Piezoresistive Membrane Surface Stress Sensors for Characterization of Breath Samples of Head and Neck Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
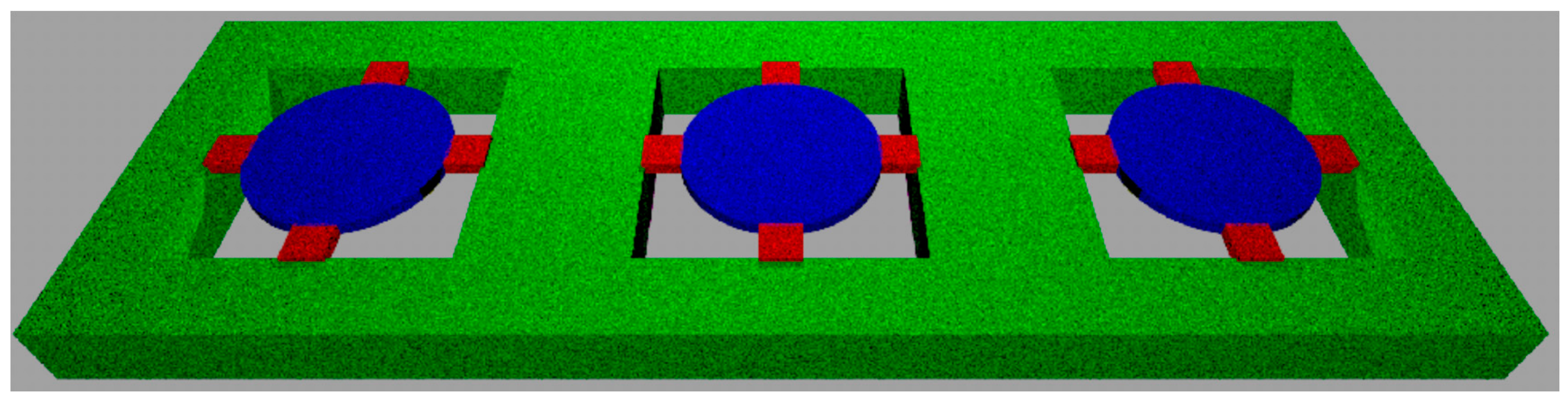
2.1. Microfabrication
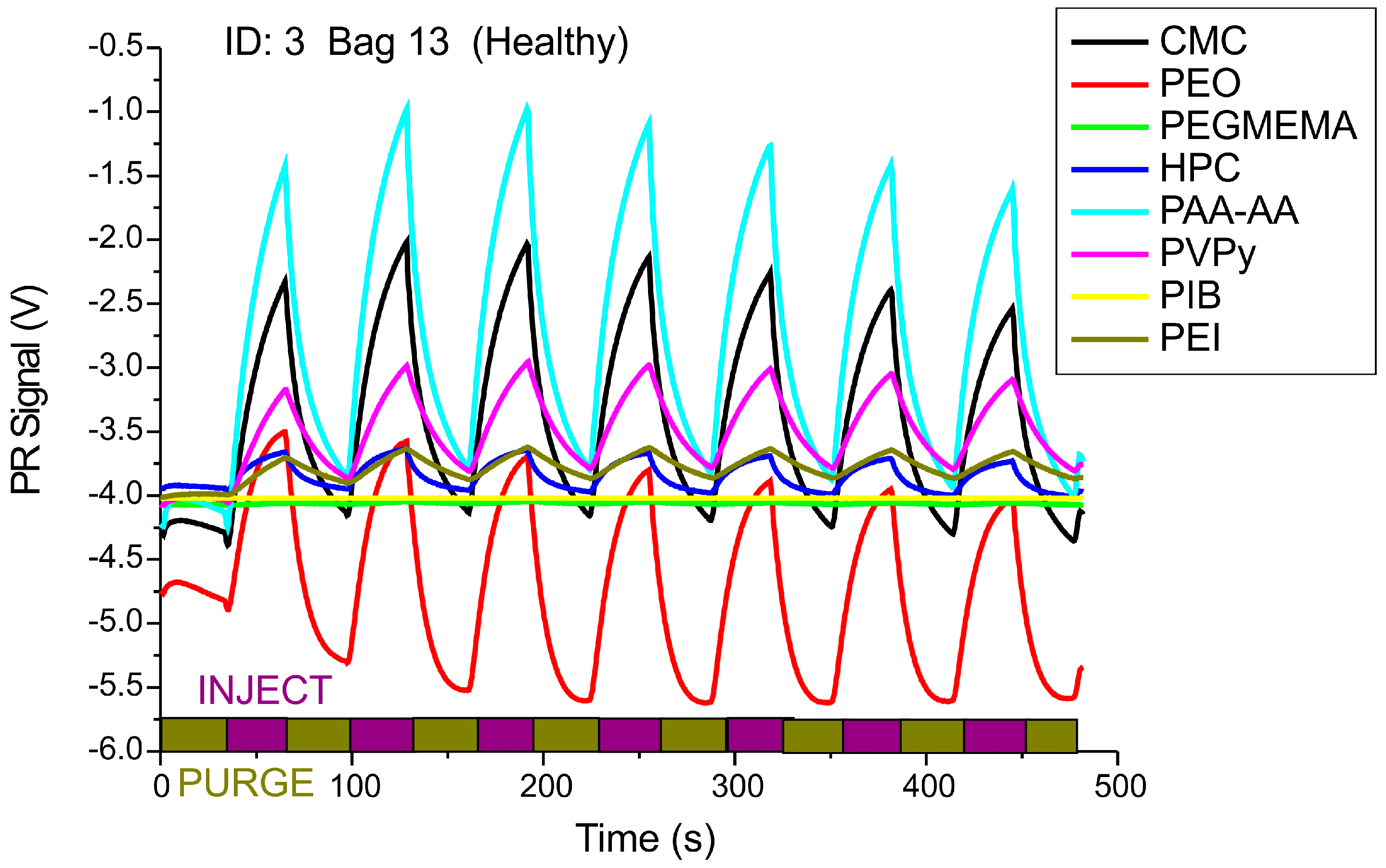
2.2. Membrane Functionalization
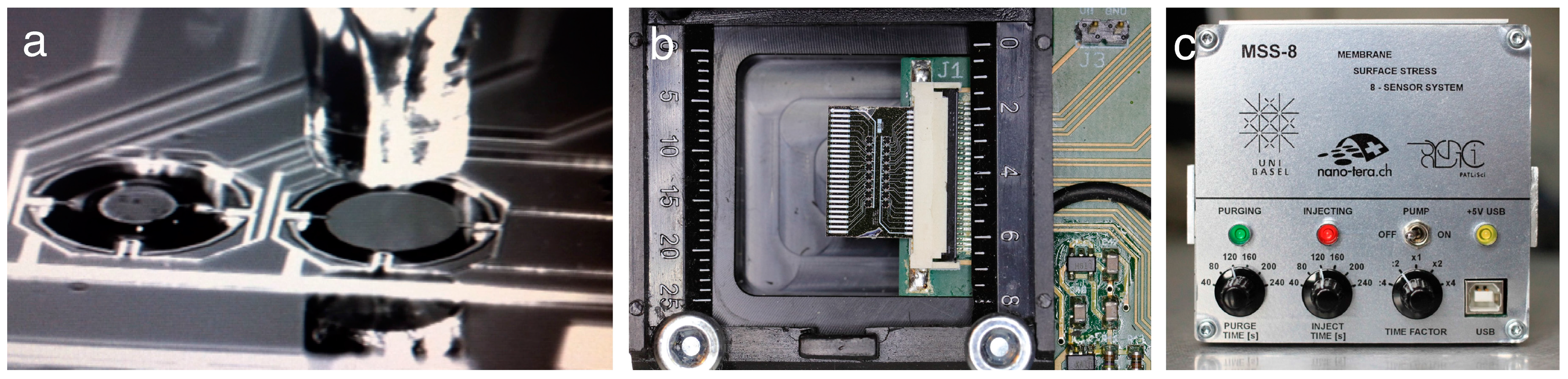
2.3. Clinical Pilot Study
- Previous history of squamous cell carcinoma of the lung or upper aerodigestive tract.
- Synchronous lesions of another histological type.
- Heart disease (NYHA Class III or IV).
- Serious pathology, such as infections requiring antibiotic use, uncontrolled peptic ulcers, gastrointestinal bleeding, central nervous system disorders.
- History of immunodeficiency or autoimmune pathologies.
- Metastases in the central nervous system, not treated and progressing.
- Chemotherapy, radiotherapy, immunotherapy less than 4 weeks prior to entry into the study (6 weeks for nitrosoureas).
- Concomitant treatment with steroids and antihistamines. Topical or inhaled steroid application is allowed.
- Psychiatric disorders or dependencies that could prevent informed consent.
- Kidney dysfunction with creatinine >2× upper limit of normal value.
- Diabetes.
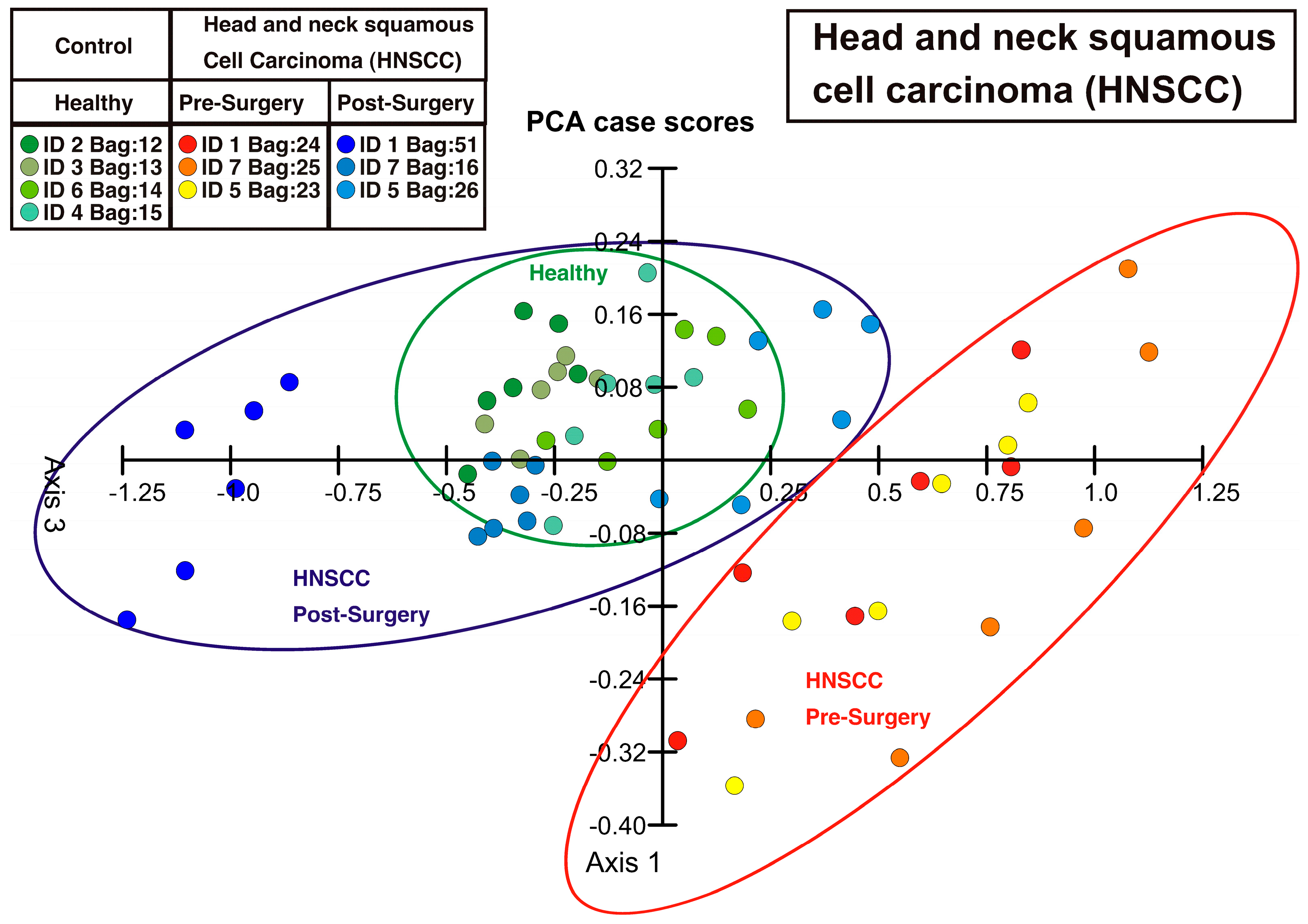
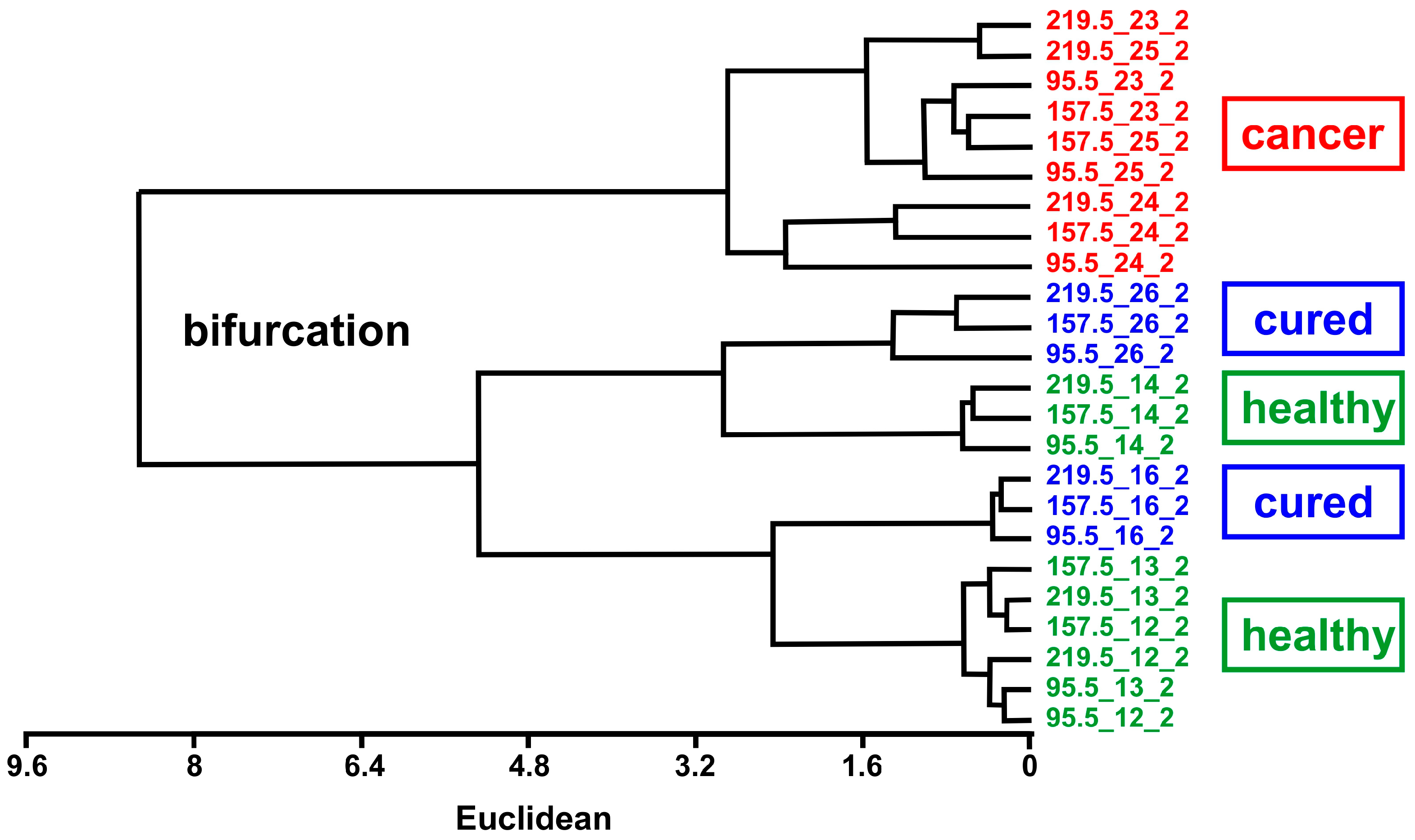
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MSS | Membrane Surface stress Sensor |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| PCA | Principal Component Analysis |
| PR | Piezoresistive |
| UPGMA | Unweighted Pair Group Method with Arithmethic Mean |
| VOC | Volatile Organic Compound |
References
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haic, H. Diagnosis of head-and-neck cancer from exhaled breath. Brit. J. Cancer 2011, 104, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.; Tisch, U.; Jeries, R.; Amal, H.; Hakim, M.; Ronen, O.; Marshak, T.; Zimmerman, D.; Israel, A.E.; Doweck, I.; et al. Analysis of exhaled breath for diagnosing head and neck squamous cell carcinoma: A feasibility study. Brit. J. Cancer 2014, 111, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Okunieff, P.; Fenton, B.; Chen, Y. Past, present, and future of oxygen in cancer research. Adv. Exp. Med. Biol. 2005, 566, 213–222. [Google Scholar] [PubMed]
- Schmutzhard, J.; Rieder, J.; Deibl, M.; Schwentner, I.M.; Schmid, S.; Lirk, P.; Abraham, I.; Gunkel, A.R. Pilot study: Volatile organic compounds as a diagnostic marker for head and neck tumors. Head Neck 2008, 30, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M.; Szidon, J.P.; Krotoszynski, B.K.; Gibbons, R.D.; O’Neill, H.J. Volatile organic-compounds in exhaled air from patients with lung-cancer. Clin. Chem. 1985, 31, 1278–1282. [Google Scholar] [PubMed]
- Phillips, M.; Cataneo, R.N.; Cummin, A.R.C.; Gagliardi, A.J.; Gleeson, K.; Greenberg, J.; Maxfield, R.A.; Rom, W.N. Detection of lung cancer with volatile markers in the breath. Chest 2003, 123, 2115–2123. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Sensors for breath testing: From nanomaterials to comprehensive disease detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Haick, H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef] [PubMed]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar] [CrossRef] [PubMed]
- Vishinkin, R.; Haick, H. Nanoscale sensor technologies for disease detection via volatolomics. Small 2015, 46, 6142–6164. [Google Scholar] [CrossRef] [PubMed]
- Peled, N.; Barash, O.; Tisch, U.; Ionescu, R.; Broza, Y.Y.; Illouze, M.; Mattei, J.; Bunn, P.A.; Hirsch, F.R.; Haick, H. Volatile fingerprints of cancer specific genetic mutations. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Barash, O.; Peled, N.; Tisch, U.; Bunn Jr., P.A.; Hirsch, F.R.; Haick, H. Classification of lung cancer histology by gild nanoparticle sensors. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Kremer, R.; Tisch, U.; Gevorkyan, A.; Shiban, A.; Best, L.A.; Haick, H. A nanomaterial-based breath test for the short-term follow-up after lung tumor resection. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bachar, N.; Liberman, L.; Muallem, F.; Feng, X.; Müllen, K.; Haick, H. Sensor arrays based on polycyclic aromatic hydrocarbons: Chemoresistors versus quartz-crystal microbalance. ACS Appl. Mater. Interfaces 2013, 5, 11641–11653. [Google Scholar] [CrossRef] [PubMed]
- Nakhleh, M.K.; Amal, H.; Awad, H.; Gharra, A.; Abu-Sahel, N.; Jeries, R.; Haick, H.; Abassi, Z. Sensor arrays based on nanoparticles for early detection of kidney injury by breath samples. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Baller, M.K.; Lang, H.P.; Fritz, J.; Gerber, C.; Gimzewski, J.K.; Drechsler, U.; Rothuizen, H.; Despont, M.; Vettiger, P.; Battiston, F.M.; et al. A cantilever array-based artficial nose. Ultramicroscopy 2000, 82, 1–9. [Google Scholar] [CrossRef]
- Fritz, J.; Baller, M.K.; Lang, H.P.; Rothuizen, H.; Vettiger, P.; Meyer, E.; Güntherodt, H.-J.; Gerber, C.; Gimzewski, J.K. Translating biomolecular recognition into nanomechanics. Science 2000, 288, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Backmann, N.; Zahnd, C.; Huber, F.; Bietsch, A.; Plückthun, A.; Lang, H.P.; Güntherodt, H.-J.; Hegner, M.; Gerber, C. A label-free immunosensor array using single-chain antibody fragments. Proc. Natl. Soc. Sci. USA 2005, 102, 14587–14592. [Google Scholar] [CrossRef] [PubMed]
- Huber, F.; Lang, H.P.; Backmann, N.; Rimoldi, D.; Gerber, C. Direct detection of a BRAF mutation in total RNA from melanoma cells using cantilever arrays. Nat. Nanotechnol. 2013, 8, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, G.; Akiyama, T.; Gautsch, S.; Vettiger, P.; Rohrer, H. Nanomechanical membrane-type Surface Stress Sensor. Nano Lett. 2011, 11, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Loizeau, F.; Akiyama, T.; Gautsch, S.; Vettiger, P.; Yoshikawa, G.; de Rooij, N. Membrane-type surface stress sensor with piezoresistive readout. In Proceedings of the 26th European Conference on Solid-State Transducers, Taipei, Taiwan, 20−24 January 2013; pp. 1085–1088.
- Loizeau, F.; Lang, H.P.; Akiyama, T.; Gautsch, S.; Vettiger, P.; Tonin, A.; Yoshikawa, G.; Gerber, Ch.; de Rooij, N. Piezoresistive membrane-type surface stress sensors arranged in arrays for cancer diagnosis through breath analysis. In Proceedings of the 26th IEEE International Conference on Micro Electro Mechanical Systems (MEMS 2013), Taipei, Taiwan, 20–24 January 2013; pp. 621–624.
- Phillips, M.; Altorki, N.; Austin, J.H.; Cameron, R.B.; Cataneo, R.N.; Greenberg, J.; Kloss, R.; Maxfield, R.A.; Munawar, M.I.; Pass, H.I.; et al. Prediction of lung cancer using volatile biomarkers in breath. Cancer Biomark. 2007, 3, 95–109. [Google Scholar] [PubMed]
- Schmid, D.; Lang, H.P.; Marsch, S.; Gerber, Ch.; Hunziker, P. Diagnosing disease by nanomechanical olfactory sensors-system design and clinical validation. Eur. J. Nanomed. 2008, 1, 44–47. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, H.P.; Loizeau, F.; Hiou-Feige, A.; Rivals, J.-P.; Romero, P.; Akiyama, T.; Gerber, C.; Meyer, E. Piezoresistive Membrane Surface Stress Sensors for Characterization of Breath Samples of Head and Neck Cancer Patients. Sensors 2016, 16, 1149. https://doi.org/10.3390/s16071149
Lang HP, Loizeau F, Hiou-Feige A, Rivals J-P, Romero P, Akiyama T, Gerber C, Meyer E. Piezoresistive Membrane Surface Stress Sensors for Characterization of Breath Samples of Head and Neck Cancer Patients. Sensors. 2016; 16(7):1149. https://doi.org/10.3390/s16071149
Chicago/Turabian StyleLang, Hans Peter, Frédéric Loizeau, Agnès Hiou-Feige, Jean-Paul Rivals, Pedro Romero, Terunobu Akiyama, Christoph Gerber, and Ernst Meyer. 2016. "Piezoresistive Membrane Surface Stress Sensors for Characterization of Breath Samples of Head and Neck Cancer Patients" Sensors 16, no. 7: 1149. https://doi.org/10.3390/s16071149
APA StyleLang, H. P., Loizeau, F., Hiou-Feige, A., Rivals, J.-P., Romero, P., Akiyama, T., Gerber, C., & Meyer, E. (2016). Piezoresistive Membrane Surface Stress Sensors for Characterization of Breath Samples of Head and Neck Cancer Patients. Sensors, 16(7), 1149. https://doi.org/10.3390/s16071149




