Sustainable Stewardship of the Landrace Diversity
Abstract
:1. Introduction
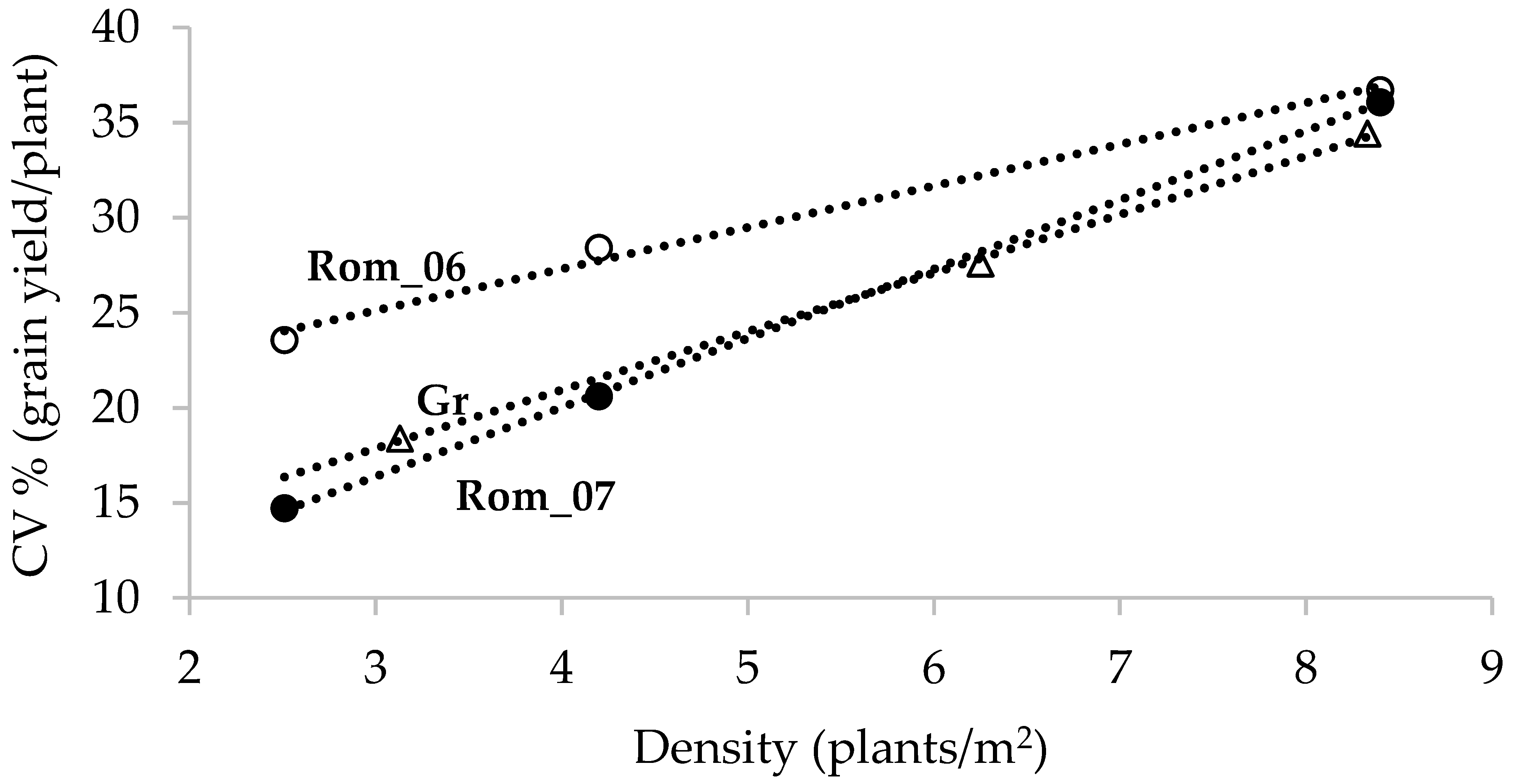
2. Crop Stand Variation and Resource Use Efficiency
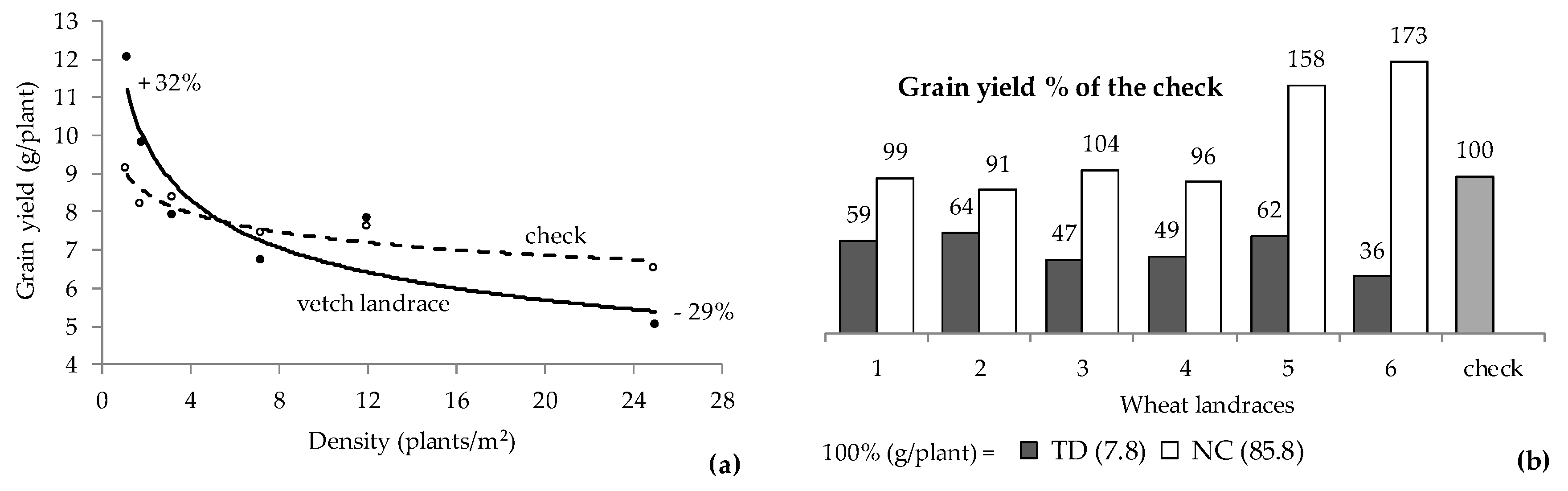
3. Competitive vs. Yielding Ability
4. Mechanisms of New Variation
5. Stewardship of the Diversity
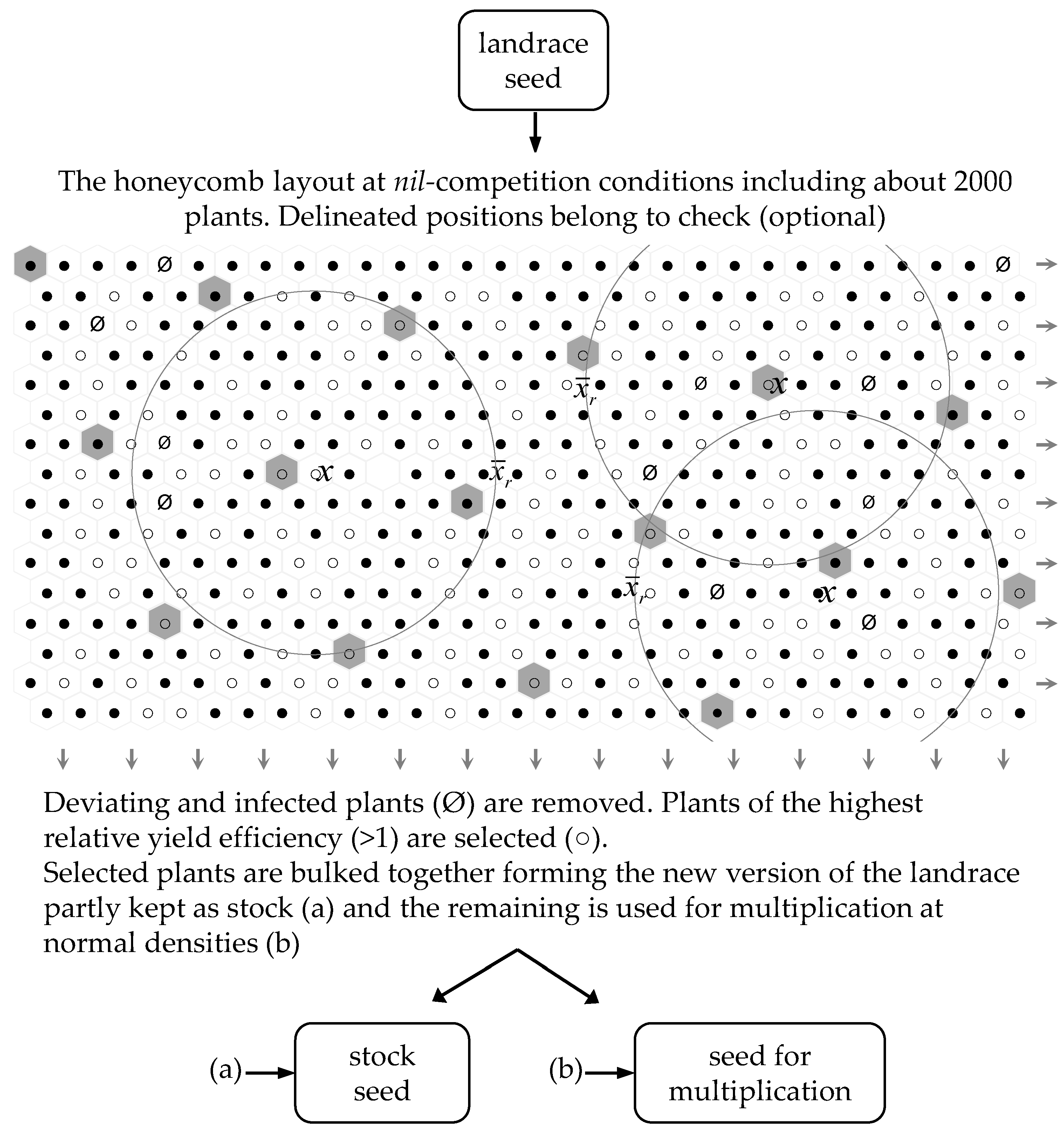
5.1. General Approach of Breeding at Nil-Competition
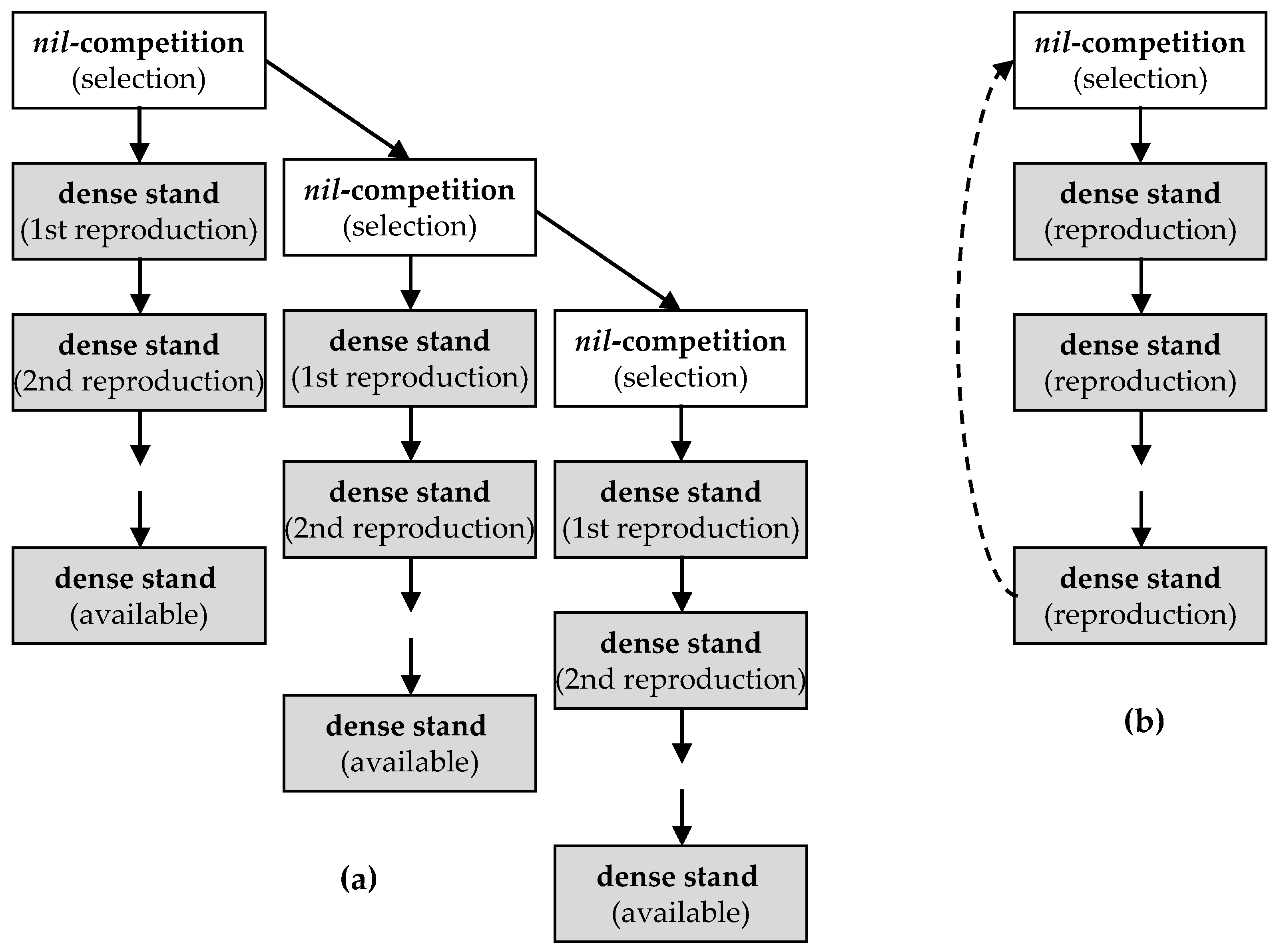
5.2. Bulk Selection to Replenish the Landrace Seed
6. Discussion
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Bota, J.; Conesa, M.A.; Ochogavia, J.M.; Medrano, H.; Francis, D.M.; Cifre, J. Characterization of a landrace collection for Tomàtiga de Ramellet (Solanum lycopersicum L.) from the Balearic Islands. Genet. Res. Crop Evol. 2014, 61, 1131–1146. [Google Scholar] [CrossRef]
- Villa, T.C.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and identifying crop landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar] [CrossRef]
- Gepts, P. A comparison between crop domestication, classical plant breeding, and genetic engineering. Crop Sci. 2002, 42, 1780–1790. [Google Scholar] [CrossRef]
- Gouesnard, B.; Zanetto, A.; Welcker, C. Identification of adaptation traits to drought in collections of maize landraces from southern Europe and temperate regions. Euphytica 2016, 209, 565–584. [Google Scholar] [CrossRef]
- Zeven, A.C. Traditional maintenance breeding of landraces: 2. Practical and theoretical considerations on maintenance of variation of landraces by farmers and gardeners. Euphytica 2002, 123, 147–158. [Google Scholar] [CrossRef]
- Koutsika-Sotiriou, M.; Mylonas, I.G.; Ninou, E.; Traka-Mavrona, E. The cultivation revival of a landrace: Pedigree and analytical breeding. Euphytica 2010, 176, 15–24. [Google Scholar] [CrossRef]
- Parlevliet, J.E. How to maintain improved cultivars. Euphytica 2007, 153, 353–362. [Google Scholar] [CrossRef]
- McClintock, B. The significance of the responses of the genome to challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, D.C.; Phillips, R.L. Plant breeding progress and genetic diversity from de novo variation and elevated epistasis. Crop Sci. 1997, 37, 303–310. [Google Scholar] [CrossRef]
- Mohana, D.C.; Prasad, P.; Vijaykumar, V.; Raveesha, K.A. Plant extract effect on seed-borne pathogenic fungi from seeds of paddy grown in Southern India. J. Plant Protection Res. 2011, 51, 101–106. [Google Scholar] [CrossRef]
- Ahmed, M.; Hossain, M.; Hassan, K.; Dash, C.K. Efficacy of different plant extract on reducing seed borne infection and increasing germination of collected rice seed sample. Univ. J. Plant Sci. 2013, 1, 66–73. [Google Scholar]
- Kumari, S.G.; Larsen, R.; Makkouk, K.M.; Bashir, M. Virus diseases and their management. In The Lentil: Botany, Production and Uses; Erskine, W., Muehlbauer, F.J., Sarker, A., Sharma, B., Eds.; CAB International, Wallingford: Oxfordshire, UK, 2009; pp. 306–325. [Google Scholar]
- Dawson, W.A.J.M.; Bateman, G.L. Fungal communities on roots of wheat and barley and effects of seed treatments containing fluquinconazole applied to control take-all. Plant Pathol. 2001, 50, 75–82. [Google Scholar] [CrossRef]
- Majumder, D.; Rajesh, T.; Suting, E.G.; Debbarma, A. Detection of seed borne pathogens in wheat: Recent trends. Aust. J. Crop Sci. 2013, 7, 500–507. [Google Scholar]
- Elmer, W.H. Seeds as vehicles for pathogen importation. Biol. Invasions 2002, 3, 263–271. [Google Scholar] [CrossRef]
- Jones, R.A.C. Using epidemiological information to develop effective integrated virus disease management strategies. Virus Res. 2004, 100, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Coutts, B.A.; Prince, R.T.; Jones, R.A.C. Quantifying effects of seedborne inoculum on virus spread, yield losses, and seed infection in the Pea seed-borne mosaic virus-field pea pathosystem. Phytopathology 2009, 99, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Gravois, K.A.; Helms, R.S. Seeding rate effects on rough rice yield, head rice, and total milled rice. Agron. J. 1996, 88, 82–84. [Google Scholar] [CrossRef]
- Martin, K.L.; Hodgen, P.J.; Freeman, K.W.; Melchiori, R.; Arnall, D.B.; Teal, R.K.; Mullen, R.W.; Desta, K.; Phillips, S.B.; Solie, J.B.; et al. Plant-to-plant variability in corn product ion. Agron. J. 2005, 97, 1603–1611. [Google Scholar] [CrossRef]
- Pan, X.Y.; Wang, G.X.; Yang, H.M.; Wei, X.P. Effect of water deficits on within-plot variability in growth and grain yield of spring wheat in northwest China. Field Crops Res. 2003, 80, 195–205. [Google Scholar] [CrossRef]
- Taylor, S.L.; Payton, M.E.; Raun, W.R. Relationships between mean yield, coefficient of variation, mean square error, and plot size in wheat field experiments. Commun. Soil Sci. Plant Anal. 1999, 30, 1439–1447. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Koutroubas, S.D. A review study of the maize hybrids’ dependence on high plant populations and its implications on crop yield stability. Field Crops Res. 2004, 88, 103–114. [Google Scholar] [CrossRef]
- Tollenaar, M.; Wu, J. Yield improvement in temperate maize is attributable to greater stress tolerance. Crop Sci. 1999, 39, 1597–1604. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Has, V.; Mylonas, I.; Has, I.; Evgenidis, G.; Melidis, V.; Copandean, A.; Ninou, E. Density effects on environmental variance and expected response to selection in maize (Zea mays L.). Euphytica 2010, 174, 283–291. [Google Scholar] [CrossRef]
- Fasoula, V.A.; Tokatlidis, I.S. Development of crop cultivars by honeycomb breeding. Agron. Sustain. Dev. 2012, 32, 161–180. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Koutsika-Sotiriou, M.; Tamoutsidis, E. Benefits from using maize density-independent hybrids. Maydica 2005, 50, 9–17. [Google Scholar]
- Fasoula, D.A.; Fasoula, V.A. Competitive ability and plant breeding. Plant Breed. Rev. 1997, 14, 89–138. [Google Scholar]
- Kyriakou, D.T.; Fasoulas, A.C. Effects of competition and selection pressure on yield response in winter rye (Secale cereale L.). Euphytica 1985, 34, 883–895. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Acevedo, E.; Sayre, K.D.; Fischer, R.A. Yield potential in modern wheat cultivars: Its association with a less competitive ideotype. Field Crops Res. 1994, 37, 149–160. [Google Scholar] [CrossRef]
- Sedgley, R.H. An appraisal of the Donald ideotype after 21 years. Field Crops Res. 1991, 26, 93–112. [Google Scholar] [CrossRef]
- Thomas, J.B.; Schaalje, G.B.; Grant, M.N. Height, competition and yield potential in winter wheat. Euphytica 1994, 74, 9–17. [Google Scholar] [CrossRef]
- Fasoula, D.A. Correlations between auto-, allo- and nill-competition and their implications in plant breeding. Euphytica 1990, 50, 57–62. [Google Scholar] [CrossRef]
- Atuahene-Amankwa, G.; Beatie, A.D.; Michaels, T.E.; Falk, D.E. Cropping system evaluation and selection of common bean genotypes for a maize/bean intercrop. Afr. Crop Sci. J. 2004, 12, 105–113. [Google Scholar] [CrossRef]
- Gebeyehu, S.; Simane, B.; Kirkby, R. Genotype x cropping system interaction in climbing beans (Phaseoulus vulgaris L.) grown as sole crop and in association with maize (Zea mays L.). Eur. J. Agron. 2006, 24, 396–403. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Jenssen, E.S. Evaluating pea and barley cultivars for complementary in intercropping at different levels of soil N availability. Field Crops Res. 2001, 72, 185–196. [Google Scholar] [CrossRef]
- O’Leary, N.; Smith, M.E. Uncovering corn adaptation to intercrop with bean by selecting for system yield in the intercrop environment. J. Sustain. Agric. 2004, 24, 109–121. [Google Scholar] [CrossRef]
- Santalla, M.; Rodiño, A.P.; Casquero, P.A.; de Ron, A.M. Interactions of bush bean intercropped with field and sweet maize. Eur. J. Agron. 2001, 15, 185–196. [Google Scholar] [CrossRef]
- Tefera, T.; Tana, T. Agronomic performance of sorghum and groundnut cultivars in sole and intercrop cultivation under semiarid conditions. J. Agron. Crop Sci. 2002, 188, 212–218. [Google Scholar] [CrossRef]
- Chatzoglou, T.; Tokatlidis, I.S. Decision on germplasm choice to apply breeding within a local population of common vetch is affected by crowding. Span. J. Agric. Res. 2012, 10, 752–755. [Google Scholar] [CrossRef]
- Ninou, E.G.; Mylonas, I.G.; Tsivelikas, A.; Ralli, P.; Dordas, C.; Tokatlidis, I.S. Wheat landraces are better qualified as potential gene pools at ultraspaced rather than densely grown conditions. Sci. World J. 2014, 957472. [Google Scholar] [CrossRef] [PubMed]
- Tokatlidis, I.S. Conservation breeding of elite cultivars. Crop Sci. 2015, 55, 2417–2434. [Google Scholar] [CrossRef]
- Goldringer, I.; Paillard, S.; Enjalbert, J.; David, J.L.; Brabant, P. Divergent evolution of wheat populations conducted under recurrent selection and dynamic management. Agronomie 1998, 18, 413–425. [Google Scholar] [CrossRef]
- Horneburg, B.; Becker, H.C. Crop Adaptation in On-Farm Management by genesand Conscious Selection: A Case Study with Lentil. Crop Sci. 2008, 48, 203–212. [Google Scholar] [CrossRef]
- Ossowski, S.; Schneeberger, K.; Lucas-Lledó, J.I.; Warthmann, N.; Clark, R.M.; Shaw, R.G.; Weigel, D.; Lynch, M. The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science 2010, 327, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.G.; Byers, D.L.; Darmo, E. Spontaneous mutational effects on reproductive traits of Arabidopsis thaliana. Genetics 2000, 155, 369–378. [Google Scholar] [PubMed]
- Cullis, C.A. Mechanisms and control of rapid genomic change in flax. Ann. Bot. 2005, 95, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Morgante, M.; Brunner, S.; Pea, G.; Fengler, K.; Zuccolo, A.; Rafalski, A. Gene duplication and exon shuffling by helitron-like transposons generate intraspecies diversity in maize. Nat. Genet. 2005, 37, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.A. Longevity of active transposons in corn breeding populations. Maydica 2008, 53, 173–180. [Google Scholar]
- Sinapidou, E.; Tokatlidis, I.S. Genetic mechanisms enhancing plant biodiversity. In Genetics, Biofuels and Local Farming System, Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Washington, DC, USA, 2011; Volume 7, pp. 51–83. [Google Scholar]
- Tsaftaris, A.S.; Polidoros, A.N.; Kapazoglou, A.; Tani, E.; Kovaćevic, N.M. Epigenetics and Plant Breeding. Plant Breed. Rev. 2008, 30, 49–176. [Google Scholar]
- Kidwell, M.G.; Lisch, D. Transposable elements as sources of genomic variation. In Mobile DNA II; Craig, N.L., Craigie, R., Gellert, M., Lambowitz, A.M., Eds.; American Society for Microbiology Press: Washington, DC, USA, 2002; pp. 59–90. [Google Scholar]
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.-K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lister, R.; O’Malley, R.C.; Tonti-Filippini, J.; Gregory, B.D.; Berry, C.C.; Millar, A.H.; Ecker, J.R. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 2008, 133, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Lolle, S.; Victor, J.L.; Young, J.M.; Pruitt, R.E. Genome-wide non-mendelian inheritance of etra-genomic information in Arabidopsis. Nature 2005, 434, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Springer, N.M.; Stupar, R.M. Allelic variation and heterosis in maize: How do two halves make more than a whole? Genome Res. 2007, 17, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, K.; Boelter, J.; Lolle, S.; Hopkins, M.; Goggi, S.; Palmer, R.G.; Sandhu, D. Evaluation of spontaneous generation of allelic variation in soybean in response to sexual hybridization and stress. Can. J. Plant Sci. 2015, 95, 405–415. [Google Scholar] [CrossRef]
- Haun, W.J.; Hyten, D.L.; Xu, W.W.; Gerhardt, D.J.; Albert, T.J.; Richmond, T.; Jeddeloh, J.A.; Jia, G.; Springer, N.M.; Vance, C.P.; et al. The composition and origins of genomic variation among individuals of the soybean reference cultivar Williams 82. Plant Physiol. 2011, 155, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Lasky, J.R.; Upadhyaya, H.D.; Ramu, P.; Deshpande, S.; Hash, C.T.; Bonnette, J.; Juenger, T.E.; Hyma, K.; Acharya, C.; Mitchell, S.E.; et al. Genome-environment associations in sorghum landraces predict adaptive traits. Sci. Adv. 2015, 1, e1400218. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, P.; Piano, E. Effect of selection under cultivation on morphological traits and yields of Ladino white clover landraces. Genet. Res. Crop Evol. 1997, 44, 405–410. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Dordas, C.; Papathanasiou, F.; Papadopoulos, I.; Pankou, C.; Gekas, F.; Ninou, E.; Mylonas, I.; Tzantarmas, C.; Petrevska, J.K.; et al. Improved plant yield efficiency is essential for maize rainfed production. Agron. J. 2015, 107, 1011–1018. [Google Scholar] [CrossRef]
- Tsaftaris, A.S. Apostolos Fasoulas, a laudation. Maydica 2005, 50, 3–8. [Google Scholar]
- Fasoulas, A.C. A moving block evaluation technique for improving the efficiency of pedigree selection. Euphytica 1987, 36, 473–478. [Google Scholar] [CrossRef]
- Fasoulas, A.C.; Fasoula, V.A. Honeycomb selection designs. Plant Breed. Rev. 1995, 13, 87–139. [Google Scholar]
- Tollenaar, M. Is low plant density a stress in maize? Maydica 1992, 37, 305–311. [Google Scholar]
- Kargiotidou, A.; Chatzivassiliou, E.; Tzantarmas, C.; Tokatlidis, I.S. Seed propagation at low density facilitated the selection of healthy plants to produce seeds with a reduced virus load in a lentil landrace. Seed Sci. Technol. 2015, 43, 31–39. [Google Scholar] [CrossRef]
- Vlachostergios, N.D.; Lithourgidis, A.S.; Roupakias, D.G. Effectiveness of single-plant selection at low density under organic environment: A field study with lentil. Crop Sci. 2011, 51, 41–51. [Google Scholar] [CrossRef]
- Mauromoustakos, A.; Fasoula, V.A.; Thompson, K. Honeycomb designs computing and analysis. In Proceedings of the ENAR Spring Meeting, International Biometric Society, Eastern North American Region, Tampa, FL, USA, 26–29 March 2006; p. 63.
- Fasoula, V.A. Prognostic breeding: A new paradigm for crop improvement. Plant Breed. Rev. 2013, 37, 297–347. [Google Scholar]
- Kargiotidou, A.; Vlachostergios, D.N.; Tzantarmas, C.; Mylonas, I.; Foti, C.; Menexes, G.; Polidoros, A.; Tokatlidis, I.S. Addressing huge spatial heterogeneity induced by virus infections in lentil breeding trials. J. Biol. Res. Thessalon. 2016, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Rodomiro, O. Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C.; Akar, T.; Baresel, J.P.; Bebeli, P.J.; Bettencourt, E.; Bladenopoulos, K.V.; Czembor, J.H.; Fasoula, D.A.; Katsiotis, A.; Koutis, K.; et al. Cereal landraces for sustainable agriculture. A review. Agron. Sustain. Dev. 2010, 30, 237–269. [Google Scholar] [CrossRef]
- Kotzamanidis, S.T.; Lithourgidis, A.S.; Roupakias, D.G. Plant density effect on the individual plant to plant yield variability expressed as coefficient of variation in barley. Span. J. Agric. Res. 2009, 7, 607–610. [Google Scholar] [CrossRef]
- Makkouk, K.M.; Kumari, S.G. Epidemiology and integrated management of persistently transmitted aphid-borne viruses of legume and cereal crops in West Asia and North Africa. Virus Res. 2009, 141, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tokatlidis, I.S. Addressing the yield by density interaction is a prerequisite to bridge the yield gap of rainfed wheat. Ann. Appl. Biol. 2014, 165, 27–42. [Google Scholar] [CrossRef]
- Abraham, B.; Araya, H.; Berhe, T.; Edwards, S.; Gujja, B.; Khadka, R.B.; Koma, Y.S.; Sen, D.; Sharif, A.; Styger, E.; Uphoff, N.; et al. The system of crop intensification: Reports from the field on improving agricultural production, food security, and resilience to climate change for multiple crops. Agric. Food Chem. 2014, 3, 4. [Google Scholar] [CrossRef]
- Fitzgerald, T.L.; Waters, D.L.; Brooks, L.O.; Henry, R.J. Fragrance in rice (Oryza sativa) is associated with reduced yield under salt treatment. J. Exp. Bot. 2010, 68, 292–300. [Google Scholar] [CrossRef]
- Dhillon, B.S.; Dua, R.P.; Brahmi, P.; Bisht, I.S. On farm conservation of plant genetic resources for food and agriculture. Curr. Sci. 2014, 87, 557–559. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokatlidis, I.; Vlachostergios, D. Sustainable Stewardship of the Landrace Diversity. Diversity 2016, 8, 29. https://doi.org/10.3390/d8040029
Tokatlidis I, Vlachostergios D. Sustainable Stewardship of the Landrace Diversity. Diversity. 2016; 8(4):29. https://doi.org/10.3390/d8040029
Chicago/Turabian StyleTokatlidis, Ioannis, and Dimitrios Vlachostergios. 2016. "Sustainable Stewardship of the Landrace Diversity" Diversity 8, no. 4: 29. https://doi.org/10.3390/d8040029
APA StyleTokatlidis, I., & Vlachostergios, D. (2016). Sustainable Stewardship of the Landrace Diversity. Diversity, 8(4), 29. https://doi.org/10.3390/d8040029





