang, H.; Tong, F. A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of staphylococcus aureus. Biosens. Bioelectron. 2015, 65, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Syaifudin, A.; Jayasundera, K.; Mukhopadhyay, S. A novel planar interdigital sensor based sensing and instrumentation for detection of dangerous contaminated chemical in seafood. In Proceedings of the IEEE Instrumentation and Measurement Technology Conference, I2MTC′09, Singapore, 5–7 May 2009; pp. 701–706.
- Ramanathan, M.; Patil, M.; Epur, R.; Yun, Y.; Shanov, V.; Schulz, M.; Heineman, W.R.; Datta, M.K.; Kumta, P.N. Gold-coated carbon nanotube electrode arrays: Immunosensors for impedimetric detection of bone biomarkers. Biosens. Bioelectron. 2016, 77, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Buller, S.; Karden, E.; Kok, D.; Doncker, R.W.D. Modeling the dynamic behavior of supercapacitors using impedance spectroscopy. In Proceedings of the IEEE Thirty-Sixth IAS Annual Meeting, Chicago, IL, USA, 30 September–4 October 2001; Volume 2504, pp. 2500–2504.
- Hammar, A.; Venet, P.; Lallemand, R.; Coquery, G.; Rojat, G. Study of accelerated aging of supercapacitors for transport applications. IEEE Trans. Ind. Electron. 2010, 57, 3972–3979. [Google Scholar] [CrossRef]
- Duarte, R.; Castela, A.; Neves, R.; Freire, L.; Montemor, M. Corrosion behavior of stainless steel rebars embedded in concrete: An electrochemical impedance spectroscopy study. Electrochim. Acta 2014, 124, 218–224. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB 1 in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zuo, P.; Ye, B.-C. Label-free electrochemical impedance spectroscopy biosensor for direct detection of cancer cells based on the interaction between carbohydrate and lectin. Biosens. Bioelectron. 2013, 43, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Ohno, R.; Ohnuki, H.; Wang, H.; Yokoyama, T.; Endo, H.; Tsuya, D.; Izumi, M. Electrochemical impedance spectroscopy biosensor with interdigitated electrode for detection of human immunoglobulin A. Biosens. Bioelectron. 2013, 40, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.C.; Gooneratne, C.P. A novel planar-type biosensor for noninvasive meat inspection. IEEE Sens. J. 2007, 7, 1340–1346. [Google Scholar] [CrossRef]
- Rahman, M.S.A.; Mukhopadhyay, S.C.; Yu, P.-L.; Goicoechea, J.; Matias, I.R.; Gooneratne, C.P.; Kosel, J. Detection of bacterial endotoxin in food: New planar interdigital sensors based approach. J. Food Eng. 2013, 114, 346–360. [Google Scholar] [CrossRef]
- Li, X.; Toyoda, K.; Ihara, I. Coagulation process of soymilk characterized by electrical impedance spectroscopy. J. Food Eng. 2011, 105, 563–568. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Bhattacharya, A.; Watts, N.B.; Schulz, M.J. A label-free electronic biosensor for detection of bone turnover markers. Sensors 2009, 9, 7957–7969. [Google Scholar] [CrossRef] [PubMed]
- Putzbach, W.; Ronkainen, N.J. Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: A review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef] [PubMed]
- Nojdelov, R.; Nihtianov, S. Capacitive-sensor interface with high accuracy and stability. IEEE Trans. Instrum. Meas. 2009, 58, 1633–1639. [Google Scholar] [CrossRef]
- Hsu, C.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]

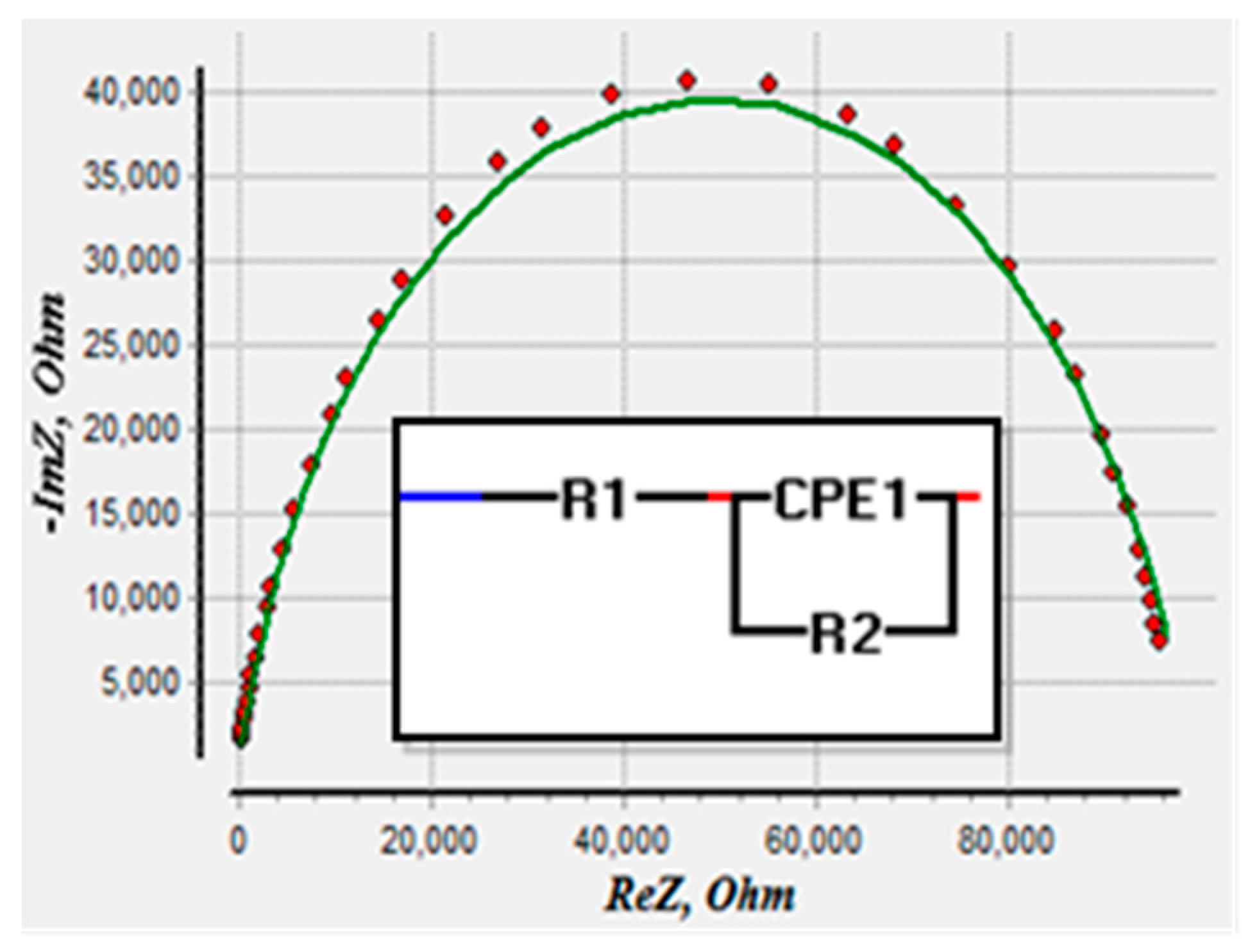

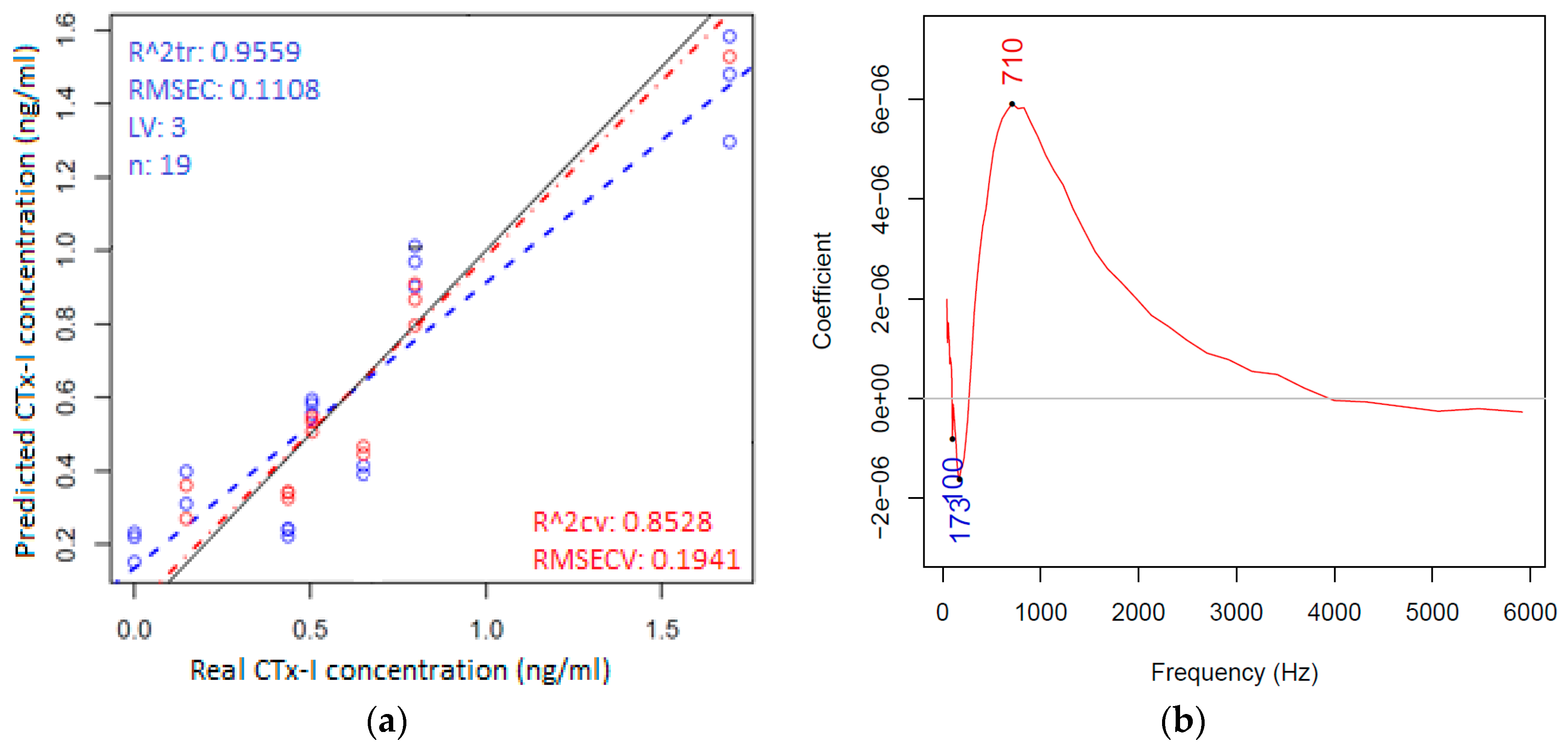
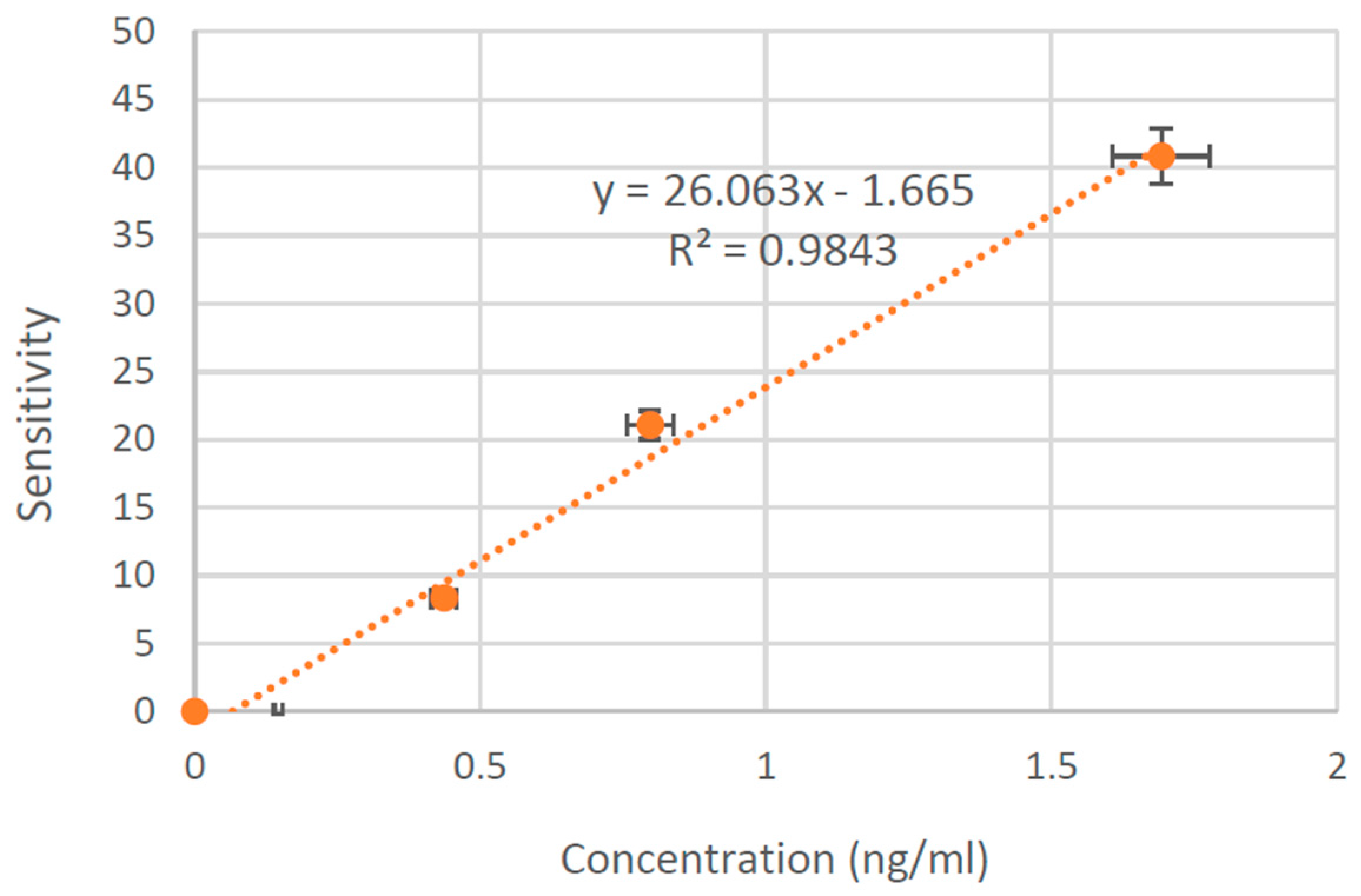
| Component Parameters | 0.147 ng/mL | 0.437 ng/mL | 0.798 ng/mL | 1.693 ng/mL |
|---|---|---|---|---|
| R1(Ω) | 1.790 × 10−14 | 1.686 × 10−14 | 1.657 × 10−14 | 1.660 × 10−12 |
| R2(Ω) | 1.153 × 105 | 1.347 × 105 | 1.480 × 105 | 1.596 × 105 |
| P1 | 1.535 × 10−8 | 1.588 × 10−8 | 1.036 × 10−8 | 6.464 × 10−9 |
| n1 | 0.7944 | 0.7944 | 0.8345 | 0.8823 |
| 0.0133 | 0.0100 | 0.0161 | 0.0246 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afsarimanesh, N.; Zia, A.I.; Mukhopadhyay, S.C.; Kruger, M.; Yu, P.-L.; Kosel, J.; Kovacs, Z. Smart Sensing System for the Prognostic Monitoring of Bone Health. Sensors 2016, 16, 976. https://doi.org/10.3390/s16070976
Afsarimanesh N, Zia AI, Mukhopadhyay SC, Kruger M, Yu P-L, Kosel J, Kovacs Z. Smart Sensing System for the Prognostic Monitoring of Bone Health. Sensors. 2016; 16(7):976. https://doi.org/10.3390/s16070976
Chicago/Turabian StyleAfsarimanesh, Nasrin, Asif I. Zia, Subhas Chandra Mukhopadhyay, Marlena Kruger, Pak-Lam Yu, Jurgen Kosel, and Zoltan Kovacs. 2016. "Smart Sensing System for the Prognostic Monitoring of Bone Health" Sensors 16, no. 7: 976. https://doi.org/10.3390/s16070976
APA StyleAfsarimanesh, N., Zia, A. I., Mukhopadhyay, S. C., Kruger, M., Yu, P.-L., Kosel, J., & Kovacs, Z. (2016). Smart Sensing System for the Prognostic Monitoring of Bone Health. Sensors, 16(7), 976. https://doi.org/10.3390/s16070976