New Flexible Silicone-Based EEG Dry Sensor Material Compositions Exhibiting Improvements in Lifespan, Conductivity, and Reliability
Abstract
:1. Introduction
2. Materials and Processing
2.1. Materials and Processing
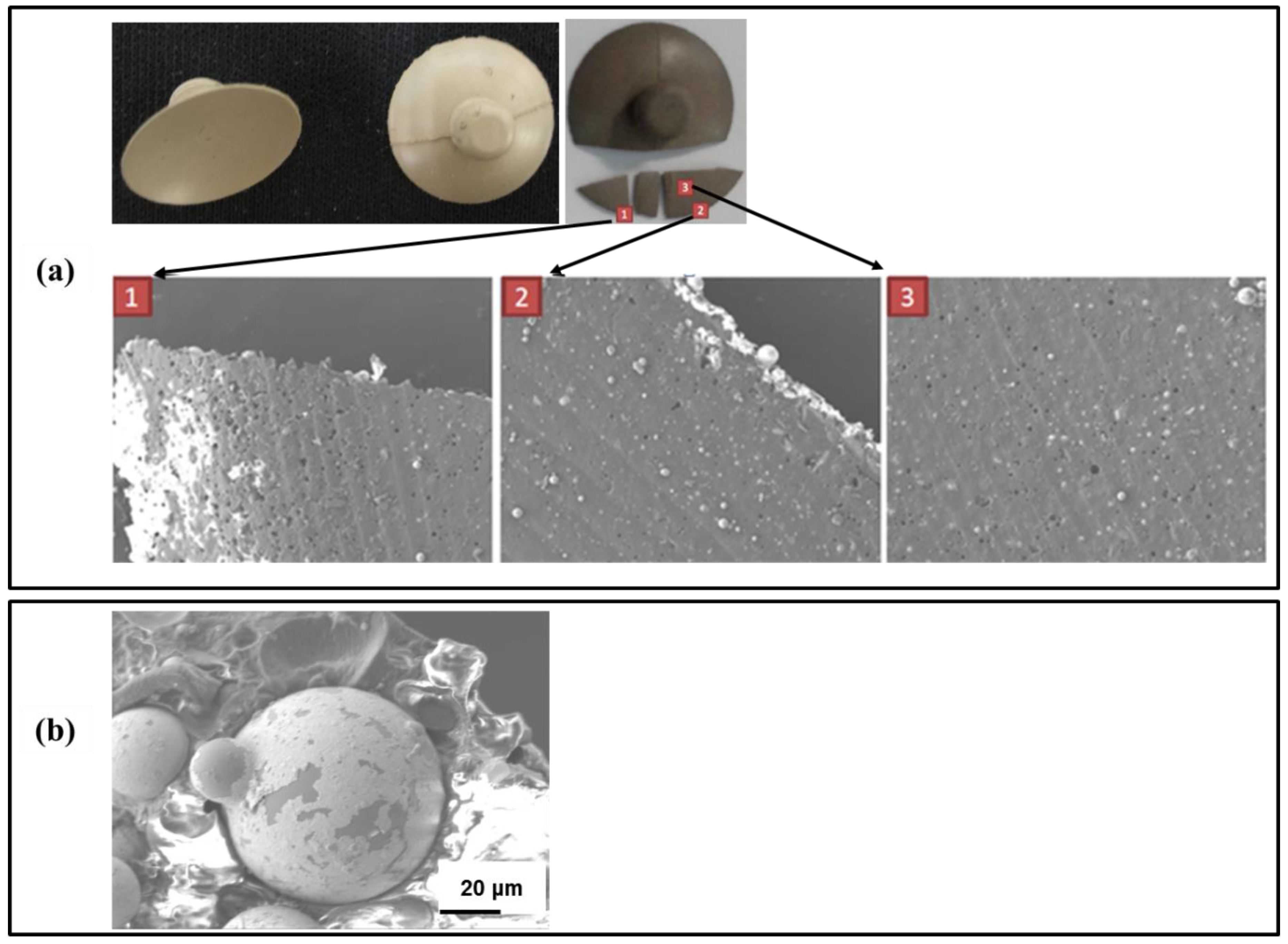
2.2. Scanning Electron Microscopy Characterization
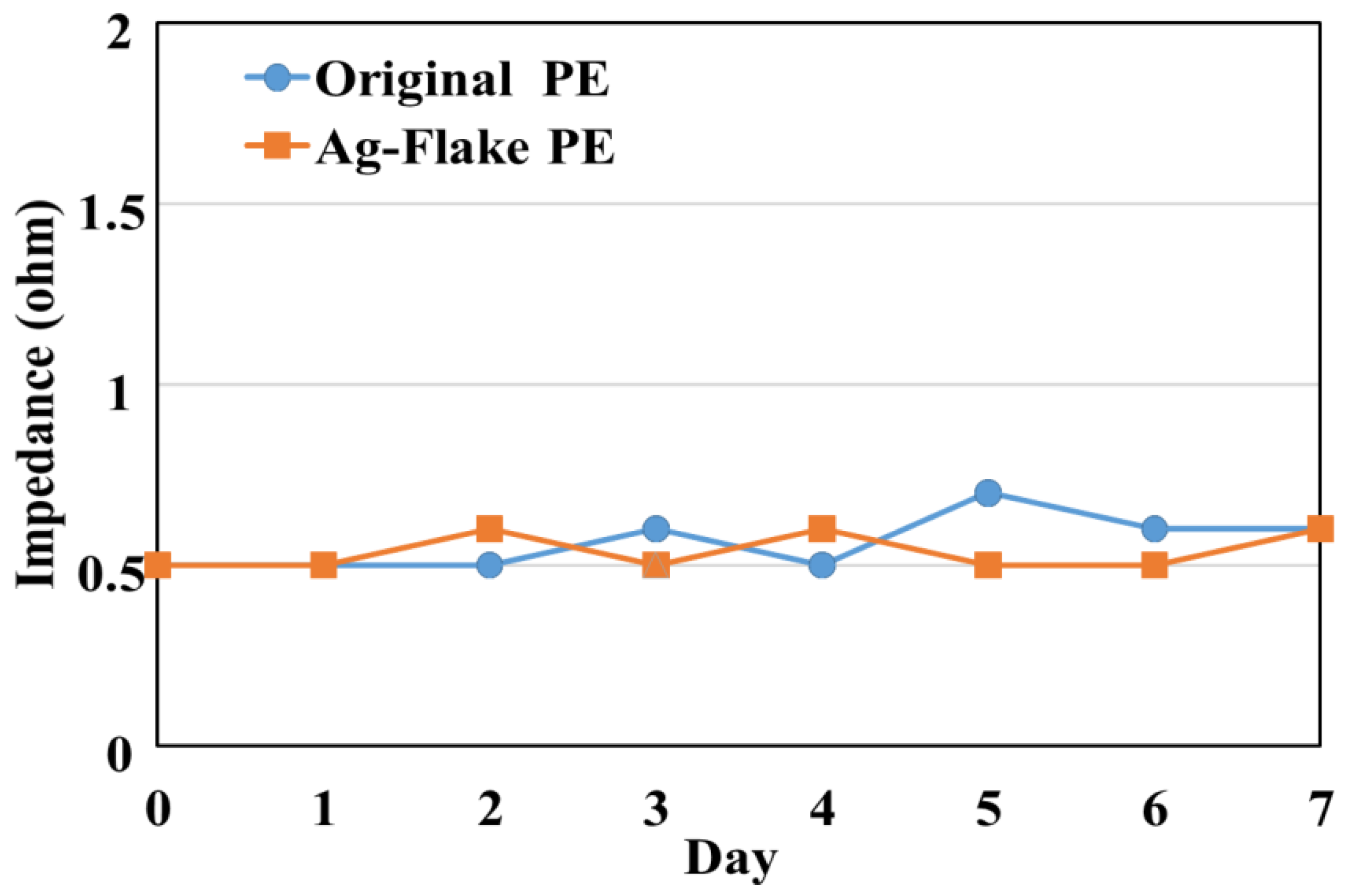
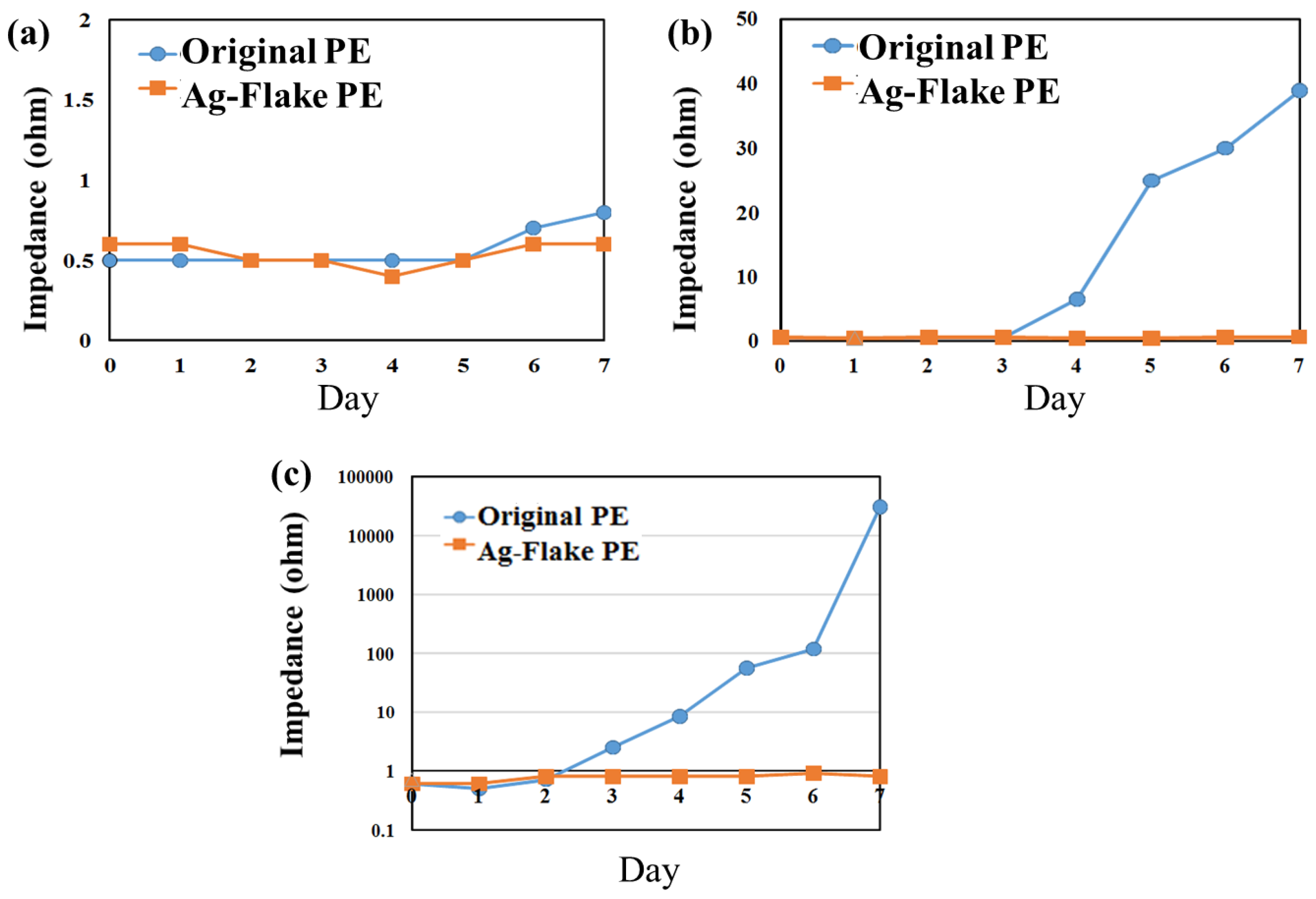
2.3. Environment Testing
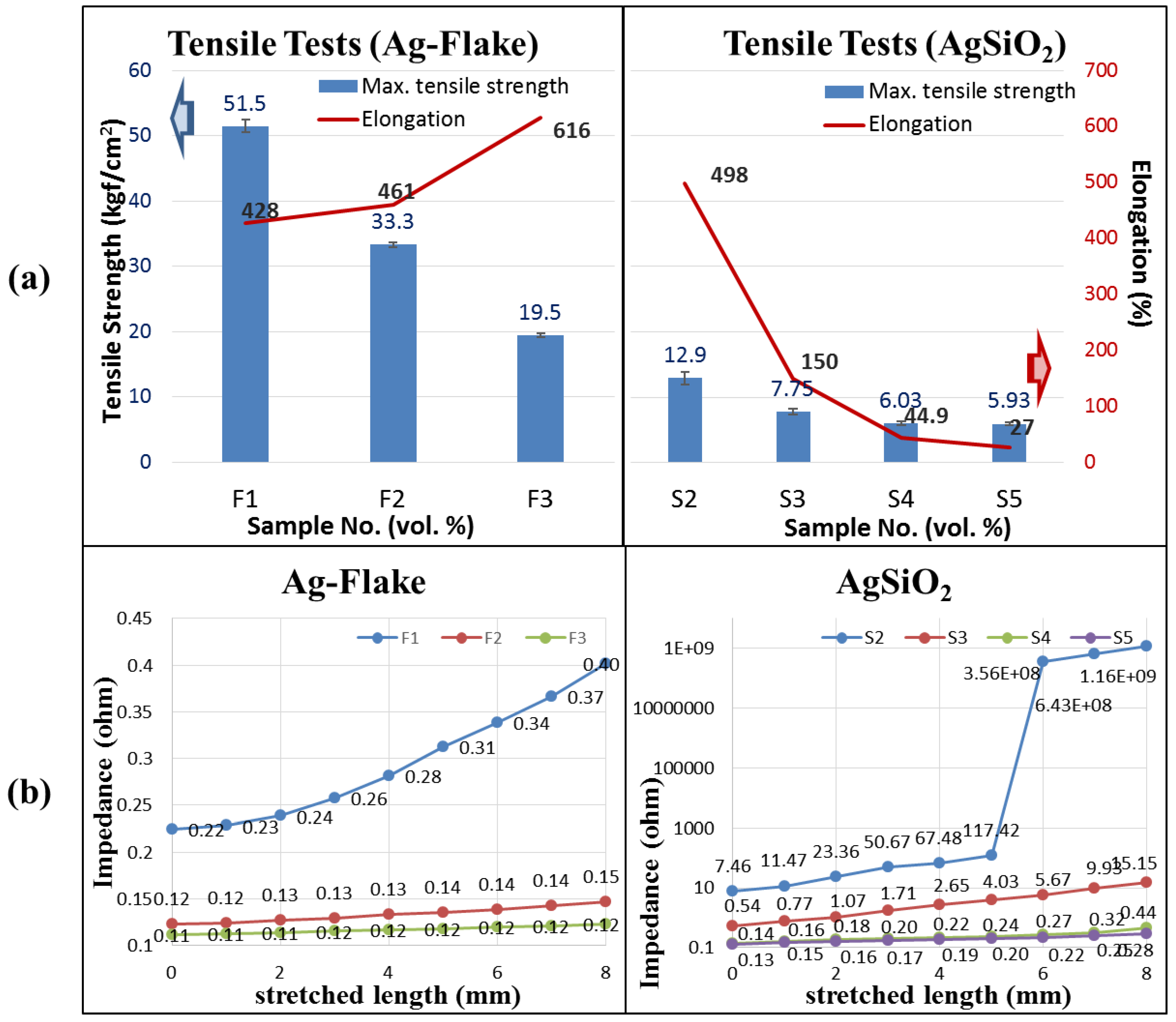
2.4. Tensile Testing
2.5. Salt Spray Testing
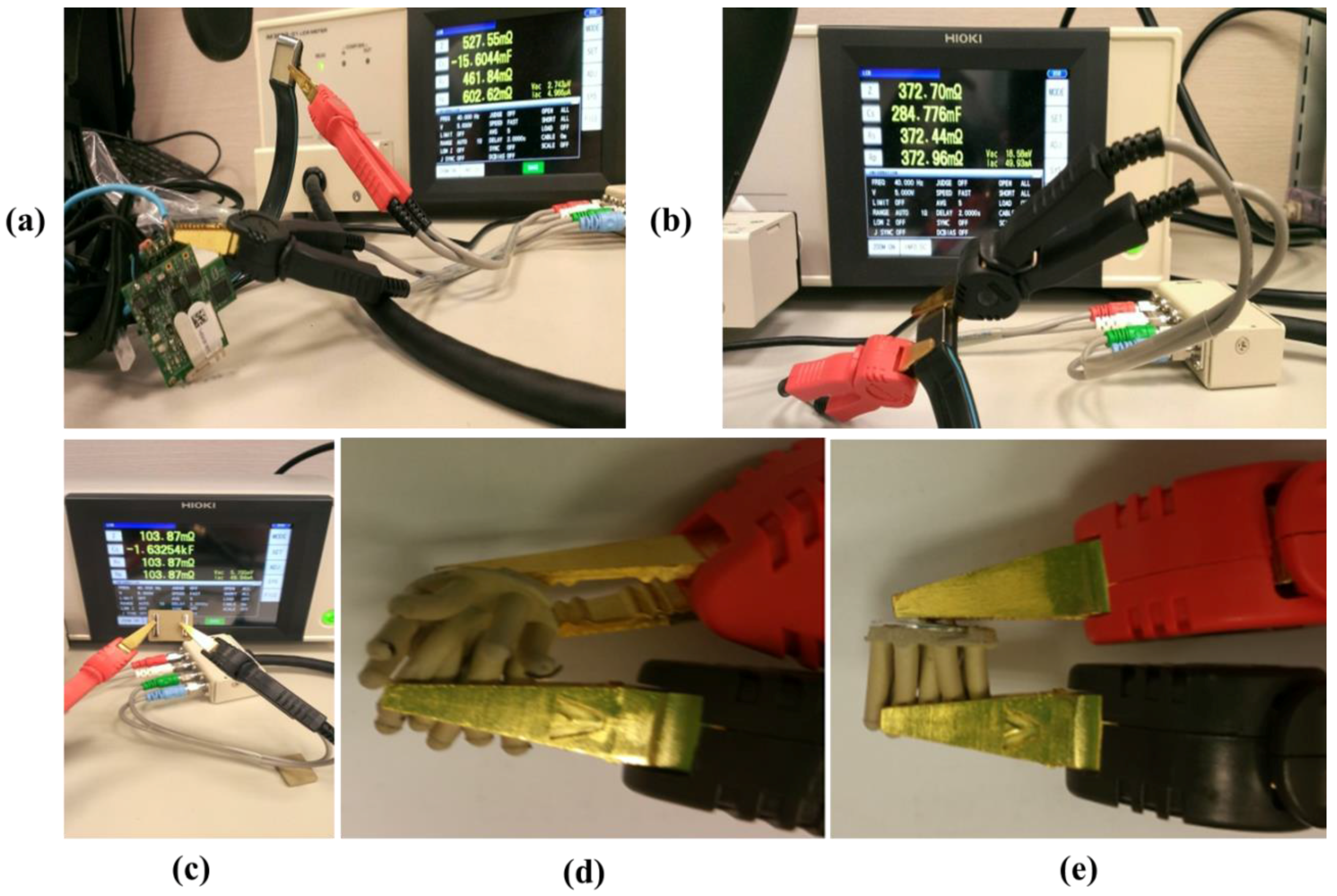
2.6. Impedance Tests of Dry Electrode and PEs
2.7. Assessment of EEG Data Quality
3. Results and Discussion
3.1. Result of Materials Basic Study
3.2. Result of Environment Tests for PEs
3.3. Result of Impedance Tests for Neruosky Dry Flat Electrorde and the Proposed Electrode
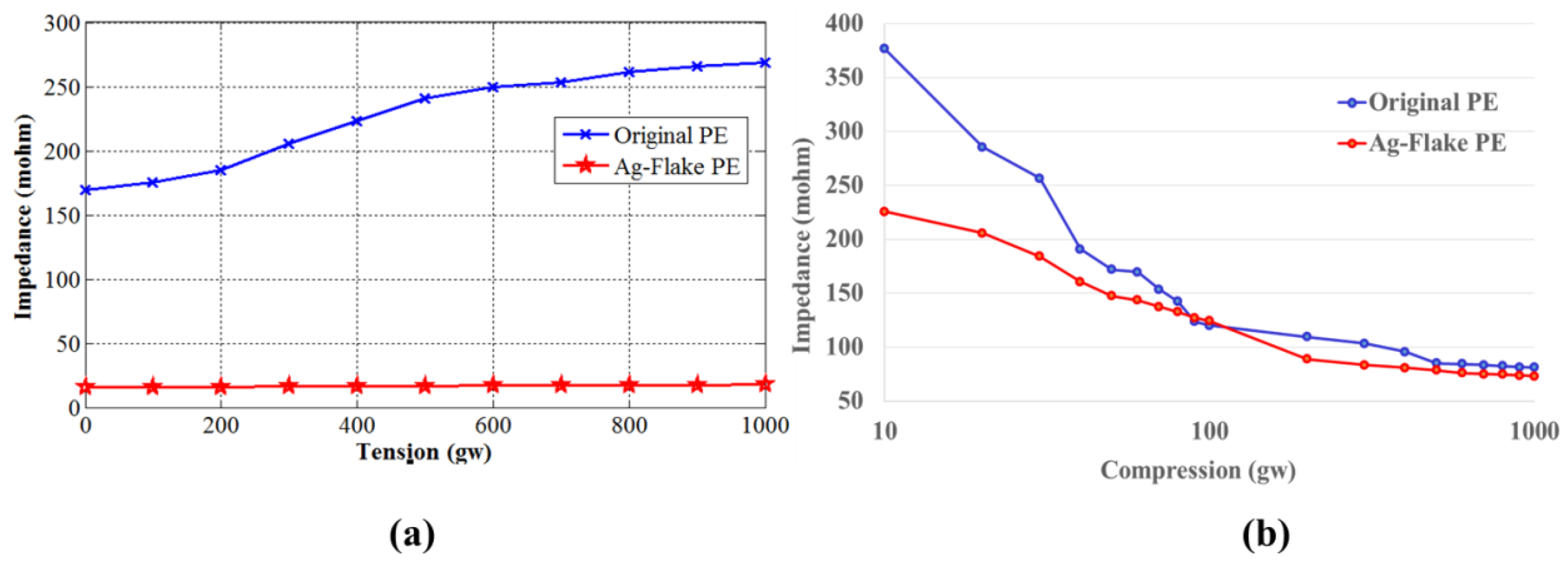
3.4. Result of Impedance Tests under Different Tensile Stresses and Compressions
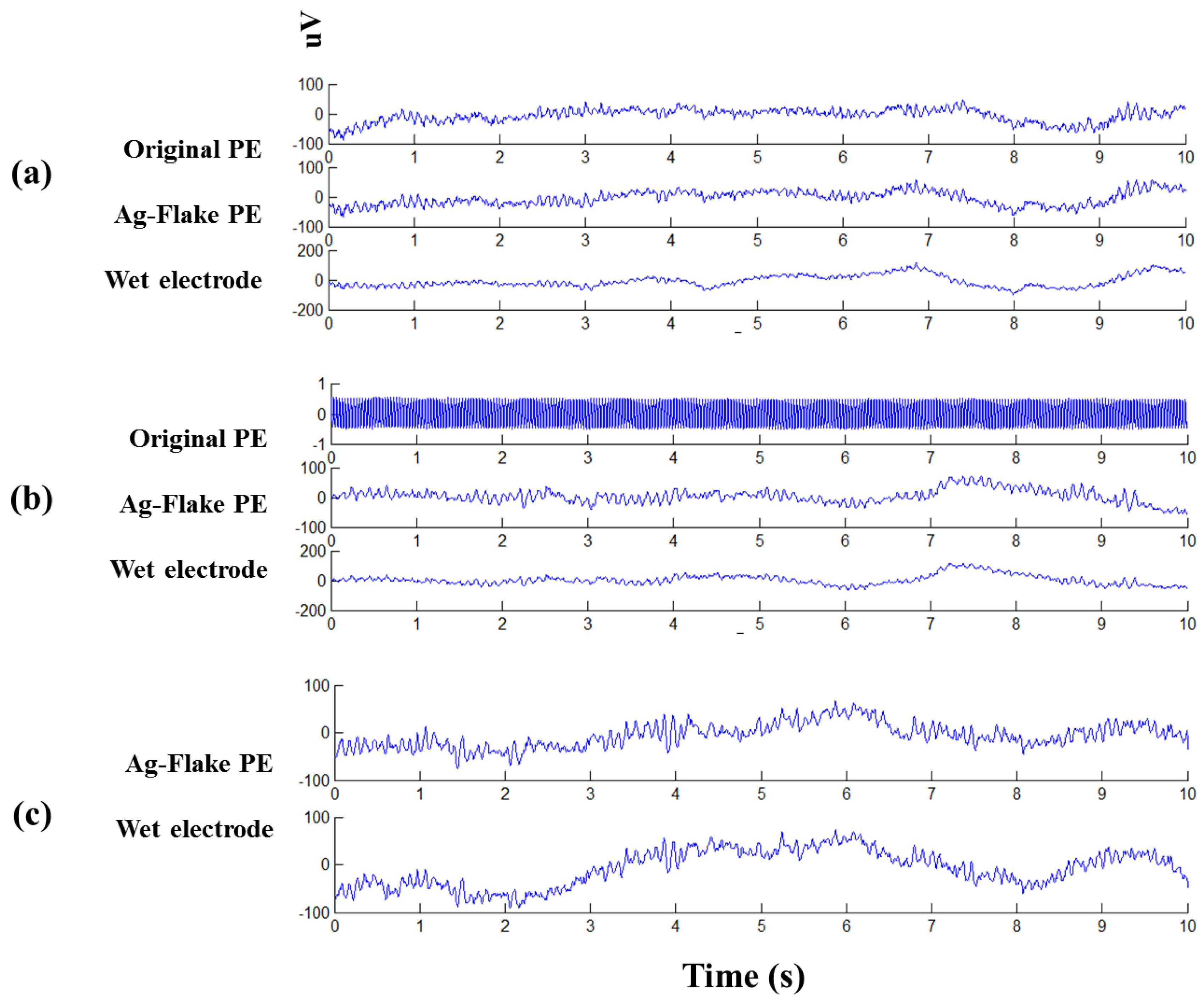
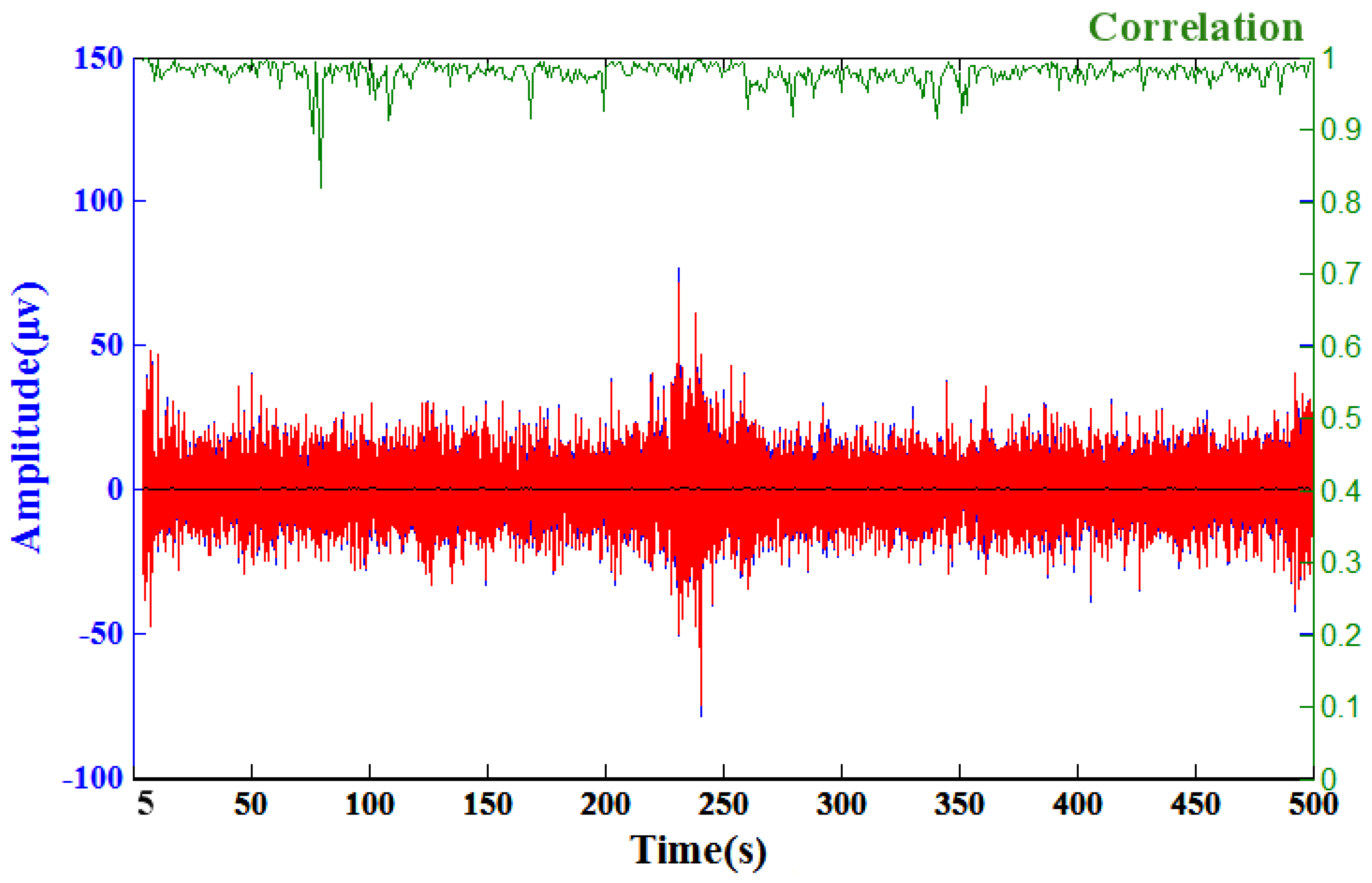
3.5. Physiological Potentials and EEG Signal Tests
3.6. Modified EEG Sensors Prototype Signal Tests
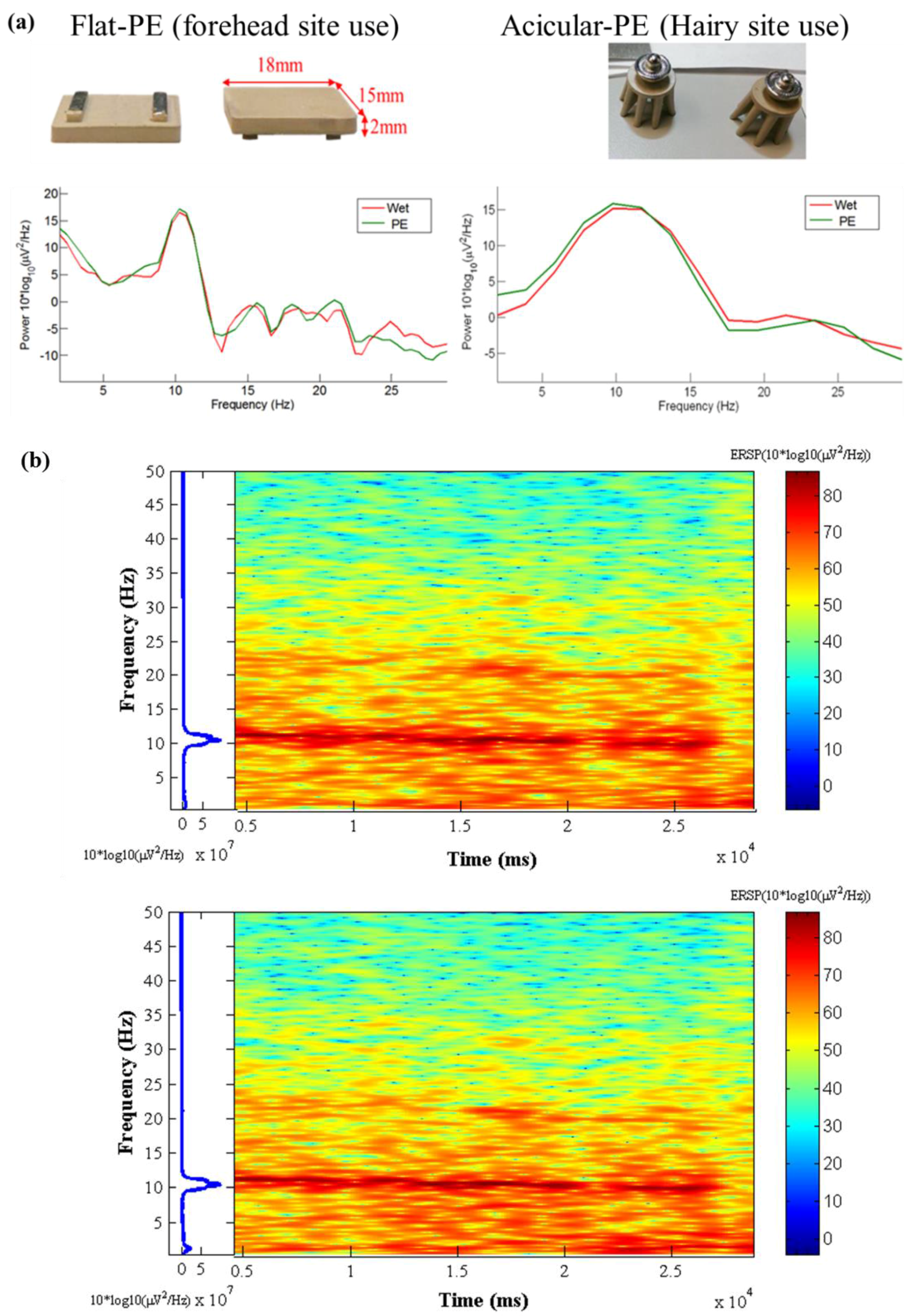
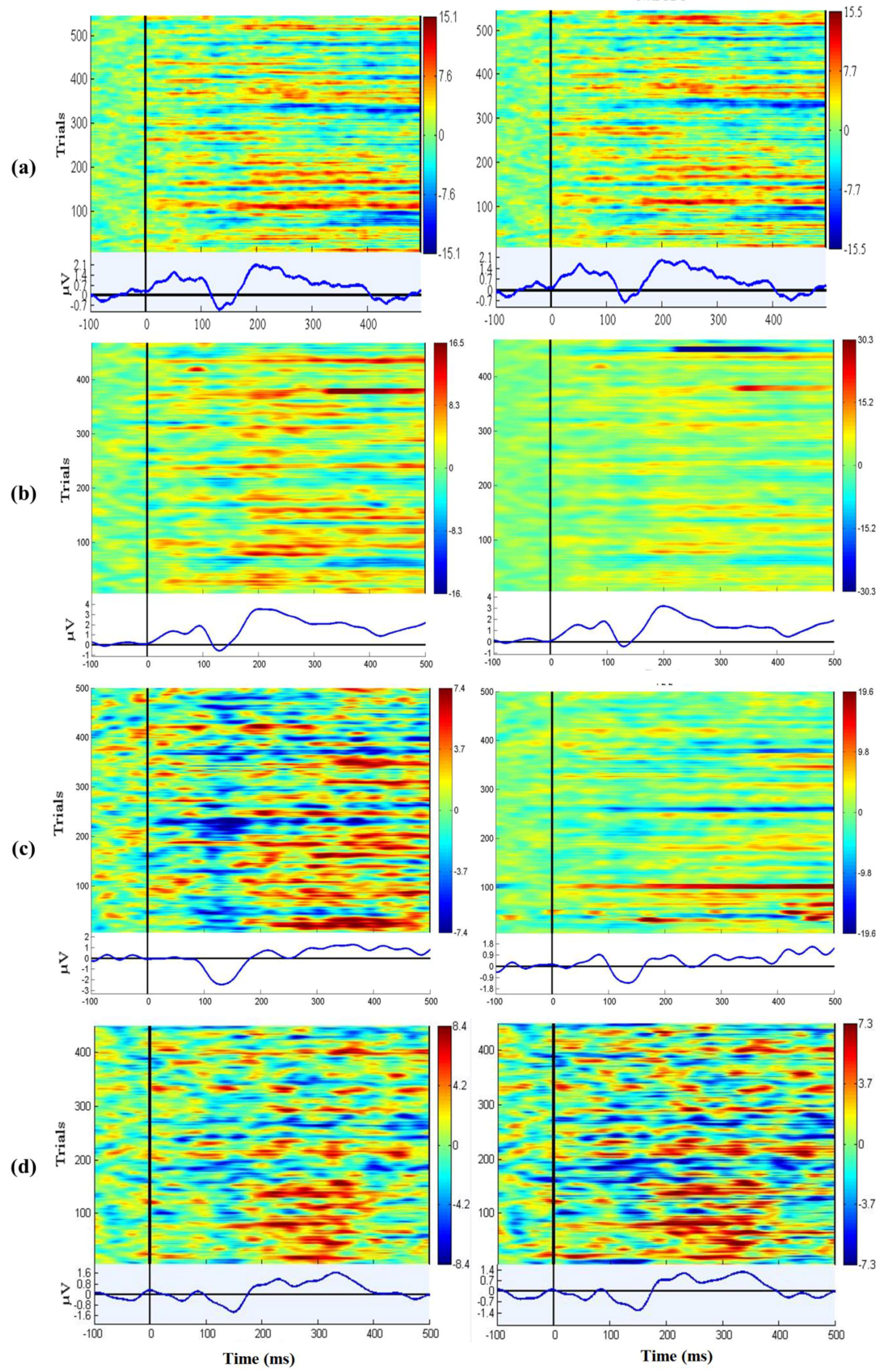
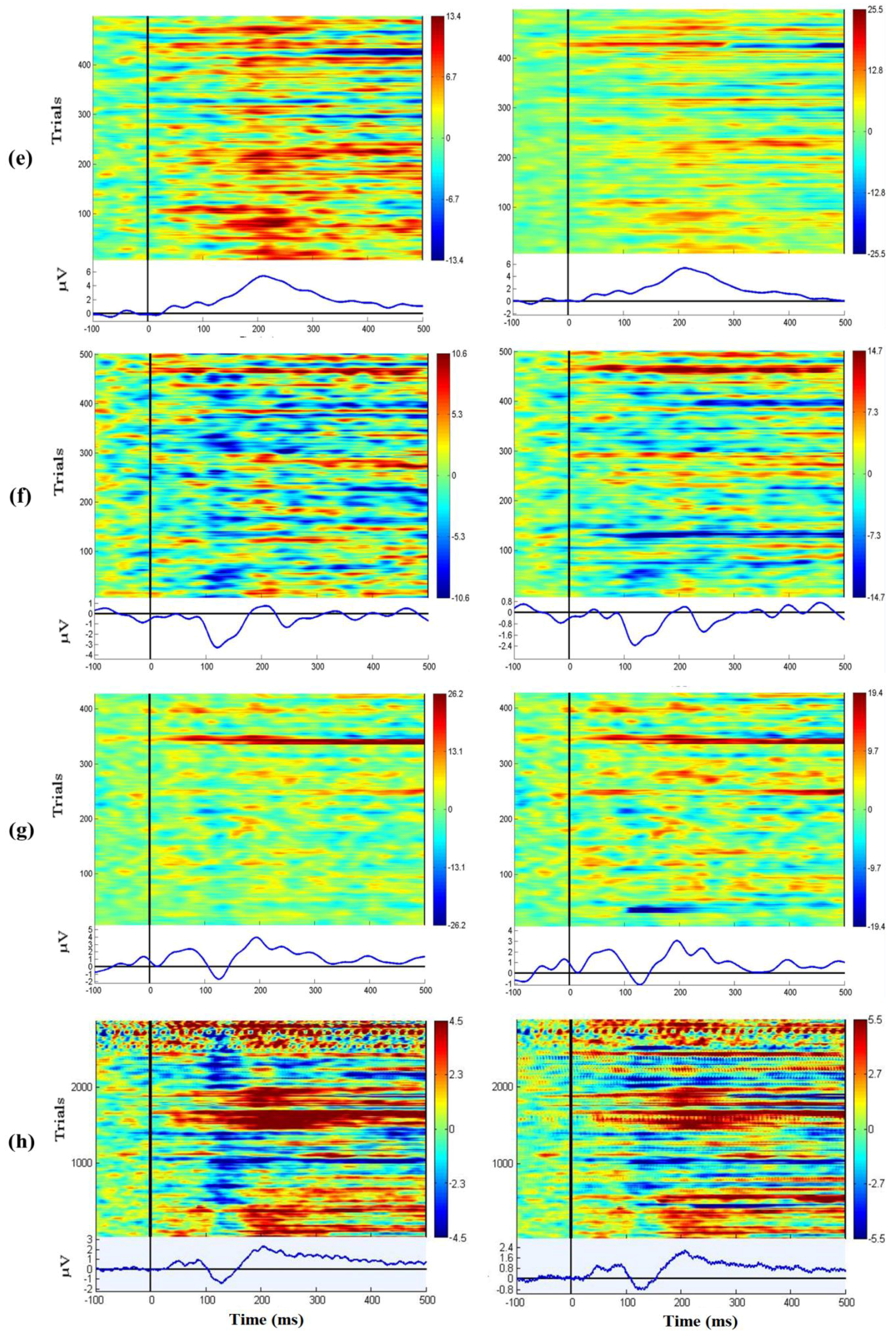
3.7. EEG Oddball Experiment for Quality Verification
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Langenhove, L.V. Smart Textiles for Medicine and Healthcare: Materials, Systems and Applications; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Paul, L.; Nunez, R.S. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Moser, E.; Meyerspeer, M.; Fischmeister, F.P.S.; Grabner, G.; Bauer, H.; Trattnig, S. Windows on the human body—In vivo high-field magnetic resonance research and applications in medicine and psychology. Sensors 2010, 10, 5724–5757. [Google Scholar] [CrossRef] [PubMed]
- Gevins, A.; Le, J.; Martin, N.K.; Brickett, P.; Desmond, J.; Reutter, B. High-resolution EEG-124-channel recording, spatial deblurring and mri integration methods. Electroen. Clin. Neuro. 1994, 90, 337–358. [Google Scholar] [CrossRef]
- Lin, C.T.; Chen, S.A.; Chiu, T.T.; Lin, H.Z.; Ko, L.W. Spatial and temporal EEG dynamics of dual-task driving performance. J. Neuroeng. Rehabil. 2011, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Lin, C.L.; Ko, L.W.; Wang, Y.K.; Liang, J.W.; Lin, C.T. Automatic sleep stage classification gui with a portable EEG device. In Hci International 2013-Posters’ Extended Abstracts; Stephanidis, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 373, pp. 613–617. [Google Scholar]
- Lin, C.T.; Liao, L.D.; Liu, Y.H.; Wang, I.J.; Lin, B.S.; Chang, J.Y. Novel dry polymer foam electrodes for long-term EEG measurement. IEEE Trans. Biomed. Eng. 2011, 58, 1200–1207. [Google Scholar] [PubMed]
- Ferree, T.C.; Luu, P.; Russell, G.S.; Tucker, D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef]
- Gargiulo, G.; Calvo, R.A.; Bifulco, P.; Cesarelli, M.; Jin, C.; Mohamed, A.; van Schaik, A. A new EEG recording system for passive dry electrodes. Clin. Neurophysiol. 2010, 121, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R. The electrode-skin interface and optimal detection of bioelectric signals. Physiol. Meas. 2010, 31. [Google Scholar] [CrossRef]
- David Hairston, W.; Whitaker, K.W.; Ries, A.J.; Vettel, J.M.; Cortney Bradford, J.; Kerick, S.E.; McDowell, K. Usability of four commercially-oriented EEG systems. J. Neural Eng. 2014, 11, 046018. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.D.; Wang, I.J.; Chen, S.F.; Chang, J.Y.; Lin, C.T. Design, fabrication and experimental validation of a novel dry-contact sensor for measuring electroencephalography signals without skin preparation. Sensors 2011, 11, 5819–5834. [Google Scholar] [CrossRef] [PubMed]
- Grozea, C.; Voinescu, C.D.; Fazli, S. Bristle-sensors—Low-cost flexible passive dry EEG electrodes for neurofeedback and BCI applications. J. Neural Eng. 2011, 8, 025008. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Lu, S.W.; Liao, L.D.; Lin, C.T. Design, fabrication, and experimental validation of novel flexible silicon-based dry sensors for electroencephalography signal measurements. IEEE J. Transl. Eng. Health Med. 2014, 2, 1–7. [Google Scholar]
- Yuan, T.; Chen, D.; Chen, J.; Chen, X.; Wang, X.; Lu, B. A novel MEMS elastic-based dry electrode for electroencephalography measurement. Microsyst. Technol. 2014, 20, 1125–1129. [Google Scholar] [CrossRef]
- Chiou, J.C.; Ko, L.W.; Lin, C.T.; Hong, C.T.; Jung, T.P.; Liang, S.F.; Jeng, J.L. Using Novel MEMS EEG Sensors in Detecting Drowsiness Application. In Proceedings of the Biomedical Circuits and Systems Conference (BioCAS 2006), London, UK, 29 November–1 December 2006; pp. 33–36.
- Mcdowell, K.; Lin, C.T.; Oie, K.S.; Jung, T.P.; Gordon, S.; Whitaker, K.W.; Li, S.Y.; Lu, S.W.; Hairston, W.D. Real-world neuroimaging technologies. IEEE Access. 2013, 1, 131–149. [Google Scholar] [CrossRef]
- Lance, B.J.; Kerick, S.E.; Ries, A.J.; Oie, K.S.; McDowell, K. Brain-computer interface technologies in the coming decades. Proc. IEEE 2012, 100, 1585–1599. [Google Scholar] [CrossRef]
- Brett-Green, B.A.; Miller, L.J.; Gavin, W.J.; Davies, P.L. Multisensory integration in children: A preliminary ERP study. Brain Res. 2008, 1242, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, F.; Silveira, C.; Almeida, P.R.; Palha, A.; Barbosa, F.; Marques-Teixeira, J. The auditory p200 is both increased and reduced in schizophrenia? A meta-analytic dissociation of the effect for standard and target stimuli in the oddball task. Clin. Neurophysiol. 2012, 123, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Macy, A.J. The Handbook of Human Physiological Recording. Available online: http://www.alanmacy.com/HPR-Chapter10.html (accessed on 29 October 2016).
- American Clinical Neurophysiology Society. Guideline 3: Minimum technical standards for EEG recording in suspected cerebral death. Clin. Neurophysiol. 2006, 23, 97. [Google Scholar]
- Gargiulo, G.; Bifulco, P.; Cesarelli, M.; Fratini, A.; Romano, M. Problems in assessment of novel biopotential front-end with dry electrode: A brief review. Machines 2014, 2, 87. [Google Scholar] [CrossRef]
- Mueller-Putz, G.; Scherer, R.; Brunner, C.; Leeb, R.; Pfurtscheller, G. Better than random: A closer look on BCI results. Int. J. Bioelectromag. 2008, 10, 52–55. [Google Scholar]
- Huang, T.H.; Huang, H.P.; Liu, Y.H.; Kang, Z.H.; Kuan, J.Y. Development of a brain-controlled rehabilitation system (BCRS). J. Neurosci. Neuroeng. 2013, 2, 79–89. [Google Scholar] [CrossRef]
- Liao, L.D.; Chen, C.Y.; Wang, I.J.; Chen, S.F.; Li, S.Y.; Chen, B.W.; Chang, J.Y.; Lin, C.T. Gaming control using a wearable and wireless EEG-based brain-computer interface device with novel dry foam-based sensors. J. Neuroeng. Rehabil. 2012, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zander, T.; Lehne, M.; Ihme, K.; Jatzev, S.; Correia, J.; Kothe, C.; Picht, B.; Nijboer, F. A dry EEG-system for scientific research and brain–computer interfaces. Front. Neurosci. 2011, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Makeig, S.; Onton, J. ERP Features and EEG Dynamics: An ICA Perspective; Oxford University Press: Oxford, UK, 2009; Volume 51. [Google Scholar]
- Delorme, A.; Makeig, S. Eeglab: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]

| Ag/SiO2 | Ag-Flake | |||||
|---|---|---|---|---|---|---|
| (~2.75 g/mL) | (10.5 g/mL) | |||||
| Volume Percentage | Volume Percentage | |||||
| Sample No. | Silicone | Ag/SiO2 | Sample No. | Silicone | Ag-Flake | |
| More | S5 | 40% | 60% | F5 | 55% | 45% |
| ↑ | S4 | 44% | 56% | F4 | 65% | 35% |
| Additives | (origin) | |||||
| ↓ | S3 | 50% | 50% | F3 | 75% | 25% |
| Less | S2 | 55% | 45% | F2 | 85% | 15% |
| S1 | 60% | 40% | F1 | 95% | 5% | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-H.; Chen, S.-H.; Chang, C.-L.; Lin, C.-T.; Hairston, W.D.; Mrozek, R.A. New Flexible Silicone-Based EEG Dry Sensor Material Compositions Exhibiting Improvements in Lifespan, Conductivity, and Reliability. Sensors 2016, 16, 1826. https://doi.org/10.3390/s16111826
Yu Y-H, Chen S-H, Chang C-L, Lin C-T, Hairston WD, Mrozek RA. New Flexible Silicone-Based EEG Dry Sensor Material Compositions Exhibiting Improvements in Lifespan, Conductivity, and Reliability. Sensors. 2016; 16(11):1826. https://doi.org/10.3390/s16111826
Chicago/Turabian StyleYu, Yi-Hsin, Shih-Hsun Chen, Che-Lun Chang, Chin-Teng Lin, W. David Hairston, and Randy A. Mrozek. 2016. "New Flexible Silicone-Based EEG Dry Sensor Material Compositions Exhibiting Improvements in Lifespan, Conductivity, and Reliability" Sensors 16, no. 11: 1826. https://doi.org/10.3390/s16111826
APA StyleYu, Y.-H., Chen, S.-H., Chang, C.-L., Lin, C.-T., Hairston, W. D., & Mrozek, R. A. (2016). New Flexible Silicone-Based EEG Dry Sensor Material Compositions Exhibiting Improvements in Lifespan, Conductivity, and Reliability. Sensors, 16(11), 1826. https://doi.org/10.3390/s16111826






