NiCu Alloy Nanoparticle-Loaded Carbon Nanofibers for Phenolic Biosensor Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Synthesis of NiCuCNFs
2.3. Preparation of Biosensors
2.4. Structure Characterization
2.5. Performance Evaluation
3. Results and Discussion
3.1. Characterization
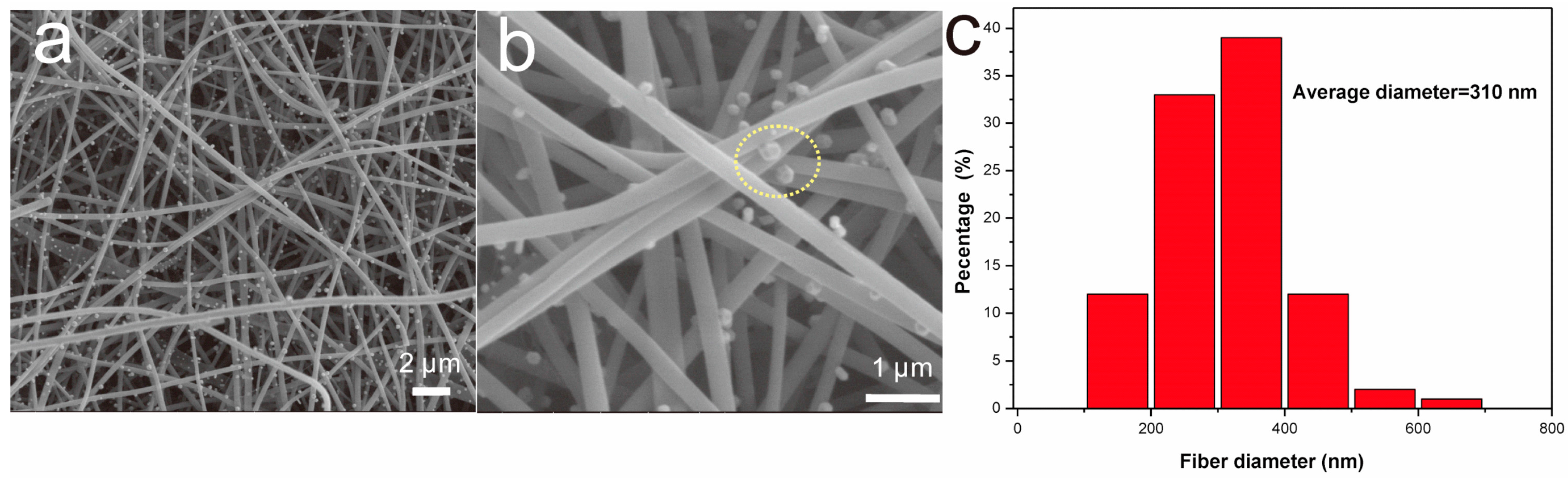
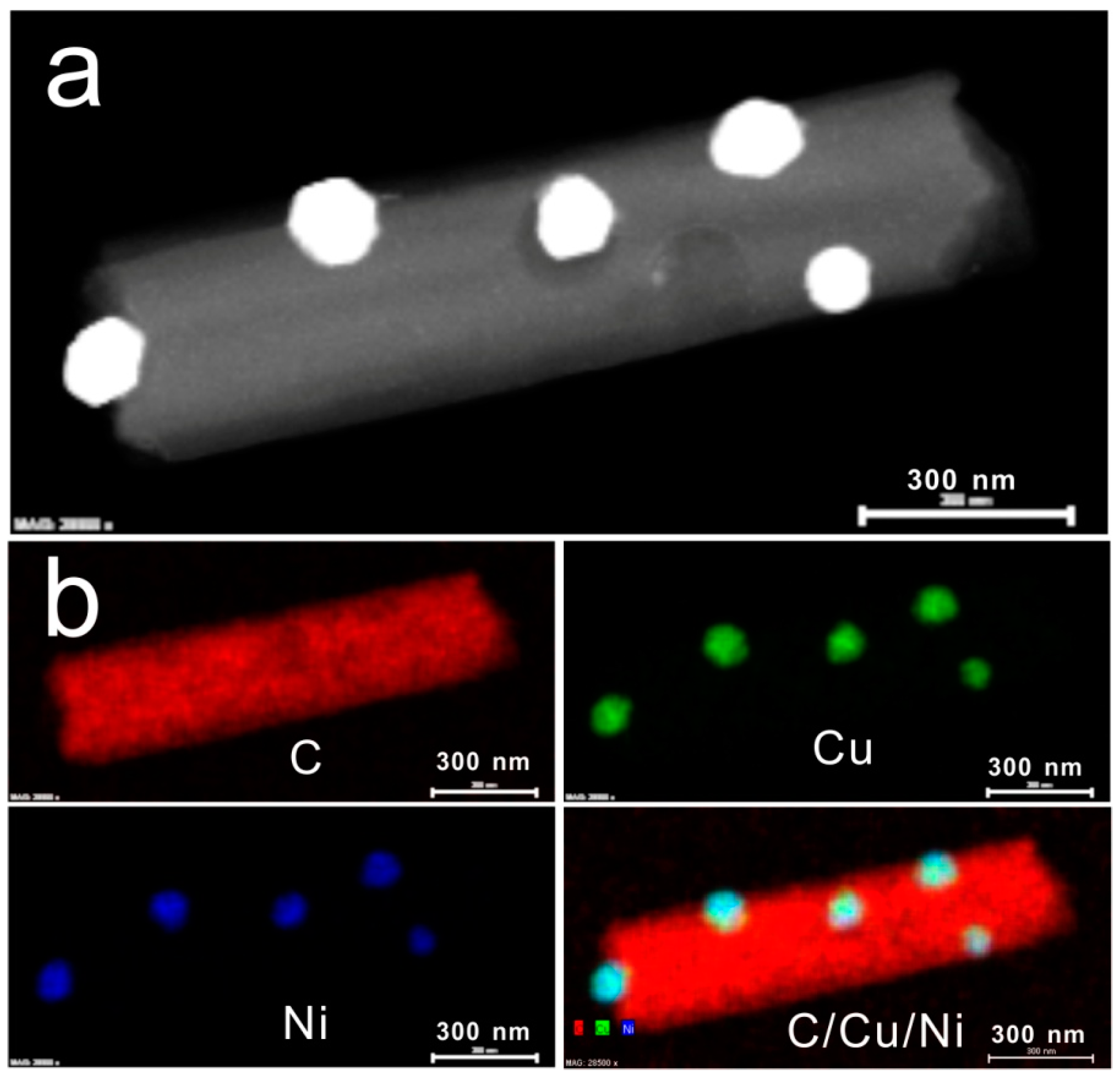
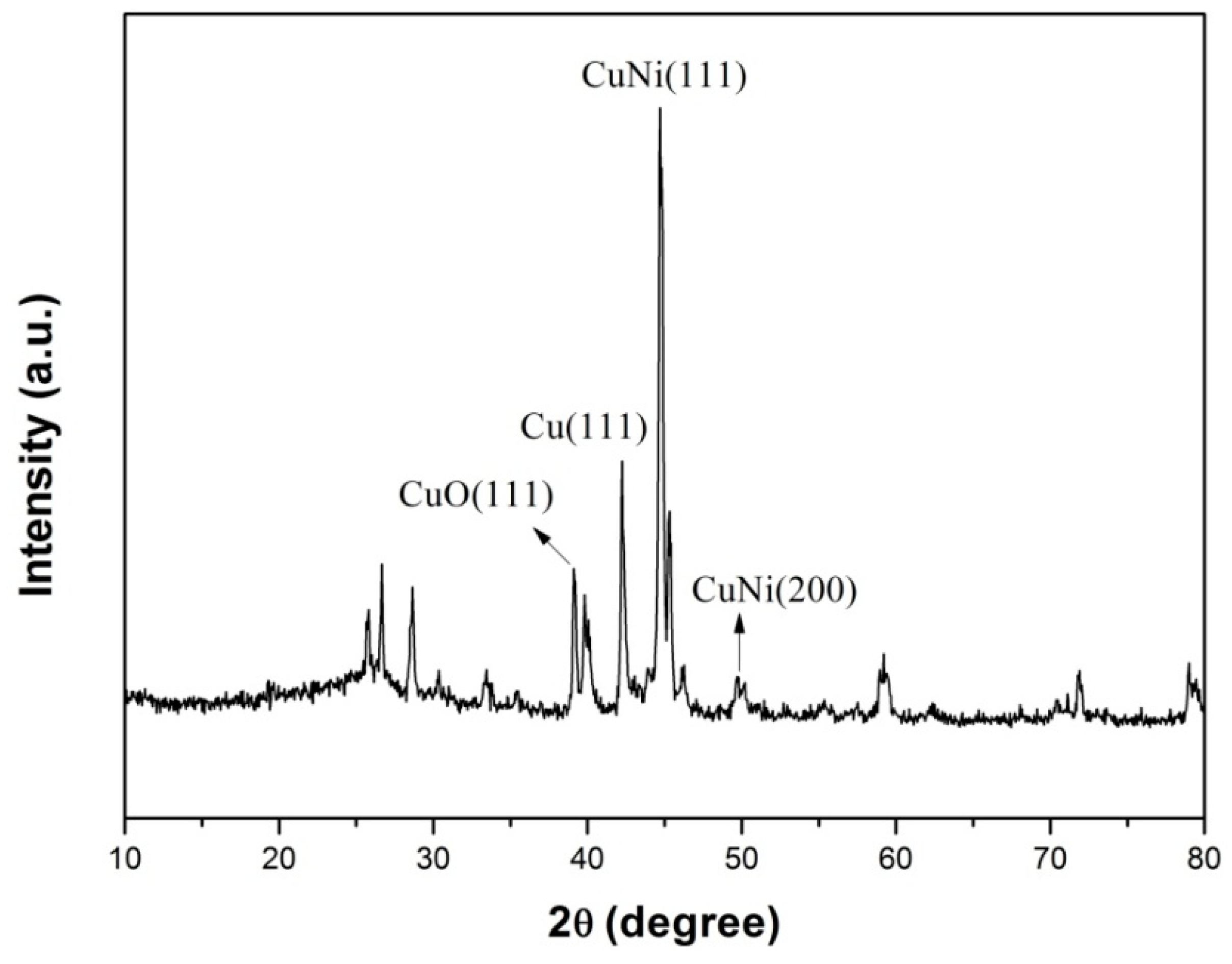

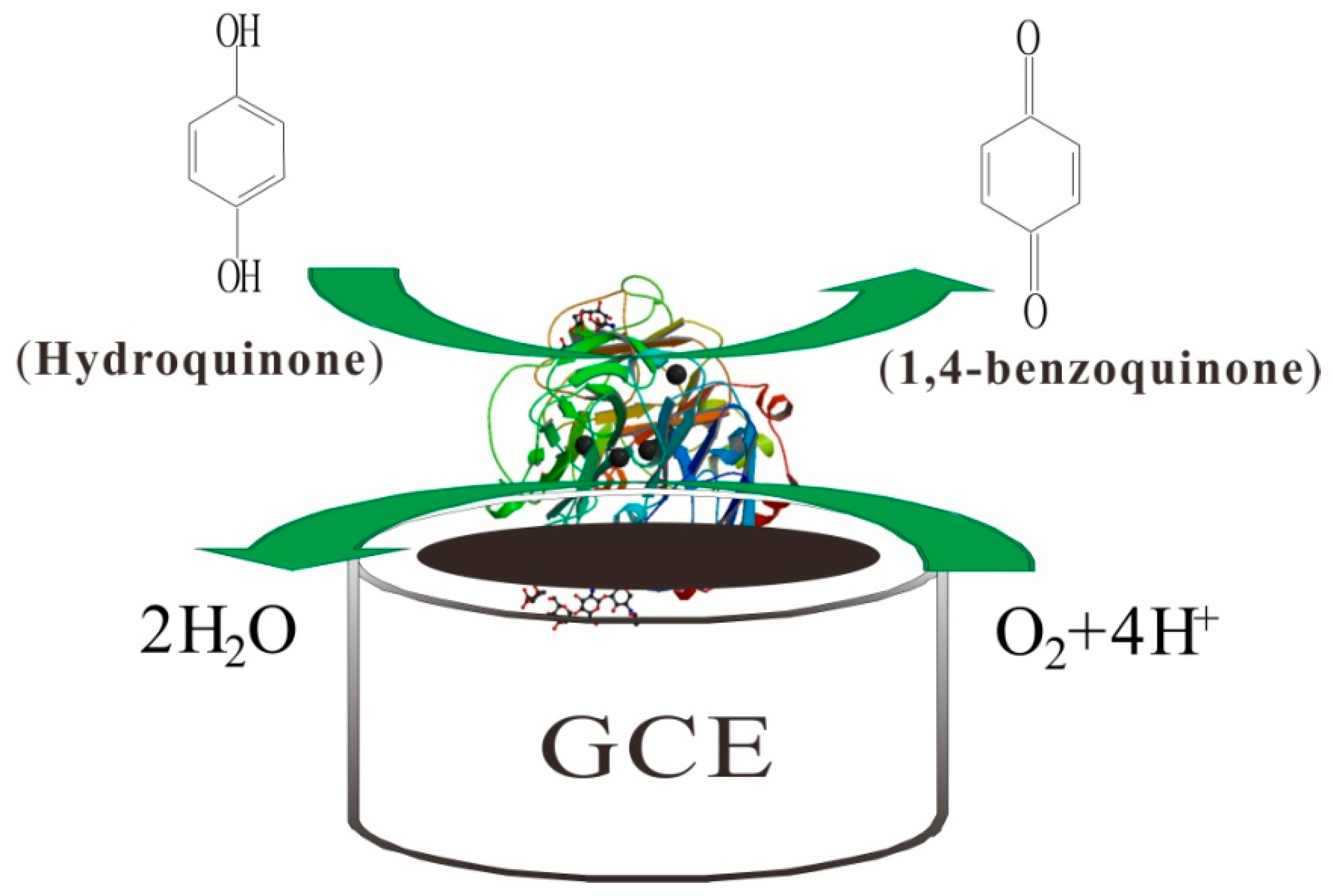
3.2. Direct Electron Transfer of Lac-NiCuCNF-Nafion/GCE
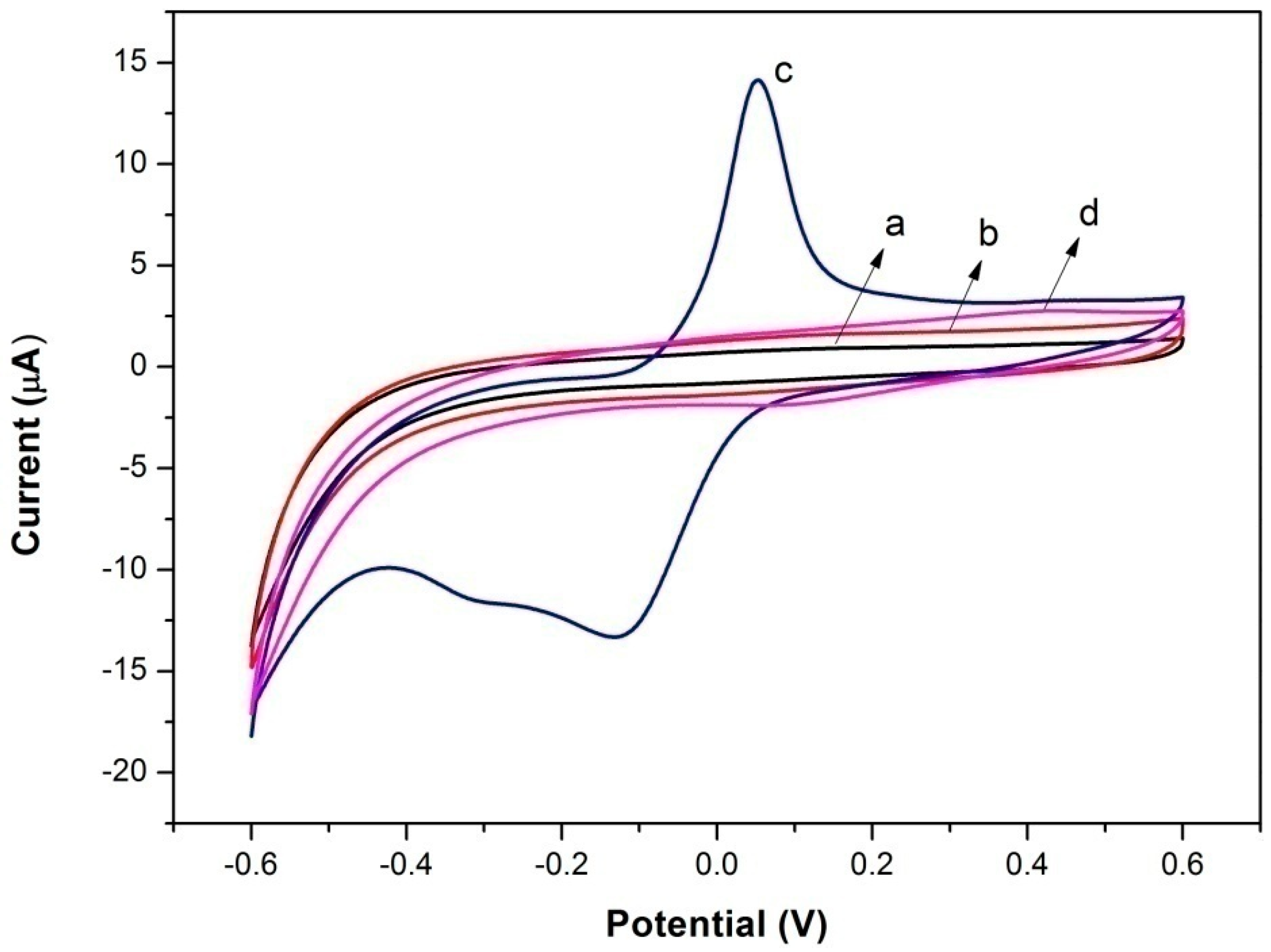

3.3. Electrocatalysis of Different Electrodes
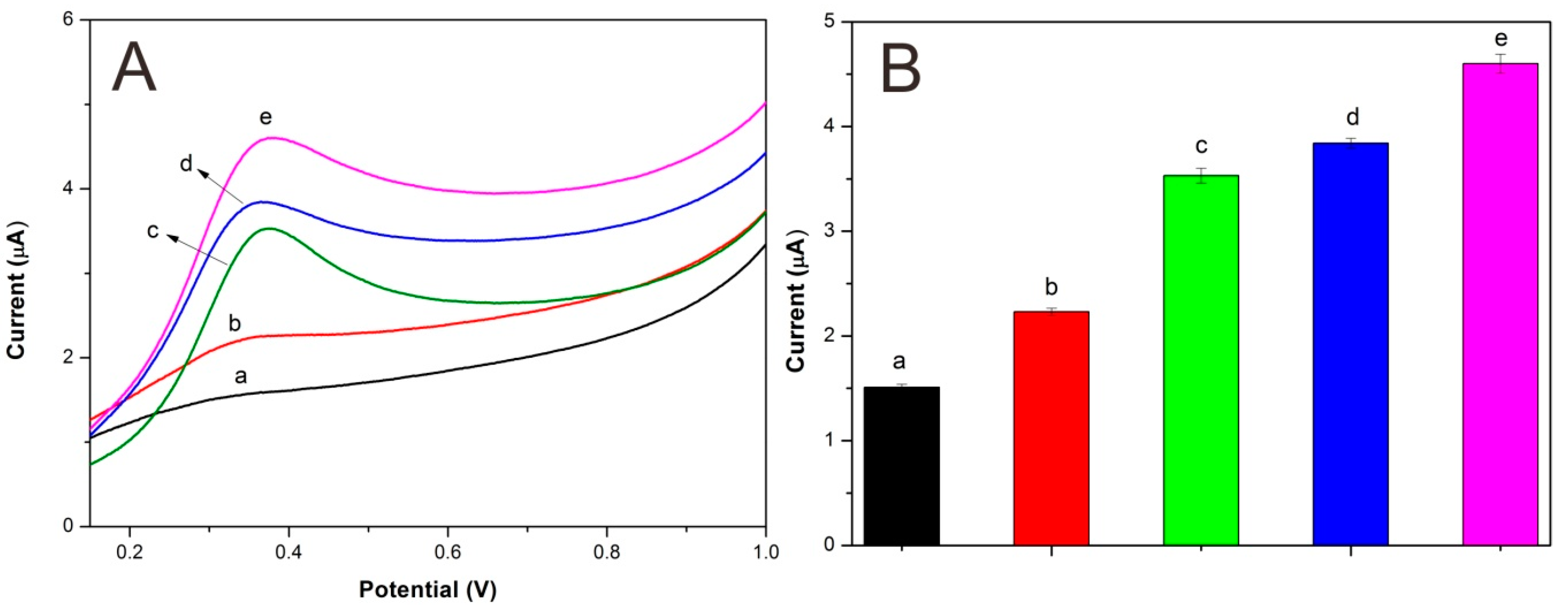
3.4. Analytical Characteristics of Lac-NiCuCNF-Nafion/GCE
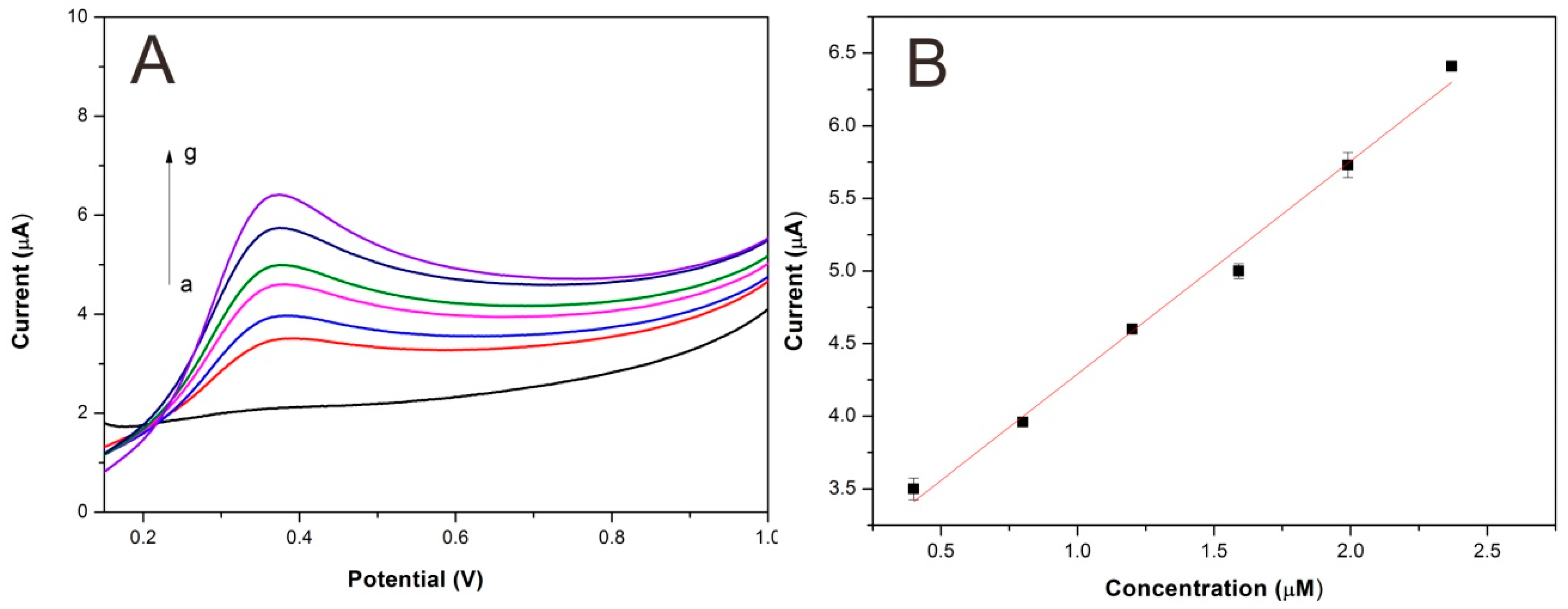
| Electrode | Detection Limit (µM) | Linear Range (µM) | Reference |
|---|---|---|---|
| GCE/MWCNT/CoPc electrode | 0.1600 | 0.99–8.30 | [35] |
| Au-SAMmix-HRP electrode | 1.2600 | 5.00–30.00 | [36] |
| dsDNA/PANI/CTS/GCE | 0.9600 | 1.25–320.00 | [24] |
| GCE-PEI-AuNP-LAC | 0.2100 | 2.90–22.00 | [37] |
| Lac-NiCuCNF-Nafion/GCE | 0.0900 | 0.40–2.37 | this work |
3.5. Repeatability, Reproducibility, Anti-Interference and Stability of Lac-NiCuCNF-Nafion/GCE
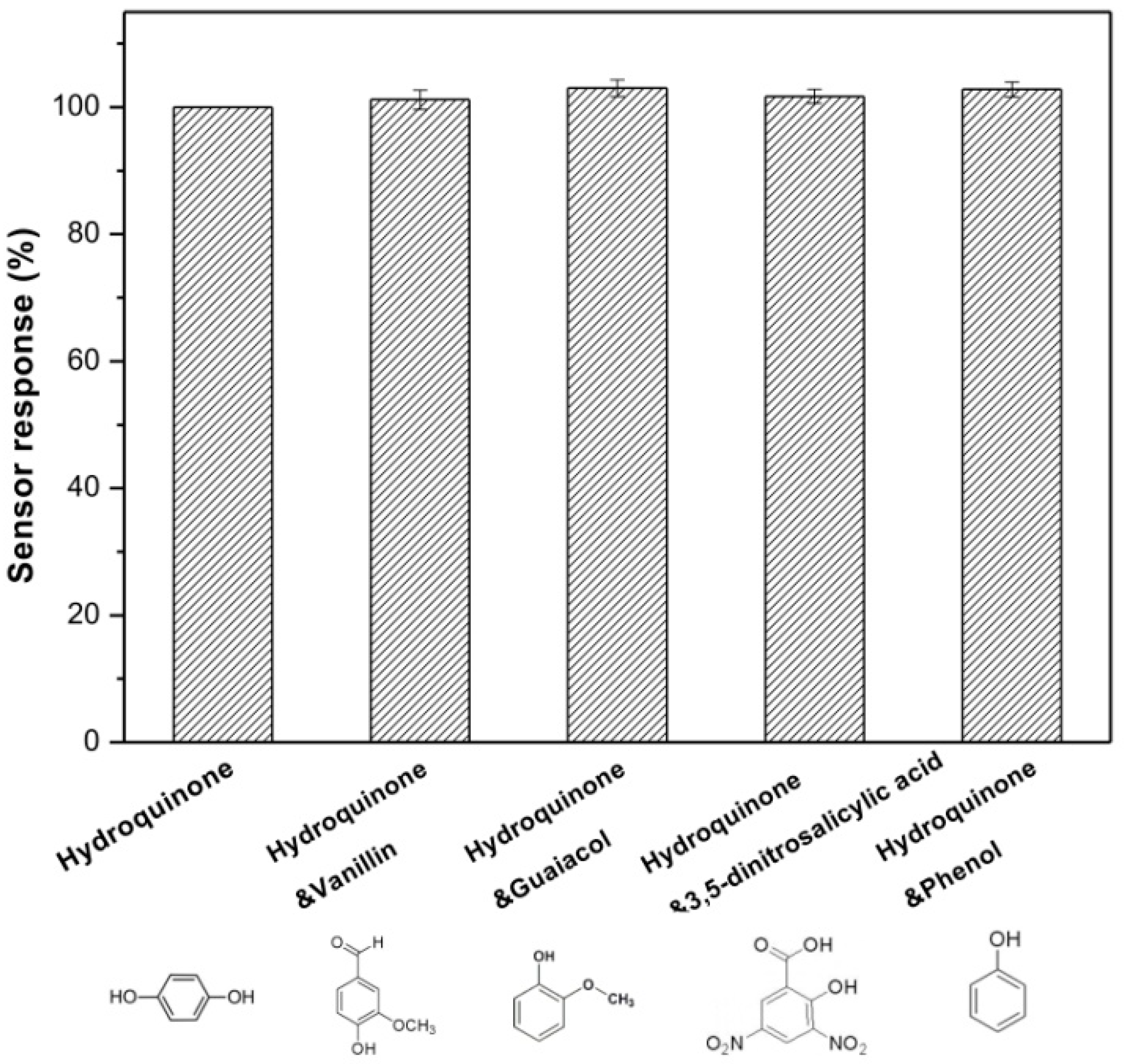
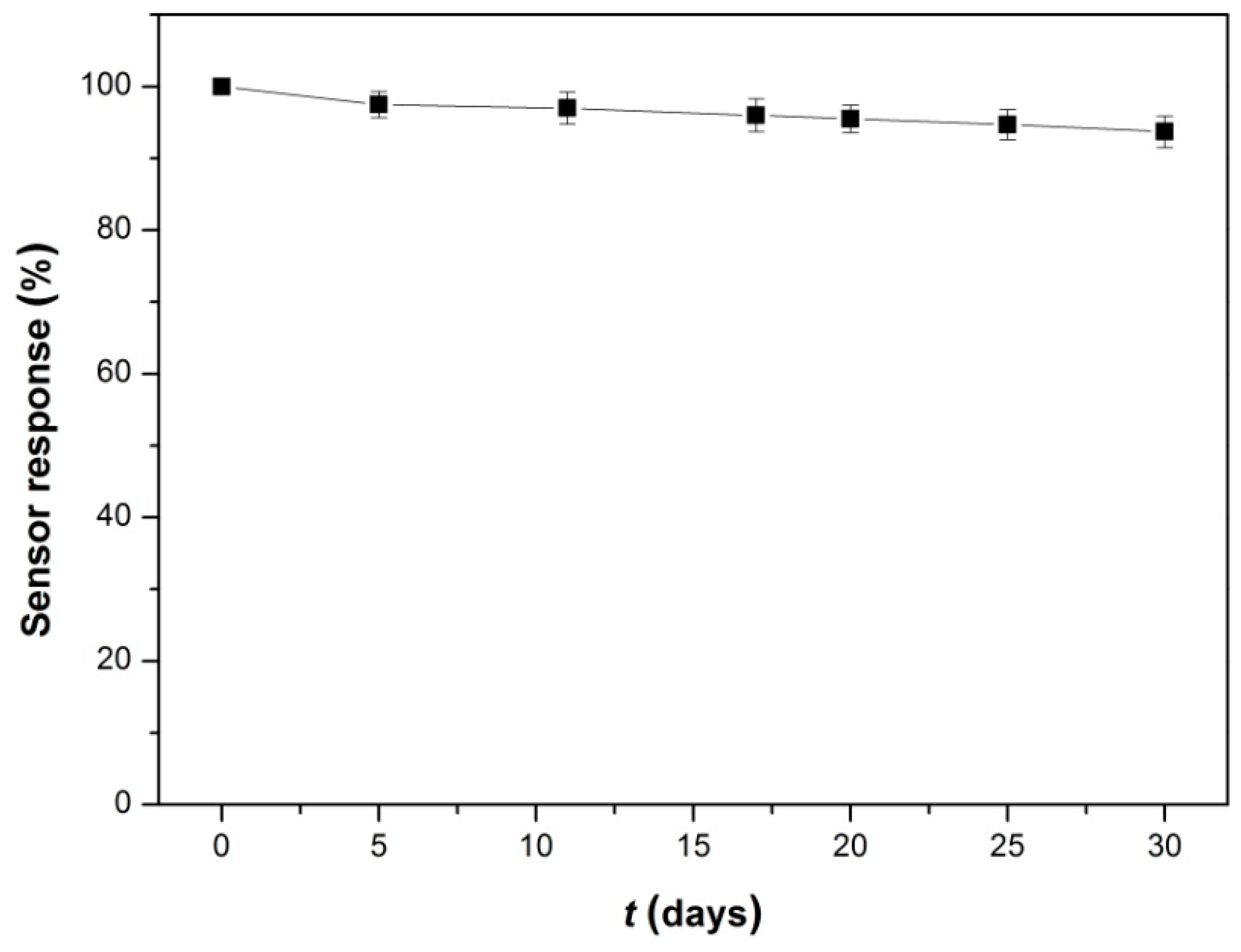
3.6. Real Water Sample Analysis
| Sample a | Cadded (µM) | Cfound (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 1.50 | 1.43 | 95.30 | 3.70 |
| 1.52 | 101.30 | |||
| 1.46 | 97.30 | |||
| 1.38 | 92.00 | |||
| 1.41 | 94.00 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siangproh, W.; Dungchai, W.; Rattanarat, P.; Chailapakul, O. Nanoparticle-based electrochemical detection in conventional and miniaturized systems and their bioanalytical applications: A review. Anal. Chim. Acta 2011, 690, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Lee, N.; Park, J.; Kim, B.H.; Yi, Y.-W.; Kim, T.; Kim, T.K.; Lee, I.H.; Paik, S.R.; Hyeon, T. Ni/NiO core/shell nanoparticles for selective binding and magnetic separation of histidine-tagged proteins. J. Am. Chem. Soc. 2006, 128, 10658–10669. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Sharifi, E.; Noorbakhsh, A.; Soltanian, S. Direct voltammetry and electrocatalytic properties of hemoglobin immobilized on a glassy carbon electrode modified with nickel oxide nanoparticles. Electrochem. Commun. 2006, 8, 1499–1508. [Google Scholar] [CrossRef]
- Salimi, A.; Sharifi, E.; Noorbakhsh, A.; Soltanian, S. Immobilization of glucose oxidase on electrodeposited nickel oxide nanoparticles: Direct electron transfer and electrocatalytic activity. Biosens. Bioelectron. 2007, 22, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xiong, H.; Zhang, X.; Wang, S. A novel tyrosinase biosensor based on chitosan-carbon-coated nickel nanocomposite film. Bioelectrochemistry 2012, 84, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Lata, S.; Sharma, M.; Pundir, C.S. An acrylamide biosensor based on immobilization of hemoglobin onto multiwalled carbon nanotube/copper nanoparticles/polyaniline hybrid film. Anal. Biochem. 2013, 433, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Rawal, R.; Pundir, C.S. Fabrication of polyphenol biosensor based on laccase immobilized on copper nanoparticles/chitosan/multiwalled carbon nanotubes/polyaniline-modified gold electrode. J. Biotechnol. 2011, 156, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Lei, C.Y.; Shen, Q.P.; Li, L.J.; Wang, M.; Guo, M.L.; Huang, Y.; Nie, Z.; Yao, S.Z. Analysis of copper nanoparticles toxicity based on a stress-responsive bacterial biosensor array. Nanoscale 2013, 5, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Li, C.Y.; Su, C.; Ji, X.G.; Zheng, J.; Tinnefeldc, P.; He, Z.K. Enzymatic polymerization of poly(thymine) for the synthesis of copper nanoparticles with tunable size and their application in enzyme sensing. Chem. Commun. 2015, 51, 8644–8647. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, J.; Liu, F.; He, H.; Zhou, M.; Mao, N.; Xiao, P.; Zhang, Y. Nickel/Copper nanoparticles modified TiO2 nanotubes for non-enzymatic glucose biosensors. Sens. Actuators B 2013, 181, 501–508. [Google Scholar] [CrossRef]
- Tong, S.; Xu, Y.; Zhang, Z.; Song, W. Dendritic bimetallic nanostructures supported on self-assembled titanate films for sensor application. J. Phys. Chem. C 2010, 114, 20925–20931. [Google Scholar] [CrossRef]
- Tian, X.-K.; Zhao, X.-Y.; Yang, C.; Pi, Z.-B.; Zhang, S.-X. Performance of ethanol electro-oxidation on Ni–Cu alloy nanowires through composition modulation. Nanotechnology 2008, 19, 215711. [Google Scholar] [CrossRef] [PubMed]
- Van Ingen, R.; Fastenau, R.; Mittemeijer, E. Laser ablation deposition of Cu–Ni and Ag–Ni films: Nonconservation of alloy composition and film microstructure. J. Appl. Phys. 1994, 76, 1871–1883. [Google Scholar] [CrossRef]
- Marzik, J.V.; Carreiro, L.G.; Davies, G. Cu–Ni alloy formation by reduction in hydrogen of a polyheterometallic complex. J. Mater. Sci. Lett. 1988, 7, 833–835. [Google Scholar] [CrossRef]
- Bahlawane, N.; Premkumar, P.A.; Tian, Z.; Hong, X.; Qi, F.; Kohse-Höinghaus, K. Nickel and nickel-based nanoalloy thin films from alcohol-assisted chemical vapor deposition. Chem. Mater. 2009, 22, 92–100. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Xu, G.; Li, S.; Lu, Y.; Toprakci, O.; Zhang, X. High-capacity Li2Mn0.8Fe0.2SiO4/carbon composite nanofiber cathodes for lithium-ion batteries. J. Power Sources 2012, 213, 10–15. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, Z.; Ji, L.; Li, Y.; Xu, G.; Xue, L.; Li, S.; Lu, Y.; Toprakci, O.; Zhang, X. Cr-doped Li2MnSiO4/carbon composite nanofibers as high-energy cathodes for Li-ion batteries. J. Mater. Chem. 2012, 22, 14661–14666. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, D.; Zhang, X.; Li, L.; Hou, H.; Niwa, O.; You, T. Pd–Ni Alloy Nanoparticle/Carbon Nanofiber Composites: Preparation, Structure, and Superior Electrocatalytic Properties for Sugar Analysis. Anal. Chem. 2014, 86, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- Vamvakaki, V.; Tsagaraki, K.; Chaniotakis, N. Carbon nanofiber-based glucose biosensor. Anal. Chem. 2006, 78, 5538–5542. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, L.; Pang, Z.; Ding, L.; Wang, Q.; Ke, H.; Huang, F.; Wei, Q. Novel Phenolic Biosensor Based on a Magnetic Polydopamine-Laccase-Nickel Nanoparticle Loaded Carbon Nanofiber Composite. ACS Appl. Mater. Interfaces 2014, 6, 5144–5151. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhou, J.; Lu, W.; Liu, Q.; Li, J. Carbon nanofiber-based composites for the construction of mediator-free biosensors. Biosens. Bioelectron. 2008, 23, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Qiao, H.; Li, D.; Luo, L.; Chen, K.; Wei, Q. Laccase biosensor based on electrospun copper/carbon composite nanofibers for catechol detection. Sensors 2014, 14, 3543–3556. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, G.; Lv, P.; Ullah, N.; Wang, C.; Wang, Q.; Zhang, X.; Wei, Q. Preparation of a graphene-loaded carbon nanofiber composite with enhanced graphitization and conductivity for biosensing applications. RSC Adv. 2015, 5, 30602–30609. [Google Scholar] [CrossRef]
- Tang, W.W.; Zhang, M.; Li, W.H.; Zeng, X.P. An electrochemical sensor based on polyaniline for monitoring hydroquinone and its damage on DNA. Talanta 2014, 127, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, A.J.S.; Rahman, M.M.; Xu, G.R.; Kim, S.; Lee, J.J. Highly sensitive and simultaneous determination of hydroquinone and catechol at poly(thionine) modified glassy carbon electrode. Electrochim. Acta 2011, 56, 5266–5271. [Google Scholar] [CrossRef]
- Babadostu, A.; Guldu, O.K.; Demirkol, D.O.; Medine, E.I.; Unak, P.; Timur, S. Affinity Based Laccase Immobilization on Modified Magnetic Nanoparticles: Biosensing Platform for the Monitoring of Phenolic Compounds. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 260–266. [Google Scholar] [CrossRef]
- Zeng, Z.M.; Tian, L.J.; Li, Z.; Jia, L.N.; Zhang, X.Y.; Xia, M.M.; Hu, Y.G. Whole-cell method for phenol detection based on the color reaction of phenol with 4-aminoantipyrine catalyzed by CotA laccase on endospore surfaces. Biosens. Bioelectron. 2015, 69, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.-P.; Feng, J.-J.; Wu, L.; Zhou, J.-Y.; Chen, J.-R.; Wang, A.-J. Novel phenol biosensor based on laccase immobilized on reduced graphene oxide supported palladium-copper alloyed nanocages. Biosens. Bioelectron. 2015, 74, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, D.; Li, G.; Luo, L.; Ullah, N.; Wei, Q.; Huang, F. Facile fabrication of gold nanoparticle on zein ultrafine fibers and their application for catechol biosensor. Appl. Surf. Sci. 2015, 328, 444–452. [Google Scholar] [CrossRef]
- Mu, J.B.; Shao, C.L.; Guo, Z.C.; Zhang, Z.Y.; Zhang, M.Y.; Zhang, P.; Chen, B.; Liu, Y. High Photocatalytic Activity of ZnO-Carbon Nanofiber Heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 3, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Zhang, X.L.; Qiao, R.; Li, Y.; Kim, Y.I.; Kang, Y.S. CuNi dendritic material: Synthesis, mechanism discussion, and application as glucose sensor. Chem. Mater. 2007, 19, 4174–4180. [Google Scholar] [CrossRef]
- Wang, K.; Tang, J.; Zhang, Z.; Gao, Y.; Chen, G. Laccase on Black Pearl 2000 modified glassy carbon electrode: Characterization of direct electron transfer and biological sensing properties for pyrocatechol. Electrochim. Acta 2012, 70, 112–117. [Google Scholar] [CrossRef]
- Committee, A.M. Recommendations for the definition, estimation and use of the detection limit. Analyst 1987, 112, 199–204. [Google Scholar] [CrossRef]
- Cesarino, I.; Moraes, F.C.; Ferreira, T.C.R.; Lanza, M.R.V.; Machado, S.A.S. Real-time electrochemical determination of phenolic compounds after benzene oxidation. J. Electroanal. Chem. 2012, 672, 34–39. [Google Scholar] [CrossRef]
- Mossanha, R.; Ramos, M.K.; Santos, C.S.; Pessoaz, C.A. Mixed Self-Assembled Monolayers of Mercaptoundecanoic Acid and Thiolactic Acid for the Construction of an Enzymatic Biosensor for Hydroquinone Determination. J. Electrochem. Soc. 2015, 162, B145–B151. [Google Scholar] [CrossRef]
- Tan, Y.M.; Deng, W.F.; Ge, B.; Xie, Q.J.; Huang, J.H.; Yao, S.Z. Biofuel cell and phenolic biosensor based on acid-resistant laccase-glutaraldehyde functionalized chitosan-multiwalled carbon nanotubes nanocomposite film. Biosens. Bioelectron. 2009, 24, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Brondani, D.; de Souza, B.; Souza, B.S.; Neves, A.; Vieira, I.C. PEI-coated gold nanoparticles decorated with laccase: A new platform for direct electrochemistry of enzymes and biosensing applications. Biosens. Bioelectron. 2013, 42, 242–247. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Lv, P.; Zhu, J.; Lu, Y.; Chen, C.; Zhang, X.; Wei, Q. NiCu Alloy Nanoparticle-Loaded Carbon Nanofibers for Phenolic Biosensor Applications. Sensors 2015, 15, 29419-29433. https://doi.org/10.3390/s151129419
Li D, Lv P, Zhu J, Lu Y, Chen C, Zhang X, Wei Q. NiCu Alloy Nanoparticle-Loaded Carbon Nanofibers for Phenolic Biosensor Applications. Sensors. 2015; 15(11):29419-29433. https://doi.org/10.3390/s151129419
Chicago/Turabian StyleLi, Dawei, Pengfei Lv, Jiadeng Zhu, Yao Lu, Chen Chen, Xiangwu Zhang, and Qufu Wei. 2015. "NiCu Alloy Nanoparticle-Loaded Carbon Nanofibers for Phenolic Biosensor Applications" Sensors 15, no. 11: 29419-29433. https://doi.org/10.3390/s151129419
APA StyleLi, D., Lv, P., Zhu, J., Lu, Y., Chen, C., Zhang, X., & Wei, Q. (2015). NiCu Alloy Nanoparticle-Loaded Carbon Nanofibers for Phenolic Biosensor Applications. Sensors, 15(11), 29419-29433. https://doi.org/10.3390/s151129419






