Exploring Cladocera Assemblage and Responses to Land Use Patterns
Abstract
1. Introduction
2. Materials and Methods
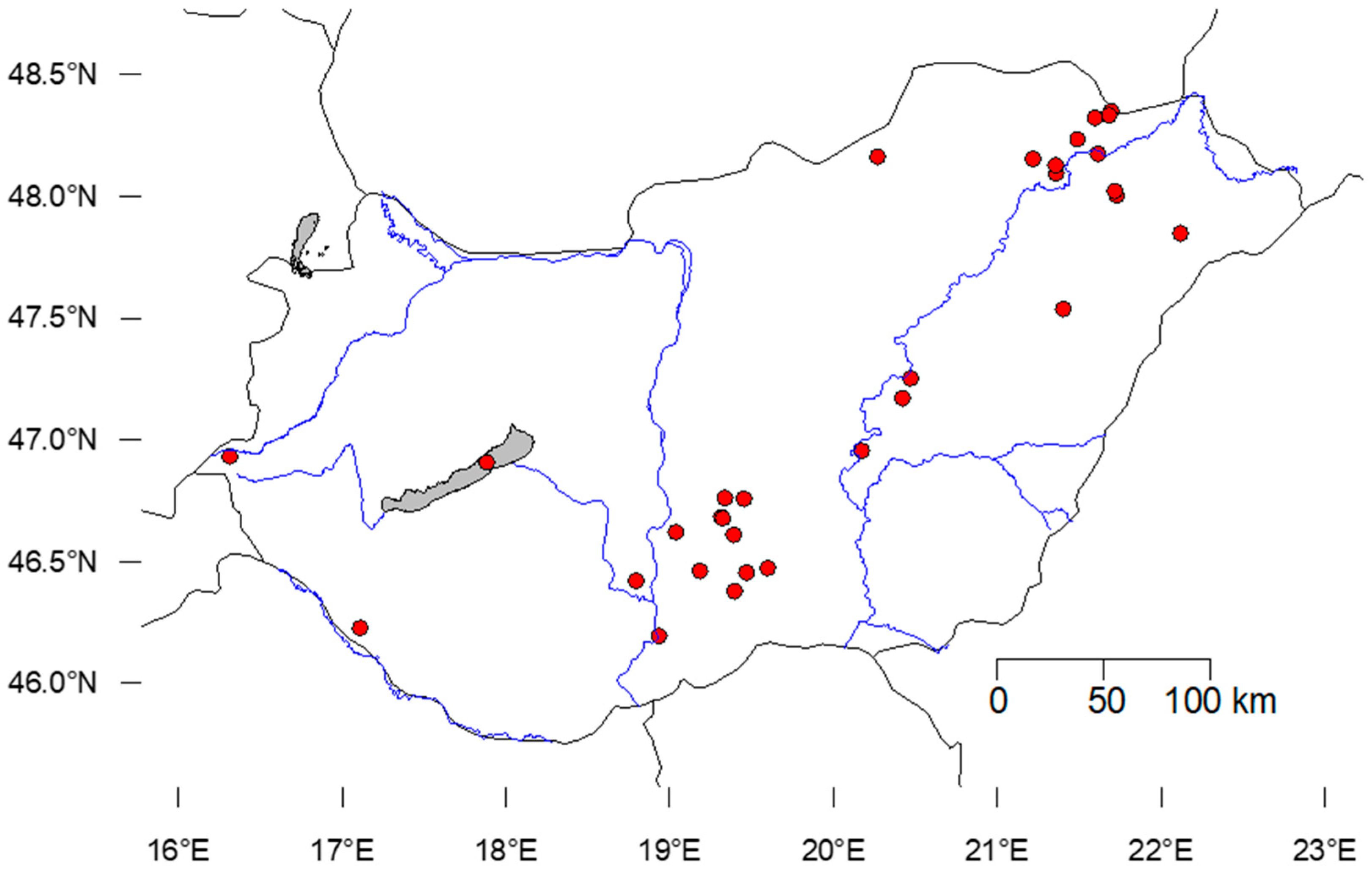
2.1. Study Area and the Measured Environmental Variables
2.2. Land Cover and Use Classification
2.3. Statistical Analysis
3. Results
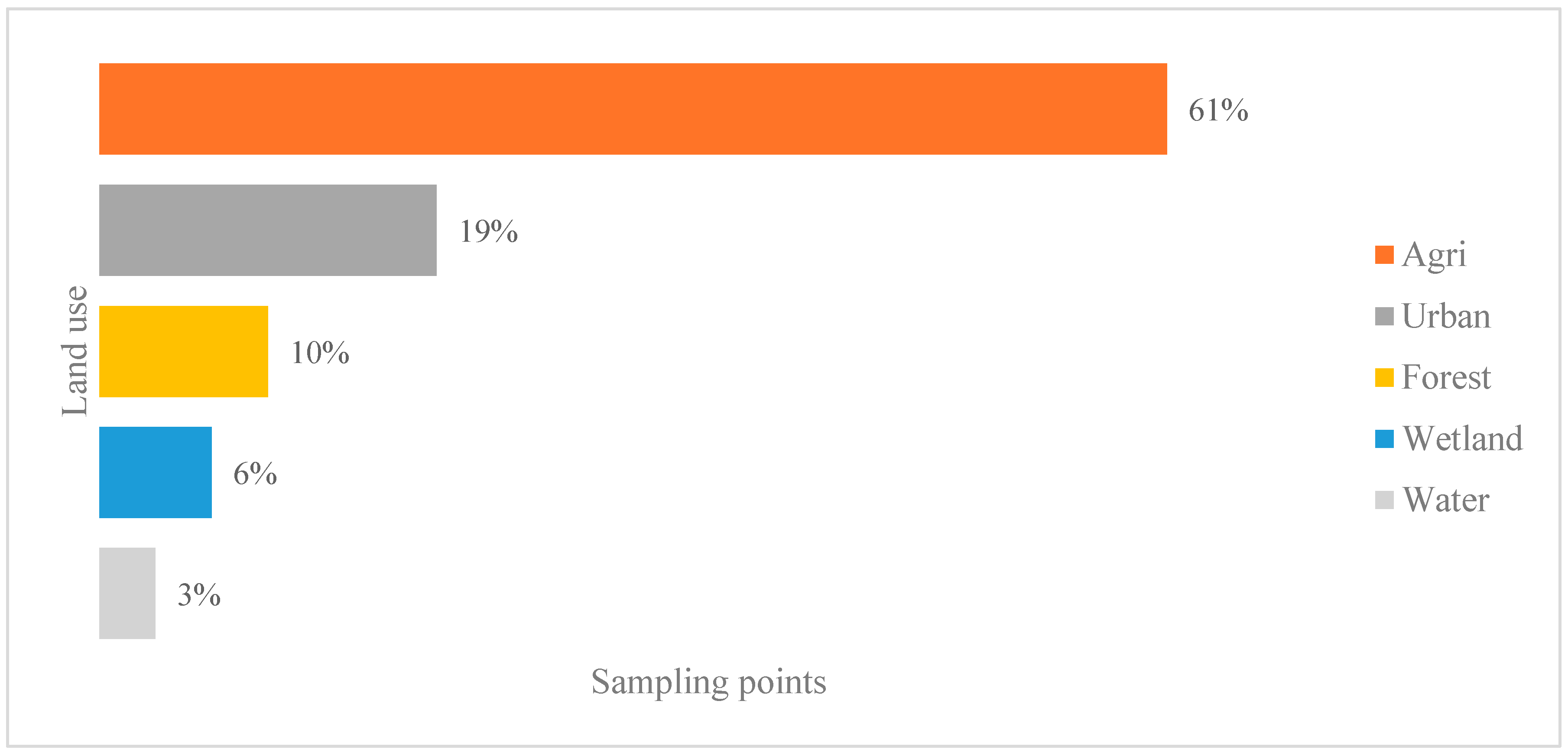
3.1. Land Use Description
3.2. Environmental Variables
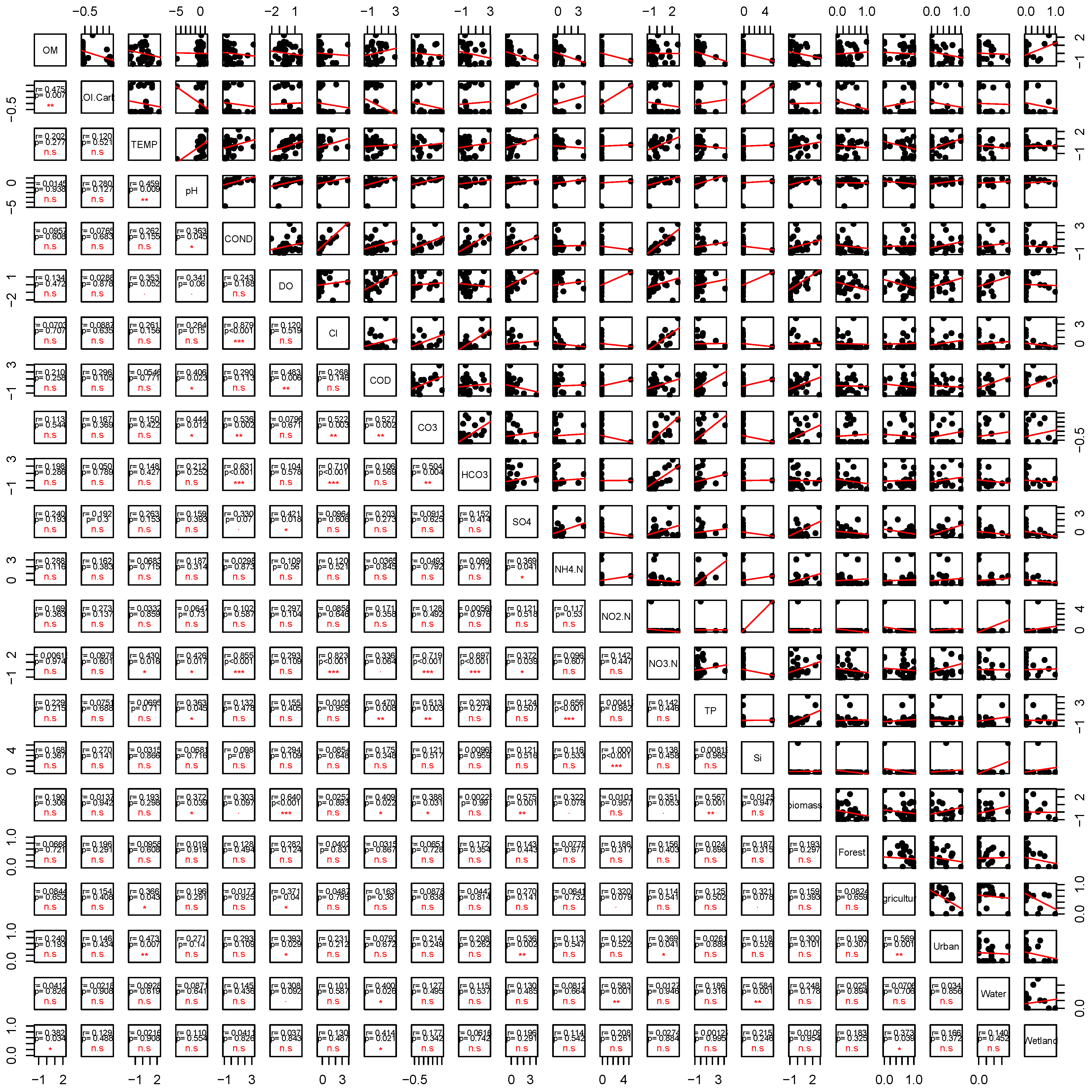
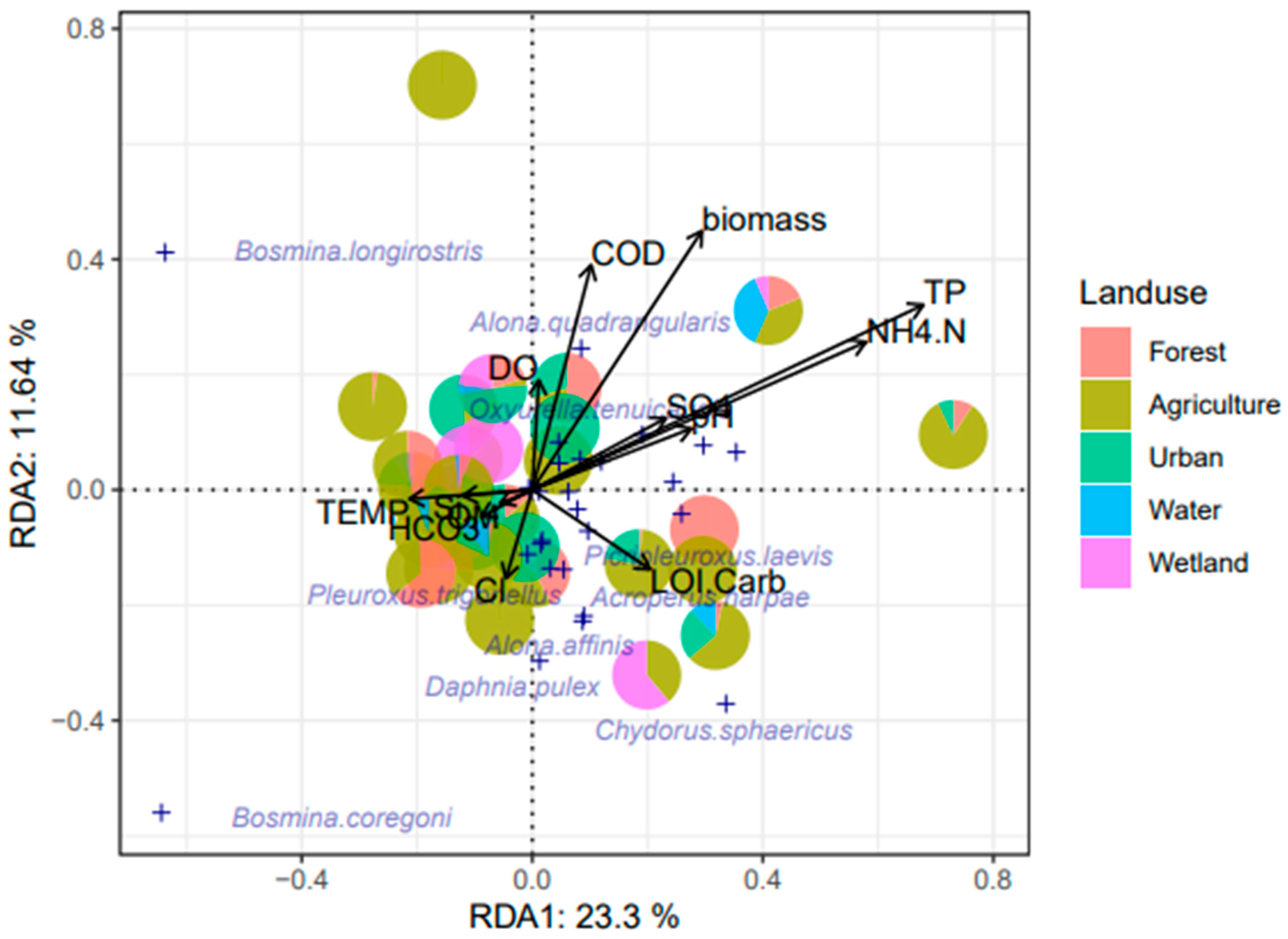
3.3. Correlation Between Environmental and Land Use Variables
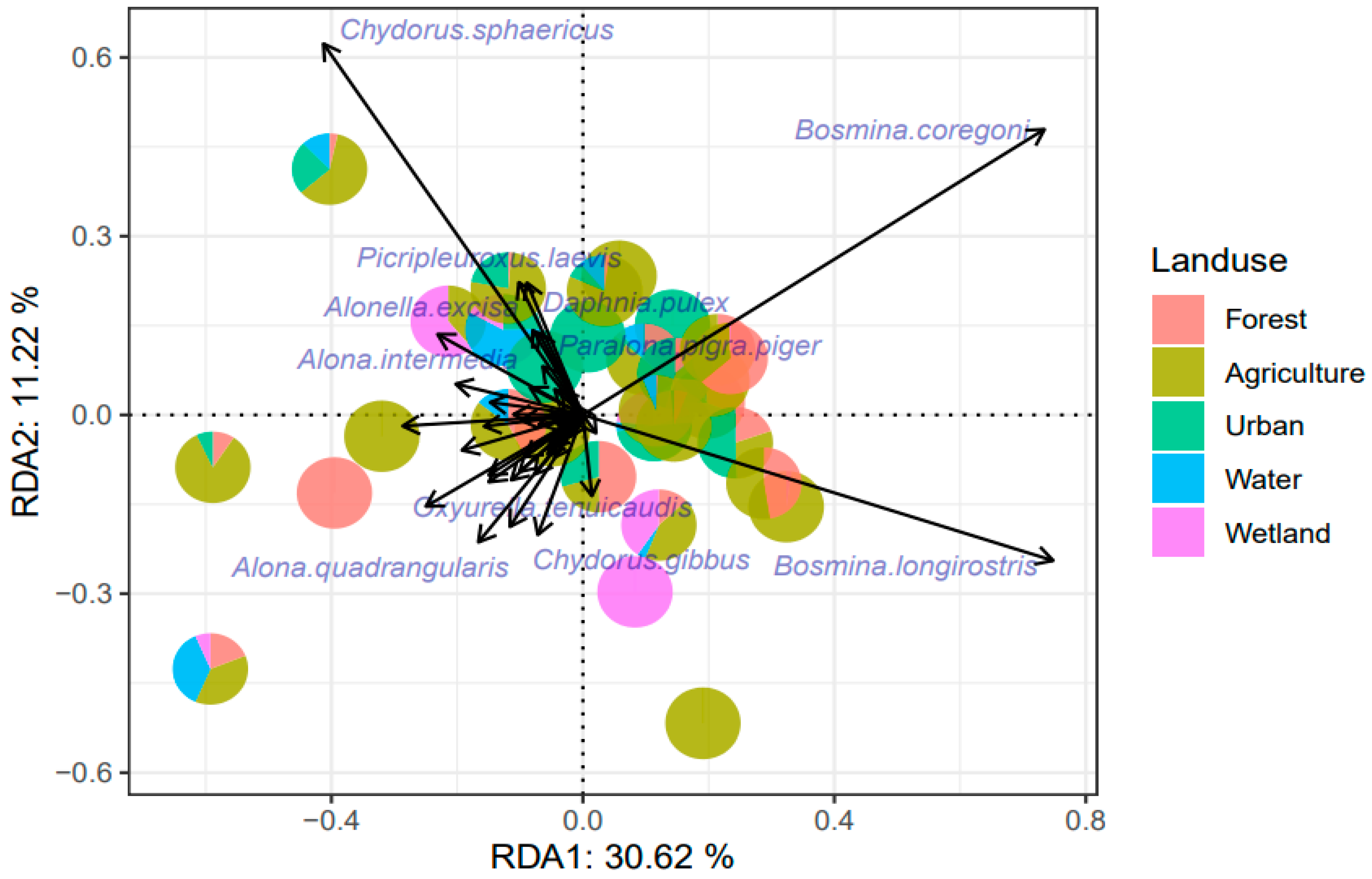
3.4. Cladocera Communities and Diversity Metrics
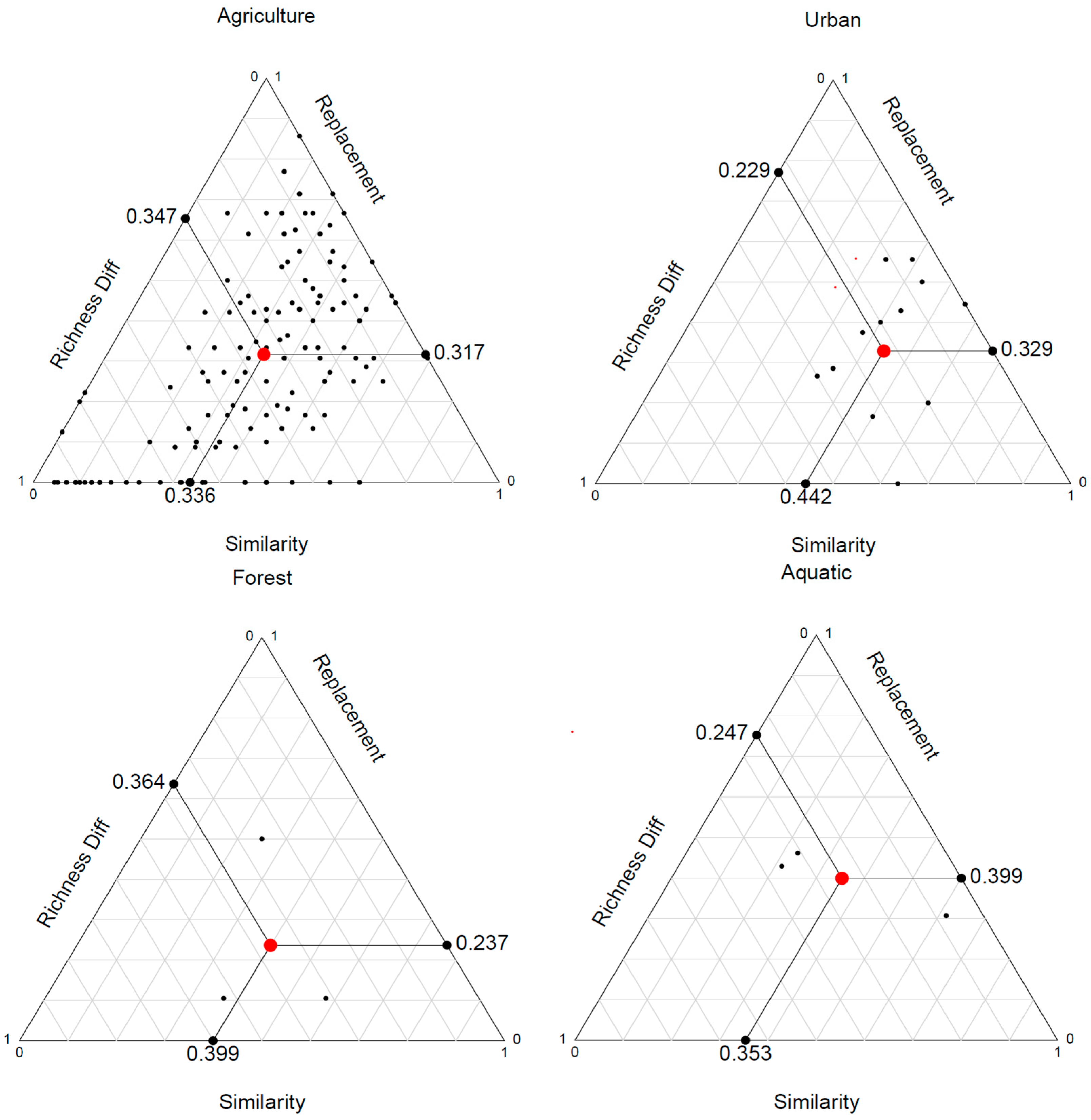
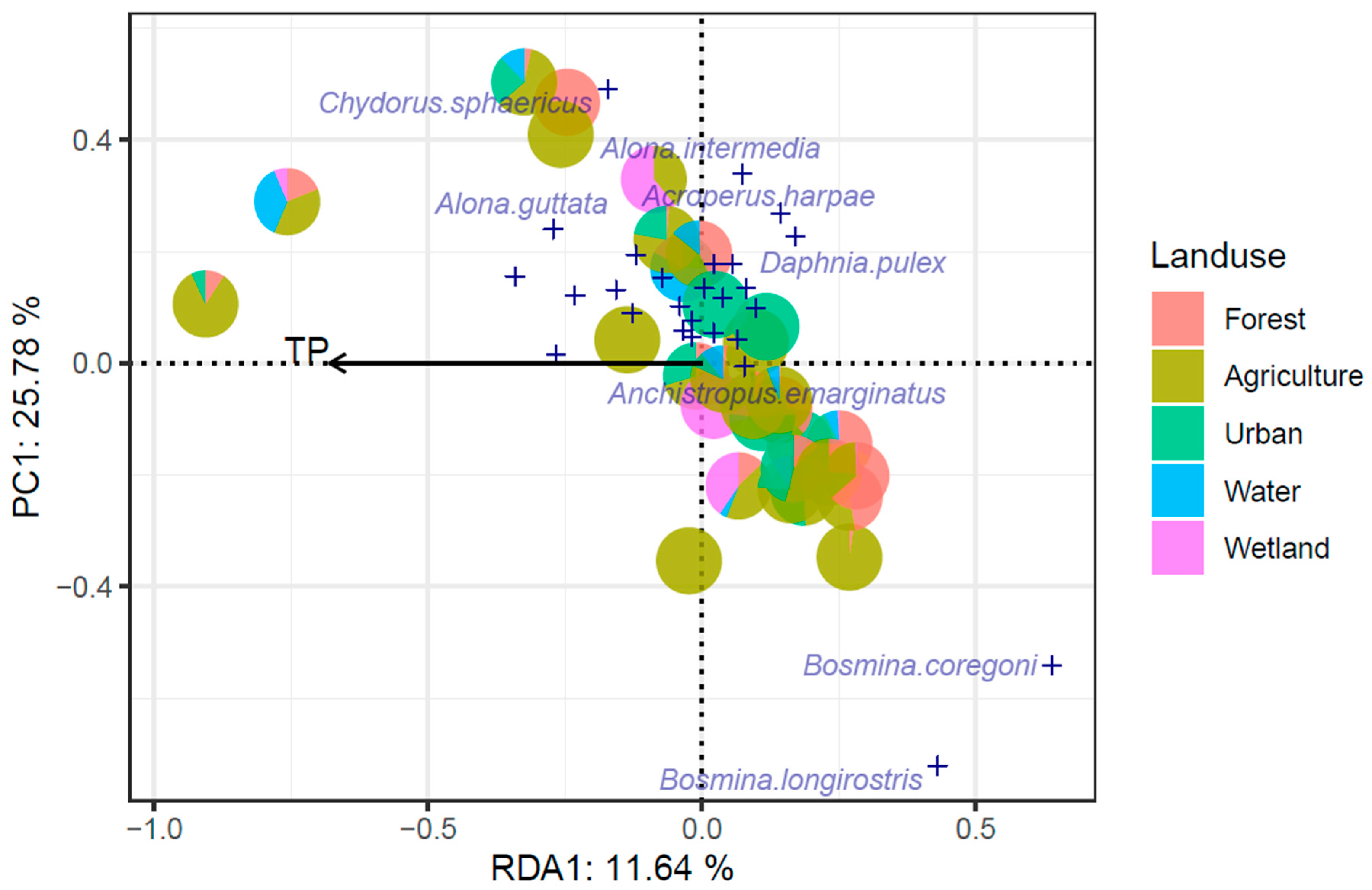
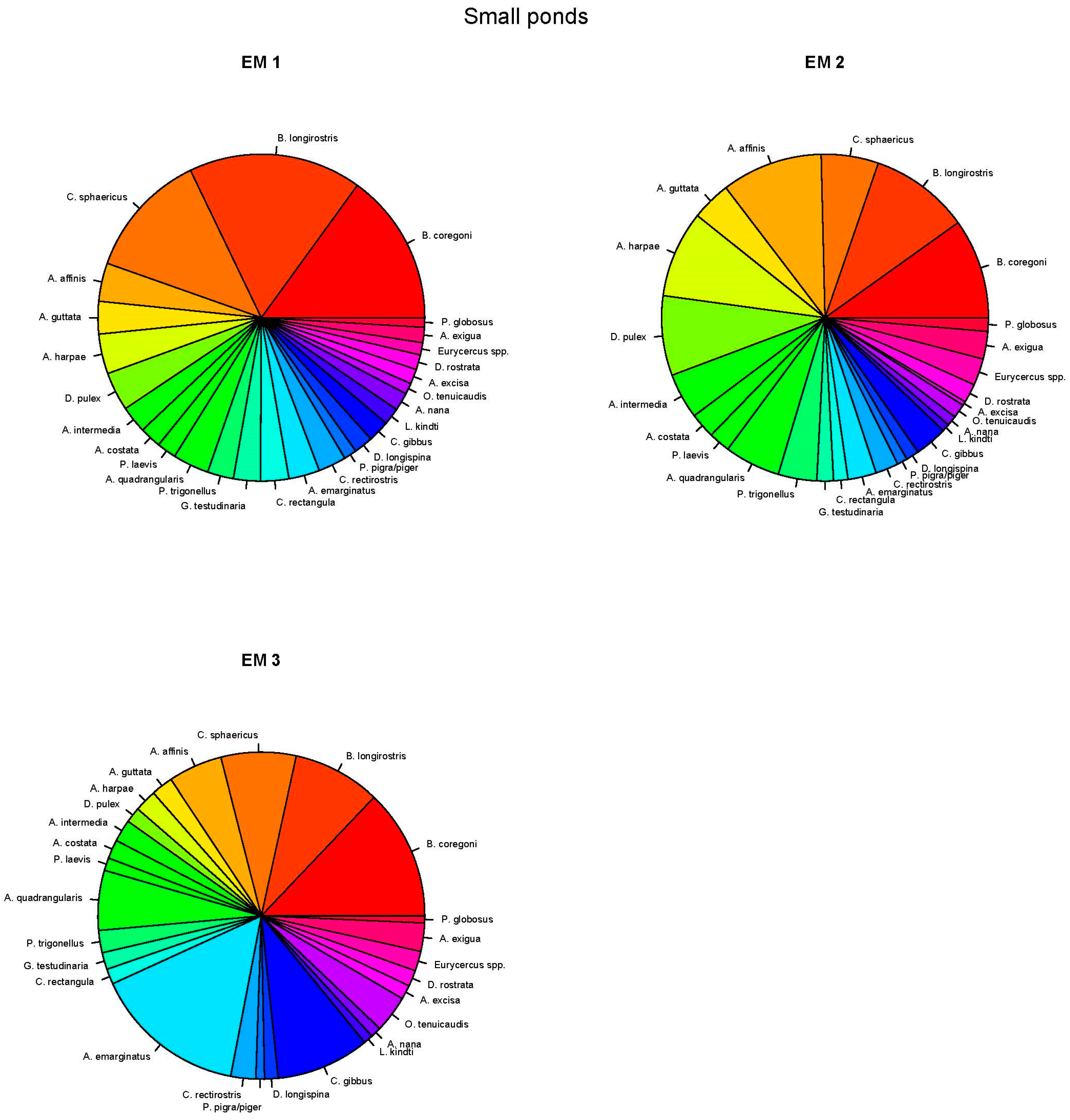
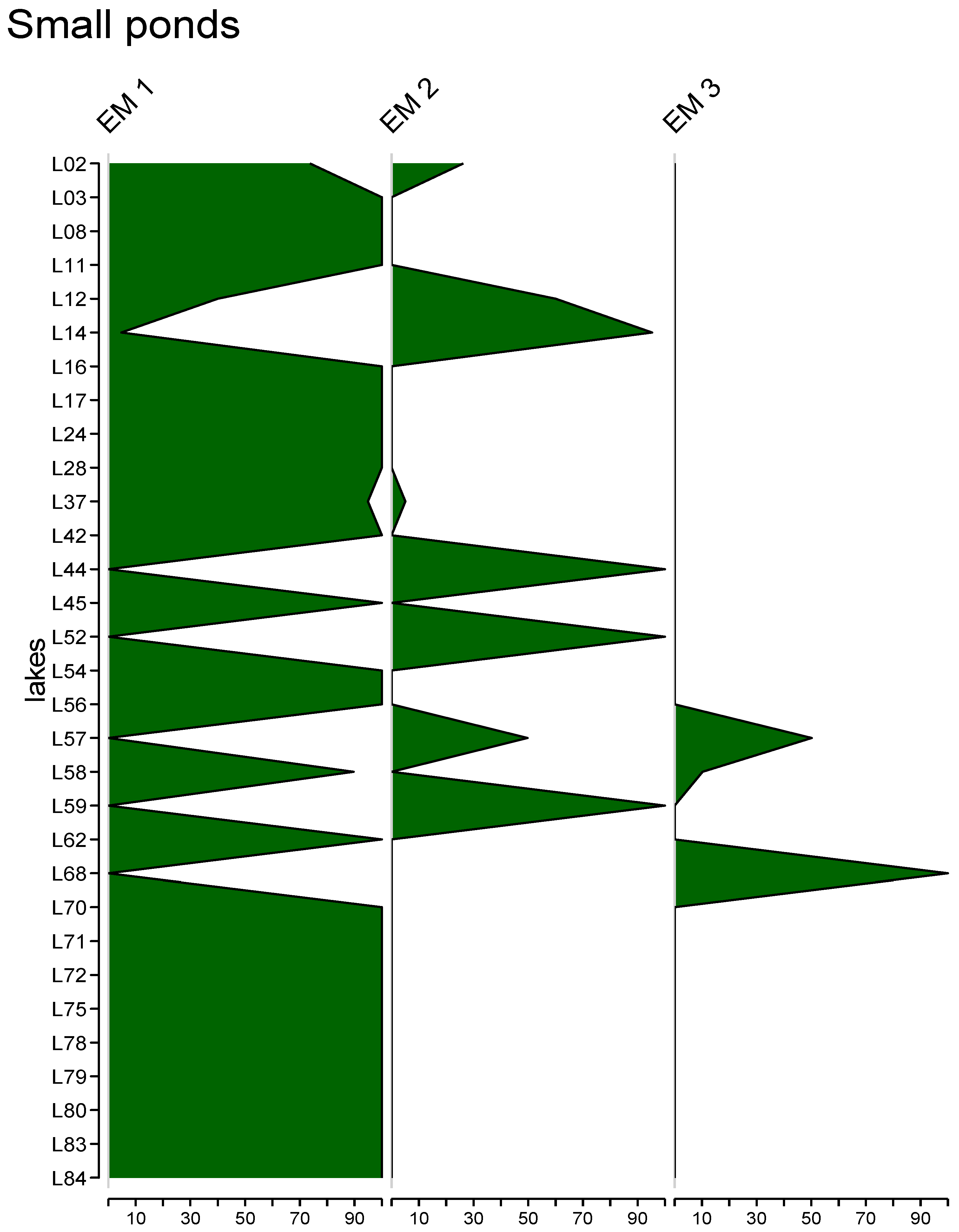
3.5. End-Member Mixing Analysis (EMMA) Results
4. Discussions
4.1. Environmental Variables and Land Use Types
4.2. Cladocera Analysis and Responses to Land Use Types
4.3. End-Member Analysis (EM1)
4.4. End-Member Analysis (EM2)
4.5. End-Member Analysis (EM3)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters | N | Mean | SD | Min | Max | Median | Skew | Kurtosis |
|---|---|---|---|---|---|---|---|---|
| organic matter (%) | 31 | 18.22 | 11.58 | 1.96 | 44.3 | 14.79 | 0.33 | −1.1 |
| carbohydrates | 31 | 26.05 | 37.85 | 0.01 | 91.93 | 0.15 | 0.83 | −1.26 |
| temperature (°C) | 31 | 25.65 | 2.57 | 21.1 | 31.5 | 25.6 | −0.13 | −0.57 |
| pH | 31 | 8.5 | 1.19 | 7.8 | 9.99 | 8.65 | −3.46 | 14.25 |
| conductivity (µS cm−1) | 31 | 684.97 | 528.06 | 129.7 | 2410 | 483 | 1.59 | 2.16 |
| dissolved oxygen | 31 | 9.4 | 3.32 | 2.51 | 15.24 | 8.93 | 0.09 | −0.77 |
| chloride | 31 | 7.3 | 11.41 | 0.35 | 51.5 | 2.75 | 2.32 | 5.25 |
| COD | 31 | 5.82 | 4.37 | 0.25 | 18.6 | 5.15 | 0.89 | 0.32 |
| carbonate | 31 | 0.23 | 0.32 | 0 | 1.05 | 0 | 1.24 | 0.18 |
| bicarbonate | 31 | 2.36 | 1.55 | 0.5 | 6.85 | 1.9 | 1.3 | 0.94 |
| sulphate | 31 | 19.38 | 26.88 | 1.06 | 128.41 | 12.39 | 2.45 | 6.52 |
| ammonium-N | 31 | 0.08 | 0.13 | 0.01 | 0.59 | 0.02 | 2.67 | 6.67 |
| nitrite-N | 31 | 39.03 | 213.1 | 0.08 | 1187.26 | 0.42 | 5.04 | 24.19 |
| nitrate-N | 31 | 1.14 | 0.8 | 0.23 | 3.39 | 0.9 | 1.16 | 0.42 |
| total phosphorus | 31 | 258.67 | 294.97 | 11.3 | 1492.18 | 159.54 | 2.84 | 8.39 |
| silicate | 31 | 44.14 | 224.57 | 0 | 1254 | 2.46 | 5.04 | 24.18 |
| biomass | 31 | 15,691.99 | 15,866.42 | 44.37 | 61,750.02 | 12,702.83 | 1.22 | 0.85 |
Appendix B
| Variable 1 | Variable 2 | Correlation (r) | p-Value |
|---|---|---|---|
| Cl | COND | 0.879 | <0.001 |
| NO3-N | COND | 0.855 | <0.001 |
| NO3-N | Cl | 0.823 | <0.001 |
| NO3-N | HCO3− | 0.697 | <0.001 |
| NH4-N | TP | 0.656 | <0.001 |
| DO | Biomass | 0.640 | <0.001 |
Appendix C
| Sampling Sites | Code | GPS_X | GPS_Y | Land Use | |
|---|---|---|---|---|---|
| 1 | Törökszentmiklós tó | L2 | 20.419521 | 47.17156 | Urban |
| 2 | Cibakházi Holt-Tisza | L3 | 20.17159 | 46.95589 | Agri |
| 3 | Verba tanya horgásztó | L8 | 21.7793 | 48.02191 | Agri |
| 4 | Kengyel tó | L11 | 21.35726 | 48.0941 | Agri |
| 5 | Bányató | L12 | 21.35691 | 48.12721 | Agri |
| 6 | Csónakázó-tó | L14 | 21.72837 | 48.0038 | Urban |
| 7 | Fegyverneki Holt-Tisza, Fegyvernek | L16 | 20.46954 | 47.25305 | Agri |
| 8 | Morotva | L17 | 21.61376 | 48.17478 | Forest |
| 9 | Szerencs, Homokos tó | L24 | 21.21629 | 48.15429 | Urban |
| 10 | Arlói-tó | L28 | 20.26854 | 48.16256 | Forest |
| 11 | Szelidi-tó | L37 | 19.03723 | 46.62177 | Agri |
| 13 | Vörösmocsár | L44 | 19.183946 | 46.4629 | Wetland |
| 14 | Csárda-szék magántó | L45 | 19.452948 | 46.758252 | Agri |
| 15 | Tasskertes halastótelep | L47 | 19.09431 | 47.01221 | Agri |
| 16 | Hársas-tó | L52 | 16.31281 | 46.93279 | Forest |
| 17 | Szt. István csatorna-tó | L54 | 18.93404 | 46.19504 | Urban |
| 18 | Kráter tó | L56 | 21.40256 | 47.53836 | Agri |
| 19 | Harkai tó | L57 | 19.59742 | 46.47481 | Agri |
| 20 | Sós-tó, strand | L58 | 19.46833 | 46.45547 | Agri |
| 21 | Kunfehértó horgásztó | L59 | 19.39552 | 46.37941 | Water |
| 22 | Szalma-tó | L62 | 19.314.694 | 46.683259 | Agri |
| 23 | Kolon-tó | L68 | 19.336312 | 46.762232 | Wetland |
| 24 | Zis-tó | L70 | 17.11058 | 46.22813 | Agri |
| 25 | Pék-tó | L71 | 19.324414 | 46.67826 | Agri |
| 26 | Vadkerti-tó, strand | L72 | 19.39031 | 46.61083 | Urban |
| 27 | Szénaréti tó | L75 | 22.11773 | 47.84809 | Urban |
| 28 | BSZ | L78 | 21.694857 | 48.3494 | Agri |
| 29 | VISS | L79 | 21.490396 | 48.23441 | Agri |
| 30 | KBNM | L80 | 21.596925 | 48.323261 | Agri |
| 31 | Sulc | L83 | 21.681958 | 48.332417 | Agri |
Appendix D
Appendix E
| Species Abbreviations | Scientific Name |
|---|---|
| A. harpae | Acroperus harpae |
| A. guttata | Alona guttata |
| A. quadrangularis | Alona quadrangularis |
| A. affinis | Alona affinis |
| A. intermedia | Alona intermedia |
| A. costata | Alona costata |
| A. rustica | Alona rustica |
| A. excisa | Alonella excisa |
| A. nana | Alonella nana |
| A. exigua | Alonella exigua |
| A. emarginatus | Anchistropus emarginatus |
| B. coregoni | Bosmina coregoni |
| B. longirostris | Bosmina longirostris |
| C. rectirostris | Camptocerus rectirostris |
| C.fennicus | Camptocerus fennicus |
| C. sphaericus | Chydorus sphaericus |
| C. gibbus | Chydorus gibbus |
| C. rectangula | Coronatella rectangula |
| C. lilljeborgi | Camptocercus lilljeborgi |
| D. pulex | Daphnia pulex |
| D. longispina | Daphnia longispina |
| D. brachyurum | Diaphanosoma brachyurum |
| D. rostrata | Disparalona rostrata |
| Eurycercus spp. | Eurycercus spp. |
| G. testudinaria | Graptoleberis testudinaria |
| L. acathocercoides | Leydigia acanthocercoides |
| L. kindtii | Leptodora kindtii |
| P. globusus | Pseudochydorus globosus |
| P. laevis | Pleuroxus laevis |
| P. trigonellus | Pleuroxus trigonellus |
| P. uncinatus | Pleuroxus uncinatus |
| O. tenuicaudis | Oxyurella tenuicaudis |
| M. dispar | Monospilus dispar |
Appendix F
| Code | Richness | Shannon’s | Simpson |
|---|---|---|---|
| L02 | 7 | 4.298 | 3.123 |
| L03 | 7 | 3.161 | 2.524 |
| L08 | 8 | 3.149 | 2.359 |
| L11 | 12 | 5.106 | 3.211 |
| L12 | 30 | 17.830 | 13.329 |
| L14 | 12 | 6.908 | 5.103 |
| L16 | 10 | 5.857 | 4.199 |
| L17 | 9 | 2.994 | 2.142 |
| L24 | 17 | 10.030 | 7.271 |
| L28 | 15 | 7.524 | 4.944 |
| L37 | 8 | 3.806 | 2.922 |
| L42 | 10 | 5.509 | 4.090 |
| L44 | 11 | 9.288 | 8.472 |
| L45 | 1 | 1.000 | 1.000 |
| L52 | 25 | 18.114 | 13.212 |
| L54 | 7 | 3.012 | 2.445 |
| L56 | 7 | 4.948 | 4.155 |
| L57 | 22 | 17.357 | 13.868 |
| L58 | 11 | 6.248 | 4.320 |
| L59 | 11 | 8.791 | 7.735 |
| L62 | 10 | 7.043 | 5.597 |
| L68 | 7 | 5.515 | 4.654 |
| L70 | 6 | 2.573 | 2.246 |
| L71 | 12 | 7.303 | 5.402 |
| L72 | 10 | 6.027 | 4.325 |
| L75 | 14 | 4.550 | 2.940 |
| L78 | 14 | 8.245 | 6.296 |
| L79 | 6 | 2.727 | 2.341 |
| L80 | 8 | 4.670 | 3.651 |
| L83 | 24 | 15.493 | 11.503 |
| L84 | 11 | 5.666 | 3.430 |
References
- Meyer, S.T.; Ebeling, A.; Eisenhauer, N.; Hertzog, L.; Hillebrand, H.; Milcu, A.; Pompe, S.; Abbas, M.; Bessler, H.; Buchmann, N.; et al. Effects of biodiversity strengthen over time as ecosystem functioning declines at low and increases at high biodiversity. Ecosphere 2016, 7, e01619. [Google Scholar] [CrossRef]
- Leira, M.; Cantonati, M. Effects of water-level fluctuations on lakes: An annotated bibliography. In Ecological Effects of Water-Level Fluctuations in Lakes Springer Netherlands; Springer: Dordrecht, The Netherlands, 2008; pp. 171–184. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Zhang, X.; Li, S.; Wu, H. Incorporating carbon sequestration into lake management: A potential perspective on climate change. Sci. Total Environ. 2023, 895, 164939. [Google Scholar] [CrossRef]
- Meyers, P.; Teranes, J. Sediment Organic Matter. In Tracking Environmental Change Using Lake Sediments; Kluwer: Dordrecht, The Netherlands, 2006; pp. 239–269. [Google Scholar] [CrossRef]
- Schmieder, K. European lake shores in danger-concepts for a sustainable development. Limnologica 2004, 34, 3–14. [Google Scholar] [CrossRef]
- Rawcliffe, R.; Sayer, C.D.; Woodward, G.; Grey, J.; Davidson, T.A.; Iwan Jones, J. Back to the future: Using palaeolimnology to infer long-term changes in shallow lake food webs. Freshw. Biol. 2010, 55, 600–613. [Google Scholar] [CrossRef]
- Dos Santos, N.G.; Chiarelli, L.J.; Morari, P.H.R.; de Souza, M.E.T.; Calixto, G.; Kato, B.E.D.; Rodrigues, G.L.D.P.; Figueira, L.C.; Castilho-Noll, M.S.M. How land use affects freshwater zooplankton communities: A global overview. Hydrobiologia 2024, 852, 2555–2580. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Oliver, T.H.; Morecroft, M.D. Interactions between climate change and land use change on biodiversity: Attribution problems, risks, and opportunities. Wiley Interdiscip. Rev. Clim. Change 2014, 5, 317–335. [Google Scholar] [CrossRef]
- Li, Y.; Xie, P.; Zhao, D.; Zhu, T.; Guo, L.; Zhang, J. Eutrophication strengthens the response of zooplankton to temperature changes in a high-altitude lake. Ecol. Evol. 2016, 6, 6690–6701. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Clinton, S.; Jefferson, A.; Manda, A.; McMillan, S. Urbanization effects on watershed hydrology and in-stream processes in the southern United States. Water 2010, 2, 605–648. [Google Scholar] [CrossRef]
- Semenova, A.S.; Tchougounov, V.K. The Distribution of Moina micrura Kurz, 1875 (Crustacea: Moinidae) in the Russian Part of the Vistula Lagoon (Baltic Sea). Russ. J. Biol. Invasions 2018, 9, 175–183. [Google Scholar] [CrossRef]
- Eggermont, H.; Martens, K. Preface: Cladocera crustaceans: Sentinels of environmental change. Hydrobiologia 2011, 676, 1–7. [Google Scholar] [CrossRef]
- Forró, L.; Korovchinsky, N.; Kotov, A.; Petrusek, A. Global diversity of cladocerans (Cladocera; Crustacea) in freshwater. Hydrobiologia 2008, 595, 177–184. [Google Scholar] [CrossRef]
- Kotov, A.; Forró, L.; Korovchinsky, N.M.; Petrusek, A. World Checklist of freshwater Cladocera Species; Catalogue of Life: Leiden, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Chen, G.; Dalton, C.; Taylor, D. Cladocera as indicators of trophic state in Irish lakes. J. Paleolimnol. 2010, 44, 465–481. [Google Scholar] [CrossRef]
- Gyulai, I.; Korponai, J.; Wamugi, S.M.A.; Jakab, J.; Kawu, U.A.; Soltész, A.G.; Karches, T.; Tumurtogoo, U. Cladocera and Geochemical Variables from Core Sediments Show Different Conditions of Hungarian Lakes. Water 2024, 16, 1310. [Google Scholar] [CrossRef]
- Shumate, B.C.; Schelske, C.L.; Crisman, T.L.; Kenney, W.F. Response of the cladoceran community to trophic state change in Lake Apopka, Florida. J. Paleolimnol. 2002, 27, 71–77. [Google Scholar] [CrossRef]
- Amsinck, S.L.; Jeppesen, E.; Landkildehus, F. Relationships between environmental variables and zooplankton subfossils in the surface sediments of 36 shallow coastal brackish lakes with special emphasis on the role of fish. J. Paleolimnol. 2005, 1, 39–51. [Google Scholar] [CrossRef]
- Bjerring, R.; Becares, E.; Declerck, S.; Gross, E.M.; Hansson, L.-A.; Kairesalo, T.; Nykänen, M.; Halkiewicz, A.; Kornijów, R.; Conde-Porcuna, J.M.; et al. Subfossil Cladocera in relation to contemporary environmental variables in 54 PanEuropean lakes. Freshw. Biol. 2009, 54, 2401–2417. [Google Scholar] [CrossRef]
- Korhola, A.; Rautio, M. Cladocera and Other Branchiopod Crustaceans. In Tracking Environmental Change Using Lake Sediments; Volume 4: Zoological Indicators; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; Volume 4, pp. 5–41. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as indicators in lakes: A scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Castanho Amaral, D.; de Fátima Bomfim, F.; Amodêo Lansac-Tôha, F. Drivers of zooplankton functional and taxonomic β-diversity in two neotropical floodplains: Implications for conservation. Biodivers. Conserv. 2024, 33, 3905–3922. [Google Scholar] [CrossRef]
- Gálvez, Á.; Castillo-Escrivà, A.; Magurran, A.; Alambiaga, I.; Bonilla, F.; Camacho, A.; García-Roger, E.M.; Iepure, S.; Miralles-Lorenzo, J.; Monrós, J.S.; et al. Higher alpha and gamma, but not beta diversity in tropical than in Mediterranean temporary ponds: A multi-taxon spatiotemporal approach. Limnol. Oceanogr. 2023, 68, 2402–2414. [Google Scholar] [CrossRef]
- Nevalainen, L.; Luoto, T. Relationship between cladoceran (Crustacea) functional diversity and lake trophic gradients. Funct. Ecol. 2016, 31, 488–498. [Google Scholar] [CrossRef]
- Selmeczy, G.B.; Tapolczai, K.; Padisák, J. Catchment land use drivers are weak predictors of lakes’ phytoplankton assemblage structure at functional group level. Hydrobiologia 2023, 850, 2075–2088. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.; Lemcke, G. Loss on Ignition as a Method for Estimating Organic and Carbonate Content in Sediments: Reproducibility and Comparability of Results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Frey, D.G. The Ecological Significance of Cladoceran Remains in Lake Sediments. Ecology 1960, 41, 684–699. [Google Scholar] [CrossRef]
- Szeroczynska, K.; Samaj A-Korj, K.; Szeroczyfiska, K.; Sarmaja-Korjonen, K. Atlas of Subfossil Cladocera from Central and Northern Europe; Friends of the Lower Vistula Society: Swiecie, Polska, 2007. [Google Scholar]
- Copernicus Land Monitoring Service. Corine Land Cover (CLC) Datasets. 2018. Available online: https://doi.org/10.2909/71c95a07-e296-44fc-b22b-415f42acfdf0 (accessed on 21 August 2025).
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Telford, R.J.; Birks, H.J.B. A novel method for assessing the statistical significance of quantitative reconstructions inferred from biotic assemblages. Quat. Sci. Rev. 2011, 30, 1272–1278. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Podani, J.; Schmera, D. A new conceptual and methodological framework for exploring and explaining pattern in presence-absence data. Oikos 2011, 120, 1625–1638. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Norsk Institutt for Naturforskning: Trondheim, Norway, 2018. [Google Scholar]
- Oksanen, J.; Gavin, L.; Guillaume Blanchet, S.; Kindt, R.; Legendre, P.; Peter, R.; Minchin, R.B.; O’Hara; Peter Solymos, M.; Henry, H.; et al. Ordination Methods, Diversity Analysis, and Other Functions for Community and Vegetation Ecologists. 2016. Available online: https://vegandevs.github.io/vegan/ (accessed on 21 August 2025).
- Hungarian Central Statistical Office. Agriculture Database; Hungarian Central Statistical office: Budapest, Hungary.
- Zawisza, E.; Zawiska, I.; Correa-Metrio, A. Cladocera Community Composition as a Function of Physicochemical and Morphological Parameters of Dystrophic Lakes in NE Poland. Wetlands 2016, 36, 1131–1142. [Google Scholar] [CrossRef]
- Hu, S.; Niu, Z.; Chen, Y.; Li, L.; Zhang, H. Global wetlands: Potential distribution, wetland loss, and status. Sci. Total Environ. 2017, 586, 319–327. [Google Scholar] [CrossRef]
- Cheng, L.; Xue, B.; Yao, S.; Liu, J. Response of Cladocera fauna to environmental change based on sediments from Shengjin Lake, a Yangtze River-connected lake in China. Quat. Int. 2020, 536, 52–59. [Google Scholar] [CrossRef]
- Keatley, B.E.; Bennett, E.M.; MacDonald, G.K.; Taranu, Z.E.; Gregory-Eaves, I. Land-use legacies are important determinants of lake eutrophication in the anthropocene. PLoS ONE 2011, 6, e15913. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Bu, X.; Chen, J.; Zhou, F.; Chen, L.; Liu, M.; Tan, X.; Yu, T.; Zhang, W.; Mi, Z.; et al. Estimation of nutrient discharge from the Yangtze River to the East China Sea and the identification of nutrient sources. J. Hazard. Mater. 2017, 321, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Lanka, A.; Dimante-Deimantovica, I.; Saarni, S.; Stivrins, N.; Tylmann, W.; Zawiska, I.; Veski, S. Urbanization-driven Cladocera community shifts in the lake-a case study from Baltic region, Europe. Anthropocene 2024, 46, 100439. [Google Scholar] [CrossRef]
- DeSellas, A.M.; Paterson, A.M.; Sweetman, J.N.; Smol, J.P. Cladocera assemblages from the surface sediments of south-central Ontario (Canada) lakes and their relationships to measured environmental variables. Hydrobiologia 2008, 600, 105–119. [Google Scholar] [CrossRef]
- Ji, D.; Han, Y.; Long, L.; Xin, X.; Xu, H.; Qiu, S.; Meng, J.; Zhao, X.; Huang, Y.; Liu, D. Hypoxia and its feedback response to algal blooms and CH4 emissions in subtropical reservoirs. Front. Ecol. Evol. 2023, 11, 1297047. [Google Scholar] [CrossRef]
- Sarmaja-Korjonen, K.; Hakojärvi, M.; Korhola, A. Subfossil remains of an unknown chydorid (Anomopoda: Chydoridae) from Finland. Hydrobiologia 2000, 436, 165–169. [Google Scholar] [CrossRef]
- Norwegian Biodiversity Information Centre. Small Crustaceans in Fresh Waters. March 2017. Available online: https://biodiversity.no/Pages/214484/ (accessed on 21 August 2025).
- Ngo Ndje, A.C.N.; Mfayakouo, C.B.; Fadil-Djenabou, S.; Ndjigui, P.D. Multi-method characterization of the recent sediment from the Dibi subsidence lake in the tropical Adamawa region (central Cameroon): Implications for the palaeoenvironmental reconstruction. Int. J. Sediment Res. 2024, 39, 110–130. [Google Scholar] [CrossRef]
- Lanka, A.; Poska, A.; Bakumenko, V.; Dimante-Deimantovica, I.; Liiv, M.; Stivrins, N.; Zagars, M.; Veski, S. Subfossil Cladocera as indicators of pH, trophic state and conductivity: Separate and combined effects in hemi boreal freshwater lakes. Ecol. Indic. 2024, 167, 112592. [Google Scholar] [CrossRef]
- Bledzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Creed, R.P.; Cherry, R.P.; Pflaum, J.R.; Wood, C.J. Dominant species can produce a negative relationship between species diversity and ecosystem function. Oikos 2009, 118, 723–732. [Google Scholar] [CrossRef]
- Gliwicz, Z.M.; Szymanska, E.; Wrzosek, D. Body size distribution in Daphnia populations as an effect of prey selectivity by planktivorous fish. Hydrobiologia 2010, 643, 5–19. [Google Scholar] [CrossRef]
- Zawiska, I.; Correa-Metrio, A.; Rzodkiewicz, M.; Wolski, J. Cladocera assemblages indicate environmental gradients of lake productivity and morphometry in central Europe. Boreas 2025, 54, 258–272. [Google Scholar] [CrossRef]


| Min | Max | Median | Mean | |
|---|---|---|---|---|
| Richness | 1 | 30 | 10 | 12 |
| Shannon’s index | 1 | 14 | 6 | 7 |
| Simpson index | 1 | 18 | 4 | 5 |
| Inertia | Proportion | |
|---|---|---|
| Total | 0.3402 | 1.0000 |
| Constrained | 0.0669 | 0.1966 |
| Unconstrained | 0.2733 | 0.8034 |
| Inertia | Proportion | |
|---|---|---|
| Conditioned | 0.06687 | 0.1966 |
| Constrained | 0.16192 | 0.4759 |
| Unconstrained | 0.11142 | 0.3275 |
| Inertia | Proportion | |
|---|---|---|
| Total | 0.3402 | 1.0000 |
| Constrained | 0.0396 | 0.1164 |
| Unconstrained | 0.3006 | 0.8836 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wamugi, S.M.A.; Gyulai, I.; Jakab, J.; Kawu, U.A.; Soltész, A.G.; Böjthe, A.; Sajtos, Z.; Selmeczy, G.; Korponai, J. Exploring Cladocera Assemblage and Responses to Land Use Patterns. Diversity 2025, 17, 642. https://doi.org/10.3390/d17090642
Wamugi SMA, Gyulai I, Jakab J, Kawu UA, Soltész AG, Böjthe A, Sajtos Z, Selmeczy G, Korponai J. Exploring Cladocera Assemblage and Responses to Land Use Patterns. Diversity. 2025; 17(9):642. https://doi.org/10.3390/d17090642
Chicago/Turabian StyleWamugi, Sheila Mumbi A., István Gyulai, Jázmin Jakab, Umar Abba Kawu, Andor G. Soltész, Andrea Böjthe, Zsófi Sajtos, Géza Selmeczy, and Janos Korponai. 2025. "Exploring Cladocera Assemblage and Responses to Land Use Patterns" Diversity 17, no. 9: 642. https://doi.org/10.3390/d17090642
APA StyleWamugi, S. M. A., Gyulai, I., Jakab, J., Kawu, U. A., Soltész, A. G., Böjthe, A., Sajtos, Z., Selmeczy, G., & Korponai, J. (2025). Exploring Cladocera Assemblage and Responses to Land Use Patterns. Diversity, 17(9), 642. https://doi.org/10.3390/d17090642









