Reduced Graphene Oxide/Au Nanocomposite for NO2 Sensing at Low Operating Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of GO
2.3. Preparation of rGO/Au Nanocomposite
2.4. Characterizations
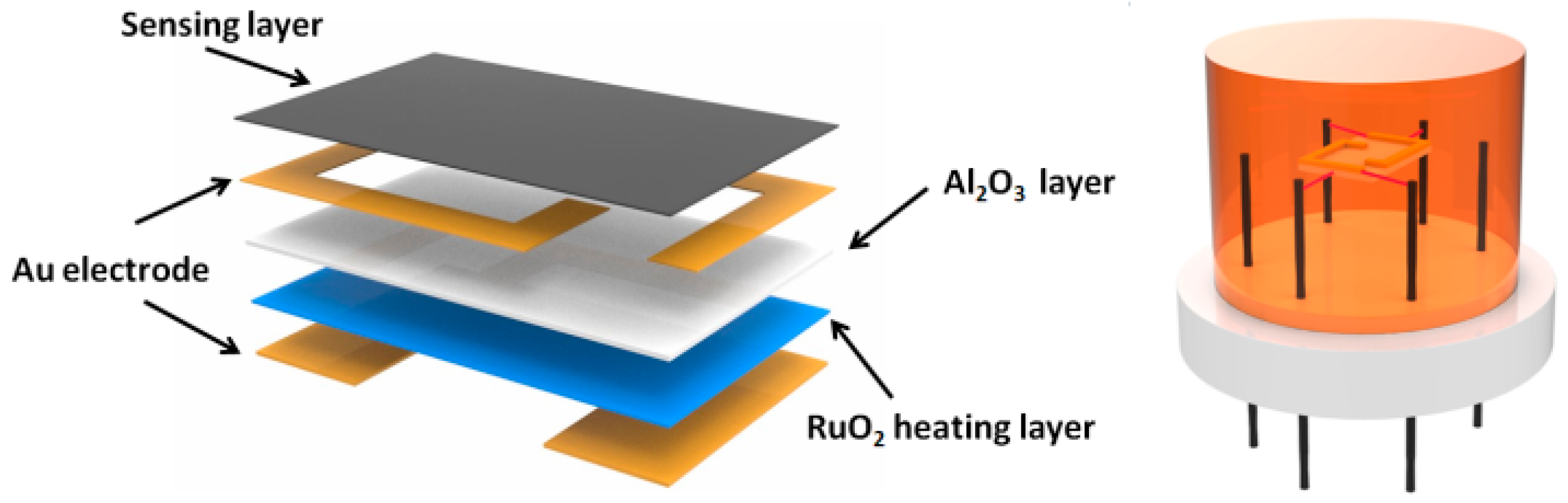
2.5. Fabrication and Gas Sensing Measurements
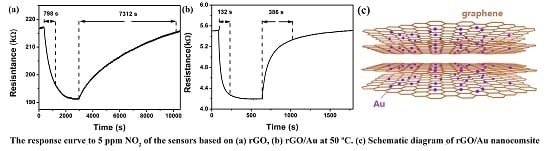
3. Results
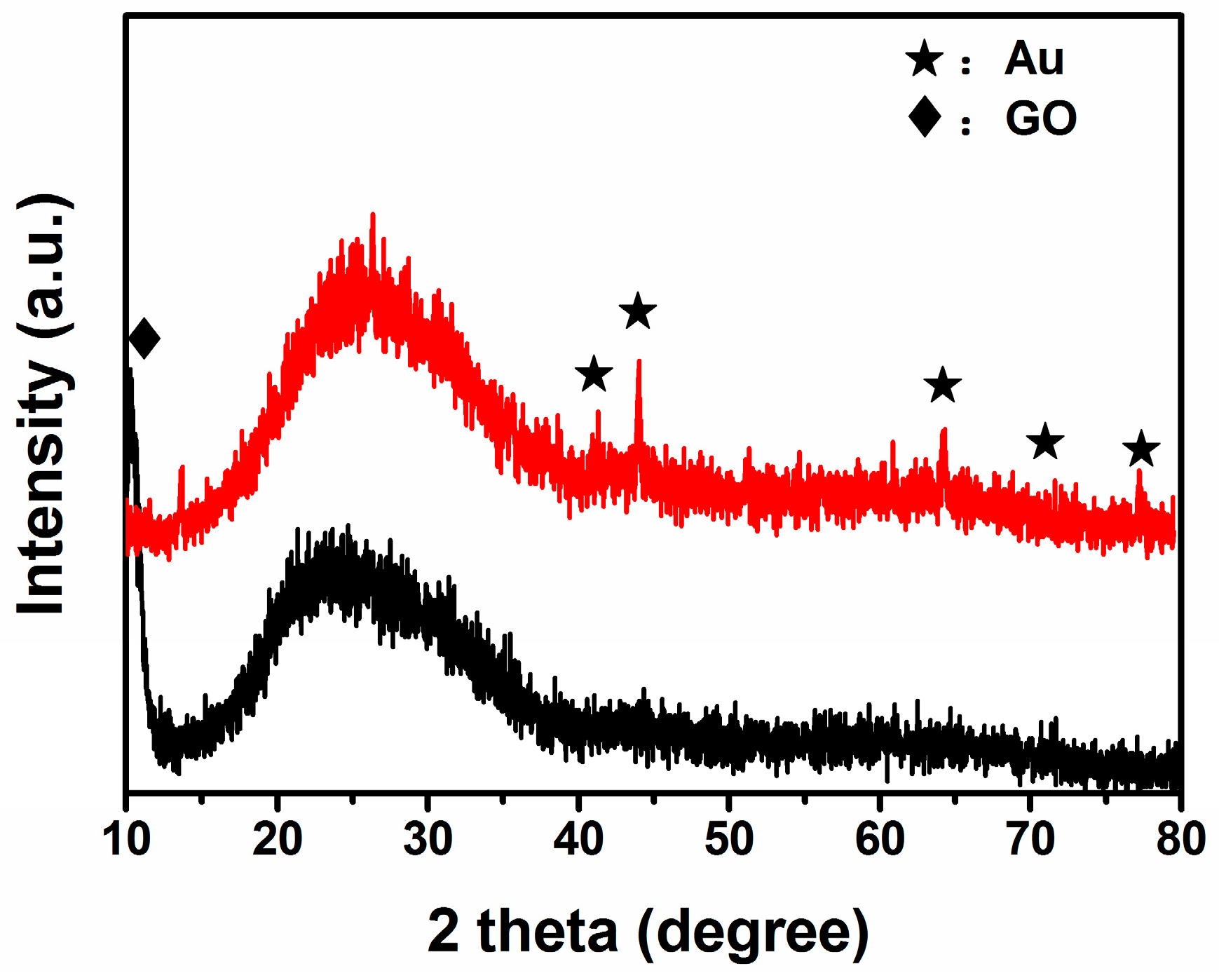
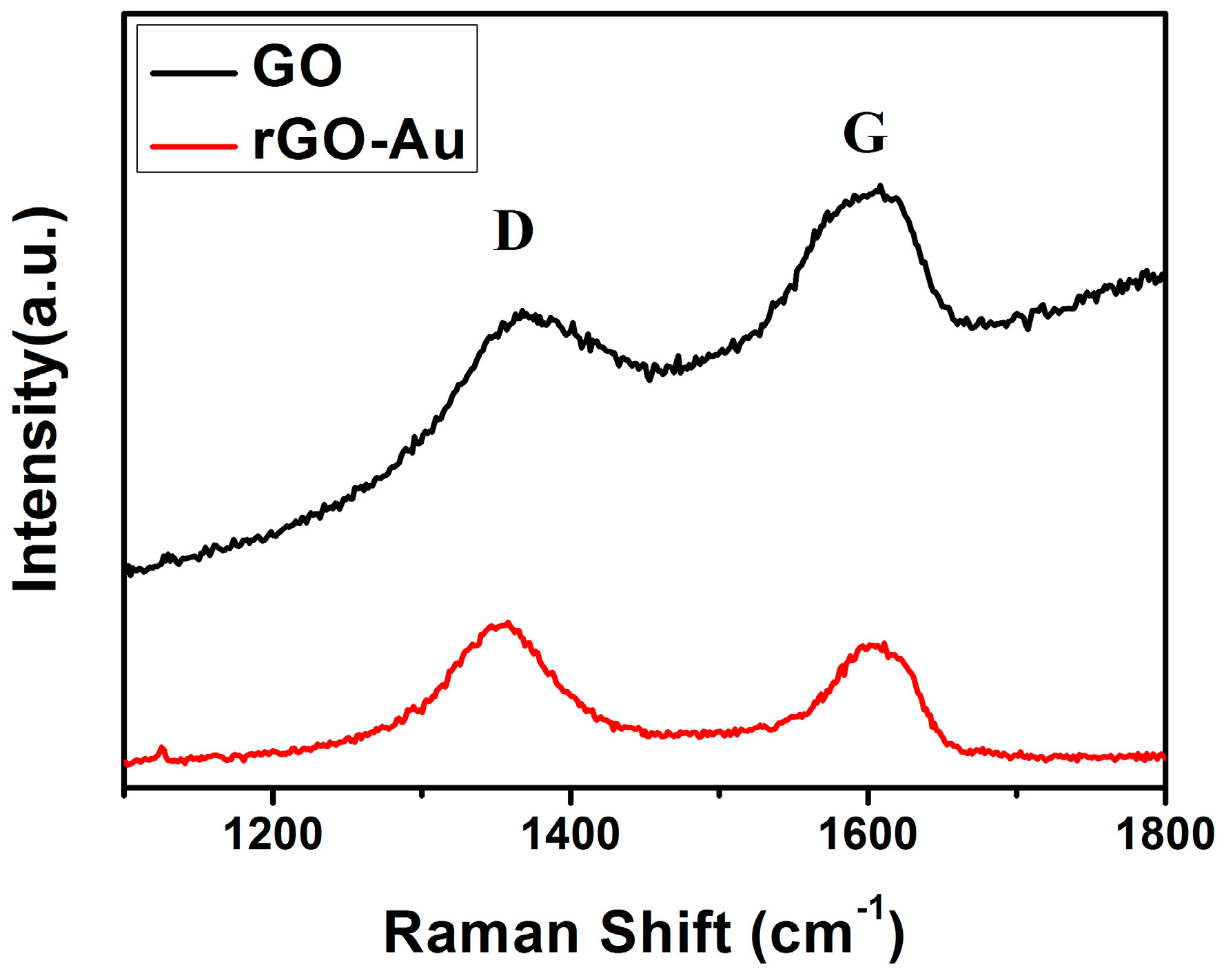
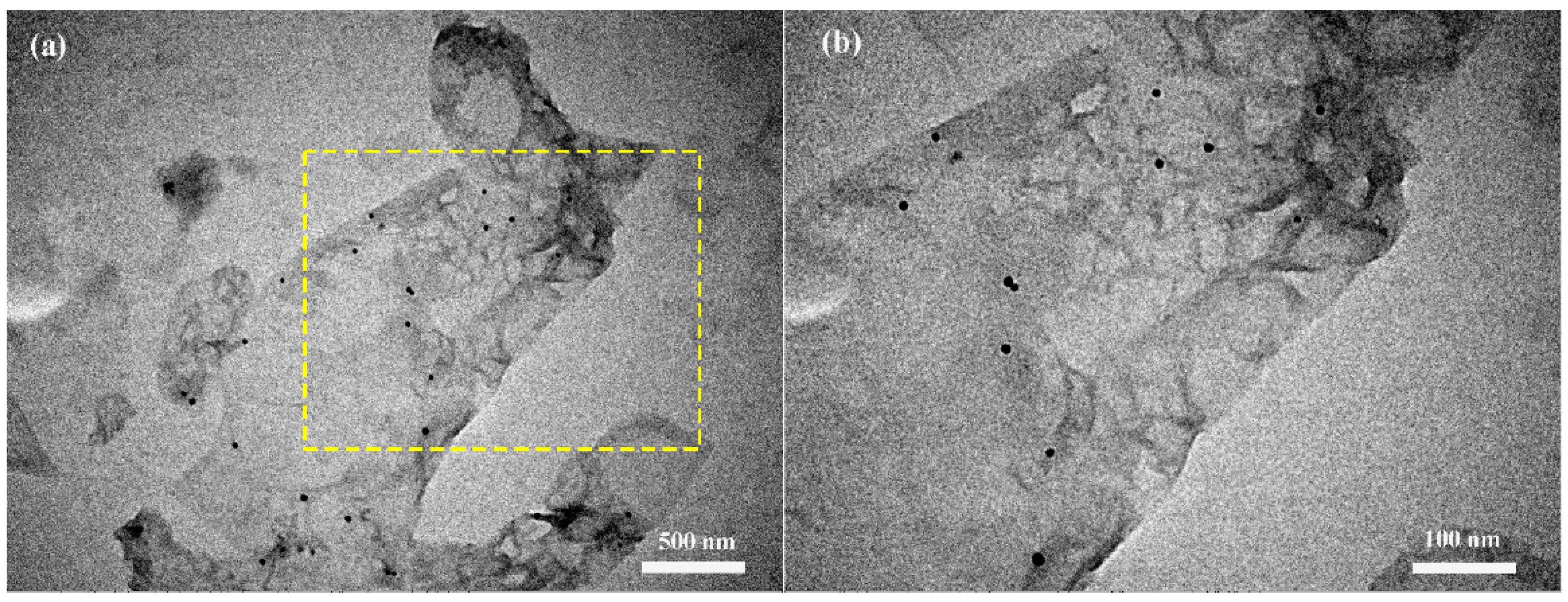
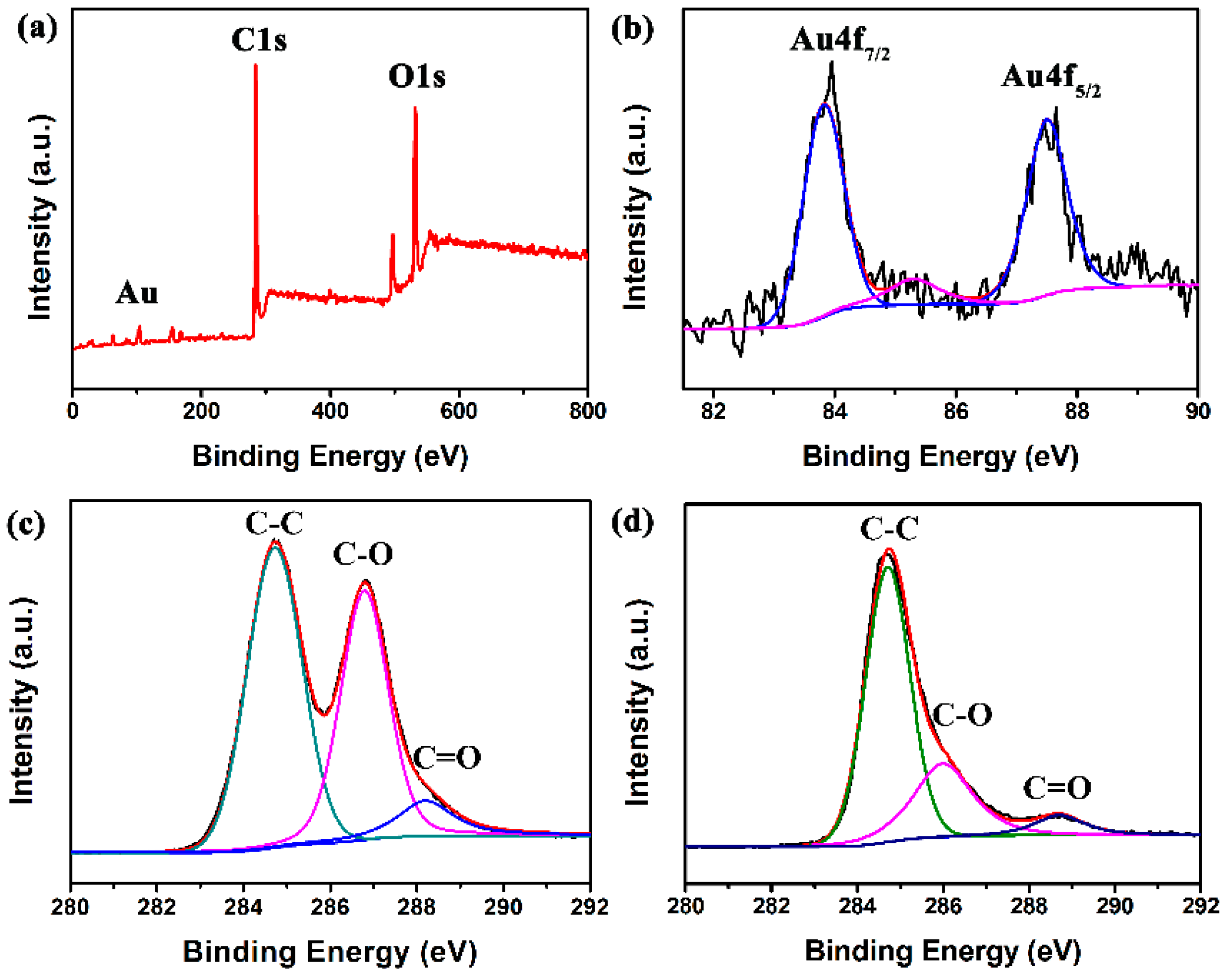
3.1. Structural and Morphological Characteristics
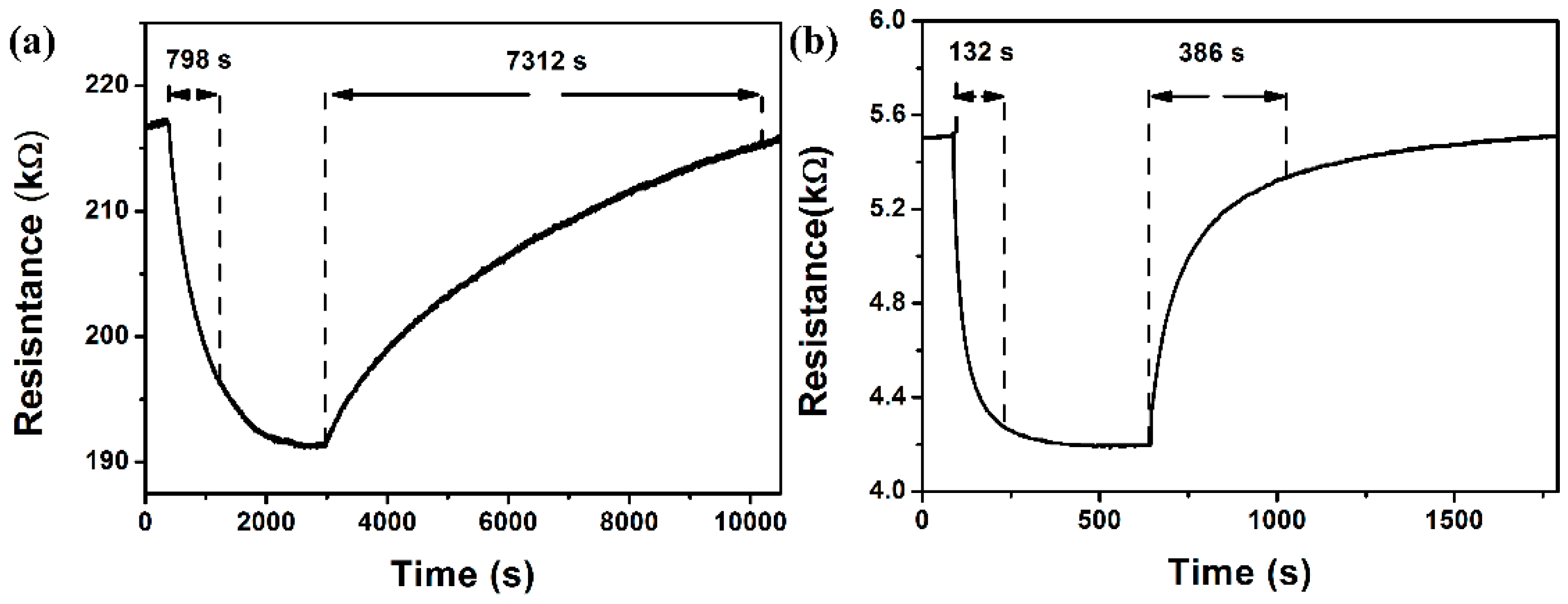
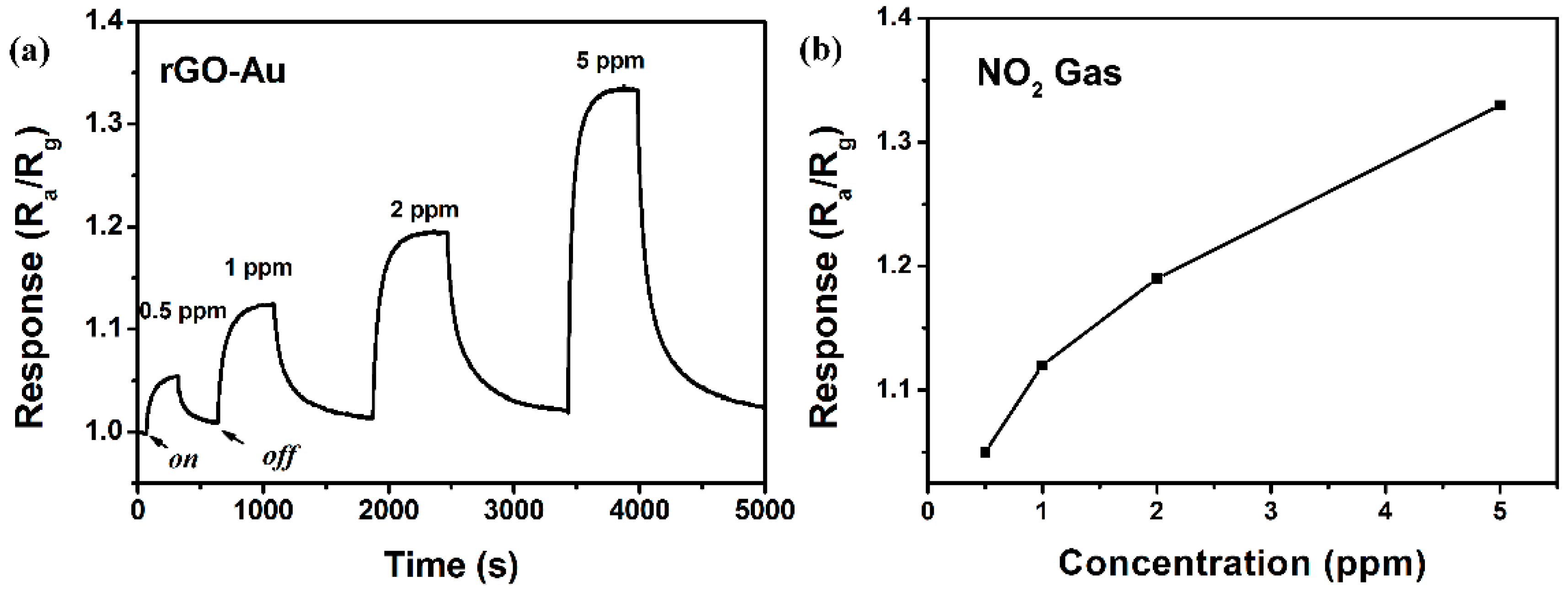
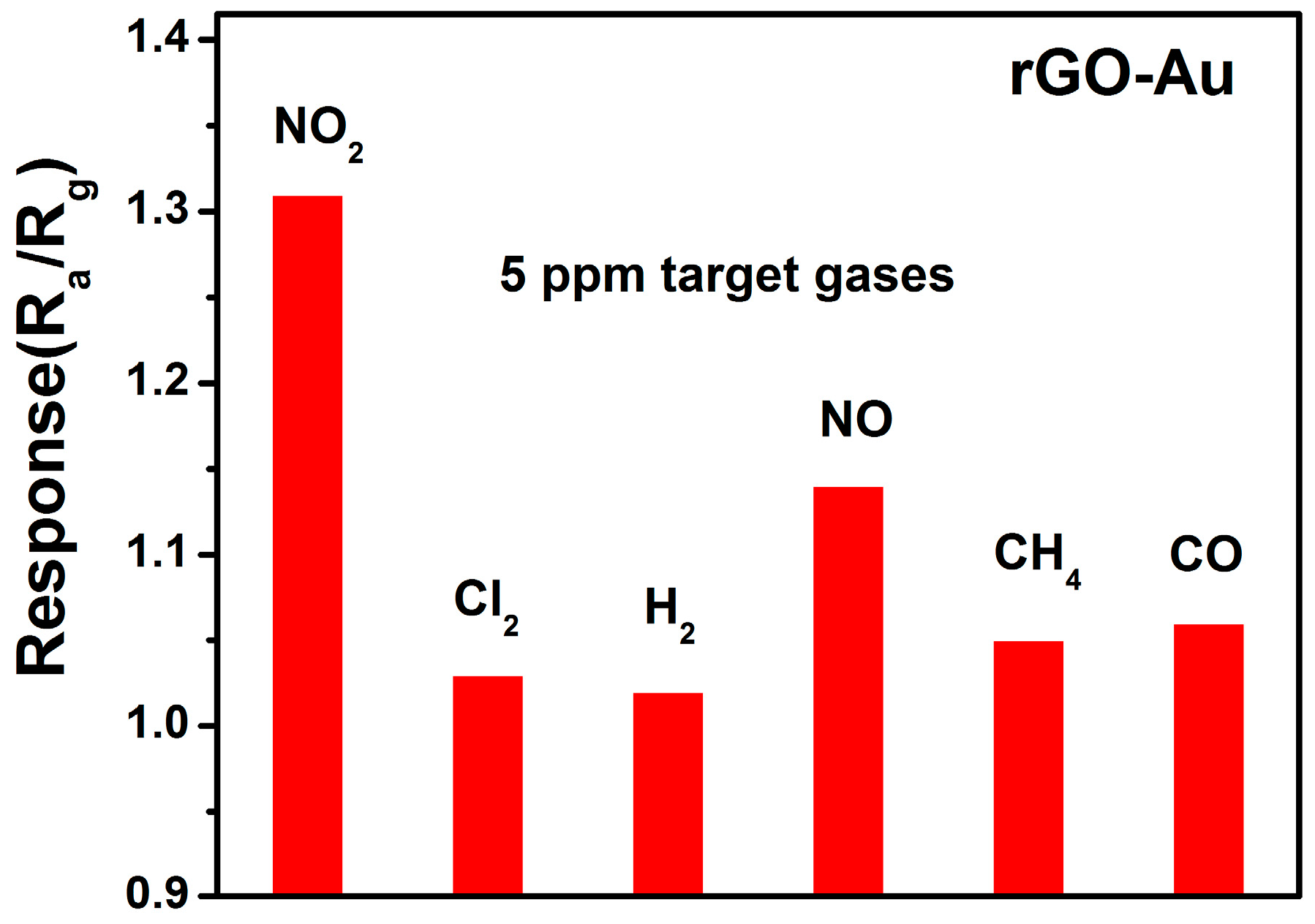
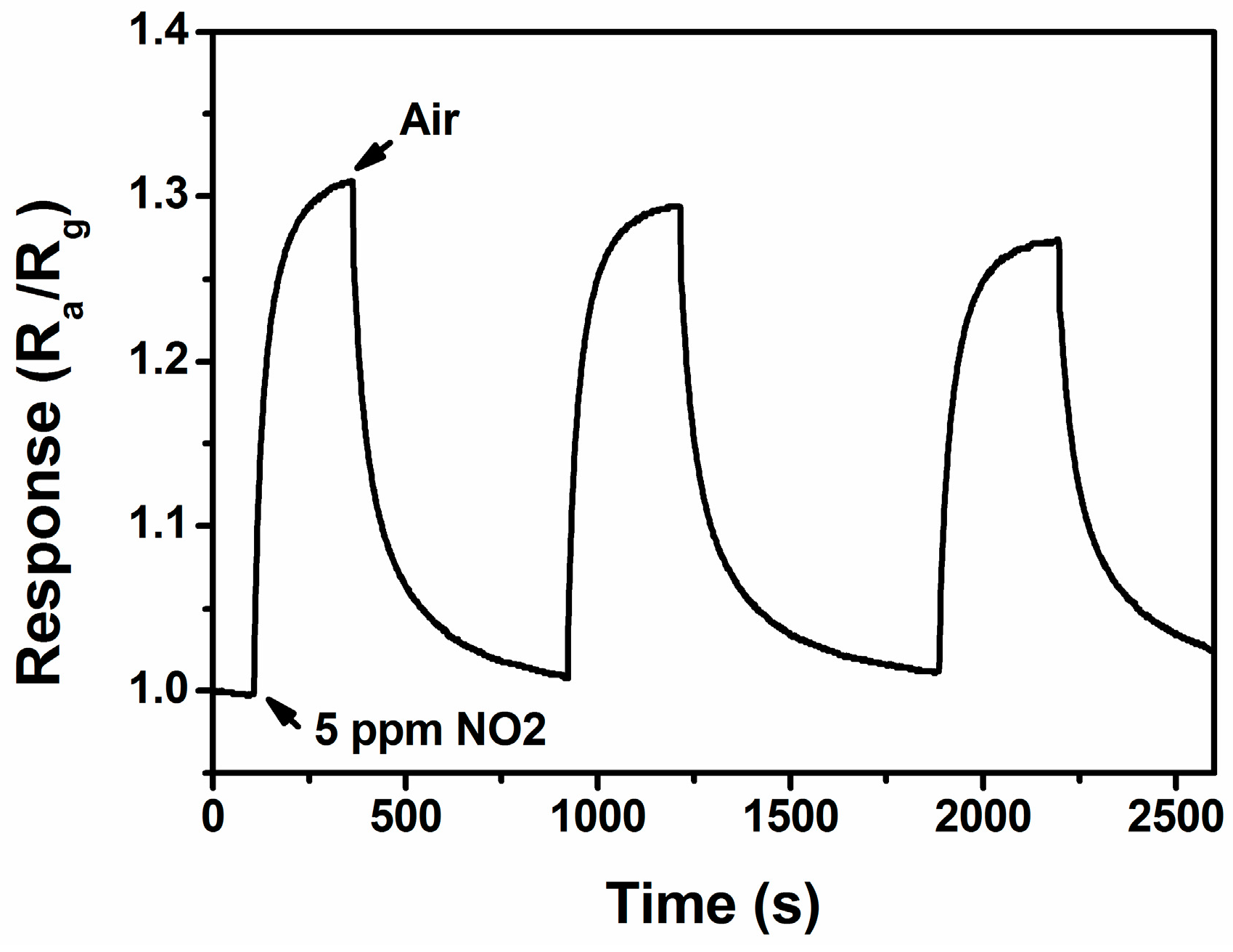
3.2. NO2 Sensing Properties
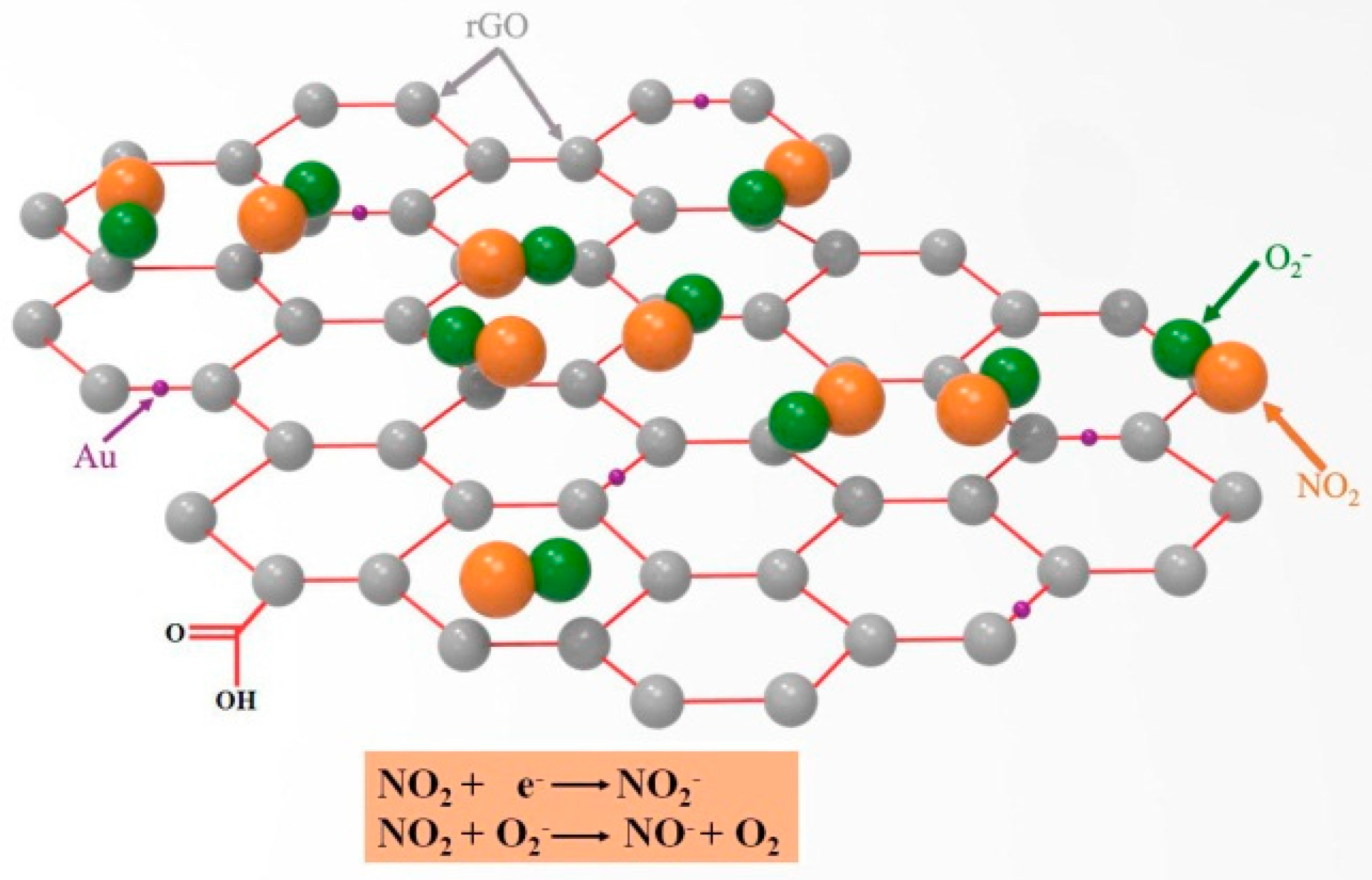
4. Gas Sensing Mechanism of rGO/Au
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, X.; Zhang, J.; Guo, X.; Wu, S.; Wang, S. Amino acid-assisted one-pot assembly of Au, Pt nanoparticles onto one-dimensional ZnO microrods. Nanoscale 2010, 2, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Wang, L.; Yang, Y.; Guo, X.; Wu, S.; Wang, S. 3D hierarchically porous ZnO structures and their functionalization by Au nanoparticles for gas sensors. J. Mater. Chem. 2011, 21, 349–356. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Yang, T.; Guo, X.; Wu, S.; Wang, S. Synthesis of Pt nanoparticles functionalized WO3 nanorods and their gas sensing properties. Sens. Actuators B Chem. 2011, 156, 918–923. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Xu, M.; Guo, X.; Wu, S.; Zhang, S.; Wang, S. Pt clusters supported on WO3 for ethanol detection. Sens. Actuators B Chem. 2010, 2, 185–190. [Google Scholar] [CrossRef]
- Gundiah, G.; Govindarj, A.; Rao, C.N.R. Nanowires, nanobelts and related nanostructures of Ga2O3. Chem. Phys. Lett. 2002, 351, 189–194. [Google Scholar] [CrossRef]
- Gao, Y.H.; Bando, Y.; Sato, T. Synthesis, Raman scattering and defects of β-Ga2O3 nanorods. Appl. Phys. Lett. 2002, 81, 2267–2269. [Google Scholar] [CrossRef]
- Kim, H.; Jin, C.; Park, S.; Kim, S.; Lee, C. H2S gas sensing properties of bare and Pd-functionalized CuO nanorods. Sens. Actuators B Chem. 2012, 161, 594–599. [Google Scholar] [CrossRef]
- Arnold, S.P.; Prokes, S.M.; Perkins, K.; Zaghloul, M.E. Design and performance of a simple, room-temperature Ga2O3 nanowire gas sensor. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Choi, Y.J.; Hwang, I.S.; Park, J.G.; Choi, K.J.; Park, J.H.; Lee, J.H. Novel fabrication of a SnO2 nanowire gas sensor with high sensitivity. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kolmakov, A.; Lilach, Y.; Moskovits, M. Electronic control of chemistry and catalysis at the surface of an individual Tin Oxide nanowire. J. Phys. Chem. B 2005, 109, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Faglia, G.; Sberveglieri, G. UV light activation of tin oxide thin films for NO2 sensing at low temperatures. Sens. Actuators B Chem. 2001, 78, 73–77. [Google Scholar] [CrossRef]
- Prades, J.D.; Diaz, R.J.; Ramirez, F.H.; Barth, S.; Cirera, A.; Rodriguez, A.R.; Mathur, S.; Morante, R. Equivalence between thermal and room temperature UV light-modulated responses of gas sensors based on individual SnO2 nanowires. Sens. Actuators B Chem. 2009, 140, 337–341. [Google Scholar] [CrossRef]
- Fan, S.W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Fan, S.W.; Srivastava, A.K.; Dravid, V.P. Nanopatterned polycrystalline ZnO for room temperature gas sensing. Sens. Actuators B Chem. 2010, 144, 159–163. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.H.; Chai, X.S.; Hu, Z.S.; Deng, Y.L. UV and visible light controllable depletion zone of ZnO-polyaniline p–n junction and its application in a photoresponsive sensor. J. Phys. Chem. C 2010, 114, 1293–1298. [Google Scholar] [CrossRef]
- Cao, C.L.; Hu, C.G.; Wang, X.; Wang, S.X.; Tian, Y.S.; Zhang, H.L. UV sensor based on TiO2 nanorod arrays on FTO thin film. Sens. Actuators B Chem. 2011, 156, 114–119. [Google Scholar] [CrossRef]
- Lupana, O.; Chowa, L.; Chaid, G. A single ZnO tetrapod-based sensor. Sens. Actuators B 2009, 141, 511–517. [Google Scholar] [CrossRef]
- Lu, G.; Xu, J.; Sun, J.; Yu, Y.; Zhang, Y.; Liu, F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuators B Chem. 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Graphene−Metal Particle Nanocomposite. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Kamat, P.V. Graphene−Semiconductor Nanocomposites: Excited-State interactions between ZnO nanoparticles and graphene oxide. Langmuir 2009, 25, 13869–13873. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Ng, W.B.; Jackman, J.A.; Cho, N.-J. Graphene Functionalized Natural Microcapsules: Modular Building Blocks for Ultrahigh Sensitivity Bioelectronic Platforms. Adv. Funct. Mater. 2016, 26, 2097–2103. [Google Scholar] [CrossRef]
- Yang, D.X.; Velamakann, A.; Bozoklu, G.; Park, S.J.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas Sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.C.; Cha, K.; Hall, A.S.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R.B. A One-Step, Solvothermal reduction method for producing reduced graphene oxide dispersions in organic solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bao, Q.; Tang, L.A.L.; Zhong, Y.; Loh, K.P. Hydrothermal Dehydration for the “Green” Reduction of Exfoliated Graphene Oxide to Graphene and Demonstration of Tunable Optical Limiting Properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Paek, S.M.; Yoo, E.; Honma, I. Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three-dimensionally delaminated flexible structure. Nano Lett. 2009, 9, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Xu, Y.; Zhang, W.; Zhao, J.; Tang, X.; Tsai, C.H.; Li, L.J. Electrical and spectroscopic characterizations of ultra-Large reduced graphene oxide monolayers. Chem. Mater. 2009, 21, 5674–5680. [Google Scholar] [CrossRef]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, M.H. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Fowler, J.D.; Allen, M.J.; Tung, V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical chemical sensors from chemically derived graphene. ACS Nano 2009, 3, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Kolmakov, A.; Klenov, D.; Lilach, Y.; Stemmer, S. Enhanced gas sensing by Individual SnO2 nanowires and nanobelts functionalized with Pd Catalyst Particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Sonnichsen, C.; Franzl, T.; Wilk, T.; Vonplessen, G.; Feldmann, J. Drastic reduction of plasmon damping in gold nanorods. Phys. Rev. Lett. 2002, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Ren, X. Effects of localized surface plasmons on the photoluminescence properties of Au-coated ZnO films. Opt. Exp. 2009, 17, 8735–8740. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, Q.; Huang, J.; Du, Y.; Ruan, S.C. Reduced Graphene Oxide/Au Nanocomposite for NO2 Sensing at Low Operating Temperature. Sensors 2016, 16, 1152. https://doi.org/10.3390/s16071152
Zhang H, Li Q, Huang J, Du Y, Ruan SC. Reduced Graphene Oxide/Au Nanocomposite for NO2 Sensing at Low Operating Temperature. Sensors. 2016; 16(7):1152. https://doi.org/10.3390/s16071152
Chicago/Turabian StyleZhang, Hao, Qun Li, Jinyu Huang, Yu Du, and Shuang Chen Ruan. 2016. "Reduced Graphene Oxide/Au Nanocomposite for NO2 Sensing at Low Operating Temperature" Sensors 16, no. 7: 1152. https://doi.org/10.3390/s16071152
APA StyleZhang, H., Li, Q., Huang, J., Du, Y., & Ruan, S. C. (2016). Reduced Graphene Oxide/Au Nanocomposite for NO2 Sensing at Low Operating Temperature. Sensors, 16(7), 1152. https://doi.org/10.3390/s16071152