Marine Highways and Barriers: A Case Study of Limacina helicina Phylogeography Across the Siberian Arctic Shelf Seas
Abstract
1. Introduction
1.1. Biology and Ecology of L. helicina
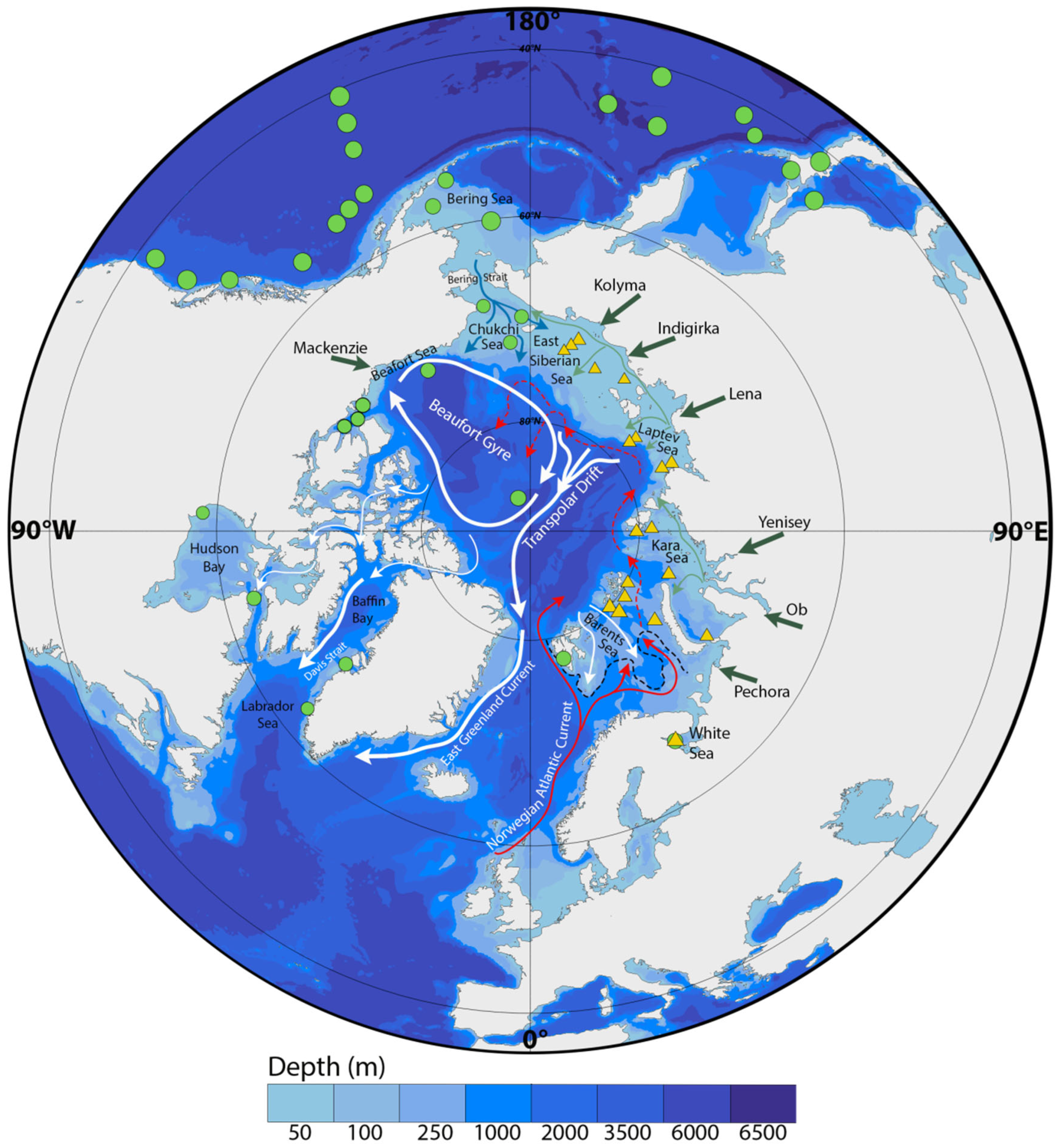
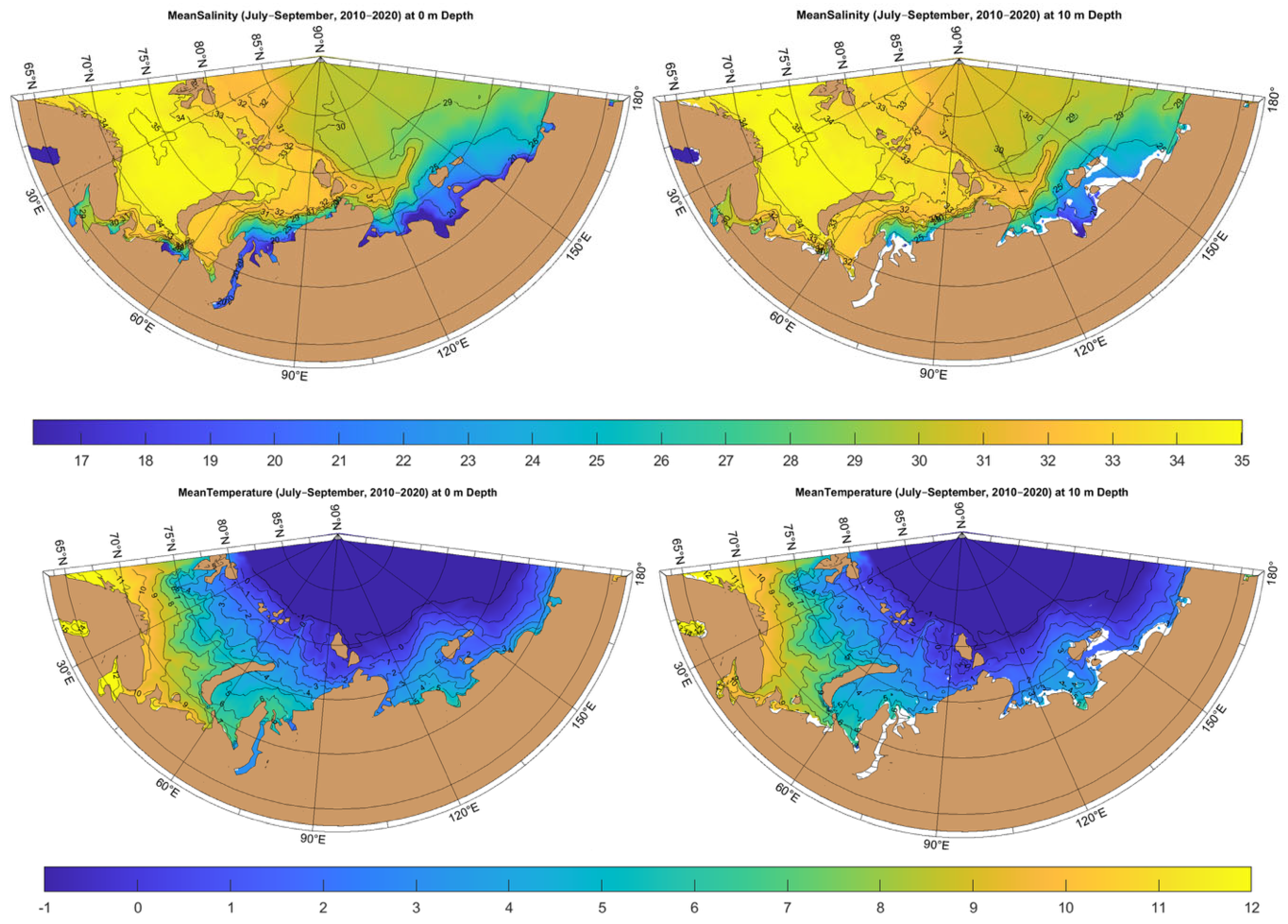
1.2. Regional Setting and Oceanographic Context of the Siberian Arctic Seas
2. Materials and Methods
2.1. Collection of Samples
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Phylogenetic Analysis
3. Results
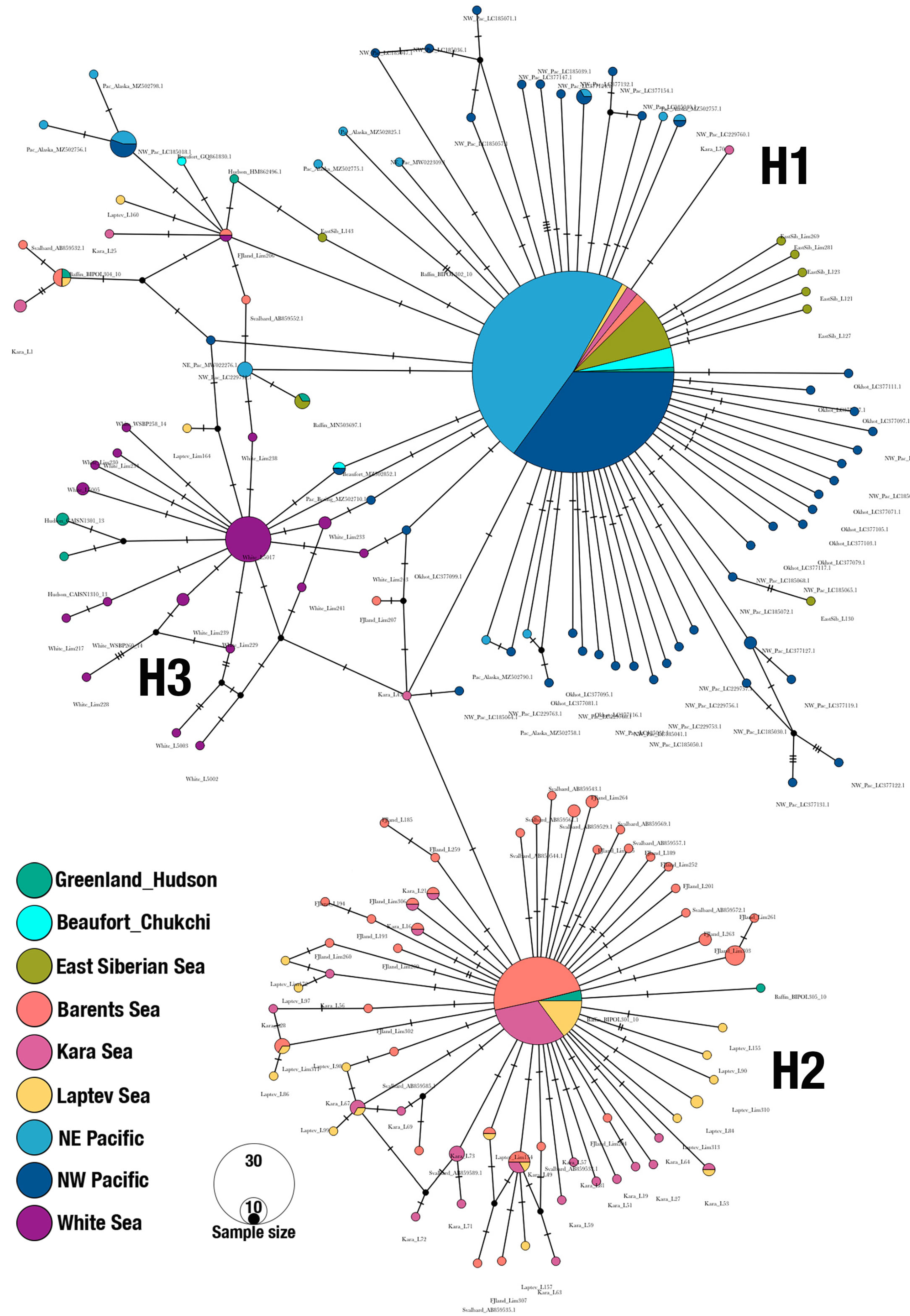
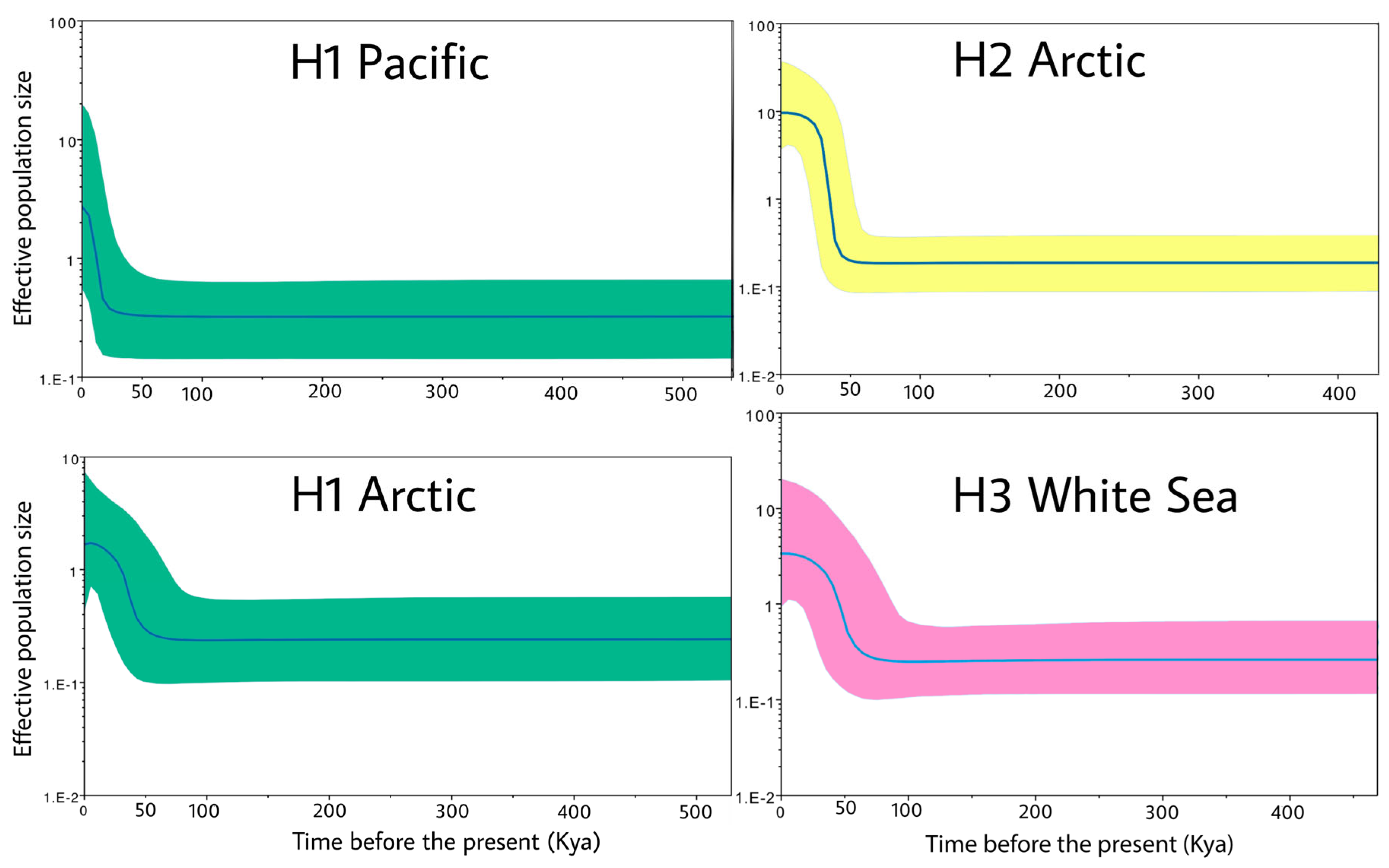
3.1. Population Structure and Demographic Patterns
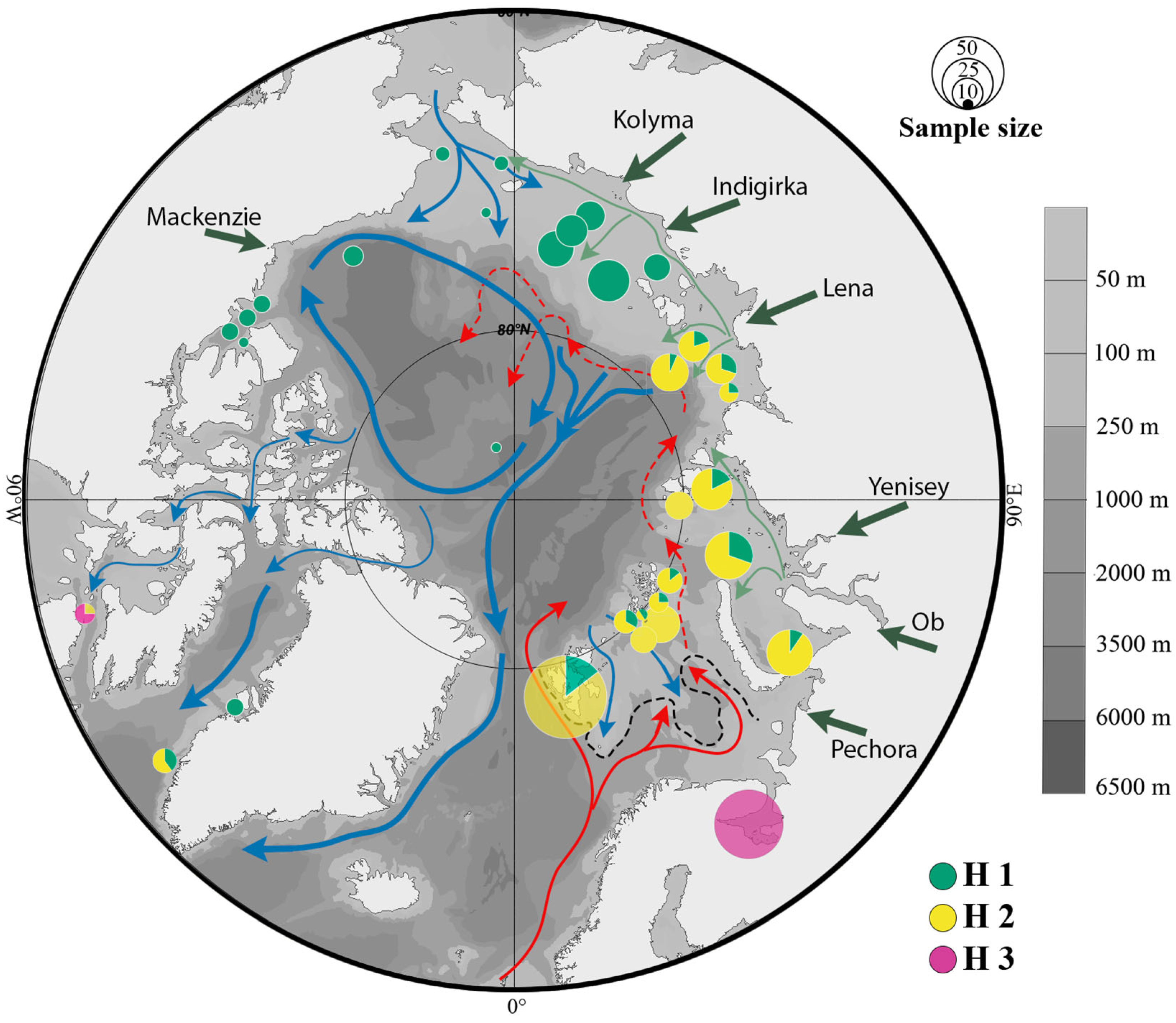
3.2. Haplogroups of Limacina helicina and Their Geographical Ranges
4. Discussion
4.1. Environmental Preferences and Habitat Conditions of Limacina helicina
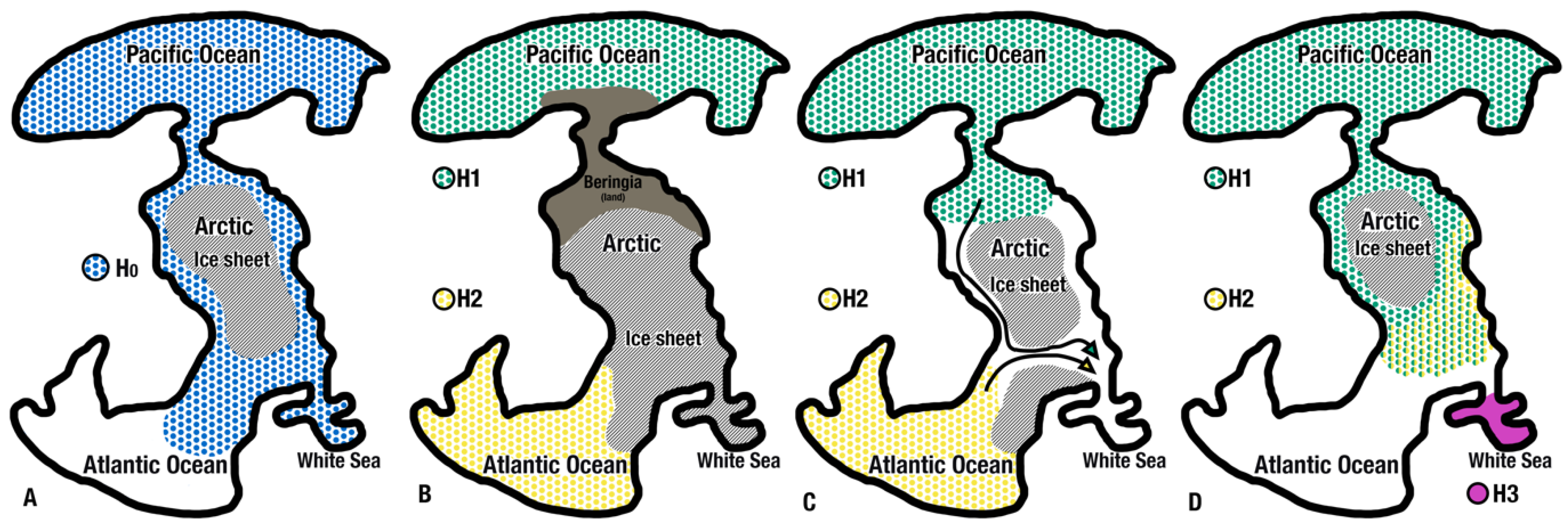
4.2. Palaeoecological Processes and Phylogeography
4.3. Contemporary Hydrographic Barriers and Their Influence on the Distribution of L. helicina
4.4. The White Sea: Isolation and Local Population Specificity
5. Conclusions: Implications for Arctic Pelagic Dynamics and Future Projections
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lalli, C.M.; Gilmer, R.W. The Thecosomes, Shelled Pteropods. In Pelagic Snails. The Biology of Holoplanktonic Gastropod Mollusks; Lalli, C.M., Gilmer, R.W., Eds.; Stanford University Press: Stanford, CA, USA, 1989; pp. 58–166. [Google Scholar]
- Hunt, B.P.V.; Pakhomov, E.A.; Hosie, G.W.; Siegel, V.; Ward, P.; Bernard, K. Pteropods in Southern Ocean Ecosystems. Prog. Oceanogr. 2008, 78, 193–221. [Google Scholar] [CrossRef]
- Doubleday, A.J.; Hopcroft, R.R. Interannual Patterns during Spring and Late Summer of Larvaceans and Pteropods in the Coastal Gulf of Alaska, and Their Relationship to Pink Salmon Survival. J. Plankton Res. 2015, 37, 134–150. [Google Scholar] [CrossRef]
- Pasternak, A.F.; Drits, A.V.; Gopko, M.V.; Flint, M.V. Influence of Environmental Factors on the Distribution of Pteropods Limacina Helicina (Phipps, 1774) in Siberian Arctic Seas. Oceanology 2020, 60, 490–500. [Google Scholar] [CrossRef]
- Gilmer, R.; Harbison, G. Diet of Limacina Helicina (Gastropoda: Thecosomata) in Arctic Waters in Midsummer. Mar. Ecol. Prog. Ser. 1991, 77, 125–134. [Google Scholar] [CrossRef]
- Bé, A.W.H.; Gilmer, R.W. A Zoogeographic and Taxonomic Review of Euthecosomatous Pteropoda. In Oceanic Micropaleontology; Academic Press: Cambridge, MA, USA, 1977; pp. 733–808. [Google Scholar]
- Guinotte, J.M.; Fabry, V.J. Ocean Acidification and Its Potential Effects on Marine Ecosystems. Ann. N. Y. Acad. Sci. 2008, 1134, 320–342. [Google Scholar] [CrossRef]
- Bednaršek, N.; Naish, K.-A.; Feely, R.A.; Hauri, C.; Kimoto, K.; Hermann, A.J.; Michel, C.; Niemi, A.; Pilcher, D. Integrated Assessment of Ocean Acidification Risks to Pteropods in the Northern High Latitudes: Regional Comparison of Exposure, Sensitivity and Adaptive Capacity. Front. Mar. Sci. 2021, 8, 671497. [Google Scholar] [CrossRef]
- Mekkes, L.; Renema, W.; Bednaršek, N.; Alin, S.R.; Feely, R.A.; Huisman, J.; Roessingh, P.; Peijnenburg, K.T.C.A. Pteropods Make Thinner Shells in the Upwelling Region of the California Current Ecosystem. Sci. Rep. 2021, 11, 1731. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic Ocean Acidification over the Twenty-First Century and Its Impact on Calcifying Organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Niemi, A.; Bednaršek, N.; Michel, C.; Feely, R.A.; Williams, W.; Azetsu-Scott, K.; Walkusz, W.; Reist, J.D. Biological Impact of Ocean Acidification in the Canadian Arctic: Widespread Severe Pteropod Shell Dissolution in Amundsen Gulf. Front. Mar. Sci. 2021, 8, 600184. [Google Scholar] [CrossRef]
- Comeau, S.; Jeffree, R.; Teyssié, J.-L.; Gattuso, J.-P. Response of the Arctic Pteropod Limacina Helicina to Projected Future Environmental Conditions. PLoS ONE 2010, 5, e11362. [Google Scholar] [CrossRef]
- Manno, C.; Bednaršek, N.; Tarling, G.A.; Peck, V.L.; Comeau, S.; Adhikari, D.; Bakker, D.C.E.; Bauerfeind, E.; Bergan, A.J.; Berning, M.I.; et al. Shelled Pteropods in Peril: Assessing Vulnerability in a High CO2 Ocean. Earth-Sci. Rev. 2017, 169, 132–145. [Google Scholar] [CrossRef]
- Buitenhuis, E.T.; Le Quéré, C.; Bednaršek, N.; Schiebel, R. Large Contribution of Pteropods to Shallow CaCO3 Export. Glob. Biogeochem. Cycles 2019, 33, 458–468. [Google Scholar] [CrossRef]
- Burridge, A.K.; Hörnlein, C.; Janssen, A.W.; Hughes, M.; Bush, S.L.; Marlétaz, F.; Gasca, R.; Pierrot-Bults, A.C.; Michel, E.; Todd, J.A.; et al. Time-Calibrated Molecular Phylogeny of Pteropods. PLoS ONE 2017, 12, e0177325. [Google Scholar] [CrossRef]
- Abyzova, G.A.; Nikitin, M.A.; Popova, O.V.; Pasternak, A.F. Genetic Population Structure of the Pelagic Mollusk Limacina Helicina in the Kara Sea. PeerJ 2018, 6, e5709. [Google Scholar] [CrossRef]
- Janssen, A.W.; Bush, S.L.; Bednaršek, N. The Shelled Pteropods of the Northeast Pacific Ocean (Mollusca: Heterobranchia, Pteropoda). Zoosymposia 2019, 13, 305–346. [Google Scholar] [CrossRef]
- Choo, L.Q.; Bal, T.M.P.; Choquet, M.; Smolina, I.; Ramos-Silva, P.; Marlétaz, F.; Kopp, M.; Hoarau, G.; Peijnenburg, K.T.C.A. Novel Genomic Resources for Shelled Pteropods: A Draft Genome and Target Capture Probes for Limacina Bulimoides, Tested for Cross-Species Relevance. BMC Genom. 2020, 21, 11. [Google Scholar] [CrossRef]
- Vidal-Miralles, J.; Kohnert, P.; Monte, M.; Salvador, X.; Schrödl, M.; Moles, J. Between Sea Angels and Butterflies: A Global Phylogeny of Pelagic Pteropod Molluscs. Mol. Phylogenet. Evol. 2024, 201, 108183. [Google Scholar] [CrossRef]
- Sromek, L.; Lasota, R.; Wolowicz, M. Impact of Glaciations on Genetic Diversity of Pelagic Mollusks: Antarctic Limacina Antarctica and Arctic Limacina Helicina. Mar. Ecol. Prog. Ser. 2015, 525, 143–152. [Google Scholar] [CrossRef]
- Shimizu, K.; Noshita, K.; Kimoto, K.; Sasaki, T. Phylogeography and Shell Morphology of the Pelagic Snail Limacina Helicina in the Okhotsk Sea and Western North Pacific. Mar. Biodivers. 2021, 51, 22. [Google Scholar] [CrossRef]
- Jakobsson, M.; Andreassen, K.; Bjarnadóttir, L.R.; Dove, D.; Dowdeswell, J.A.; England, J.H.; Funder, S.; Hogan, K.; Ingólfsson, Ó.; Jennings, A.; et al. Arctic Ocean Glacial History. Quat. Sci. Rev. 2014, 92, 40–67. [Google Scholar] [CrossRef]
- Svendsen, J.I.; Alexanderson, H.; Astakhov, V.I.; Demidov, I.; Dowdeswell, J.A.; Funder, S.; Gataullin, V.; Henriksen, M.; Hjort, C.; Houmark-Nielsen, M.; et al. Late Quaternary Ice Sheet History of Northern Eurasia. Quat. Sci. Rev. 2004, 23, 1229–1271. [Google Scholar] [CrossRef]
- Questel, J.M.; Blanco-Bercial, L.; Hopcroft, R.R.; Bucklin, A. Phylogeography and Connectivity of the Pseudocalanus (Copepoda: Calanoida) Species Complex in the Eastern North Pacific and the Pacific Arctic Region. J. Plankton Res. 2016, 38, 610–623. [Google Scholar] [CrossRef]
- DeHart, H.M.; Blanco-Bercial, L.; Passacantando, M.; Questel, J.M.; Bucklin, A. Pathways of Pelagic Connectivity: Eukrohnia Hamata (Chaetognatha) in the Arctic Ocean. Front. Mar. Sci. 2020, 7, 396. [Google Scholar] [CrossRef]
- Hirai, J.; Katakura, S.; Nagai, S. Comparisons of Genetic Population Structures of Copepods Pseudocalanus Spp. in the Okhotsk Sea: The First Record of P. Acuspes in Coastal Waters off Japan. Mar. Biodivers. 2023, 53, 12. [Google Scholar] [CrossRef]
- Weydmann, A.; Przyłucka, A.; Lubośny, M.; Walczyńska, K.S.; Serrão, E.A.; Pearson, G.A.; Burzyński, A. Postglacial Expansion of the Arctic Keystone Copepod Calanus Glacialis. Mar. Biodivers. 2018, 48, 1027–1035. [Google Scholar] [CrossRef]
- Bucklin, A.; Questel, J.M.; Batta-Lona, P.G.; Reid, M.; Frenzel, A.; Gelfman, C.; Wiebe, P.H.; Campbell, R.G.; Ashjian, C.J. Population Genetic Diversity and Structure of the Euphausiids Thysanoessa Inermis and T. Raschii in the Arctic Ocean: Inferences from COI Barcodes. Mar. Biodivers. 2023, 53, 70. [Google Scholar] [CrossRef]
- Peijnenburg, K.T.C.A.; Goetze, E. High Evolutionary Potential of Marine Zooplankton. Ecol. Evol. 2013, 3, 2765–2781. [Google Scholar] [CrossRef]
- Kulagin, D.N.; Simakova, U.V.; Lunina, A.A.; Vereshchaka, A.L. Comparative Phylogeography and Genetic Diversity of Two Co-Occurring Anti-Tropical Krill Species Hansarsia Megalops and Thysanoessa Gregaria in the Atlantic Ocean. ICES J. Mar. Sci. 2024, 82, fsae105. [Google Scholar] [CrossRef]
- Burridge, A.K.; Goetze, E.; Wall-Palmer, D.; Le Double, S.L.; Huisman, J.; Peijnenburg, K.T.C.A. Diversity and Abundance of Pteropods and Heteropods along a Latitudinal Gradient across the Atlantic Ocean. Prog. Oceanogr. 2017, 158, 213–223. [Google Scholar] [CrossRef]
- Rudels, B. Arctic Ocean Circulation and Variability—Advection and External Forcing Encounter Constraints and Local Processes. Ocean Sci. 2012, 8, 261–286. [Google Scholar] [CrossRef]
- Wang, Q.; Danilov, S. A Synthesis of the Upper Arctic Ocean Circulation During 2000–2019: Understanding the Roles of Wind Forcing and Sea Ice Decline. Front. Mar. Sci. 2022, 9, 863204. [Google Scholar] [CrossRef]
- Clement Kinney, J.; Assmann, K.M.; Maslowski, W.; Björk, G.; Jakobsson, M.; Jutterström, S.; Lee, Y.J.; Osinski, R.; Semiletov, I.; Ulfsbo, A.; et al. On the Circulation, Water Mass Distribution, and Nutrient Concentrations of the Western Chukchi Sea. Ocean Sci. 2022, 18, 29–49. [Google Scholar] [CrossRef]
- Kobayashi, H.A. Growth Cycle and Related Vertical Distribution of the Thecosomatous Pteropod Spiratella (?Limacina?) Helicina in the Central Arctic Ocean. Mar. Biol. 1974, 26, 295–301. [Google Scholar] [CrossRef]
- Falk-Petersen, S.; Leu, E.; Berge, J.; Kwasniewski, S.; Nygård, H.; Røstad, A.; Keskinen, E.; Thormar, J.; Von Quillfeldt, C.; Wold, A.; et al. Vertical Migration in High Arctic Waters during Autumn 2004. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 2275–2284. [Google Scholar] [CrossRef]
- Lischka, S.; Büdenbender, J.; Boxhammer, T.; Riebesell, U. Impact of Ocean Acidification and Elevated Temperatures on Early Juveniles of the Polar Shelled Pteropod <I>Limacina Helicina</I>: Mortality, Shell Degradation, and Shell Growth. Biogeosciences 2011, 8, 919–932. [Google Scholar] [CrossRef]
- Osadchiev, A.A.; Pisareva, M.N.; Spivak, E.A.; Shchuka, S.A.; Semiletov, I.P. Freshwater Transport between the Kara, Laptev, and East-Siberian Seas. Sci. Rep. 2020, 10, 13041. [Google Scholar] [CrossRef]
- Osadchiev, A.; Frey, D.; Spivak, E.; Shchuka, S.; Tilinina, N.; Semiletov, I. Structure and Inter-Annual Variability of the Freshened Surface Layer in the Laptev and East-Siberian Seas During Ice-Free Periods. Front. Mar. Sci. 2021, 8, 735011. [Google Scholar] [CrossRef]
- Solomon, A.; Heuzé, C.; Rabe, B.; Bacon, S.; Bertino, L.; Heimbach, P.; Inoue, J.; Iovino, D.; Mottram, R.; Zhang, X.; et al. Freshwater in the Arctic Ocean 2010–2019. Ocean Sci. 2021, 17, 1081–1102. [Google Scholar] [CrossRef]
- Alfaro-Lucas, J.M.; Chaudhary, C.; Brandt, A.; Saeedi, H. Species Composition Comparisons and Relationships of Arctic Marine Ecoregions. Deep Sea Res. Part I Oceanogr. Res. Pap. 2023, 198, 104077. [Google Scholar] [CrossRef]
- Oziel, L.; Sirven, J.; Gascard, J.-C. The Barents Sea Frontal Zones and Water Masses Variability (1980–2011). Ocean Sci. 2016, 12, 169–184. [Google Scholar] [CrossRef]
- Weydmann, A.; Coelho, N.C.; Serrão, E.A.; Burzyński, A.; Pearson, G.A. Pan-Arctic Population of the Keystone Copepod Calanus Glacialis. Polar Biol. 2016, 39, 2311–2318. [Google Scholar] [CrossRef]
- Kacprzak, P.; Panasiuk, A.; Wawrzynek, J.; Weydmann, A. Distribution and Abundance of Pteropods in the Western Barents Sea. Oceanol. Hydrobiol. Stud. 2017, 46, 393–404. [Google Scholar] [CrossRef]
- Zatsepin, A.G.; Zavialov, P.O.; Kremenetskiy, V.V.; Poyarkov, S.G.; Soloviev, D.M. The Upper Desalinated Layer in the Kara Sea. Oceanology 2010, 50, 657–667. [Google Scholar] [CrossRef]
- Osadchiev, A.A.; Frey, D.I.; Shchuka, S.A.; Tilinina, N.D.; Morozov, E.G.; Zavialov, P.O. Structure of the Freshened Surface Layer in the Kara Sea During Ice-Free Periods. JGR Ocean. 2021, 126, e2020JC016486. [Google Scholar] [CrossRef]
- Osadchiev, A.; Zabudkina, Z.; Rogozhin, V.; Frey, D.; Gordey, A.; Spivak, E.; Salyuk, A.; Semiletov, I.; Sedakov, R. Structure of the Ob-Yenisei Plume in the Kara Sea Shortly before Autumn Ice Formation. Front. Mar. Sci. 2023, 10, 1129331. [Google Scholar] [CrossRef]
- Pasternak, A.F.; Drits, A.V.; Abyzova, G.A.; Semenova, T.N.; Sergeeva, V.M.; Flint, M.V. Feeding and Distribution of Zooplankton in the Desalinated “Lens” in the Kara Sea: Impact of the Vertical Salinity Gradient. Oceanology 2015, 55, 863–870. [Google Scholar] [CrossRef]
- Drits, A.V.; Pasternak, A.F.; Arashkevich, E.G.; Amelina, A.B.; Shchuka, T.A.; Flint, M.V. Zooplankton of the Eastern Kara Sea: Response to a Short Ice-Free Period. Oceanology 2023, 63, S174–S187. [Google Scholar] [CrossRef]
- Dmitrenko, I.; Kirillov, S.; Eicken, H.; Markova, N. Wind-driven Summer Surface Hydrography of the Eastern Siberian Shelf. Geophys. Res. Lett. 2005, 32, L14613. [Google Scholar] [CrossRef]
- Golubeva, E.; Platov, G.; Malakhova, V.; Iakshina, D.; Kraineva, M. Modeling the Impact of the Lena River on the Laptev Sea Summer Hydrography and Submarine Permafrost State. Bull. Novosib. Comput. Center. Ser. Numer. Anal. 2015, 15, 13–22. [Google Scholar]
- Bauch, D.; Dmitrenko, I.; Kirillov, S.; Wegner, C.; Hölemann, J.; Pivovarov, S.; Timokhov, L.; Kassens, H. Eurasian Arctic Shelf Hydrography: Exchange and Residence Time of Southern Laptev Sea Waters. Cont. Shelf Res. 2009, 29, 1815–1820. [Google Scholar] [CrossRef]
- Arashkevich, E.G.; Drits, A.V.; Pasternak, A.F.; Flint, M.V.; Demidov, A.B.; Amelina, A.B.; Kravchishina, M.D.; Sukhanova, I.N.; Shchuka, S.A. Distribution and Feeding of Herbivorous Zooplankton in the Laptev Sea. Oceanology 2018, 58, 381–395. [Google Scholar] [CrossRef]
- Abramova, E.; Tuschling, K. A 12-Year Study of the Seasonal and Interannual Dynamics of Mesozooplankton in the Laptev Sea: Significance of Salinity Regime and Life Cycle Patterns. Glob. Planet. Change 2005, 48, 141–164. [Google Scholar] [CrossRef]
- Semiletov, I.; Dudarev, O.; Luchin, V.; Charkin, A.; Shin, K.; Tanaka, N. The East Siberian Sea as a Transition Zone between Pacific-derived Waters and Arctic Shelf Waters. Geophys. Res. Lett. 2005, 32, L14613. [Google Scholar] [CrossRef]
- Münchow, A.; Weingartner, T.J.; Cooper, L.W. The Summer Hydrography and Surface Circulation of the East Siberian Shelf Sea. J. Phys. Oceanogr. 1999, 29, 2167–2182. [Google Scholar] [CrossRef]
- Macdonald, R.W.; Harner, T.; Fyfe, J. Recent Climate Change in the Arctic and Its Impact on Contaminant Pathways and Interpretation of Temporal Trend Data. Sci. Total Environ. 2005, 342, 5–86. [Google Scholar] [CrossRef]
- Berger, V.Y.; Naumov, A.D. General Features of the White Sea: Morphology, Sediments, Hydrology, Oxygen Conditions, Nutrients and Organic Matter. Berichte Polarforsch 2000, 359, 3–5. [Google Scholar]
- Kosobokova, K.N.; Pertsova, N.M. Zooplankton of the White Sea: Structure, Dynamics, and Community Ecology. In Suspended Particulate Matter in the Biosphere of the White Sea; Nauka: Nayarit, Mexico, 2012; pp. 640–661. [Google Scholar]
- Vrijenhoek, R.; Hoeh, W.; Lutz, R.; Black, M. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. popart: Full-feature Software for Haplotype Network Construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A Computer Program to Estimate Gene Genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Tajima, F. Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (Version 3.0): An Integrated Software Package for Population Genetics Data Analysis. Evol. Bioinform. Online 2007, 1, 47–50. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Laakkonen, H.M.; Hardman, M.; Strelkov, P.; Väinölä, R. Cycles of trans-Arctic dispersal and vicariance, and diversification of the amphi-boreal marine fauna. J. Evol. Biol. 2021, 34, 73–96. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ho, S.Y.W.; Lanfear, R.; Bromham, L.; Phillips, M.J.; Soubrier, J.; Rodrigo, A.G.; Cooper, A. Time-dependent rates of molecular evolution. Mol. Ecol. 2011, 20, 3087–3101. [Google Scholar] [CrossRef]
- Budko, D.; Polyakova, Y.; Ryabchuk, D.; Sergeev, A.; Zhamoida, V.; Ulyanova, M. Postglacial sedimentation in the White Sea (northwestern Russia) reconstructed by integrated microfossil and geochemical data. Boreas 2020, 49, 37–56. [Google Scholar] [CrossRef]
- Busch, K.; Bauerfeind, E.; Nöthig, E.-M. Pteropod Sedimentation Patterns in Different Water Depths Observed with Moored Sediment Traps over a 4-Year Period at the LTER Station HAUSGARTEN in Eastern Fram Strait. Polar Biol. 2015, 38, 845–859. [Google Scholar] [CrossRef]
- van der Spoel, S.; Heyman, R.P. Some General Trends. In A Comparative Atlas of Zooplankton: Biological Patterns in the Oceans; Springer Nature: Berlin, Germany, 1983; pp. 141–166. [Google Scholar]
- Gannefors, C.; Böer, M.; Kattner, G.; Graeve, M.; Eiane, K.; Gulliksen, B.; Hop, H.; Falk-Petersen, S. The Arctic Sea Butterfly Limacina Helicina: Lipids and Life Strategy. Mar. Biol. 2005, 147, 169–177. [Google Scholar] [CrossRef]
- Skjoldal, H.R.; Bagøien, E.; Martinussen, M.B. A Mass Occurrence of Pteropods (Limacina Spp.) Drove a Pronounced Peak in Zooplankton Biomass in Atlantic Water in the Barents Sea in 1994. J. Plankton Res. 2025, 47, fbaf012. [Google Scholar] [CrossRef]
- Falk-Petersen, S. Spatial Distribution and Life-Cycle Timing of Zooplankton in the Marginal Ice Zone of the Barents Sea during the Summer Melt Season in 1995. J. Plankton Res. 1999, 21, 1249–1264. [Google Scholar] [CrossRef]
- Wang, K.; Hunt, B.P.V.; Liang, C.; Pauly, D.; Pakhomov, E.A. Reassessment of the Life Cycle of the Pteropod Limacina Helicina from a High Resolution Interannual Time Series in the Temperate North Pacific. ICES J. Mar. Sci. 2017, 74, 1906–1920. [Google Scholar] [CrossRef]
- Bednaršek, N.; Možina, J.; Vogt, M.; O’Brien, C.; Tarling, G.A. The Global Distribution of Pteropods and Their Contribution to Carbonate and Carbon Biomass in the Modern Ocean. Earth Syst. Sci. Data 2012, 4, 167–186. [Google Scholar] [CrossRef]
- Bednaršek, N.; Feely, R.A.; Reum, J.C.P.; Peterson, B.; Menkel, J.; Alin, S.R.; Hales, B. Limacina Helicina Shell Dissolution as an Indicator of Declining Habitat Suitability Owing to Ocean Acidification in the California Current Ecosystem. Proc. R. Soc. B. 2014, 281, 20140123. [Google Scholar] [CrossRef]
- Patton, H.; Hubbard, A.; Andreassen, K.; Auriac, A.; Whitehouse, P.L.; Stroeven, A.P.; Shackleton, C.; Winsborrow, M.; Heyman, J.; Hall, A.M. Deglaciation of the Eurasian Ice Sheet Complex. Quat. Sci. Rev. 2017, 169, 148–172. [Google Scholar] [CrossRef]
- Stroeven, A.P.; Hättestrand, C.; Kleman, J.; Heyman, J.; Fabel, D.; Fredin, O.; Goodfellow, B.W.; Harbor, J.M.; Jansen, J.D.; Olsen, L.; et al. Deglaciation of Fennoscandia. Quat. Sci. Rev. 2016, 147, 91–121. [Google Scholar] [CrossRef]
- Makhrov, A.A.; Bolotov, I.N.; Vinarski, M.V.; Artamonova, V.S. Origin of Glacial Relicts in Northern and Central Europe: Four Waves of Introduction of Cold-Water Species from Asia (Review). Inland Water Biol. 2022, 15, 707–728. [Google Scholar] [CrossRef]
- Maggs, C.A.; Castilho, R.; Foltz, D.; Henzler, C.; Jolly, M.T.; Kelly, J.; Olsen, J.; Perez, K.E.; Stam, W.; Väinölä, R.; et al. Evaluating signatures of glacial refugia for north atlantic benthic marine taxa. Ecology 2008, 89, S108–S122. [Google Scholar] [CrossRef]
- Schneider, C.L. Marine Refugia Past, Present, and Future: Lessons from Ancient Geologic Crises for Modern Marine Ecosystem Conservation. In Marine Conservation Paleobiology; Tyler, C.L., Schneider, C.L., Eds.; Topics in Geobiology; Springer International Publishing: Cham, Switzerland, 2018; Volume 47, pp. 163–208. ISBN 978-3-319-73793-5. [Google Scholar]
- Csapó, H.K.; Grabowski, M.; Węsławski, J.M. Coming Home-Boreal Ecosystem Claims Atlantic Sector of the Arctic. Sci. Total Environ. 2021, 771, 144817. [Google Scholar] [CrossRef]
- Choquet, M.; Hatlebakk, M.; Dhanasiri, A.K.S.; Kosobokova, K.; Smolina, I.; Søreide, J.E.; Svensen, C.; Melle, W.; Kwaśniewski, S.; Eiane, K.; et al. Genetics Redraws Pelagic Biogeography of Calanus. Biol. Lett. 2017, 13, 20170588. [Google Scholar] [CrossRef]
- Grabowski, M.; Jabłońska, A.; Weydmann-Zwolicka, A.; Gantsevich, M.; Strelkov, P.; Skazina, M.; Węsławski, J.M. Contrasting Molecular Diversity and Demography Patterns in Two Intertidal Amphipod Crustaceans Reflect Atlantification of High Arctic. Mar. Biol. 2019, 166, 155. [Google Scholar] [CrossRef]
- Bigg, G.R. Environmental Confirmation of Multiple Ice Age Refugia for Pacific Cod, Gadus Macrocephalus. Evol. Ecol. 2014, 28, 177–191. [Google Scholar] [CrossRef]
- Farmer, J.R.; Pico, T.; Underwood, O.M.; Cleveland Stout, R.; Granger, J.; Cronin, T.M.; Fripiat, F.; Martínez-García, A.; Haug, G.H.; Sigman, D.M. The Bering Strait Was Flooded 10,000 Years before the Last Glacial Maximum. Proc. Natl. Acad. Sci. USA 2023, 120, e2206742119. [Google Scholar] [CrossRef]
- Aarbakke, O.N.S.; Bucklin, A.; Halsband, C.; Norrbin, F. Comparative Phylogeography and Demographic History of Five Sibling Species of Pseudocalanus (Copepoda: Calanoida) in the North Atlantic Ocean. J. Exp. Mar. Biol. Ecol. 2014, 461, 479–488. [Google Scholar] [CrossRef]
- Neiva, J.; Assis, J.; Fragkopoulou, E.; Pearson, G.A.; Raimondi, P.T.; Anderson, L.; Krause-Jensen, D.; Marbà, N.; Want, A.; Selivanova, O.; et al. Trans-Arctic Asymmetries, Melting Pots and Weak Species Cohesion in the Low-Dispersal Amphiboreal Seaweed Fucus Distichus. Front. Ecol. Evol. 2024, 12, 1356987. [Google Scholar] [CrossRef]
- Kohnert, P.C.; Cerwenka, A.F.; Brandt, A.; Schrödl, M. Pteropods from the Kuril-Kamchatka Trench and the Sea of Okhotsk (Euopisthobranchia; Gastropoda). Prog. Oceanogr. 2020, 181, 102259. [Google Scholar] [CrossRef]
- Ershova, E.A.; Kosobokova, K.N. Cross-Shelf Structure and Distribution of Mesozooplankton Communities in the East-Siberian Sea and the Adjacent Arctic Ocean. Polar Biol. 2019, 42, 1353–1367. [Google Scholar] [CrossRef]
- Ershova, E.A.; Kosobokova, K.N.; Banas, N.S.; Ellingsen, I.; Niehoff, B.; Hildebrandt, N.; Hirche, H. Sea Ice Decline Drives Biogeographical Shifts of Key Calanus Species in the Central Arctic Ocean. Glob. Change Biol. 2021, 27, 2128–2143. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Epiplankton in the Barents Sea: Summer Variations of Mesozooplankton Biomass, Community Structure and Diversity. Cont. Shelf Res. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MS, USA, 2000. [Google Scholar]
- Skurikhina, L.A.; Oleinik, A.G.; Kukhlevsky, A.D.; Kovpak, N.E.; Frolov, S.V.; Sendek, D.S. Phylogeography and Demographic History of the Pacific Smelt Osmerus Dentex Inferred from Mitochondrial DNA Variation. Polar Biol. 2018, 41, 877–896. [Google Scholar] [CrossRef]
- Semenova, A.V.; Andreeva, A.P.; Karpov, A.K.; Novikov, G.G. An Analysis of Allozyme Variation in Herring Clupea Pallasii from the White and Barents Seas. J. Ichthyol. 2009, 49, 313–330. [Google Scholar] [CrossRef]
- Ekimova, I.A.; Nikitenko, E.; Stanovova, M.V.; Schepetov, D.M.; Antokhina, T.I.; Malaquias, M.A.E.; ValdÉs, Á. Unity in Diversity: Morphological and Genetic Variability, Integrative Systematics, and Phylogeography of the Widespread Nudibranch Mollusc Onchidoris Muricata. Syst. Biodivers. 2023, 21, 2246472. [Google Scholar] [CrossRef]
- Søreide, J.E.; Leu, E.; Berge, J.; Graeve, M.; Falk-Petersen, S. Timing of Blooms, Algal Food Quality and Calanus Glacialis Reproduction and Growth in a Changing Arctic. Glob. Change Biol. 2010, 16, 3154–3163. [Google Scholar] [CrossRef]
- Hunt, B.; Strugnell, J.; Bednarsek, N.; Linse, K.; Nelson, R.J.; Pakhomov, E.; Würzberg, L. Poles apart: The bipolar pteropod species Limacina helicina is genetically distinct between the Arctic and Antarctic oceans. PLoS ONE 2010, 5, e9835. [Google Scholar] [CrossRef]
- Layton, K.K.S.; Martel, A.L.; Hebert, P.D. Patterns of DNA Barcode Variation in Canadian Marine Molluscs. PLoS ONE 2014, 9, e95003. [Google Scholar] [CrossRef]
- Shimizu, K.; Kimoto, K.; Noshita, K.; Wakita, M.; Fujiki, T.; Sasaki, T. Phylogeography of the Pelagic Snail Limacina Helicina (Gastropoda: Thecosomata) in the Subarctic Western North Pacific. J. Molluscan Stud. 2018, 84, 30–37. [Google Scholar] [CrossRef]
- Chichvarkhin, A. Shallow Water Sea Slugs (Gastropoda: Heterobranchia) from the Northwestern Coast of the Sea of Japan, North of Peter the Great Bay, Russia. PeerJ 2016, 4, e2774. [Google Scholar] [CrossRef]
- Jennings, R.M.; Bucklin, A.; Ossenbrügger, H.; Hopcroft, R.R. Species Diversity of Planktonic Gastropods (Pteropoda and Heteropoda) from Six Ocean Regions Based on DNA Barcode Analysis. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 2199–2210. [Google Scholar] [CrossRef]
| Sample | N | BP | H | Hd | SD of Hd | π | SD of π | Tajima’s D | p-Value of Tajima’s D | Fu’s F |
|---|---|---|---|---|---|---|---|---|---|---|
| All data | 873 | 503 | 161 | 0.61 | 0.019 | 0.00365 | 0.00045 | −2.69815 | <0.001 | −358.121 |
| White sea | 48 | 555 | 18 | 0.66 | 0.08 | 0.00243 | 0.00055 | −2.363 | <0.01 | −16.524 |
| East Siberian | 53 | 625 | 10 | 0.37 | 0.086 | 0.00102 | 0.00031 | −2.31002 | <0.01 | −8.128 |
| Laptev sea | 41 | 556 | 23 | 0.86 | 0.051 | 0.0043 | 0.00064 | −2.26034 | <0.01 | −20.508 |
| Barents sea (Svalbard + Franz-Joseph land) | 110 | 559 | 46 | 0.83 | 0.037 | 0.00426 | 0.00089 | −2.6243 | <0.001 | −56.637 |
| Svalbard (data of Sromek) | 67 | 559 | 25 | 0.78 | 0.053 | 0.00313 | 0.00045 | −2.2685 | <0.01 | −23.941 |
| Franz-Joseph land | 42 | 566 | 25 | 0.89 | 0.045 | 0.00383 | 0.00051 | −2.16841 | <0.01 | −26.393 |
| Kara sea | 71 | 625 | 34 | 0.87 | 0.037 | 0.00369 | 0.00045 | −2.39642 | <0.01 | −37.389 |
| Bering sea | 31 | 503 | 4 | 0.19 | 0.093 | 0.00038 | 0.0002 | −1.73075 | 0.05 < p < 0.1 (not sign.) | −3.436 |
| Pacific Alaska | 116 | 503 | 12 | 0.23 | 0.052 | 0.00077 | 0.0002 | −2.22414 | <0.01 | −13.859 |
| Okhotsk sea | 55 | 503 | 14 | 0.42 | 0.086 | 0.00101 | 0.00025 | −2.49 | <0.01 | −18.404 |
| NW Pacific | 152 | 503 | 35 | 0.45 | 0.052 | 0.00161 | 0.00029 | −2.72983 | <0.001 | −57.789 |
| NE Pacific | 157 | 503 | 5 | 0.08 | 0.029 | 0.00015 | 0.00006 | −1.64582 | 0.05 < p < 0.1 (not sign.) | −7.212 |
| Beaufort + Chukchi sea | 19 | 503 | 3 | 0.21 | 0.119 | 0.00064 | 0.00041 | −1.7188 | 0.05 < p < 0.1 (not sign.) | −1.085 |
| Hudson + Greenland | 15 | 503 | 8 | 0.88 | 0.06 | 0.0061 | 0.00091 | −0.91604 | 0.05 < p < 0.1 (not sign.) | −1.624 |
| Hudson | 7 | 503 | 5 | 0.91 | 0.103 | 0.00682 | 0.001 | 0.26266 | 0.1 < p (not sign.) | −0.439 |
| Greenland | 8 | 503 | 5 | 0.86 | 0.108 | 0.00511 | 0.00121 | −0.80566 | 0.1 < p (not sign.) | −0.61 |
| Greenl. | Hudson | Beauf_Chuk | East_Sib | Fr_J_ land | Kara | Laptev | NE_ Pacific | NW_ Pacific | Okhot. Sea | Pacific_ Alaska | Pacific_ Bering | Svalb. | White Sea | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Greenland | 0.17117 | 0.000 | 0.000 | 0.000 | 0.000 | 0.03604 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Hudson | 0.05923 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Beauf_Chuk | 0.23189 | 0.32935 | 0.52252 | 0.000 | 0.000 | 0.000 | 0.03604 | 0.91892 | 0.80180 | 0.62162 | 0.67568 | 0.000 | 0.000 | |
| East_Sib | 0.30044 | 0.43974 | −0.00326 | 0.000 | 0.000 | 0.000 | 0.01802 | 0.12613 | 0.18919 | 0.05405 | 0.54955 | 0.000 | 0.000 | |
| Fr_J_land | 0.18536 | 0.33321 | 0.52074 | 0.59073 | 0.16216 | 0.27928 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.46847 | 0.000 | |
| Kara | 0.15107 | 0.31925 | 0.46101 | 0.51592 | 0.00631 | 0.69369 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.25225 | 0.000 | |
| Laptev | 0.12261 | 0.26661 | 0.45171 | 0.52919 | 0.00281 | −0.00502 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.42342 | 0.000 | |
| NE_Pacific | 0.71162 | 0.80169 | 0.08923 | 0.02108 | 0.79234 | 0.69333 | 0.74534 | 0.000 | 0.000 | 0.000 | 0.04505 | 0.000 | 0.000 | |
| NW_Pacific | 0.2671 | 0.41128 | −0.01495 | 0.00359 | 0.61096 | 0.54526 | 0.5589 | 0.00856 | 0.26126 | 0.28829 | 0.83784 | 0.000 | 0.000 | |
| Okhot. Sea | 0.31505 | 0.44495 | −0.00636 | 0.00293 | 0.59231 | 0.51732 | 0.53047 | 0.01745 | 0.0023 | 0.04505 | 0.90991 | 0.000 | 0.000 | |
| Pacific_Alaska | 0.39283 | 0.53688 | −0.00676 | 0.01353 | 0.67048 | 0.58849 | 0.61627 | 0.02488 | 0.00033 | 0.01132 | 0.22523 | 0.000 | 0.000 | |
| Pacific_Bering | 0.33733 | 0.44963 | −0.00895 | −0.00156 | 0.57062 | 0.49627 | 0.50389 | 0.03227 | −0.00679 | −0.00536 | 0.00468 | 0.000 | 0.000 | |
| Svalbard | 0.20313 | 0.3732 | 0.52612 | 0.57473 | −0.00258 | 0.00193 | −0.00182 | 0.74716 | 0.59125 | 0.57651 | 0.64154 | 0.56053 | 0.000 | |
| White | 0.43836 | 0.28695 | 0.45893 | 0.52389 | 0.59766 | 0.57111 | 0.5536 | 0.74588 | 0.52165 | 0.52546 | 0.59699 | 0.50967 | 0.61022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abyzova, G.A.; Neretina, T.V.; Nikitin, M.A.; Shapkina, A.O.; Vereshchaka, A.L. Marine Highways and Barriers: A Case Study of Limacina helicina Phylogeography Across the Siberian Arctic Shelf Seas. Diversity 2025, 17, 522. https://doi.org/10.3390/d17080522
Abyzova GA, Neretina TV, Nikitin MA, Shapkina AO, Vereshchaka AL. Marine Highways and Barriers: A Case Study of Limacina helicina Phylogeography Across the Siberian Arctic Shelf Seas. Diversity. 2025; 17(8):522. https://doi.org/10.3390/d17080522
Chicago/Turabian StyleAbyzova, Galina A., Tatiana V. Neretina, Mikhail A. Nikitin, Anna O. Shapkina, and Alexander L. Vereshchaka. 2025. "Marine Highways and Barriers: A Case Study of Limacina helicina Phylogeography Across the Siberian Arctic Shelf Seas" Diversity 17, no. 8: 522. https://doi.org/10.3390/d17080522
APA StyleAbyzova, G. A., Neretina, T. V., Nikitin, M. A., Shapkina, A. O., & Vereshchaka, A. L. (2025). Marine Highways and Barriers: A Case Study of Limacina helicina Phylogeography Across the Siberian Arctic Shelf Seas. Diversity, 17(8), 522. https://doi.org/10.3390/d17080522






