Towards Enhanced Gas Sensor Performance with Fluoropolymer Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fluoropolymers
2.2. MOX Sensor
- n: number of sensors
- xk': signal of sensor k
- xmax': signal maximum of all sensors
2.3. Catalytic Bead Sensor (CB)
2.4. Thermal Conductivity Sensor (TC)
2.5. Experimental Setup
3. Results and Discussion
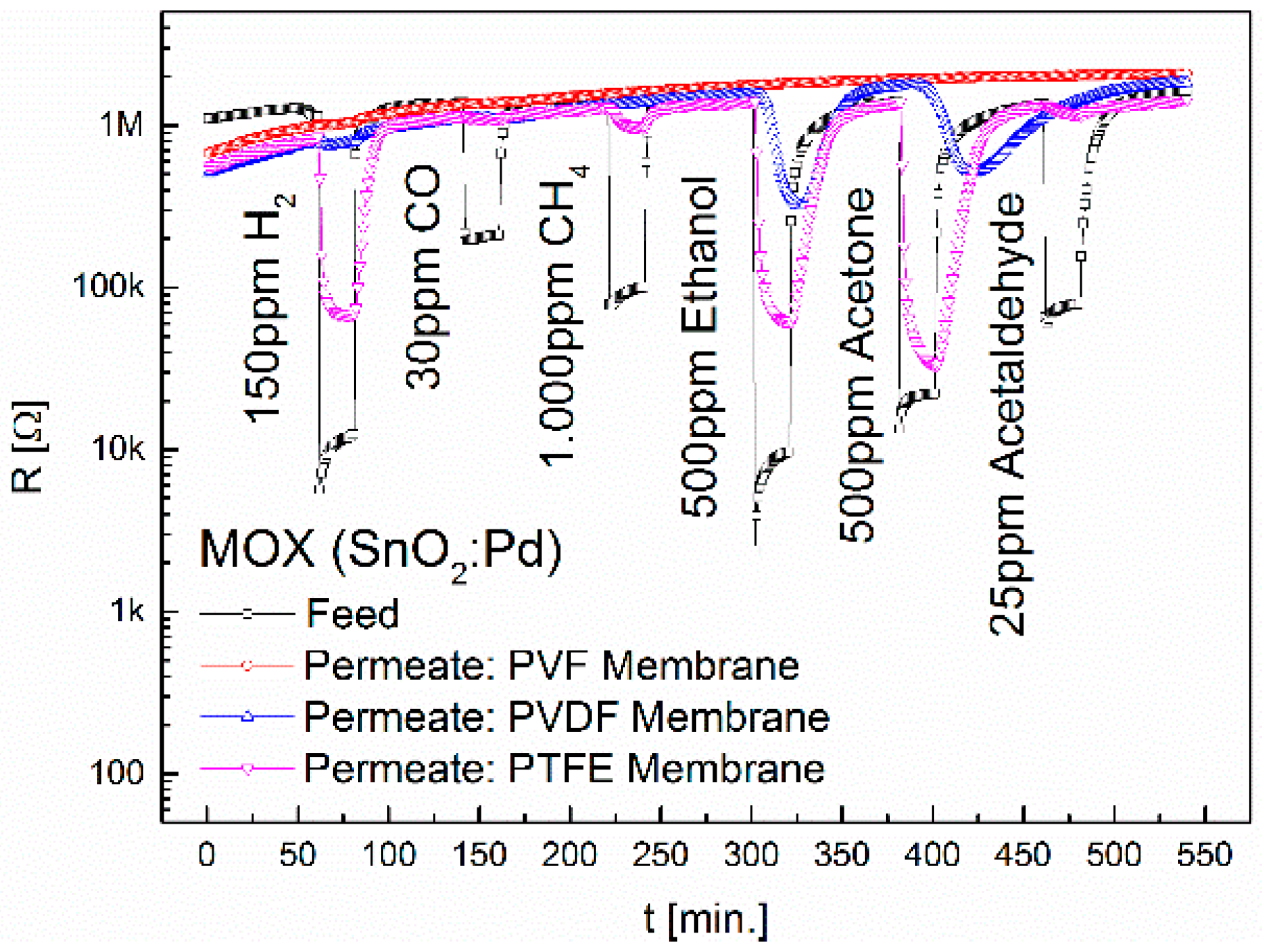
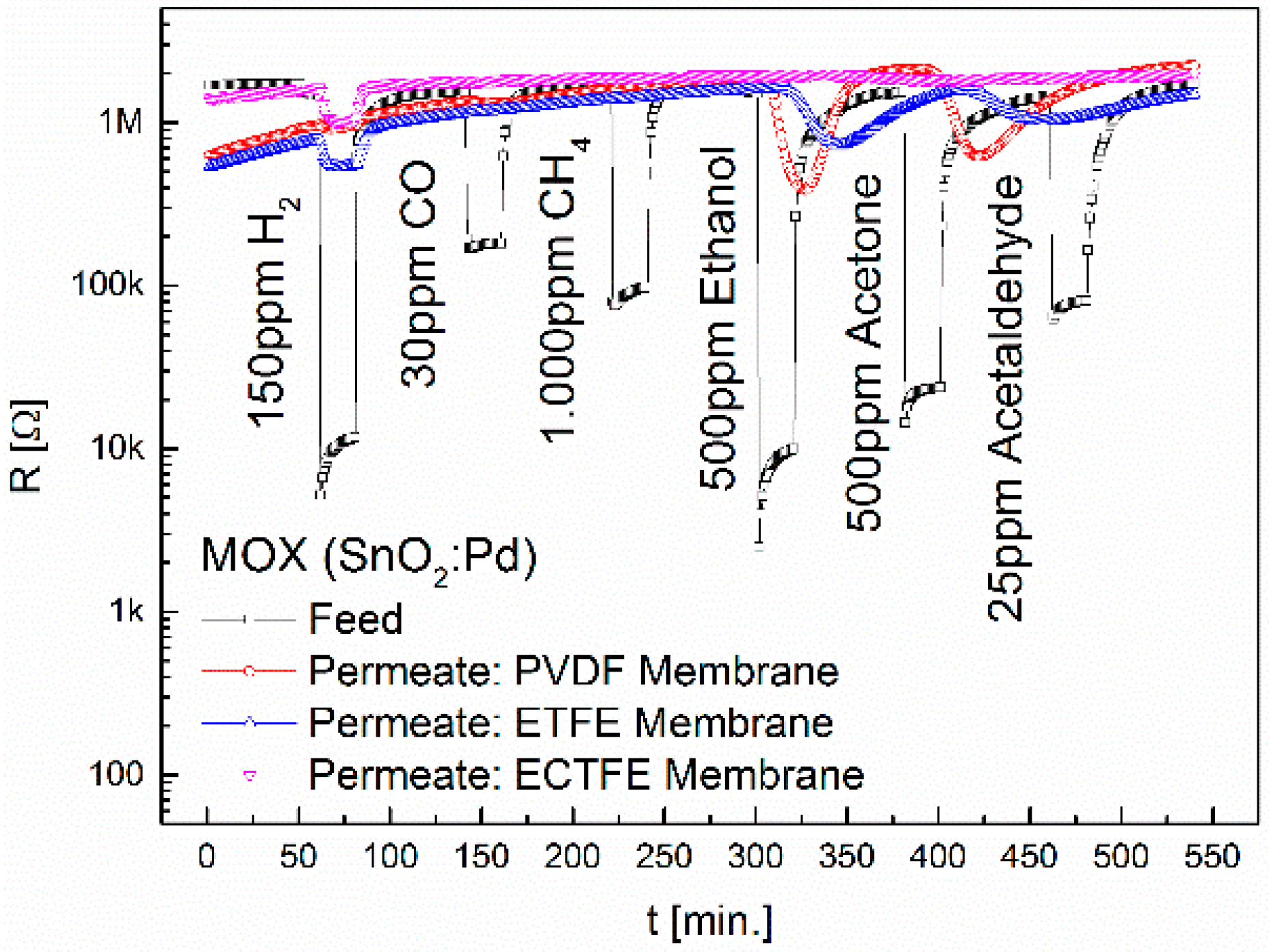
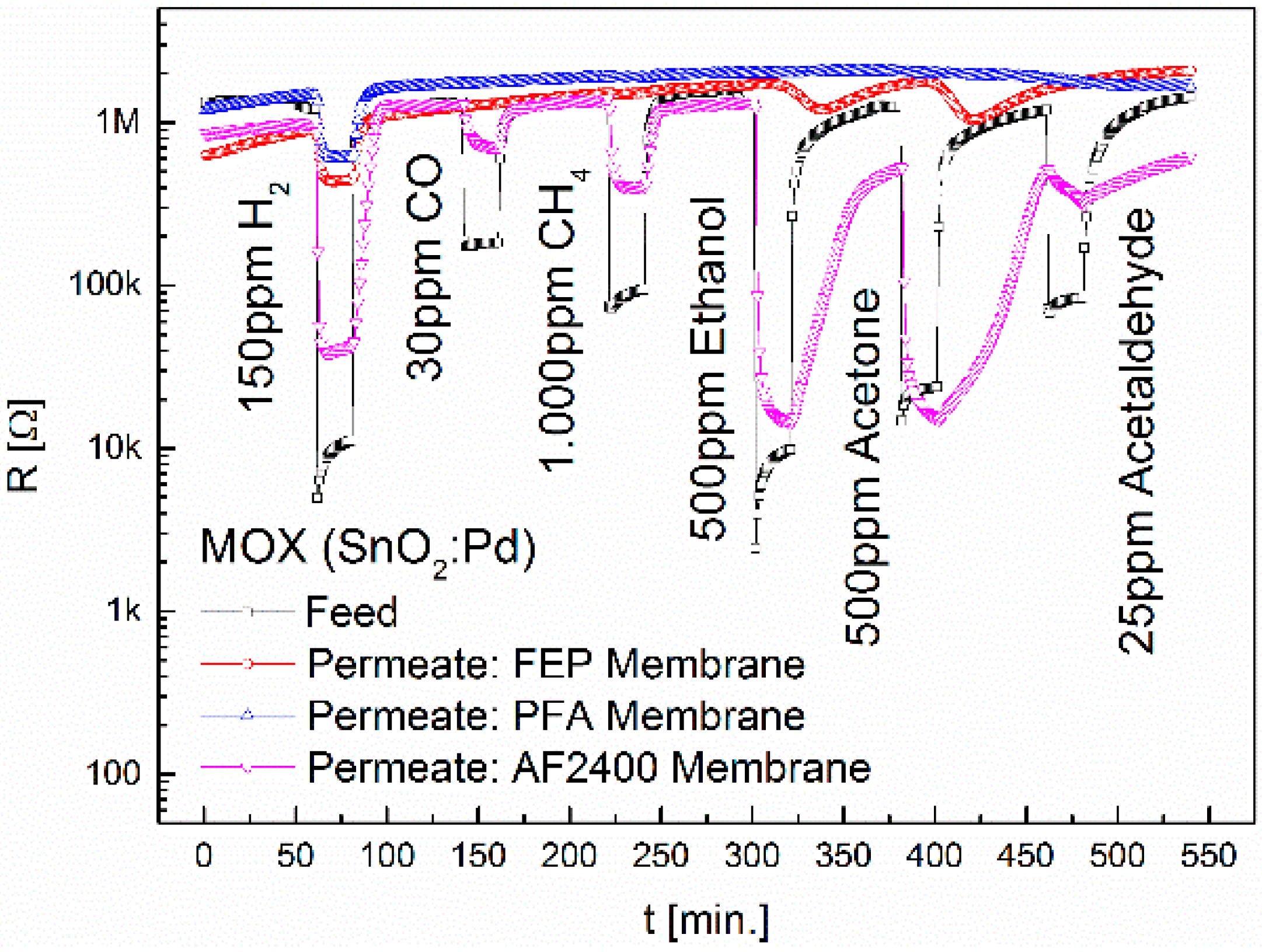
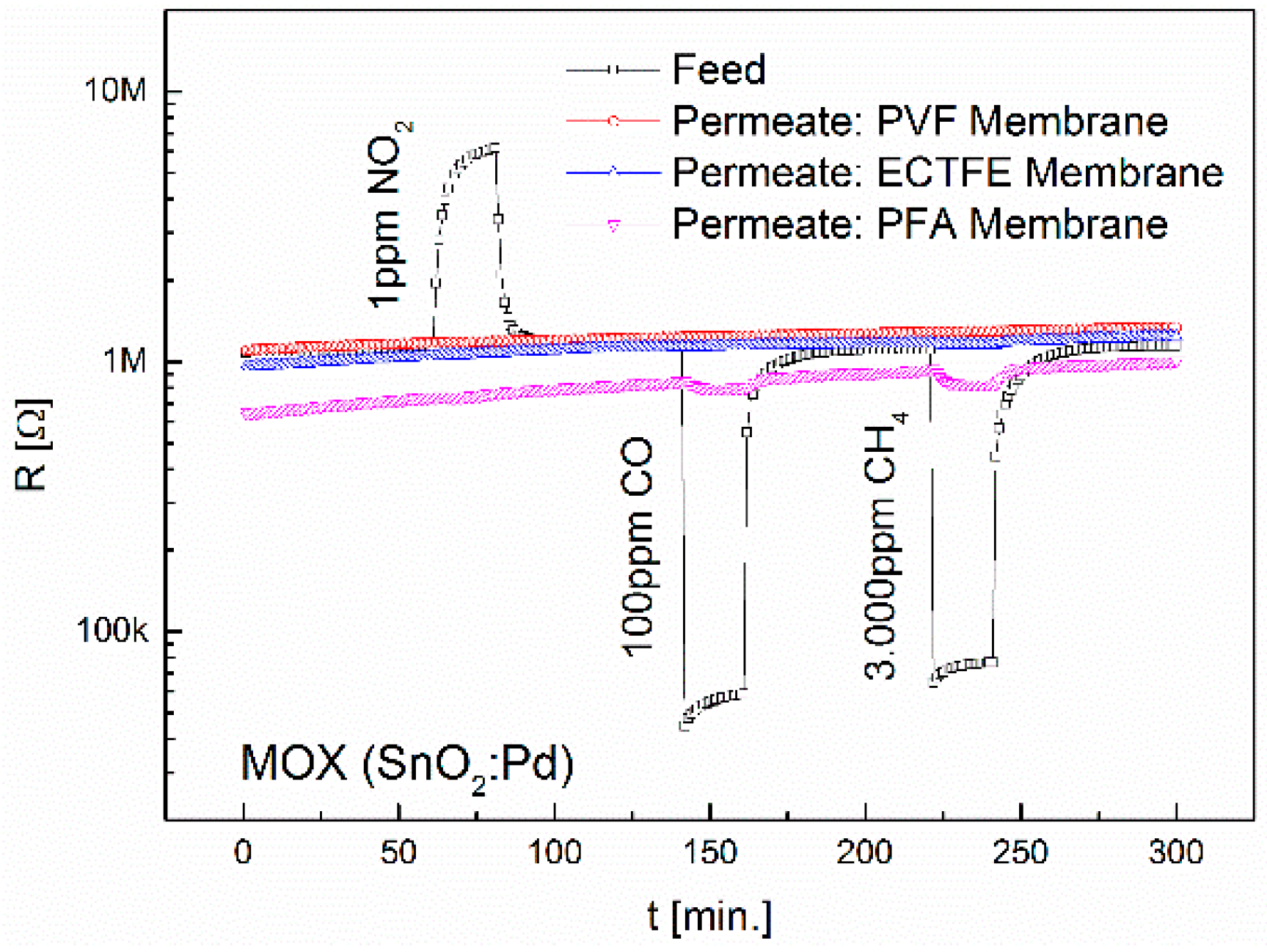
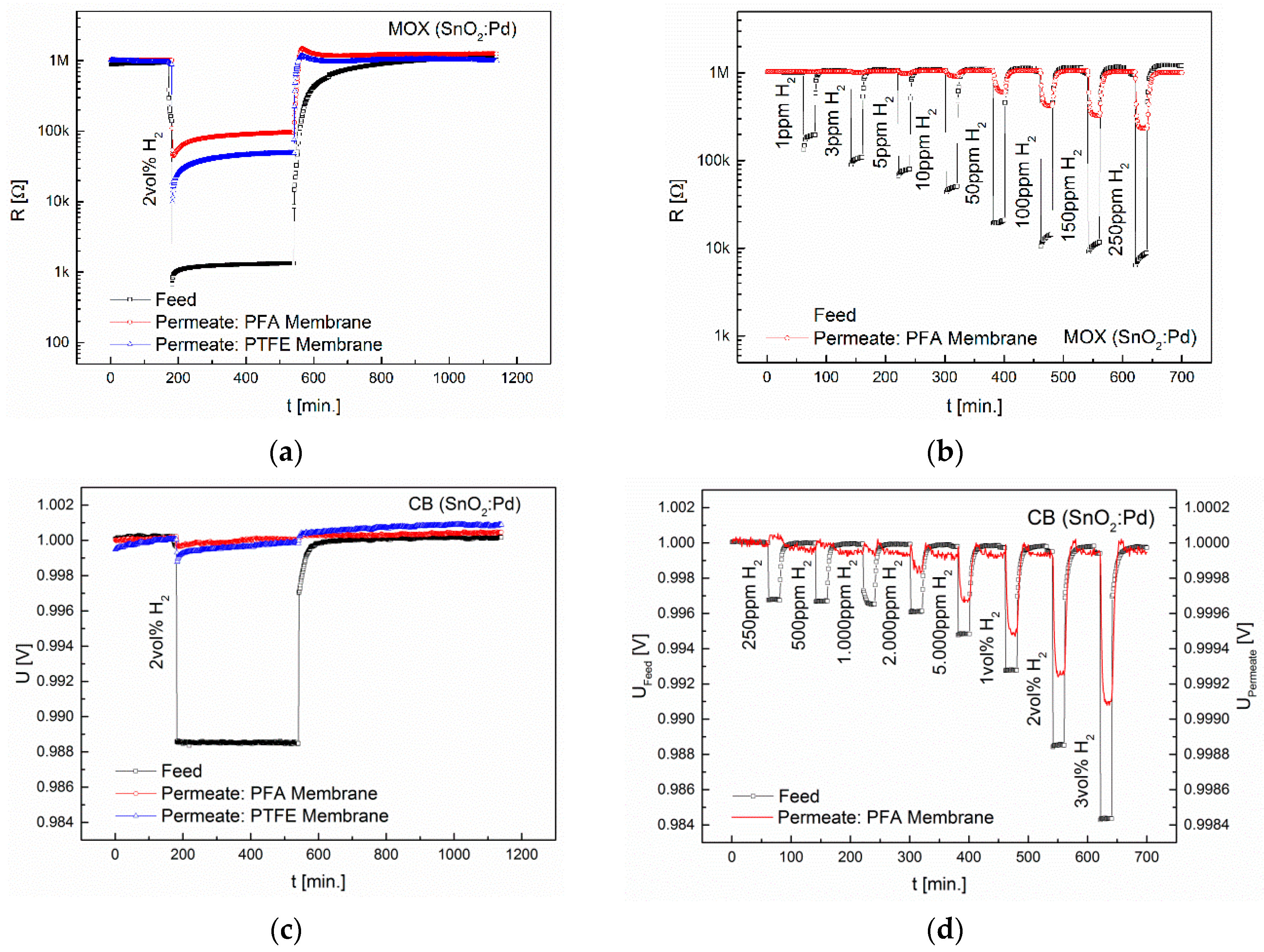
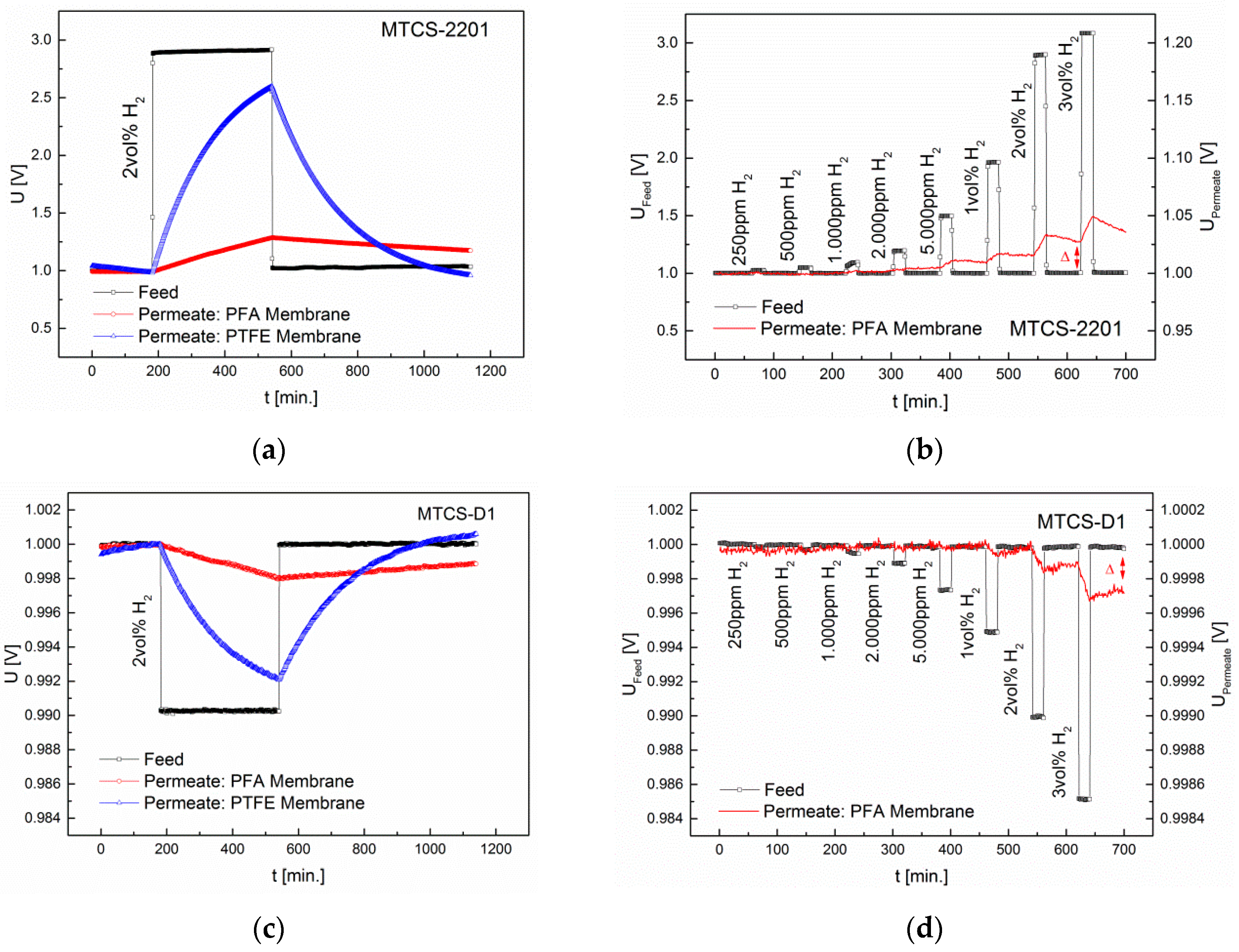
3.1. Separation Characteristics of Fluoropolymers
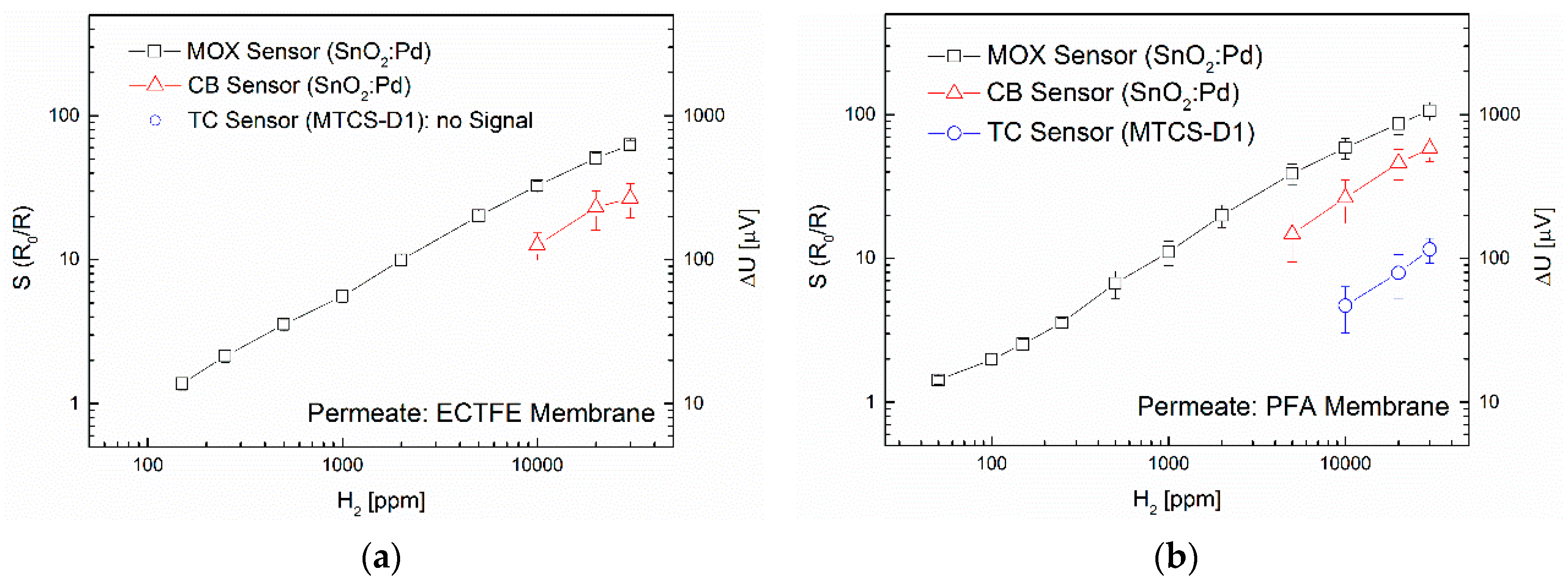
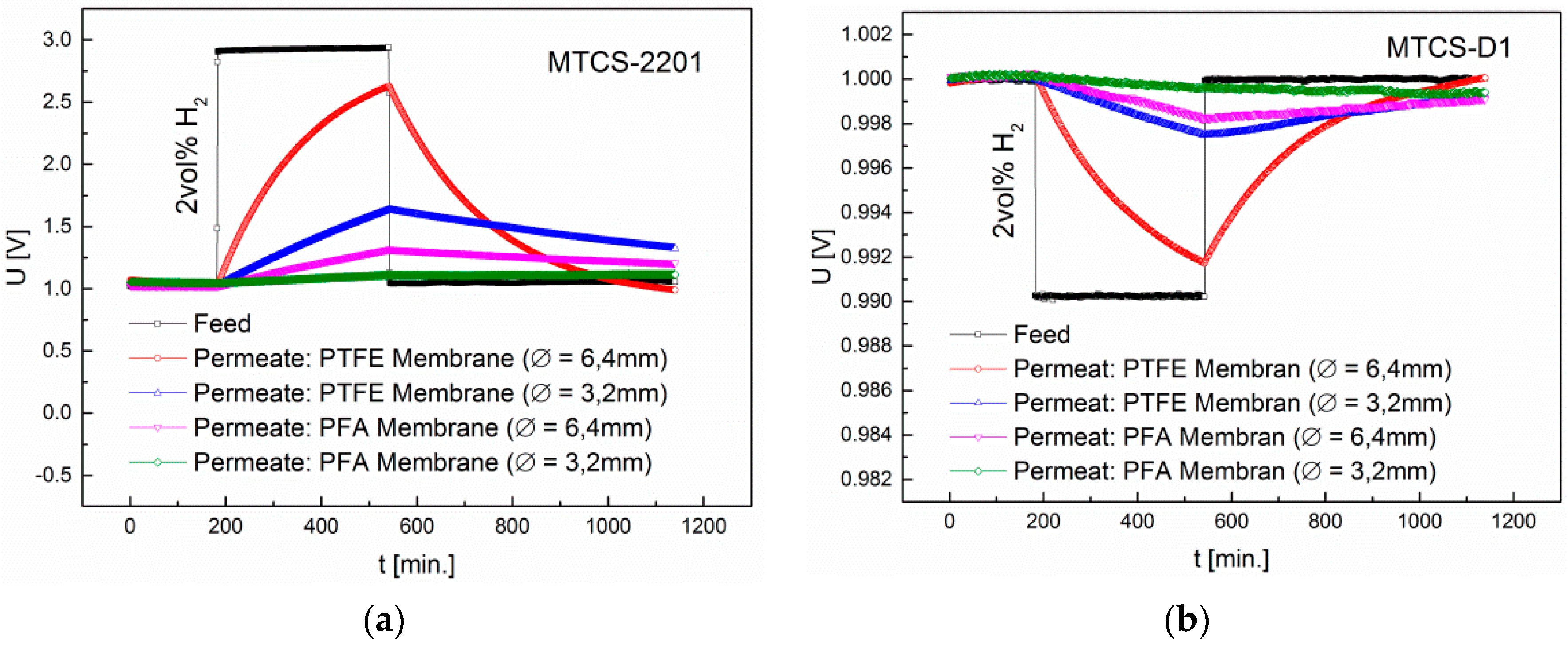
3.2. Interdependence of Diffusion and Sensor Principle
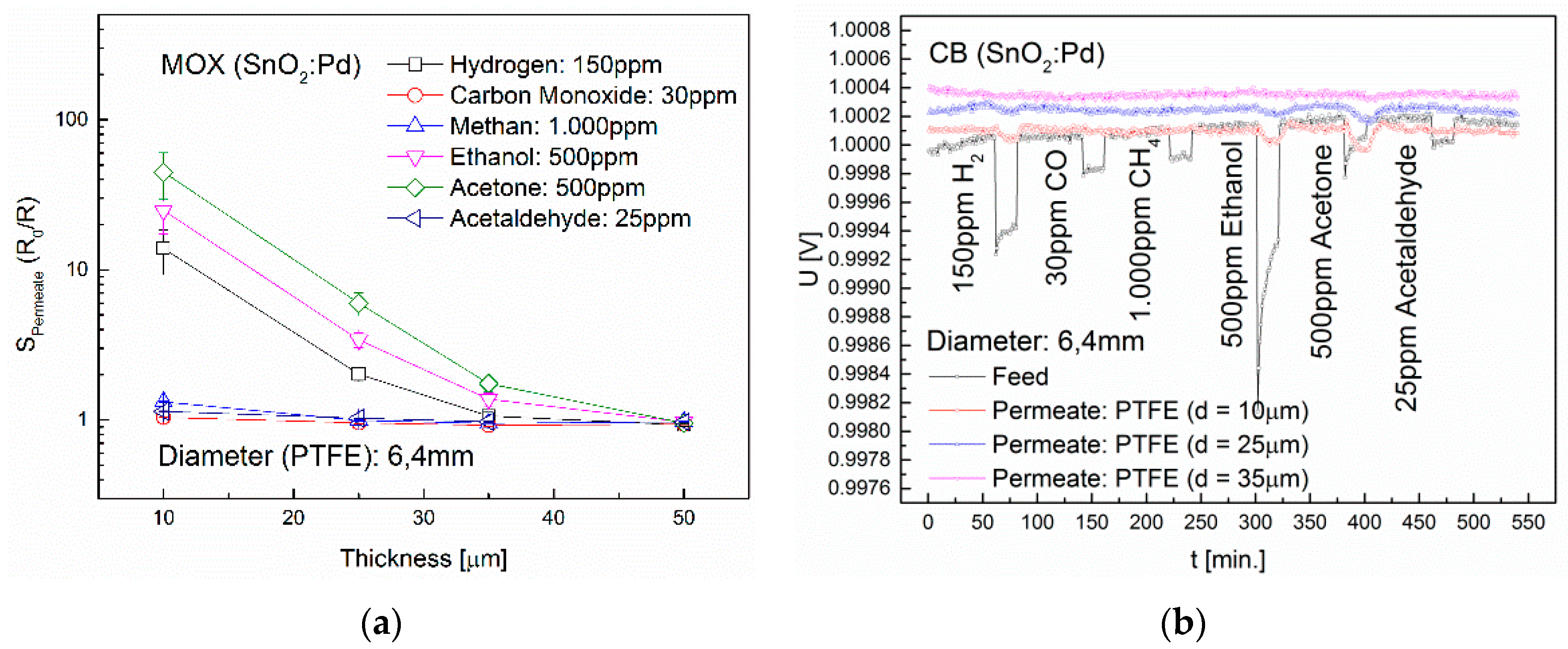
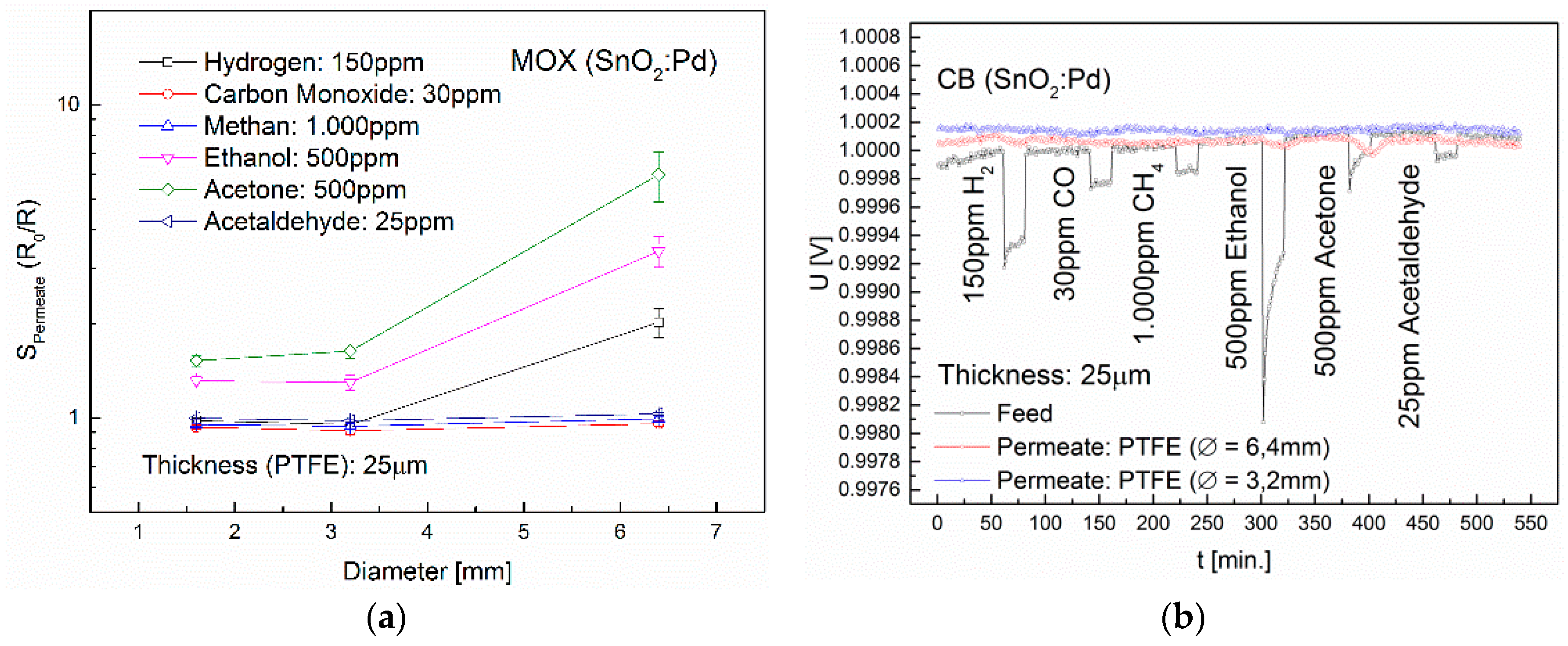
3.3. Influence of Membrane Thickness and Diameter
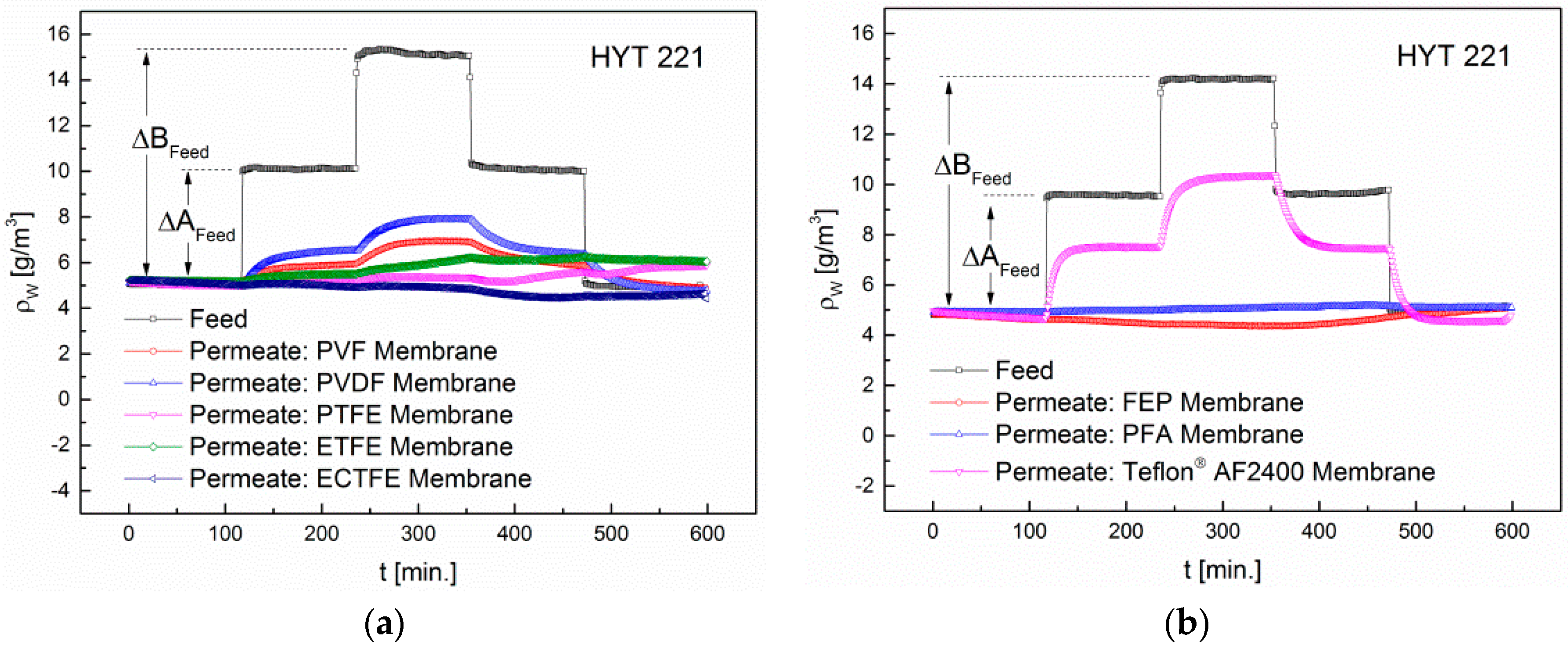
3.4. Influence of Humidity on the Retention
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ECTFE | ethylene-chlorotrifluoroethylene copolymer |
| ETFE | ethylene-tetrafluoroethylene copolymer |
| FEP | tetrafluoroethylene-hexafluoropropylene copolymer |
| FET | field effect transistor |
| LOD | limit of detection |
| MEMS | microelectromechanical system |
| MOX | metal oxide |
| PFA | tetrafluoroethylene-perfluoropropylvinyl ether copolymer, perfluoroalkoxy |
| PDD | tetrafluoroethylene-perfluorodimethyldioxol |
| PTFE | polytetrafluoroethylene |
| PVDF | polyvinylidene fluoride |
| PVF | polyvinyl fluoride |
| SCCM | Standard Cubic Centimeters per Minute |
| STEL | short-term exposure limit |
| TWA | Time-weighted average or permissible exposure limit |
| TC | thermal conductivity |
References
- Meyers, R.A. Encyclopedia of Analytical Chemistry: Applications, Theory, and Instrumentation; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Fleischer, M.; Lehmann, M. Solid State Gas Sensors—Industrial Application; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Jessel, W. Gase-Dämpfe-Gasmesstechnik: Ein Kompendium für die Praxis; Dräger Safety AG & Company KGaA: Lübeck, Germany, 2001. [Google Scholar]
- Quack, M.; Merkt, F. Handbook of High-Resolution Spectroscopy; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Gründler, P. Chemische Sensoren: Eine Einführung für Naturwissenschaftler und Ingenieure; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Hauptmann, P. Sensoren: Prinzipien und Anwendungen; Hanser: Munich/Berlin, Germany, 1991. [Google Scholar]
- Hering, E.; Schönfelder, G. Sensoren in Wissenschaft und Technik: Funktionsweise und Einsatzgebiete; Vieweg+Teubner Verlag: Wiesbaden, Germany, 2012. [Google Scholar]
- Janata, J.; Josowicz, M.; Vanýsek, P.; de Vaney, D.M. Chemical sensors. Anal. Chem. 1998, 70, 179–208. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Hoek, E.M.V.; Tarabara, V.V. Encyclopedia of Membrane Science and Technology; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Drioli, E.; Giorno, L. Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Ohlrogge, K.; Ebert, K. Membranen: Grundlagen, Verfahren und Industrielle Anwendungen; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Yampolskii, Y.; Freeman, B. Membrane Gas Separation; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Strathmann, H. Introduction to Membrane Science and Technology; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Freeman, B.; Yampolskii, Y.; Pinnau, I. Materials Science of Membranes for Gas and Vapor Separation; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Graunke, T.; Schmitt, K.; Wöllenstein, J. Organic membranes for selectivity enhancement of metal oxide gas sensors. J. Sens. 2016, 2016, 1–22. [Google Scholar] [CrossRef]
- Melin, T.; Rautenbach, R. Membranverfahren: Grundlagen der Modul- und Anlagenauslegung; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Menges, G.; Haberstroh, E.; Michaeli, W.; Schmachtenberg, E. Menges Werkstoffkunde Kunststoffe; Hanser: Munich, Germany, 2011. [Google Scholar]
- Koltzenburg, S.; Maskos, M.; Nuyken, O.; Mülhaupt, R. Polymere: Synthese, Eigenschaften und Anwendungen; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kaiser, W. Kunststoffchemie für Ingenieure: Von der Synthese bis zur Anwendung; Hanser: München/Berlin, Germany, 2011. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Materialwissenschaften und Werkstofftechnik: Eine Einführung; VCH (Imprint): Weinheim, Germany, 2012. [Google Scholar]
- Sugimoto, I.; Nakamura, M.; Kuwano, H. Organic gas sorption by chemical-sensing fluoropolymer films prepared by radio-frequency sputtering. Thin Solid Films 1994, 249, 118–125. [Google Scholar] [CrossRef]
- Samuel, J.D.J.S.; Ruther, P.; Frerichs, H.-P.; Lehmann, M.; Paul, O.; Rühe, J. A simple route towards the reduction of surface conductivity in gas sensor devices. Sens. Actuators B Chem. 2005, 110, 218–224. [Google Scholar] [CrossRef]
- Jansen, J.C.; Clariziaa, G.; Bernardoa, P.; Bazzarellia, F.; Friessb, K.; Randováa, A.; Schauerc, J.; Kubickad, D.; Kacirkováe, M.; Izake, P. Gas transport properties and pervaporation performance of fluoropolymer gel membranes based on pure and mixed ionic liquids. Sep. Purif. Technol. 2013, 109, 87–97. [Google Scholar] [CrossRef]
- Kiplinger, C.L.; Persico, D.F.; Lagow, R.J.; Paul, D.R. Gas transport in partially fluorinated low-density polyethylene. J. Appl. Polym. Sci. 1986, 31, 2617–2626. [Google Scholar] [CrossRef]
- Le Roux, J.D.; Teplyakov, V.V.; Paul, D.R. Gas transport properties of surface fluorinated poly (vinyltrimethylsilane) films and composite membranes. J. Membr. Sci. 1994, 90, 55–68. [Google Scholar] [CrossRef]
- Merkel, T.C.; Pinnau, I.; Prabhakar, R.; Freeman, B.D. Materials Science of Membranes for Gas and Vapor Separation; Wiley: Hoboken, NJ, USA, 2006; pp. 251–270. [Google Scholar] [CrossRef]
- Goodfellow GmbH: Online Katalog. Available online: http://www.goodfellow.com (accessed on 31 July 2013).
- Reichelt Chemietechnik GmbH & Co.; Thomaplast II Halbzeuge. Available online: http://www.rct-online.de (accessed on 15 October 2012).
- Ebnesajjad, S. Introduction to Fluoropolymers: Materials, Technology and Applications; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Pinnau, I.; Toy, L.G. Gas and vapor transport properties of amorphous perfluorinated copolymer membranes based on 2,2-bistrifluoromethyl-4,5-difluoro-1,3-dioxole/tetrafluoroethylene. J. Membr. Sci. 1996, 109, 125–133. [Google Scholar] [CrossRef]
- Domininghaus, H.; Elsner, P.; Eyerer, P.; Hirth, T. DOMININGHAUS—Kunststoffe: Eigenschaften und Anwendungen; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hellerich, W.; Harsch, G.; Baur, E. Werkstoff-Führer Kunststoffe: Eigenschaften, Prüfungen, Kennwerte; Hanser: Munich/Berlin, Germany, 2010. [Google Scholar]
- Baur, E.; Brinkmann, S.; Osswald, T.A.; Rudolph, N.; Schmachtenberg, E. Saechtling Kunststoff Taschenbuch; Carl Hanser Verlag GmbH & Company KG: München, Germany, 2013. [Google Scholar]
- Weimar, U. Gas Sensing with Tin Oxide: Elementary Steps and Signal Transduction. Postdoctoral Thesis, University of Tübingen, Tübingen, Germany, 2001. [Google Scholar]
- Kappler, J. Characterisation of High-Performance SnO2 Gas Sensors for CO Detection by in Situ Techniques; Shaker Verlag GmbH: Aachen, Germany, 2001. [Google Scholar]
- Göpel, W.; Hesse, J.; Zemel, J.N. Sensors: Chemical and Biochemical Sensors; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- Bonfig, K.W. Temperatursensoren: Prinzipien und Applikationen; Expert-Verlag: Renningen, Germany, 1995. [Google Scholar]
- De Coulon, Y.; Chetalat, O.; Brodard, M.; Onillon, E. Thermal Gas Sensor. U.S. Patent 8,161,795 B2, 24 April 2012. [Google Scholar]
- World Health Organization. Air Quality Guidelines for Europe, 2nd ed.; European Series; WHO Regional Publications: Copenhagen, Denmark, 2000. [Google Scholar]
- Forsch, D.; Arbeitsstoffe, D.F.K.; Zur, P.G.; Arbeitsstoffe, D.F.S.; Zur, P.G. MAK- und BAT-Werte-Liste; Wiley-VCH: Hoboken, NJ, USA, 2013. [Google Scholar]

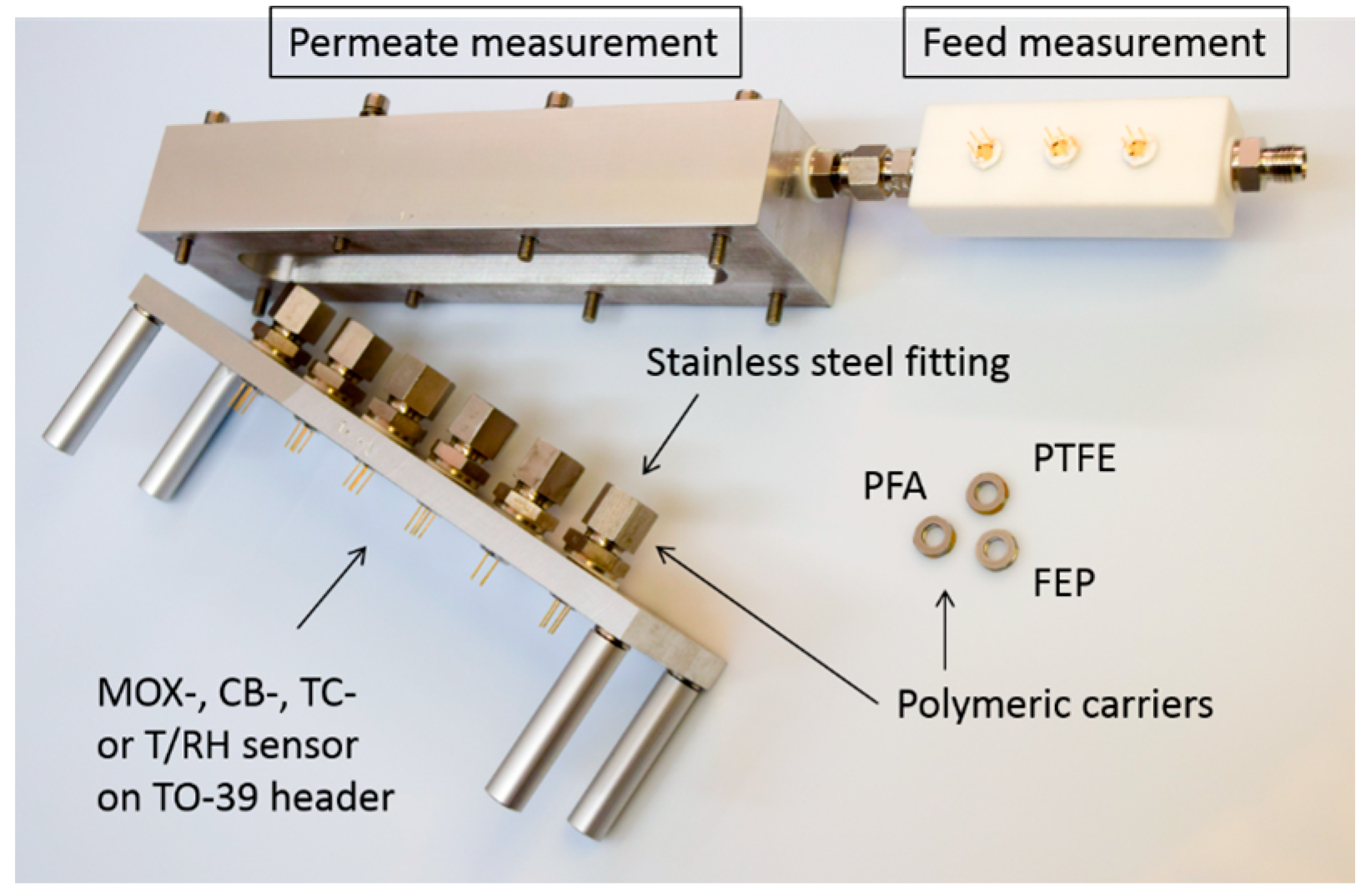
| Polymer | Structure at RT (21 °C) | TG (°C) | TM (°C) | TCO (°C) | WA (24 h, m%) | ρ (g/cm3) | z (μm) |
|---|---|---|---|---|---|---|---|
| PVF | partially crystalline | −80; −20; 50; 150 | 200 | 150–200 | 0.05 | 1.37–1.39 | 13 (GF) |
| (dc 20%–60%) | |||||||
| PVDF | partially crystalline | −70; −38; 50; 100 | 171 | 135–150 | 0.04 | 1.76 | 4.5 (GF) |
| (dc 35%–70%) | |||||||
| PTFE | partially crystalline | −97; 19; 30; 127 | 327 | 260 | 0.1 | 2.2 | 10 (RCT) |
| (dc 55%–90%) | |||||||
| ETFE | partially crystalline | −120; −25; 110 | 270 | 150–160 | ≤0.03 | 1.7 | 25 (GF) |
| (PTFE: 75%) | |||||||
| ECTFE | partially crystalline | −65; 90; 140 | 240 | 130–170 | <0.02 | 1.68 | 12.5 (GF) |
| (dc 50%–60%) | |||||||
| FEP | partially crystalline | 290 | 290 | 250 | <0.01 | 2.12–2.17 | 25 (RCT) |
| (dc 40%–50%) | |||||||
| PFA | partially crystalline | −100; −5; −30; 90 | 305 | 260 | <0.03 | 2.15 | 25 (GF) |
| (dc > 48%) | |||||||
| Teflon® AF2400 | amorphous | 240 | - | 260 | - | 1.74 | 38.1 (BG) |
| (87 mol % PDD) |
| Membrane | Sensor | H2 | CO | CH4 | Ethanol | Acetone | Acetaldehyde |
|---|---|---|---|---|---|---|---|
| PVF | MOX | - | - | - | - | - | - |
| PVDF | MOX | - | - | - | 4.2 (*5.5) | 1.1 (*4) | - |
| PTFE | MOX | 13.9 | - | 1.3 | 24.8 | 44.8 | 1.1 |
| CB | - | - | - | 68.6 | 137.3 | - | |
| ETFE | MOX | 1.4 | - | - | *2 | *1.4 | - |
| ECTFE | MOX | 1.4 | - | - | - | - | - |
| FEP | MOX | 1.9 | - | - | *1.3 | *1.6 | - |
| PFA | MOX | 3 | - | - | - | - | - |
| Teflon® AF2400 | MOX | 24 | 1.9 | 3.7 | 104.7 | 29.4 | 1.1 |
| CB | 664.9 | - | - | 1253.9 | 787.7 | - | |
| TC | 11.2 | - | - | 3.2 | 3.3 | - |
| Membrane | Sensor | H2 | CO | CH4 | Ethanol | Acetone | Acetal-Dehyde |
|---|---|---|---|---|---|---|---|
| PVF | MOX | - | - | - | - | - | - |
| PVDF | MOX | - | - | - | *0.976 | *0.965 | - |
| PTFE | MOX | 0.862 | - | 0.975 | 0.827 | 0.265 | 0.990 |
| CB | - | - | - | 0.909 | 0.194 | - | |
| ETFE | MOX | 0.997 | - | - | *0.994 | *0.994 | - |
| ECTFE | MOX | 0.997 | - | - | - | - | - |
| FEP | MOX | 0.991 | - | - | *0.998 | *0.989 | - |
| PFA | MOX | 0.997 | - | - | - | - | - |
| Teflon® AF2400 | MOX | 0.757 | 0.831 | 0.794 | 0.267 | 0.447 | 0.992 |
| TC | 0.292 | - | - | 0.699 | 0.743 | - |
| Membrane | Sensor Pair A | Sensor Pair B | Sensor Pair C |
|---|---|---|---|
| ECTFE | 0.995 ± 0.001 | 0.995 ± 0.001 | 0.995 ± 0.001 |
| PFA | 0.995 ± 0.001 | 0.995 ± 0.001 | 0.995 ± 0.001 |
| Membrane | ∆Afeed (g/m³) | ∆Bfeed (g/m³) | ∆Apermeate (g/m³) | ∆Bpermeate (g/m³) |
|---|---|---|---|---|
| PVF | 5.1 | 10 | 0.8 | 1.7 |
| PVDF | 5 | 9.8 | 1.5 | 2.8 |
| PTFE | 4.8 | 9.4 | 0.2 | 0.3 |
| ETFE | 4.9 | 10.1 | 0.3 | 1 |
| ECTFE | 4.9 | 9.7 | 0.1 | 0.2 |
| FEP | 4.8 | 9.4 | 0.2 | 0.3 |
| PFA | 5 | 10.1 | 0.1 | 0.2 |
| Teflon® AF2400 | 4.8 | 9.4 | 2.9 | 5.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graunke, T.; Schmitt, K.; Raible, S.; Wöllenstein, J. Towards Enhanced Gas Sensor Performance with Fluoropolymer Membranes. Sensors 2016, 16, 1605. https://doi.org/10.3390/s16101605
Graunke T, Schmitt K, Raible S, Wöllenstein J. Towards Enhanced Gas Sensor Performance with Fluoropolymer Membranes. Sensors. 2016; 16(10):1605. https://doi.org/10.3390/s16101605
Chicago/Turabian StyleGraunke, Thorsten, Katrin Schmitt, Stefan Raible, and Jürgen Wöllenstein. 2016. "Towards Enhanced Gas Sensor Performance with Fluoropolymer Membranes" Sensors 16, no. 10: 1605. https://doi.org/10.3390/s16101605
APA StyleGraunke, T., Schmitt, K., Raible, S., & Wöllenstein, J. (2016). Towards Enhanced Gas Sensor Performance with Fluoropolymer Membranes. Sensors, 16(10), 1605. https://doi.org/10.3390/s16101605






