Last Advances in Silicon-Based Optical Biosensors
Abstract
:1. Introduction
2. Optical Biosensors
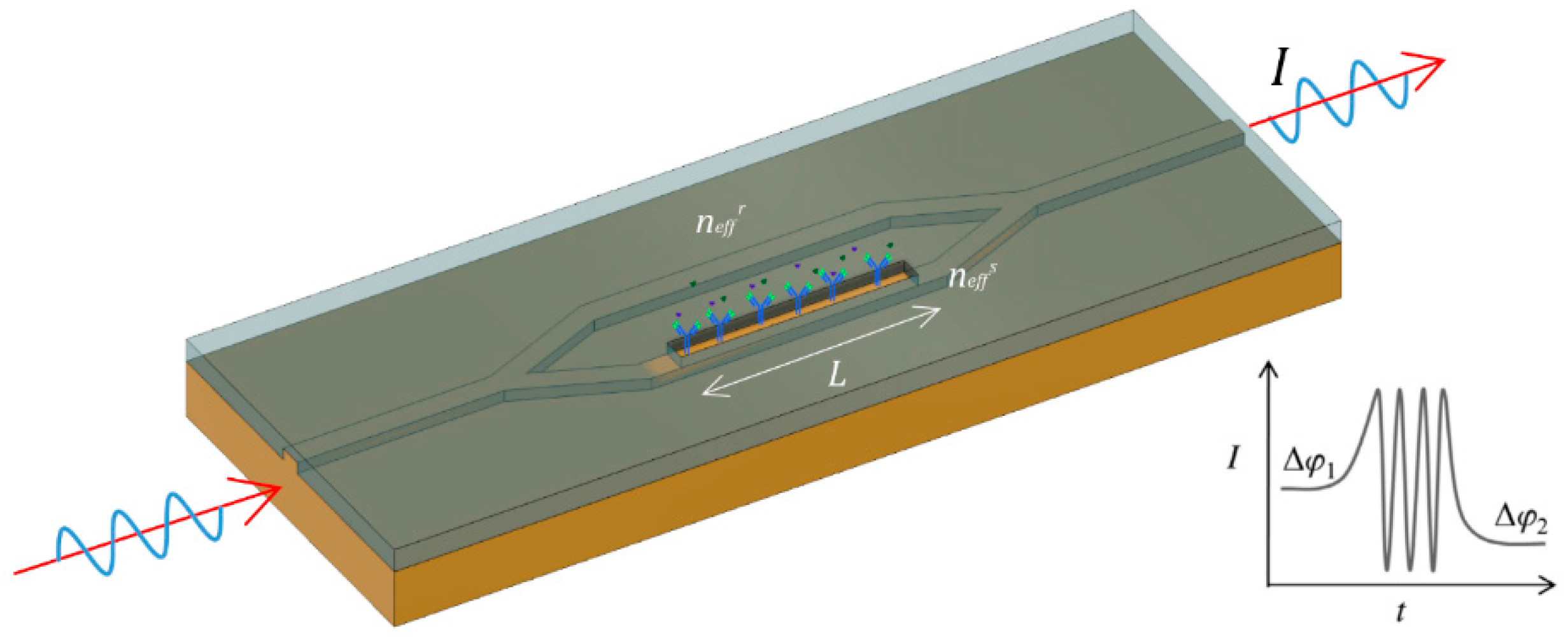
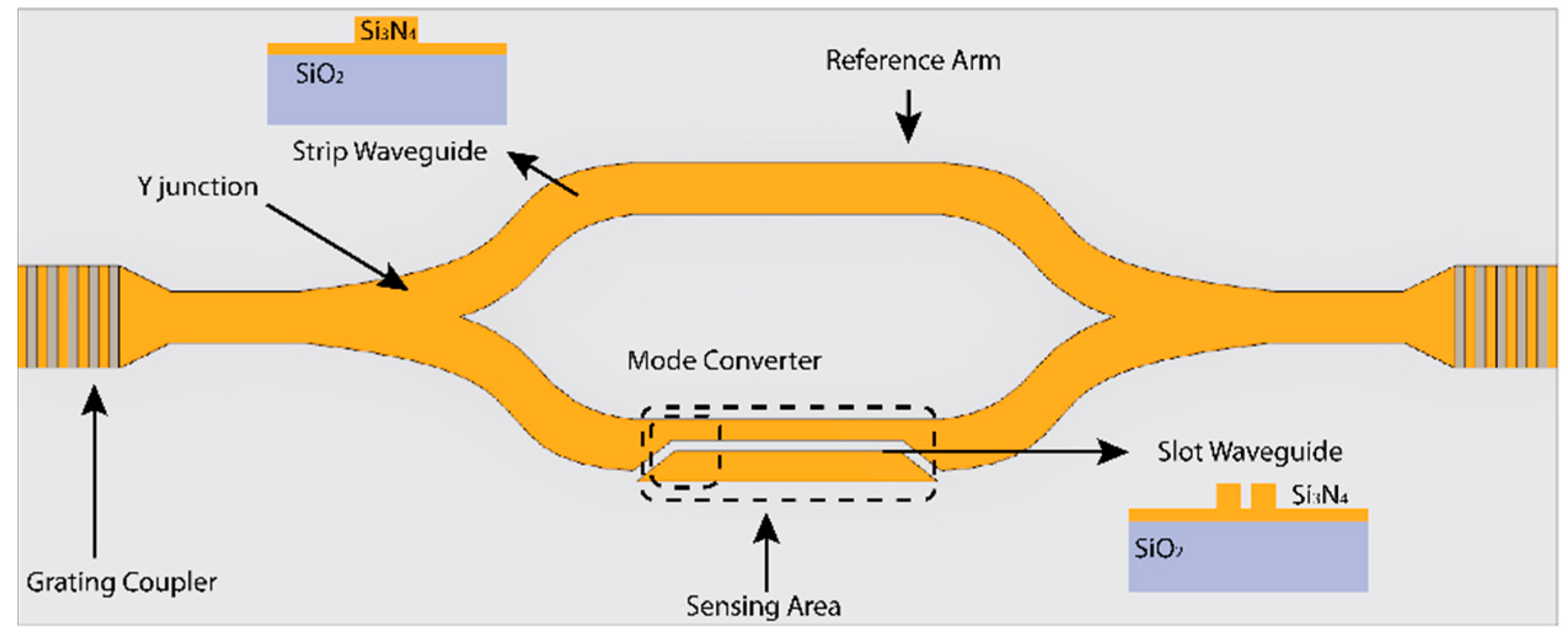
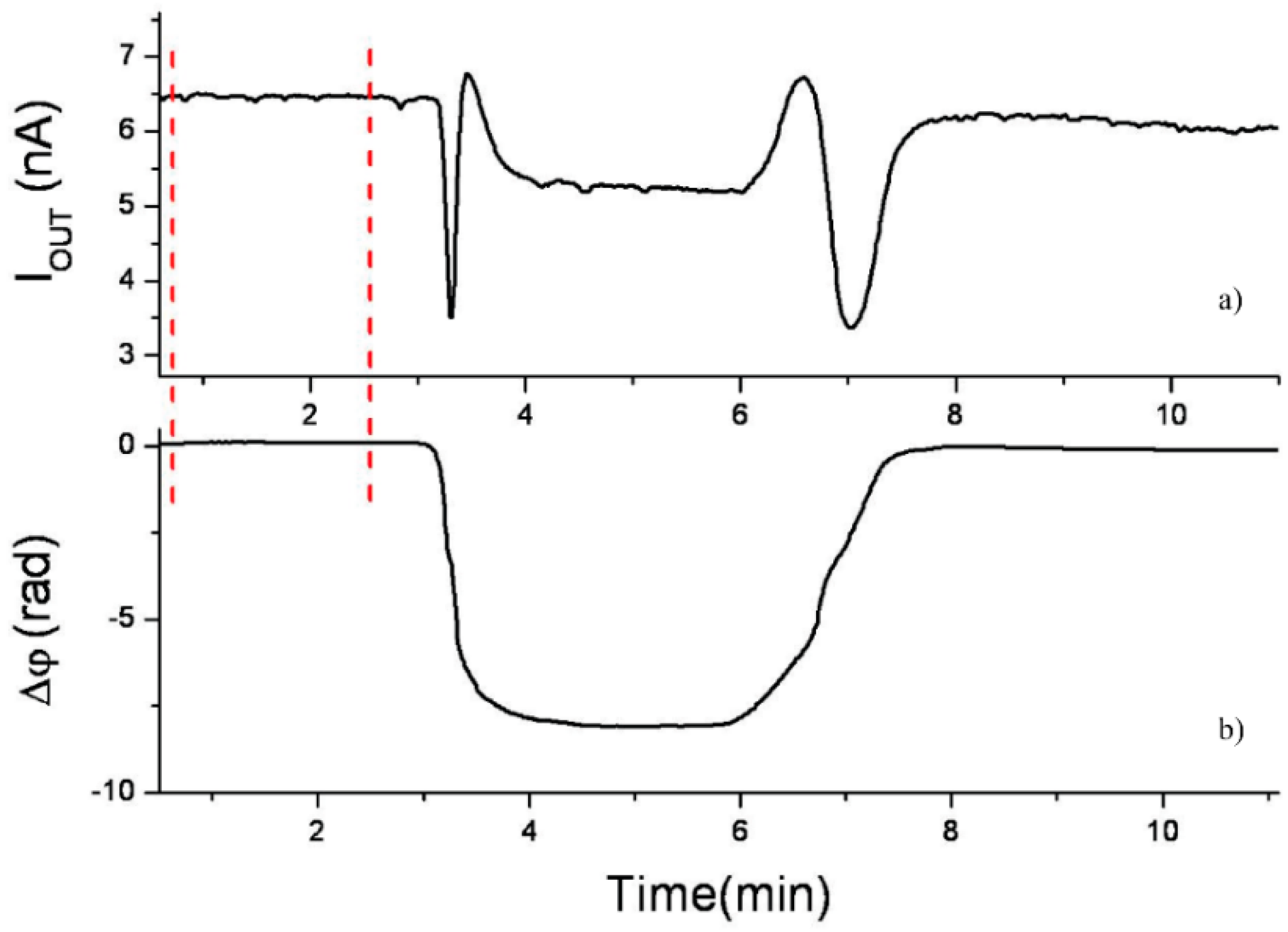
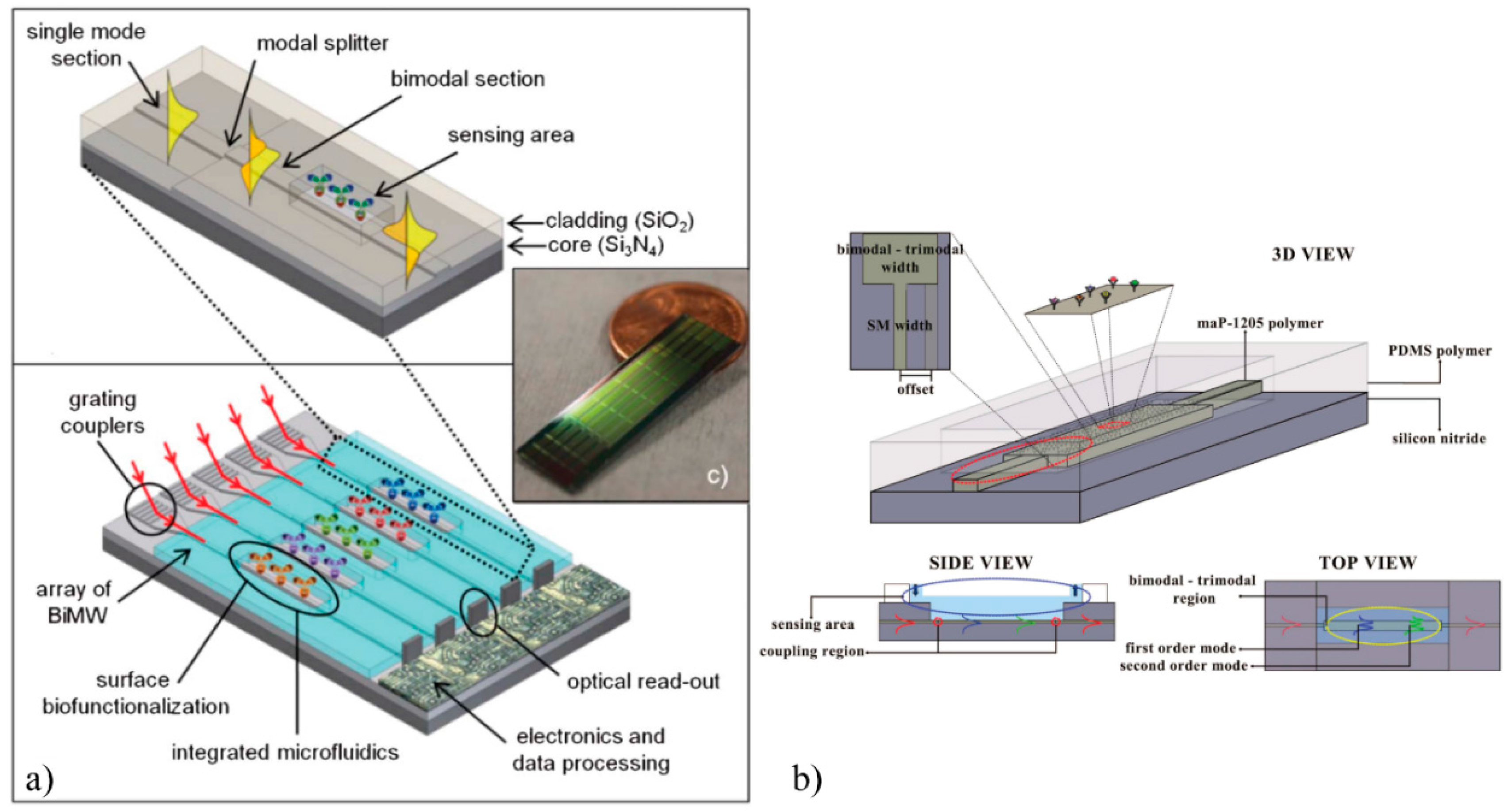
2.1. Interferometric Biosensors
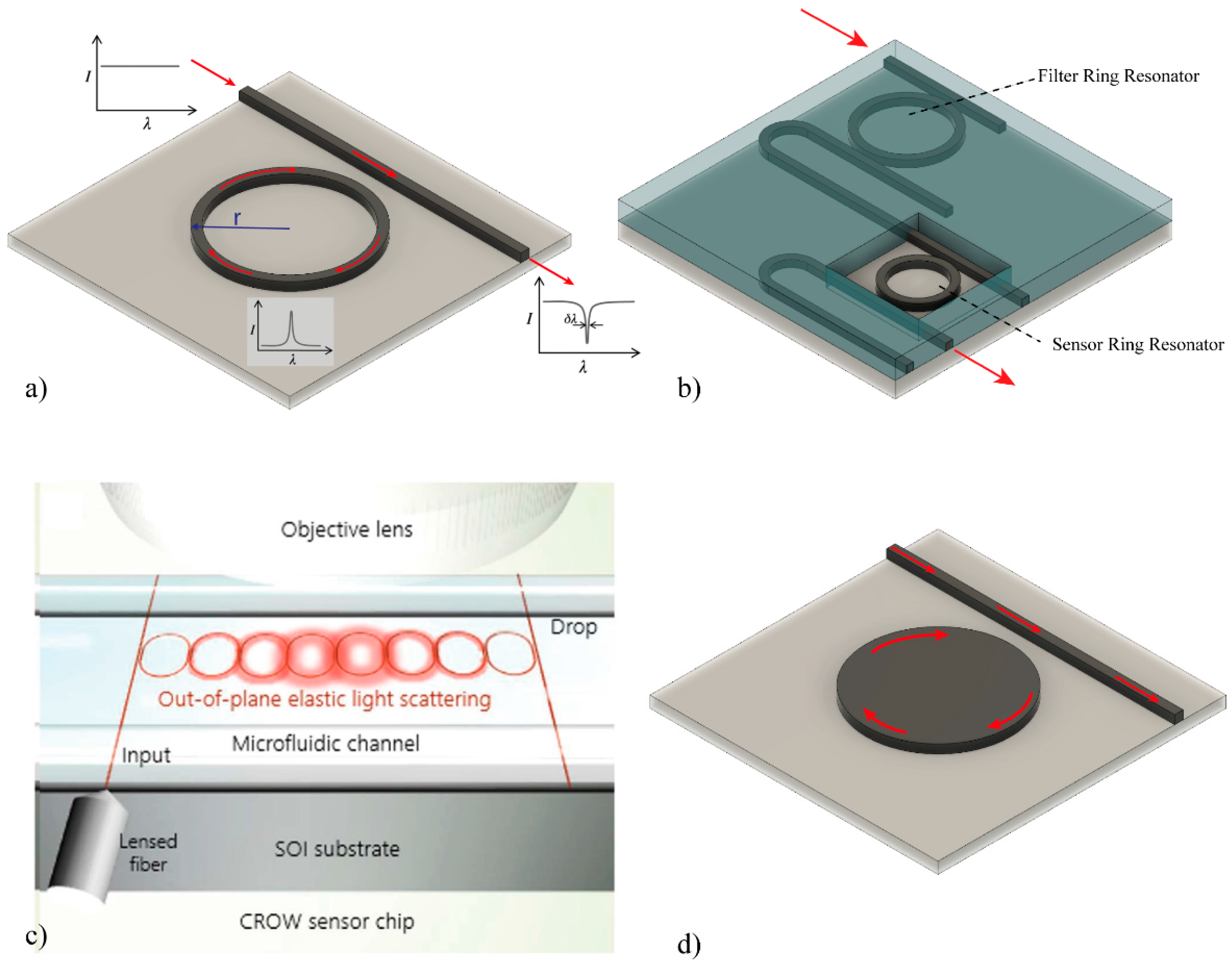
2.2. Ring Resonators-Based Biosensors
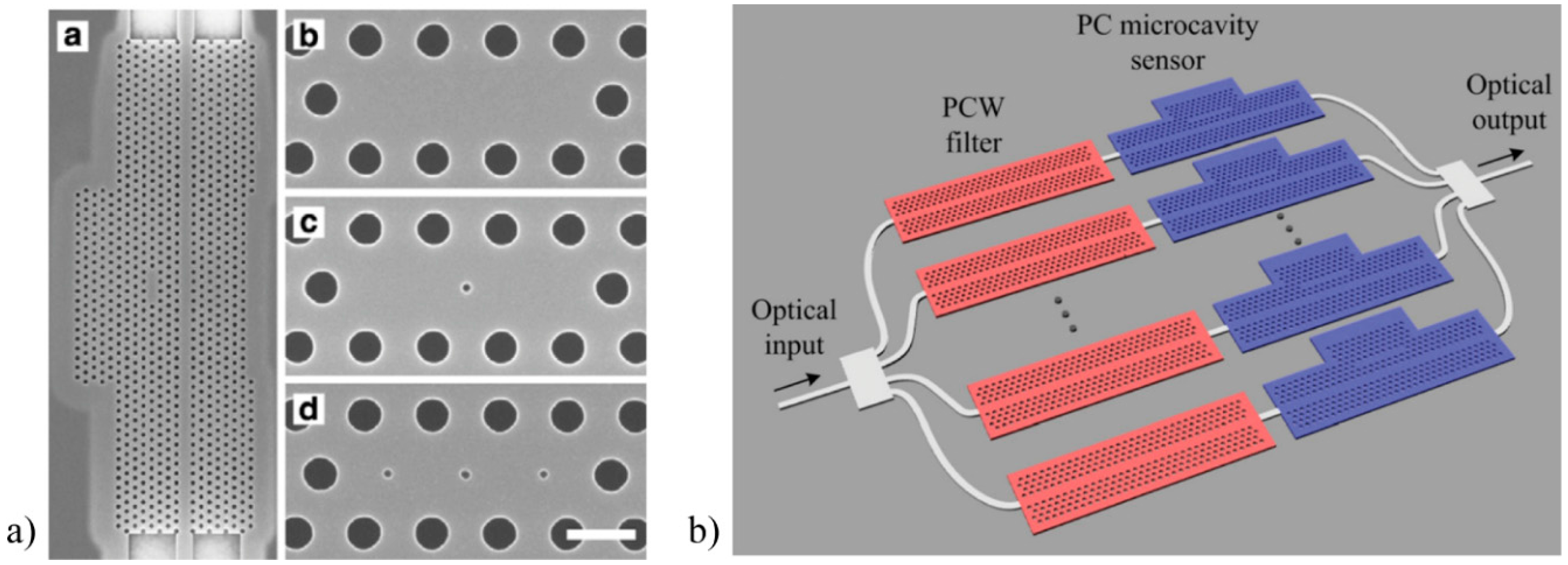
2.3. Photonic Crystals-Based Biosensors
2.4. Optical Biosensors Comparison
3. Prospects of Near Future Commercialization
Acknowledgments
Conflicts of Interest
References
- Domínguez, E.; Narváez, A. Biosensors and Modern Biospecific Analytical Techniques; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Estevez, M.C.; Alvarez, M.; Lechuga, L.M. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photon. Rev. 2012, 6, 463–487. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M. Optofluidic Microsystems for Chemical and Biological Analysis. Nat. Photonics 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Lambeck, P.V. Integrated optical sensors for the chemical domain. Meas. Sci. Technol. 2006, 17, R93–R116. [Google Scholar]
- Kussrow, A.; Enders, C.S.; Bornhop, D.J. Interferometric Methods for Label-Free Molecular Interaction Studies. Anal. Chem. 2012, 84, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Jacobson, J.W.; Bhagwandin, B.D.; Floriano, P.N.; Christodoulides, N.; Mcdevitt, J.T. Programmable Nano-Bio-Chip Sensors : Analytical Meets Clinical. Anal. Chem. 2010, 82, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Duval, D.; Lechuga, L.M. Breakthroughs in Photonics 2012: 2012 Breakthroughs in Lab-on-a-Chip and Optical Biosensors. IEEE Photonics J. 2013, 5. [Google Scholar] [CrossRef]
- Duval, D.; Lechuga, L.M. Optical Waveguide Biosensors. In Photonics: Scientific Foundations, Technology and Applications, IV; Andrews, D.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 323–365. [Google Scholar]
- De Vos, K.; Girones, J.; Popelka, S.; Schacht, E.; Baets, R.; Bienstman, P. SOI optical microring resonator with poly(ethylene glycol) polymer brush for label-free biosensor applications. Biosens. Bioelectron. 2009, 24, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.K.; Bertoncello, P.; Ding, H.; Paddeu, S.; Nicolini, C. Cholesterol biosensors prepared by layer-by-layer technique. Biosens. Bioelectron. 2001, 16, 849–856. [Google Scholar] [CrossRef]
- Cheng, Z.; Tsang, H.K.; Xu, K.; Shi, Z. Spectral hole burning in silicon waveguides with a graphene layer on top. Opt. Lett. 2013, 38, 1930–1932. [Google Scholar] [CrossRef] [PubMed]
- Rich, R.L.; Myszka, D.G. Survey of the 2009 commercial optical biosensor literature. J. Mol. Recognit. 2011, 24, 892–914. [Google Scholar] [CrossRef] [PubMed]
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Heideman, R.G.; Kooyman, R.P.H.; Greve, J. Development of an optical waveguide interferometric immunosensor. Sens. Actuators B Chem. 1991, 4, 297–299. [Google Scholar] [CrossRef]
- Lechuga, L.M.; Lenferink, A.T.M.; Kooyman, R.P.H.; Greve, J. Feasibility of evanescent wave interferometer immunosensors for pesticide detection: chemical aspects. Sens. Actuators B Chem. 1995, 25, 762–765. [Google Scholar] [CrossRef]
- Schipper, E.F.; Brugman, A.M.; Dominguez, C.; Lechuga, L.M.; Kooyman, R.P.H.; Greve, J. The realization of an integrated Mach-Zehnder waveguide immunosensor in silicon technology. Sens. Actuators B Chem. 1997, 40, 147–153. [Google Scholar] [CrossRef]
- Prieto, F.; Sepúlveda, B.; Calle, A.; Llobera, A.; Domínguez, C.; Lechuga, L.M. Integrated Mach-Zehnder interferometer based on ARROW structures for biosensor applications. Sens. Actuators B Chem. 2003, 92, 151–158. [Google Scholar] [CrossRef]
- Prieto, F.; Lechuga, L.M.; Calle, A.; Llobera, A.; Domínguez, C. Optimized silicon antiresonant reflecting optical waveguides for sensing applications. J. Light. Technol. 2001, 19, 75–83. [Google Scholar] [CrossRef]
- Prieto, F.; Sep lveda, B.; Calle, A.; Llobera, A.; Domínguez, C.; Abad, A.; Montoya, A.; Lechuga, L.M. An integrated optical interferometric nanodevice based on silicon technology for biosensor applications. Nanotechnology 2003, 14, 907–912. [Google Scholar] [CrossRef]
- Zinoviev, K.; Carrascosa, L.G.; Del Río, J.S.; Sepúlveda, B.; Domínguez, C.; Lechuga, L.M. Silicon photonic biosensors for lab-on-a-chip applications. Adv. Opt. Technol. 2008, 2008. [Google Scholar] [CrossRef]
- Densmore, A.; Xu, D.X.; Waldron, P.; Janz, S.; Cheben, P.; Lapointe, J.; Delâge, A.; Lamontagne, B.; Schmid, J.H.; Post, E. A silicon-on-insulator photonic wire based evanescent field sensor. IEEE Photonics Technol. Lett. 2006, 18, 2520–2522. [Google Scholar] [CrossRef]
- Densmore, A.; Vachon, M.; Xu, D.-X.; Janz, S.; Ma, R.; Li, Y.-H.; Lopinski, G.; Delâge, A.; Lapointe, J.; Luebbert, C.C.; et al. Silicon photonic wire biosensor array for multiplexed real-time and label-free molecular detection. Opt. Lett. 2009, 34, 3598–3600. [Google Scholar] [CrossRef] [PubMed]
- Mathesz, A.; Fábián, L.; Valkai, S.; Alexandre, D.; Marques, P.V.S.; Ormos, P.; Wolff, E.K.; Dér, A. High-speed integrated optical logic based on the protein bacteriorhodopsin. Biosens. Bioelectron. 2013, 46, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Crespi, A.; Gu, Y.; Ngamsom, B.; Hoekstra, H.J.W.M.; Dongre, C.; Pollnau, M.; Ramponi, R.; van den Vlekkert, H.H.; Watts, P.; Cerullo, G.; et al. Three-dimensional Mach-Zehnder interferometer in a microfluidic chip for spatially-resolved label-free detection. Lab Chip 2010, 10, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.J.; Lee, B.-C.; Lee, S.-M.; Park, J.H.; Shin, H.-J. Optimization of silicon oxynitrides by plasma-enhanced chemical vapor deposition for an interferometric biosensor. J. Micromech. Microeng. 2009, 19. [Google Scholar] [CrossRef]
- Choo, S.J.; Kim, J.; Lee, K.W.; Lee, D.H.; Shin, H.J.; Park, J.H. An integrated Mach-Zehnder interferometric biosensor with a silicon oxynitride waveguide by plasma-enhanced chemical vapor deposition. Curr. Appl. Phys. 2014, 14, 954–959. [Google Scholar] [CrossRef]
- Duval, D.; Osmond, J.; Dante, S.; Domínguez, C.; Lechuga, L.M. Grating couplers integrated on Mach-Zehnder interferometric biosensors operating in the visible range. IEEE Photonics J. 2013, 5. [Google Scholar] [CrossRef]
- Almeida, V.R.; Xu, Q.; Barrios, C.A.; Lipson, M. Guiding and confining light in void nanostructure. Opt. Lett. 2004, 29, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tu, X.; Kim, K.W.; Kee, J.S.; Shin, Y.; Han, K.; Yoon, Y.J.; Lo, G.Q.; Park, M.K. Highly sensitive Mach-Zehnder interferometer biosensor based on silicon nitride slot waveguide. Sens. Actuators B Chem. 2013, 188, 681–688. [Google Scholar] [CrossRef]
- Liu, Q.; Shin, Y.; Kee, J.S.; Kim, K.W.; Rafei, S.R.M.; Perera, A.P.; Tu, X.; Lo, G.-Q.; Ricci, E.; Colombel, M.; et al. Mach–Zehnder interferometer (MZI) point-of-care system for rapid multiplexed detection of microRNAs in human urine specimens. Biosens. Bioelectron. 2015, 71, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; Armelles, G.; Lechuga, L.M. Magneto-optical phase modulation in integrated Mach-Zehnder interferometric sensors. Sens. Actuators A Phys. 2007, 134, 339–347. [Google Scholar] [CrossRef]
- Lambeck, P.V. Remote opto-chemical sensing with extreme sensitivity: Design, fabrication and performance of a pigtailed integrated optical phase-modulated Mach-Zehnder interferometer system. Sens. Actuators B Chem. 1999, 61, 100–127. [Google Scholar]
- Dumais, P.; Callender, C.L.; Noad, J.P.; Ledderhof, C.J. Integrated optical sensor using a liquid-core waveguide in a Mach-Zehnder interferometer. Opt. Express 2008, 16, 18164–18172. [Google Scholar] [CrossRef] [PubMed]
- Kozma, P.; Hamori, A.; Cottier, K.; Kurunczi, S.; Horvath, R. Grating coupled interferometry for optical sensing. Appl. Phys. B Lasers Opt. 2009, 97, 5–8. [Google Scholar] [CrossRef]
- Dante, S.; Duval, D.; Sepúlveda, B.; González-Guerrero, A.B.; Sendra, J.R.; Lechuga, L.M. All-optical phase modulation for integrated interferometric biosensors. Opt. Express 2012, 20, 7195–7205. [Google Scholar] [CrossRef] [PubMed]
- Halir, R.; Vivien, L.; Le Roux, X.; Xu, D.X.; Cheben, P. Direct and sensitive phase readout for integrated waveguide sensors. IEEE Photonics J. 2013, 5. [Google Scholar] [CrossRef]
- Kitsara, M.; Misiakos, K.; Raptis, I.; Makarona, E. Integrated optical frequency-resolved Mach-Zehnder interferometers for label-free affinity sensing. Opt. Express 2010, 18, 8193–8206. [Google Scholar] [CrossRef] [PubMed]
- Misiakos, K.; Raptis, I.; Salapatas, A. Broad-band Mach-Zehnder interferometers as high performance refractive index sensors: Theory and monolithic implementation. Opt. Express 2014, 22, 8856–8870. [Google Scholar] [CrossRef] [PubMed]
- Misiakos, K.; Raptis, I.; Makarona, E.; Botsialas, A.; Salapatas, A.; Oikonomou, P.; Psarouli, A.; Petrou, P.S.; Kakabakos, S.E.; Tukkiniemi, K.; Sopanen, M.; Jobst, G. All-silicon monolithic Mach-Zehnder interferometer as a refractive index and bio-chemical sensor. Opt. Express 2014, 22, 26803–26813. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, A.; Henninger, R. Integrated optical Young interferometer. Appl. Opt. 1994, 33, 5941–5947. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, A.; Krauter, R.; Künzel, C.; Stefan, M.; Schulte, H. Interferometric sensor for detection of surface-bound bioreactions. Appl. Opt. 2000, 39, 6396–6405. [Google Scholar] [CrossRef] [PubMed]
- Brynda, E.; Houska, M.; Brandenburg, A.; Wikerstål, A. Optical biosensors for real-time measurement of analytes in blood plasma. Biosens. Bioelectron. 2002, 17, 665–675. [Google Scholar] [CrossRef]
- Ymeti, A.; Kanger, J.S.; Greve, J.; Lambeck, P.V.; Wijn, R.; Heideman, R.G. Realization of a multichannel integrated Young interferometer chemical sensor. Appl. Opt. 2003, 42, 5649–5660. [Google Scholar] [CrossRef] [PubMed]
- Ymeti, A.; Greve, J.; Lambeck, P.V.; Wink, T.; Van Novell, S.W.F.M.; Beumer, T.A.M.; Wijn, R.R.; Heideman, R.G.; Subramaniam, V.; Kanger, J.S. Fast, ultrasensitive virus detection using a young interferometer sensor. Nano Lett. 2007, 7, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Mulder, H.K.P.; Ymeti, A.; Subramaniam, V.; Kanger, J.S. Size-selective detection in integrated optical interferometric biosensors. Opt. Express 2012, 20, 20934–20950. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Schirmer, B.; Brandenburg, A. Label-free detection of biomolecules by waveguide interferometry. In Proceedings of the 17th International Conference on Optical Fibre Sensors, Bruges, Belgium, 23 May 2005.
- Schmitt, K.; Schirmer, B.; Hoffmann, C.; Brandenburg, A.; Meyrueis, P. Interferometric biosensor based on planar optical waveguide sensor chips for label-free detection of surface bound bioreactions. Biosens. Bioelectron. 2007, 22, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Cross, G.H.; Reeves, A.A.; Brand, S.; Popplewell, J.F.; Peel, L.L.; Swann, M.J.; Freeman, N.J. A new quantitative optical biosensor for protein characterisation. Biosens. Bioelectron. 2003, 19, 383–390. [Google Scholar] [CrossRef]
- Wang, M.; Hiltunen, J.; Liedert, C.; Pearce, S.; Charlton, M.; Hakalahti, L.; Karioja, P.; Myllylä, R. Highly sensitive biosensor based on UV-imprinted layered polymeric–inorganic composite waveguides. Opt. Express 2012, 20, 20309–20317. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Uusitalo, S.; Liedert, C.; Hiltunen, J.; Hakalahti, L.; Myllylä, R. Polymeric dual-slab waveguide interferometer for biochemical sensing applications. Appl. Opt. 2012, 51, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Zinoviev, K.E.; González-Guerrero, A.B.; Domínguez, C.; Lechuga, L.M. Integrated bimodal waveguide interferometric biosensor for label-free analysis. J. Light. Technol. 2011, 29, 1926–1930. [Google Scholar] [CrossRef]
- Duval, D.; González-Guerrero, A.B.; Dante, S.; Osmond, J.; Monge, R.; Fernández, L.J.; Zinoviev, K.E.; Domínguez, C.; Lechuga, L.M. Nanophotonic lab-on-a-chip platforms including novel bimodal interferometers, microfluidics and grating couplers. Lab Chip 2012, 12, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Dante, S.; Duval, D.; Fariña, D.; González-Guerrero, A.B.; Lechuga, L.M. Linear readout of integrated interferometric biosensors using a periodic wavelength modulation. Laser Photon. Rev. 2015, 9, 248–255. [Google Scholar] [CrossRef]
- Ramirez, J.C.; Lechuga, L.M.; Gabrielli, L.H.; Hernandez-Figueroa, H.E. Study of a low-cost trimodal polymer waveguide for interferometric optical biosensors. Opt. Express 2015, 23, 11985–11994. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kim, K.W.; Gu, Z.; Kee, J.S.; Park, M.K. Single-channel Mach-Zehnder interferometric biochemical sensor based on two-lateral-mode spiral waveguide. Opt. Express 2014, 22, 27910–27920. [Google Scholar] [CrossRef] [PubMed]
- Sohlstrom, H.; Oberg, M. Refractive Index Measurement Using Integrated Ring Resonators. In Proceedings of the 8th European Conference on Integrated Optics ECIO, Stockholm, Sweden, 2–4 April 1997; pp. 322–325.
- Chao, C.Y.; Fung, W.; Guo, L.J. Polymer microring resonators for biochemical sensing applications. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 134–142. [Google Scholar] [CrossRef]
- Yalçin, A.; Popat, K.C.; Aldridge, J.C.; Desai, T.A.; Hryniewicz, J.; Chbouki, N.; Little, B.E.; King, O.; Van, V.; Chu, S.; et al. Optical sensing of biomolecules using microring resonators. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 148–154. [Google Scholar]
- De Vos, K.; Bartolozzi, I.; Schacht, E.; Bienstman, P.; Baets, R. Silicon-on-Insulator microring resonator for sensitive and label-free biosensing. Opt. Express 2007, 15, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Kee, J.S.; Quah, J.Y.; Netto, V.; Song, J.; Fang, Q.; La Fosse, E.M.; Lo, G.Q. Label-free aptamer sensor based on silicon microring resonators. Sens. Actuators B Chem. 2013, 176, 552–559. [Google Scholar] [CrossRef]
- Washburn, A.L.; Gunn, L.C.; Bailey, R.C. Label-free quantitation of a cancer biomarker in complex media using silicon photonic microring resonators. Anal. Chem. 2009, 81, 9499–9506. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Gleeson, M.A.; Spaugh, B.; Tybor, F.; Gunn, W.G.; Hochberg, M.; Baehr-Jones, T.; Bailey, R.C.; Gunn, L.C. Label-free biosensor arrays based on silicon ring resonators and high-speed optical scanning instrumentation. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 654–661. [Google Scholar] [CrossRef]
- Densmore, A.; Xu, D.X.; Cheben, P.; Vachon, M.; Janz, S.; Ma, R.; Bedard, D.; Li, Y.; Lopinski, G.; Delâge, A.; et al. Integration of vertical grating couplers and microfluidic channels with silicon photonic wire biosensor arrays. In Proceedings of the 2010 IEEE Sensors, Kona, HI, USA, 1–4 November 2010; pp. 1550–1553.
- Xu, D.-X.; Vachon, M.; Densmore, A.; Ma, R.; Delâge, A.; Janz, S.; Lapointe, J.; Li, Y.; Lopinski, G.; Zhang, D.; et al. Label-free biosensor array based on silicon-on-insulator ring resonators addressed using a WDM approach. Opt. Lett. 2010, 35, 2771–2773. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-X.; Vachon, M.; Densmore, A.; Ma, R.; Janz, S.; Delâge, A.; Lapointe, J.; Cheben, P.; Schmid, J.H.; Post, E.; et al. Real-time cancellation of temperature induced resonance shifts in SOI wire waveguide ring resonator label-free biosensor arrays. Opt. Express 2010, 18, 22867–22879. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, Y.; Xu, D.-X.; Delâge, A.; Schmid, J.H.; Vachon, M.; Cheben, P.; Janz, S.; Nishiyama, N.; Arai, S. Simultaneous retrieval of fluidic refractive index and surface adsorbed molecular film thickness using silicon wire waveguide biosensors. Opt. Express 2012, 20, 26969–26977. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.A. Integrated microring resonator sensor arrays for labs-on-chips. Anal. Bioanal. Chem. 2012, 403, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Claes, T.; Bogaerts, W.; Bienstman, P. Experimental characterization of a silicon photonic biosensor consisting of two cascaded ring resonators based on the Vernier-effect and introduction of a curve fitting method for an improved detection limit. Opt. Express 2010, 18, 22747–22761. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ye, J.; Zou, J.; Li, M.; He, J.-J. Cascaded silicon-on-insulator double-ring sensors operating in high-sensitivity transverse-magnetic mode. Opt. Lett. 2013, 38, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, F.; Yang, C.; Song, J.; Tang, L.; Li, M.; He, J.-J. Label-free biosensing using cascaded double-microring resonators integrated with microfluidic channels. Opt. Commun. 2015, 344, 129–133. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Z.; Lei, T.; Poon, A.W. Silicon coupled-resonator optical-waveguide-based biosensors using light-scattering pattern recognition with pixelized mode-field-intensity distributions. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.W.; Heebner, J.E. Sensitive disk resonator photonic biosensor. Appl. Opt. 2001, 40, 5742–5747. [Google Scholar] [CrossRef] [PubMed]
- Krioukov, E.; Klunder, D.J.W.; Driessen, A.; Greve, J.; Otto, C. Sensor based on an integrated optical microcavity. Opt. Lett. 2002, 27, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Grist, S.M.; Schmidt, S.A.; Flueckiger, J.; Donzella, V.; Shi, W.; Fard, S.T.; Kirk, J.T.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon photonic micro-disk resonators for label-free biosensing. Opt. Express 2013, 21, 7994–8006. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.R.; Granda, M.G.; Gavela, A.F.; Presa, S.A.; Lastra, M.R.; Fernández, S.F. Electromagnetic Waves Scattering at interfaces Between Dielectric Waveguides: A Review on Analysis and Applications. Prog. Electromagn. Res. B 2012, 37, 103–124. [Google Scholar] [CrossRef]
- Cunningham, B.; Li, P.; Lin, B.; Pepper, J. Colorimetric resonant reflection as a direct biochemical assay technique. Sens. Actuators B Chem. 2002, 81, 316–328. [Google Scholar] [CrossRef]
- Cunningham, B.; Lin, B.; Qiu, J.; Li, P.; Pepper, J.; Hugh, B. A plastic colorimetric resonant optical biosensor for multiparallel detection of label-free biochemical interactions. Sens. Actuators B Chem. 2002, 85, 219–226. [Google Scholar] [CrossRef]
- Cunningham, B.; Qiu, J.; Li, P.; Lin, B. Enhancing the surface sensitivity of colorimetric resonant optical biosensors. Sens. Actuators B Chem. 2002, 87, 365–370. [Google Scholar] [CrossRef]
- Chan, L.L.; Cunningham, B.T.; Li, P.Y.; Puff, D. Self-referenced assay method for photonic crystal biosensors: Application to small molecule analytes. Sens. Actuators B Chem. 2007, 120, 392–398. [Google Scholar] [CrossRef]
- Chan, L.L.; Gosangari, S.L.; Watkin, K.L.; Cunningham, B.T. Label-free imaging of cancer cells using photonic crystal biosensors and application to cytotoxicity screening of a natural compound library. Sens. Actuators B Chem. 2008, 132, 418–425. [Google Scholar] [CrossRef]
- Schudel, B.R.; Choi, C.J.; Cunningham, B.T.; Kenis, P.J.A. Microfluidic chip for combinatorial mixing and screening of assays. Lab Chip 2009, 9, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.J.; Belobraydich, A.R.; Chan, L.L.; Mathias, P.C.; Cunningham, B.T. Comparison of label-free biosensing in microplate, microfluidic, and spot-based affinity capture assays. Anal. Biochem. 2010, 405, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Fauchet, P.M. Two-dimensional silicon photonic crystal based biosensing platform for protein detection. Opt. Express 2007, 15, 4530–4535. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Guillermain, E.; Sriram, R.; Miller, B.L.; Fauchet, P.M. Silicon photonic crystal nanocavity-coupled waveguides for error-corrected optical biosensing. Biosens. Bioelectron. 2011, 26, 4024–4031. [Google Scholar] [CrossRef] [PubMed]
- Dorfner, D.F.; Hürlimann, T.; Zabel, T.; Frandsen, L.H.; Abstreiter, G.; Finley, J.J. Silicon photonic crystal nanostructures for refractive index sensing. Appl. Phys. Lett. 2008, 93, 2006–2009. [Google Scholar] [CrossRef]
- Dorfner, D.; Zabel, T.; Hürlimann, T.; Hauke, N.; Frandsen, L.; Rant, U.; Abstreiter, G.; Finley, J. Photonic crystal nanostructures for optical biosensing applications. Biosens. Bioelectron. 2009, 24, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Phare, C.T.; Vlasov, Y.A.; Assefa, S.; Weiss, S.M. Photonic crystal slab sensor with enhanced surface area. Opt. Express 2010, 18, 27930–27937. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Lai, W.-C.; Zou, Y.; Drabkin, H.A.; Gemmill, R.M.; Simon, G.R.; Chin, S.H.; Chen, R.T. Multiplexed specific label-free detection of NCI-H358 lung cancer cell line lysates with silicon based photonic crystal microcavity biosensors. Biosens. Bioelectron. 2013, 43, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zou, Y.; Chakravarty, S.; Yang, C.-J.; Wang, Z.; Tang, N.; Fan, D.; Chen, R.T. Silicon on-chip bandpass filters for the multiplexing of high sensitivity photonic crystal microcavity biosensors. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef] [PubMed]
- Frascella, F.; Ricciardi, S.; Rivolo, P.; Moi, V.; Giorgis, F.; Descrovi, E.; Michelotti, F.; Munzert, P.; Danz, N.; Napione, L.; et al. A fluorescent one-dimensional photonic crystal for label-free biosensing based on BLOCH surface waves. Sensors 2013, 13, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Konopsky, V.N.; Karakouz, T.; Alieva, E.V.; Vicario, C.; Sekatskii, S.K.; Dietler, G. Photonic Crystal Biosensor based on Optical Surface Waves. Sensors 2013, 13, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Scullion, M.G.; Krauss, T.F.; di Falco, A. Slotted photonic crystal sensors. Sensors 2013, 13, 3675–3710. [Google Scholar] [CrossRef] [PubMed]
- Di Falco, A.; O’Faolain, L.; Krauss, T.F. Chemical sensing in slotted photonic crystal heterostructure cavities. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Luchansky, M.S.; Bailey, R.C. High-Q optical sensors for chemical and biological analysis. Anal. Chem. 2012, 84, 793–821. [Google Scholar] [CrossRef] [PubMed]
- Vörös, J.; Ramsden, J.J.; Csúcs, G.; Szendro, I.; de Paul, S.M.; Textor, M.; Spencer, N.D. Optical grating coupler biosensors. Biomaterials 2002, 23, 3699–3710. [Google Scholar] [CrossRef]
- Mühlsteff, J. Wearable Sensors in Syncope Management. Med. Sci. Monit. 2015, 21, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Pantelopoulos, A.; Bourbakis, N.G. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans. Syst. Man Cybern. Part. C Appl. Rev. 2010, 40, 1–12. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.R.; Poh, M.Z.; Eydgahi, H. Wearable sensors: Opportunities and challenges for low-cost health care. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1763–1766.
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait analysis using wearable sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.; Schneider, E.; Luong, J. Commercial Smartphone-Based Devices and Smart Applications for Personalized Healthcare Monitoring and Management. Diagnostics 2014, 4, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Preechaburana, P.; Suska, A.; Filippini, D. Biosensing with cell phones. Trends Biotechnol. 2014, 32, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.A.; Bonham, A.J.; White, R.J.; Zimmer, M.P.; Yadgar, R.J.; Hobza, T.M.; Honea, J.W.; Ben-Yaacov, I.; Plaxco, K.W. Cheapstat: An open-source, “do-it-yourself” potentiostat for analytical and educational applications. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]


| Device | Mass Detection Limit (pg/mm2) | RI detection Limit (RIU) | Waveguide Structure | Waveguide Material | Reference |
|---|---|---|---|---|---|
| Interferometric | |||||
| MZI | 0.06 | 1 × 10−7 | Rib | Si3N4 | [21] |
| 0.25 | ND* | Ridge | SOI | [23] | |
| Young | 0.75 | 9 × 10−8 | Rib | SixOyNz | [42] |
| 0.013 | 9 × 10−9 | Slab | Ta2O5 | [48] | |
| Bimodal WG | 0.05 | 2.5 × 10−7 | Rib | Si3N4 | [52] |
| Ring resonator | 1.5 | 7.6 × 10−7 | Ridge | SOI | [63] |
| ND | 8.3 × 10−6 | Ridge | SOI | [69] | |
| Photonic crystal | 0.42 | 3.4 × 10−5 | 2D | Si3N4 | [77] |
| ND | 7 × 10−6 | Slot | SOI | [94] |
| Company | Instrument | Technology | Webpage |
|---|---|---|---|
| Axela | DotLab | Optical grating | www.axelabiosensors.com |
| Corning | EPIC System | Resonant Gratings | www.corning.com |
| Genalyte | Maverick | Ring Resonator | www.genalyte.com |
| OWLS | OWLS210 | DPI | www.owls-sensors.com |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández Gavela, A.; Grajales García, D.; Ramirez, J.C.; Lechuga, L.M. Last Advances in Silicon-Based Optical Biosensors. Sensors 2016, 16, 285. https://doi.org/10.3390/s16030285
Fernández Gavela A, Grajales García D, Ramirez JC, Lechuga LM. Last Advances in Silicon-Based Optical Biosensors. Sensors. 2016; 16(3):285. https://doi.org/10.3390/s16030285
Chicago/Turabian StyleFernández Gavela, Adrián, Daniel Grajales García, Jhonattan C. Ramirez, and Laura M. Lechuga. 2016. "Last Advances in Silicon-Based Optical Biosensors" Sensors 16, no. 3: 285. https://doi.org/10.3390/s16030285
APA StyleFernández Gavela, A., Grajales García, D., Ramirez, J. C., & Lechuga, L. M. (2016). Last Advances in Silicon-Based Optical Biosensors. Sensors, 16(3), 285. https://doi.org/10.3390/s16030285








