Building a DNA Reference for Madagascar’s Marine Fishes: Expanding the COI Barcode Library and Establishing the First 12S Dataset for eDNA Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Sampling
2.2. Collection of Tissue Samples and Photographs
2.3. DNA Extraction, Amplification, and Sequencing
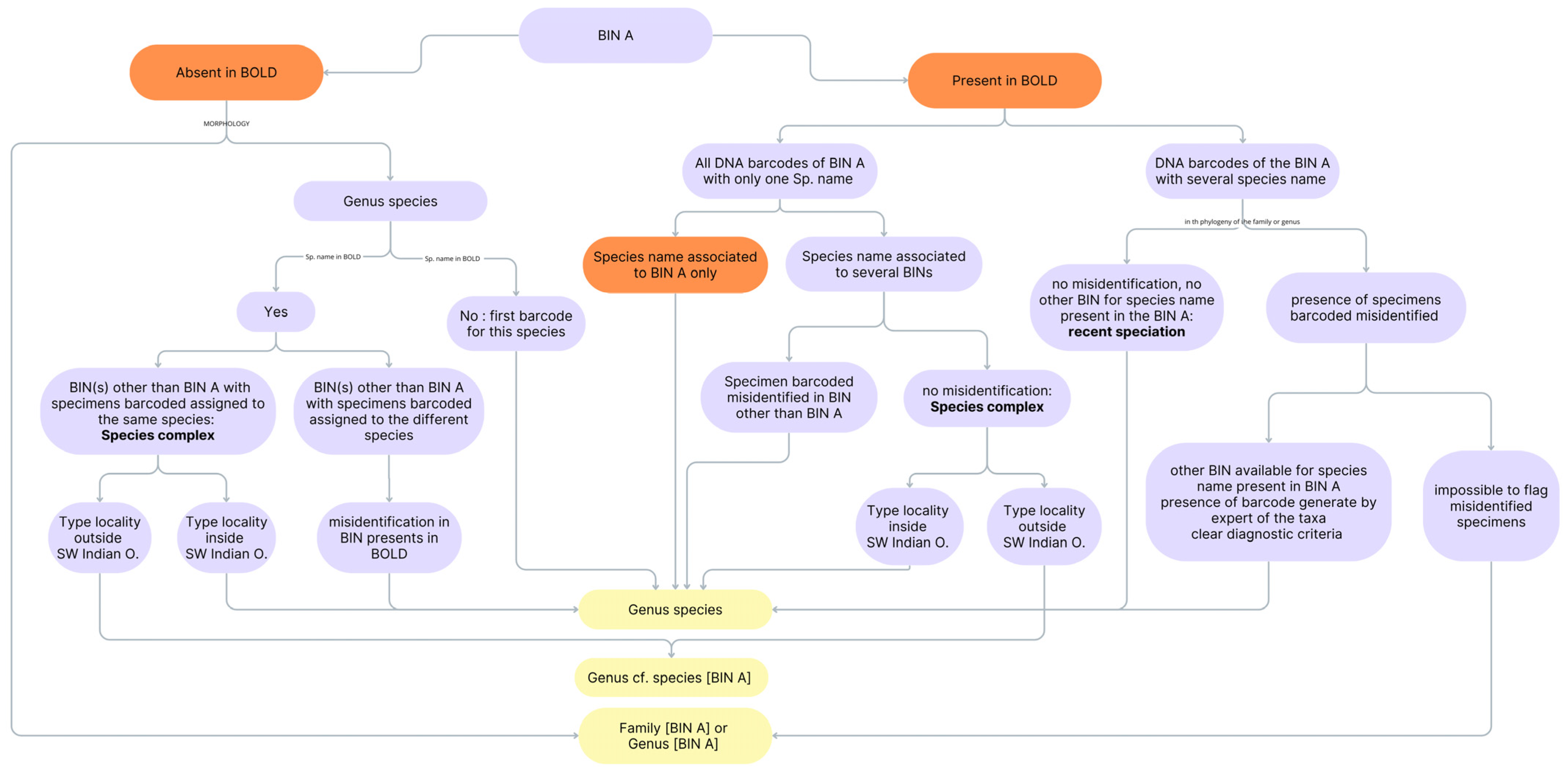
2.4. Specimen Identification and Taxonomic Curation of the DNA Barcodes Library
- when sequence identity (seq ID) was greater than 99%, the species name was retained.
- when seq ID was between 95% and 99%, if only one genus appeared within the matches, the genus name was assigned. If multiple genera were listed, the identification was limited to the family level.
- when seq ID was between 90% and 95%, only the family name was used, provided it was consistently represented among the BLAST hits.
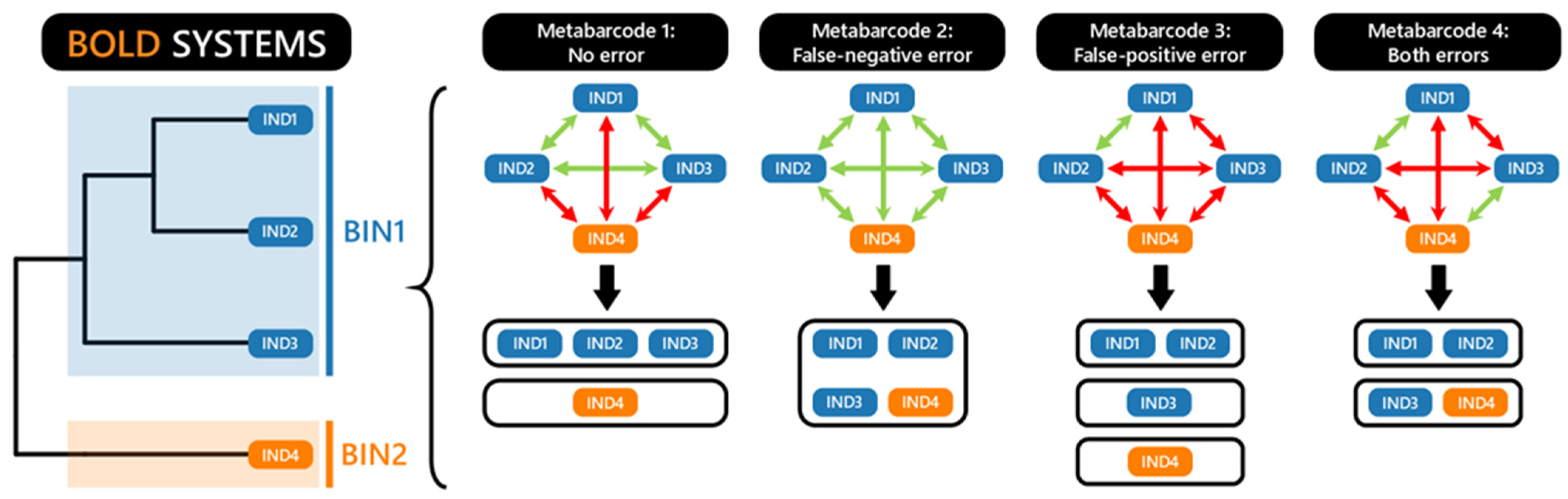
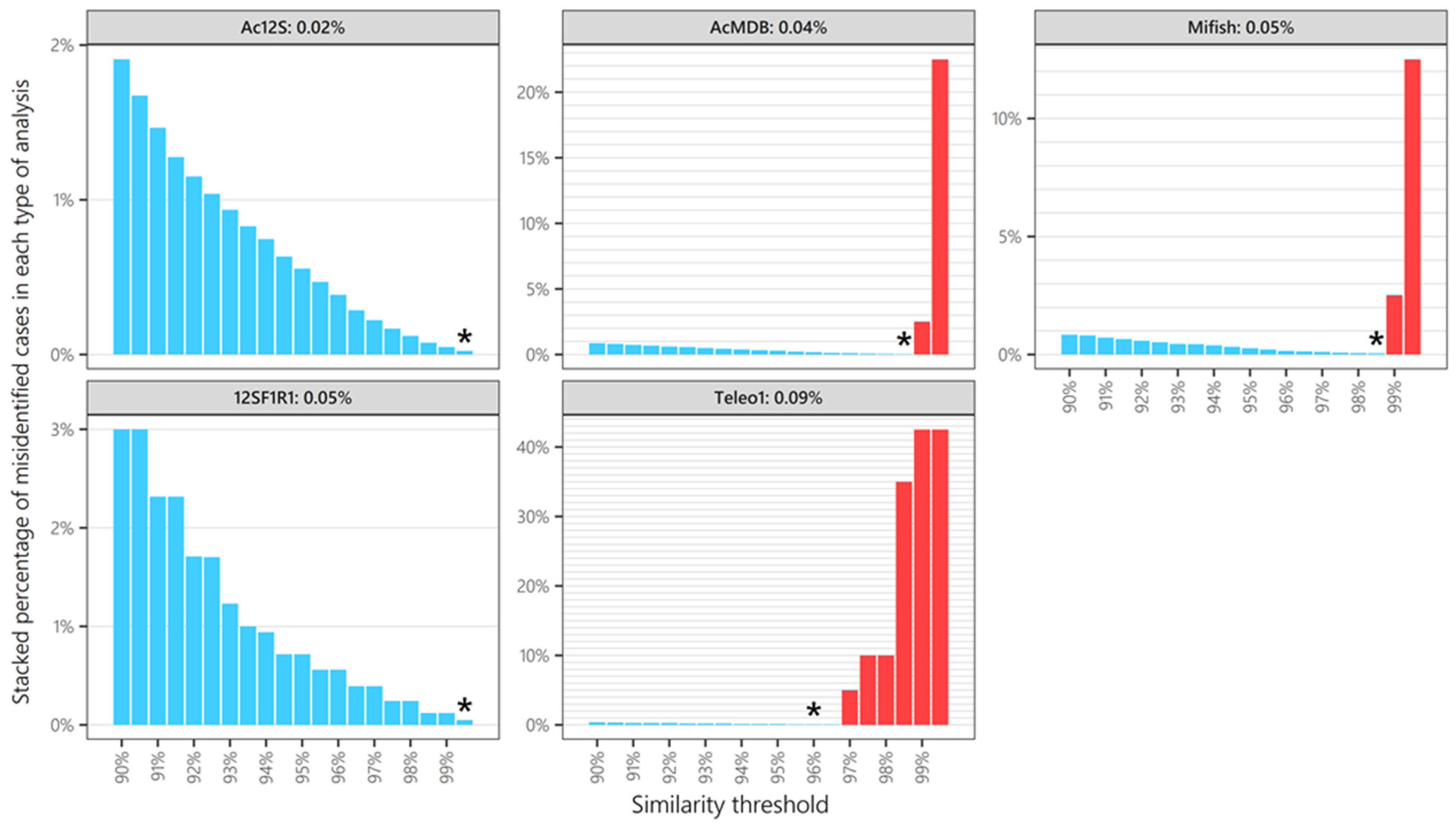
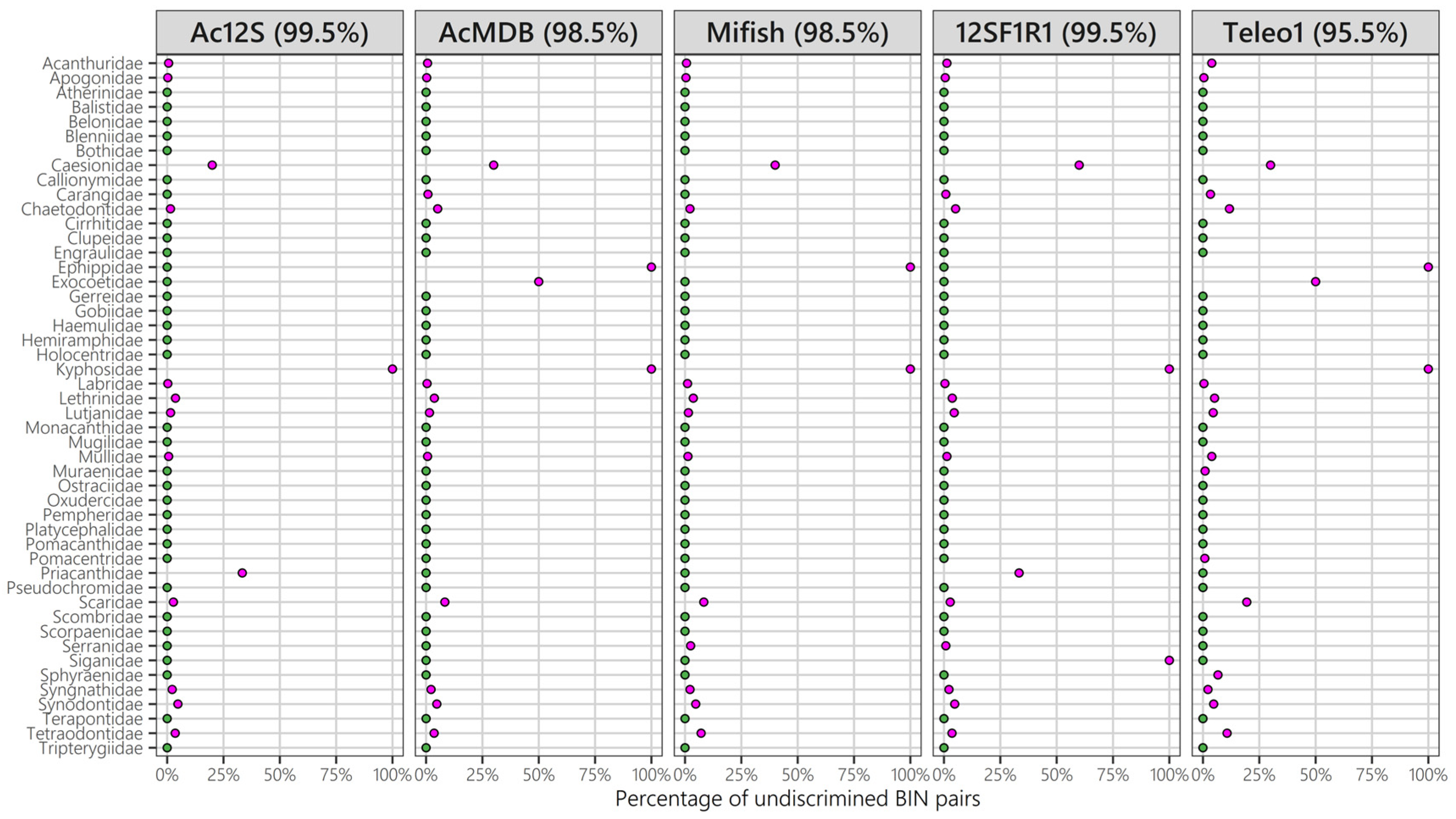
2.5. Evaluation of Taxonomic Resolution
3. Results
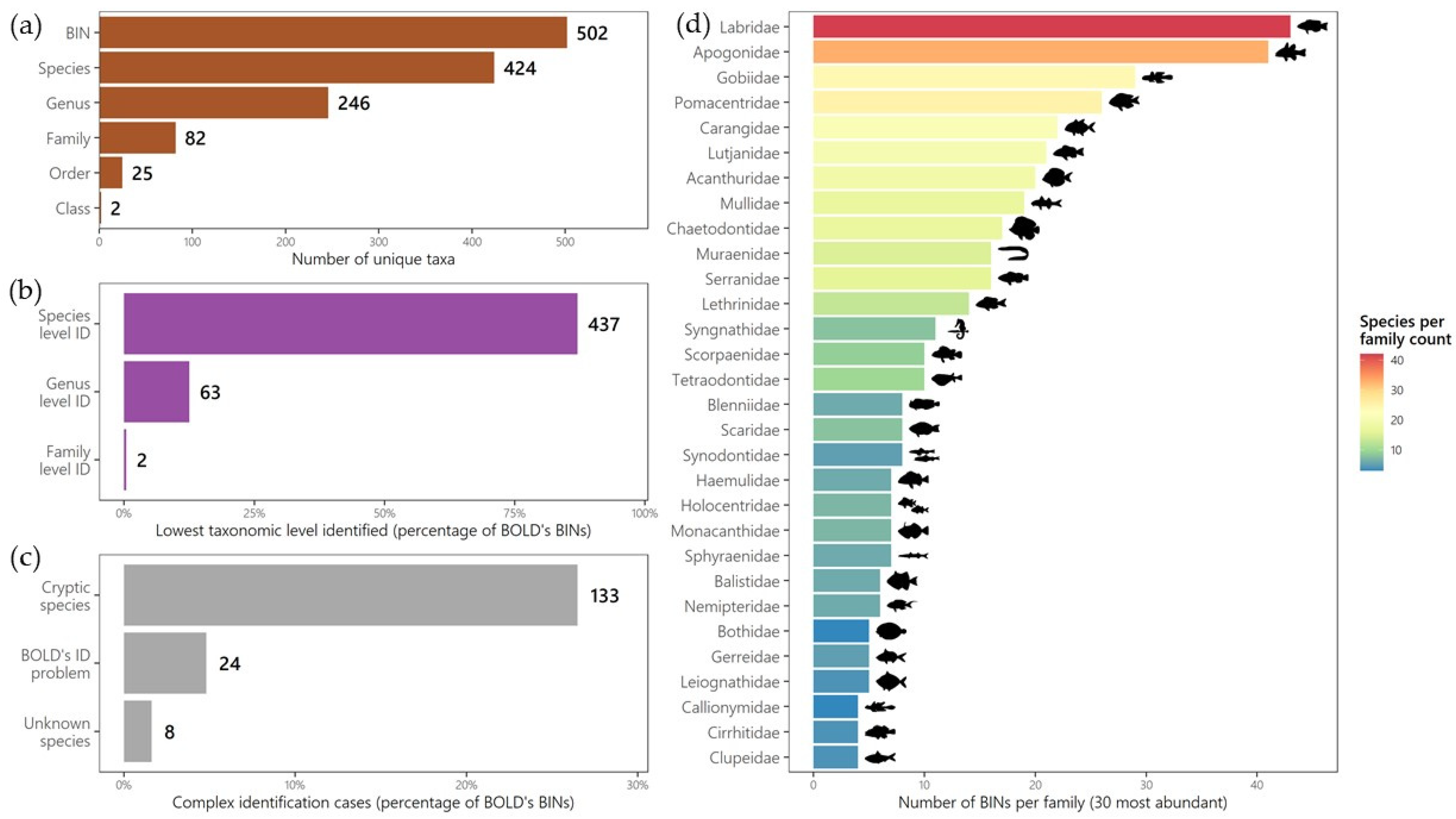
3.1. Taxonomic Coverage of COI Sequences
- 243 BINs included specimens with a unique species name (one BIN, one species name). Among them, 236 were identified at the species level. Of these species names, 125 BINs were associated with a single BIN and could therefore be directly linked to the same species name, including only one cryptic species (Ariosoma cf. meeki [BOLD:ADD3087]). The remaining 118 BINs were associated with more than one BIN. Phylogenetic reconstructions at the family or genus level revealed that 87 species most probably corresponded to species complexes, accounting for 17% of all BINs. For 24 of the remaining BINs, after verification using the phylogenetic tree, they turned out to be misidentified specimens. However, seven BINs could only be assigned at the genus level.
- 212 BINs contained specimens with different species names. Phylogenetic reconstructions and further manual verifications confirmed that these specimens were misidentified. Among these, 188 BINs had another correct BIN available for the species name and a barcode generated by a taxonomic expert, with clear diagnostic criteria. All 188 BINs were identified at the species level, including 40 cryptic species. For the remaining 24 BINs, it was not possible to resolve the misidentifications, and their identification remained at the genus or family level.
- 33 BINs were either associated with a genus name or were not identified in the BOLD database. Among them, 25 were identified at the genus level, and eight at the species level, including four cryptic species.
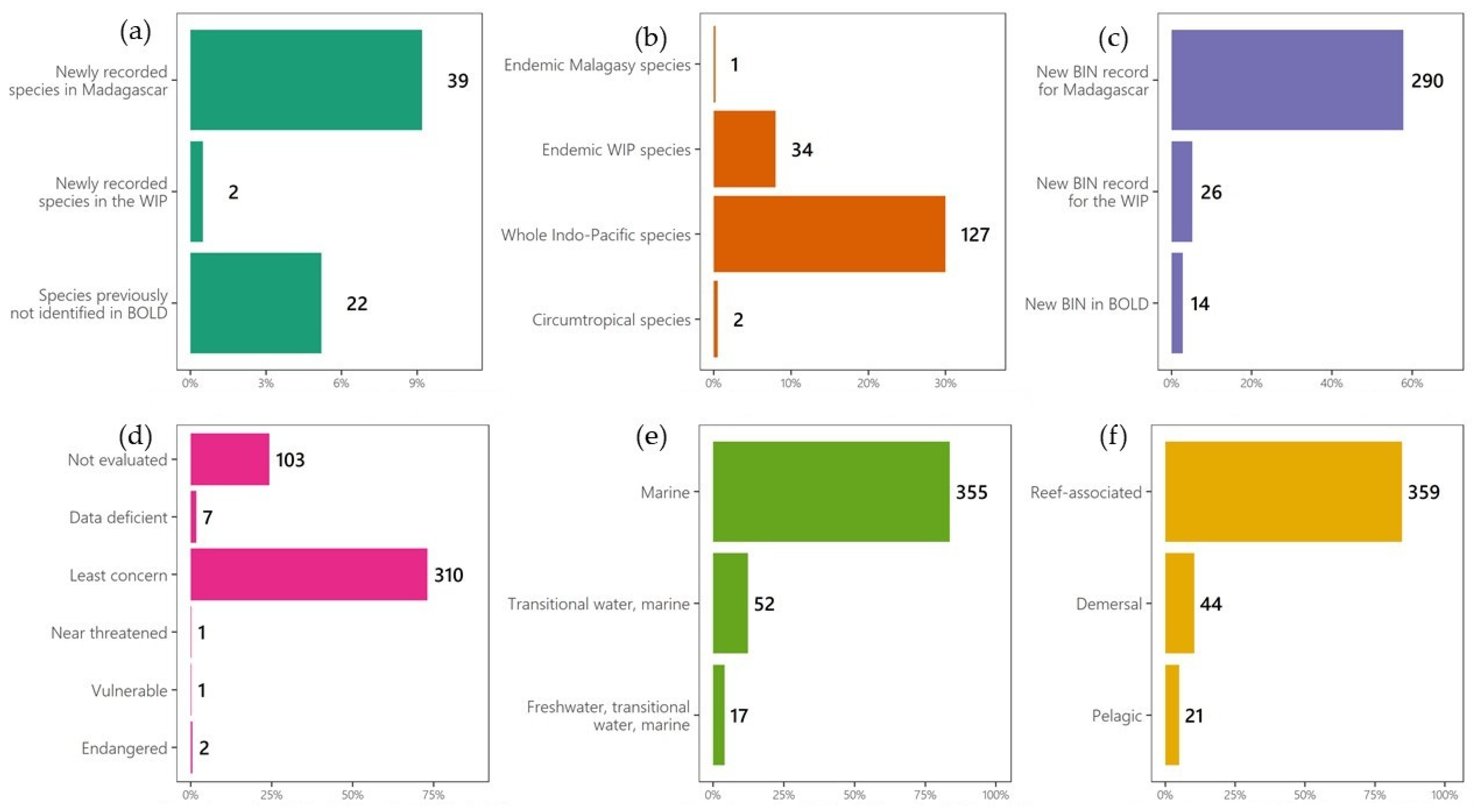
3.2. Overall Profile of Fish Species in the MADFI Database According to the Fishbase
3.3. Taxonomic Coverage of 12S Sequences
3.4. Taxonomic Resolution of Five Different 12S Primers
4. Discussion
5. Conclusions
| Class | COI | 12S | |||||
|---|---|---|---|---|---|---|---|
| Order | |||||||
| Family | |||||||
| Species | BIN | N COI in MADFI | Nsp./BIN in BOLD | NBIN/Sp. In BOLD | Sp. Complex | Allo./Symp. | N 12S in MADFI |
| Actinopterygii | |||||||
| Acanthuriformes | |||||||
| Acanthuridae | |||||||
| Acanthurus blochii | BOLD:AAF0623 | 1 | 1 | 1 | 1 | ||
| Acanthurus dussumieri | BOLD:AAE4046 | 26 | 1 | >1 | 1 | ||
| Acanthurus leucosternon | BOLD:AAB7142 | 1 | >1 | 1 | |||
| Acanthurus lineatus | BOLD:AAB3207 | 1 | >1 | 1 | |||
| Acanthurus mata | BOLD:AAE4025 | 6 | 1 | >1 | 1 | ||
| Acanthurus nigricauda | BOLD:AAB8729 | 14 | 1 | 1 | 1 | ||
| Acanthurus nigrofuscus | BOLD:AAB0201 | 3 | >1 | 1 | |||
| Acanthurus tennentii | BOLD:AAD2621 | 1 | 1 | 1 | 1 | ||
| Acanthurus triostegus | BOLD:AAA9362 | 7 | 1 | 1 | 1 | ||
| Acanthurus xanthopterus | BOLD:AAC6467 | 22 | >1 | 1 | |||
| Ctenochaetus binotatus | BOLD:AAB9166 | 2 | 1 | 1 | 1 | ||
| Ctenochaetus striatus | BOLD:AAB9167 | 13 | >1 | 2 | |||
| Naso annulatus | BOLD:AEN2475 | 7 | 1 | 1 | 2 | ||
| Naso brevirostris | BOLD:AAC1635 | 17 | 1 | 1 | 1 | ||
| Naso elegans | BOLD:AAA8635 | 1 | >1 | 1 | |||
| Naso unicornis | BOLD:AAC8042 | 3 | 1 | 1 | 1 | ||
| Paracanthurus hepatus | BOLD:AAC3227 | 1 | 1 | >1 | 1 | ||
| Zebrasoma cf. desjardinii [BOLD:AAF6311] | BOLD:AAF6311 | 2 | 1 | >1 | Y | S | 1 |
| Zebrasoma cf. desjardinii [BOLD:ACV8450] | BOLD:ACV8450 | 1 | >1 | Y | S | 1 | |
| Zebrasoma cf. scopas [BOLD:AAB3788] | BOLD:AAB3788 | 5 | 1 | >1 | Y | A | 0 |
| Zanclidae | |||||||
| Zanclus cornutus | BOLD:ADI3027 | 1 | 1 | 1 | 1 | ||
| Anguilliformes | |||||||
| Congridae | |||||||
| Ariosoma cf. meeki [BOLD:ADD3087] | BOLD:ADD3087 | 1 | 1 | 1 | Y | A | 0 |
| Ariosoma scheelei | BOLD:AAJ1709 | 14 | 1 | 1 | 1 | ||
| Conger cinereus | BOLD:AAL5915 | 6 | 1 | >1 | Y | A | 1 |
| Uroconger [BOLD:ACV7958] | BOLD:ACV7958 | 1 | NA | 0 | |||
| Muraenidae | |||||||
| Echidna nebulosa | BOLD:AAC5551 | 3 | 1 | 1 | 2 | ||
| Echidna polyzona | BOLD:AAC9046 | 4 | >1 | 3 | |||
| Gymnomuraena zebra | BOLD:AAN1246 | 1 | 1 | 1 | 1 | ||
| Gymnothorax [BOLD:ADB4048] | BOLD:ADB4048 | 1 | NA | 1 | |||
| Gymnothorax cf. chilospilus [BOLD:AAC0198] | BOLD:AAC0198 | 4 | 1 | >1 | Y | A | 2 |
| Gymnothorax cf. undulatus [BOLD:AAC5500] | BOLD:AAC5500 | 4 | >1 | Y | S | 3 | |
| Gymnothorax cf. undulatus [BOLD:AAC5502] | BOLD:AAC5502 | 1 | >1 | Y | S | 1 | |
| Gymnothorax elaineheemstrae | BOLD:AAE6825 | 2 | >1 | 3 | |||
| Gymnothorax favagineus | BOLD:ADB3872 | 3 | >1 | 1 | |||
| Gymnothorax flavimarginatus | BOLD:AAE6819 | 1 | >1 | 1 | |||
| Gymnothorax javanicus | BOLD:AAE3588 | 1 | 1 | 1 | 1 | ||
| Gymnothorax pictus | BOLD:AAE3580 | 1 | 1 | >1 | 1 | ||
| Gymnothorax richardsonii | BOLD:AAI6393 | 3 | 1 | 1 | 1 | ||
| Gymnothorax robinsi | BOLD:AAY2654 | 1 | 1 | 1 | 1 | ||
| Gymnothorax rueppelliae | BOLD:AAE3543 | 2 | >1 | 1 | |||
| Scuticaria tigrina | BOLD:AAH9730 | 1 | 1 | 1 | 0 | ||
| Ophichthidae | |||||||
| Pisodonophis cancrivorus | 0 | 1 | |||||
| Atheriniformes | |||||||
| Atherinidae | |||||||
| Atherinomorus cf. lacunosus [BOLD:ACK7521] | BOLD:ACK7521 | 3 | >1 | Y | A | 1 | |
| Hypoatherina [BOLD:AAL7563] | BOLD:AAL7563 | 1 | >1 | 1 | |||
| Hypoatherina [BOLD:ACV9758] | BOLD:ACV9758 | 5 | >1 | 1 | |||
| Aulopiformes | |||||||
| Synodontidae | |||||||
| Saurida cf. gracilis [BOLD:AAB1854] | BOLD:AAB1854 | 4 | >1 | Y | S | 1 | |
| Saurida cf. gracilis [BOLD:AAE4190] | BOLD:AAE4190 | 2 | >1 | Y | S | 2 | |
| Saurida nebulosa | BOLD:AAH0503 | 11 | 1 | >1 | Y | A | 1 |
| Synodus [BOLD:ACD1807] | BOLD:ACD1807 | 2 | NA | 2 | |||
| Synodus dermatogenys | 0 | 2 | |||||
| Synodus rubromarmoratus | 0 | 1 | |||||
| Synodus variegatus | BOLD:AAB5069 | 4 | >1 | 1 | |||
| Trachinocephalus [BOLD:AAA9578] | BOLD:AAA9578 | 2 | >1 | 0 | |||
| Trachinocephalus [BOLD:ACY8623] | BOLD:ACY8623 | 1 | 1 | >1 | 1 | ||
| Trachinocephalus cf. trachinus [BOLD:ABX6347] | BOLD:ABX6347 | 4 | >1 | Y | A | 0 | |
| Beloniformes | |||||||
| Belonidae | |||||||
| Ablennes cf. hian [BOLD:AAB9824] | BOLD:AAB9824 | 1 | >1 | Y | S | 1 | |
| Tylosurus cf. acus [BOLD:AAH7713] | BOLD:AAH7713 | 1 | 1 | >1 | Y | S | 1 |
| Tylosurus crocodilus | BOLD:AAC4148 | 7 | >1 | 1 | |||
| Exocoetidae | |||||||
| Cheilopogon [BOLD:ABZ7103] | BOLD:ABZ7103 | 1 | >1 | 1 | |||
| Cheilopogon cyanopterus | BOLD:ACK7919 | 1 | >1 | 1 | |||
| Exocoetidae [BOLD:AAK1099] | BOLD:AAK1099 | 1 | >1 | 1 | |||
| Parexocoetus brachypterus | BOLD:AAG2613 | 1 | >1 | 1 | |||
| Hemiramphidae | |||||||
| Hemiramphus cf. far [BOLD:AAC0565] | BOLD:AAC0565 | 6 | 1 | >1 | Y | A | 1 |
| Hemiramphus lutkei | BOLD:ACK7284 | 2 | >1 | >1 | 1 | ||
| Hyporhamphus affinis | BOLD:AAD0579 | 5 | 1 | 1 | |||
| Blenniiformes | |||||||
| Blenniidae | |||||||
| Aspidontus dussumieri | BOLD:AAJ3001 | 1 | 1 | 1 | 0 | ||
| Omobranchus [BOLD:ACY8418] | BOLD:ACY8418 | 1 | NA | 1 | |||
| Omobranchus elongatus | BOLD:AFC2971 | 2 | new | 1 | |||
| Petroscirtes cf. mitratus [BOLD:AAE6131] | BOLD:AAE6131 | 19 | >1 | Y | S | 1 | |
| Petroscirtes cf. mitratus [BOLD:AAE6132] | BOLD:AAE6132 | 7 | >1 | Y | S | 1 | |
| Plagiotremus tapeinosoma | BOLD:AAD0784 | 2 | 1 | 1 | 0 | ||
| Salarias [BOLD:ADN2100] | BOLD:ADN2100 | 1 | NA | 1 | |||
| Salarias cf. fasciatus [BOLD:ACV7965] | BOLD:ACV7965 | 6 | 1 | >1 | Y | A | 1 |
| Tripterygiidae | |||||||
| Enneapterygius [BOLD:ACV9383] | BOLD:ACV9383 | 2 | NA | 1 | |||
| Helcogramma [BOLD:AAT9889] | BOLD:AAT9889 | 1 | NA | 1 | |||
| Carangiformes | |||||||
| Carangidae | |||||||
| Alectis ciliaris | BOLD:AAB7827 | 2 | 1 | 1 | 1 | ||
| Atropus hedlandensis | BOLD:AAD6117 | 2 | >1 | 1 | |||
| Atropus mentalis | BOLD:AAD6118 | 1 | >1 | 1 | |||
| Atule mate | BOLD:AAB3382 | 3 | >1 | 1 | |||
| Carangichthys dinema | BOLD:AAO5886 | 1 | >1 | 1 | |||
| Caranx ignobilis | BOLD:AAB0587 | 13 | >1 | 3 | |||
| Caranx melampygus | BOLD:AAB0585 | 6 | >1 | 1 | |||
| Caranx papuensis | BOLD:ACF4541 | 2 | >1 | 1 | |||
| Caranx sexfasciatus | BOLD:AAB0584 | 5 | >1 | 1 | |||
| Caranx tille | BOLD:ACS0288 | 1 | >1 | 0 | |||
| Elagatis bipinnulata | BOLD:AAB3730 | 1 | >1 | 0 | |||
| Ferdauia ferdau | BOLD:AAE7640 | 2 | 1 | 1 | 1 | ||
| Gnathanodon speciosus | BOLD:AAB7462 | 2 | 1 | >1 | Y | S | 1 |
| Parastromateus niger | BOLD:AAB3884 | 1 | 1 | 1 | 1 | ||
| Platycaranx [BOLD:AAB4362] | BOLD:AAB4362 | 2 | >1 | 0 | |||
| Platycaranx chrysophrys | BOLD:AAB2977 | 1 | >1 | 1 | |||
| Scomberoides lysan | BOLD:AAB0512 | 11 | 1 | >1 | 0 | ||
| Selar cf. crumenophthalmus [BOLD:AAB0871] | BOLD:AAB0871 | 2 | Y | S | 1 | ||
| Seriolina [BOLD:AAB8503] | BOLD:AAB8503 | 1 | 1 | >1 | 1 | ||
| Trachinotus blochii | BOLD:ACF4014 | 1 | 1 | 1 | Y | S | 1 |
| Turrum cf. coeruleopinnatum [BOLD:AAD2297] | BOLD:AAD2297 | 6 | >1 | Y | S | 2 | |
| Turrum fulvoguttatum | BOLD:AAC2745 | 3 | 1 | 1 | 2 | ||
| Coryphaenidae | |||||||
| Coryphaena hippurus | BOLD:AAA5277 | 1 | >1 | 1 | |||
| Echeneidae | |||||||
| Echeneis naucrates | BOLD:AAB6121 | 2 | >1 | 1 | |||
| Sphyraenidae | |||||||
| Sphyraena [BOLD:AAF8783] | BOLD:AAF8783 | 1 | >1 | 1 | |||
| Sphyraena [BOLD:ACV9716] | BOLD:ACV9716 | 5 | NA | 1 | |||
| Sphyraena barracuda | BOLD:AAA6100 | 6 | >1 | 1 | |||
| Sphyraena chrysotaenia | BOLD:AAD0400 | 2 | >1 | 1 | |||
| Sphyraena flavicauda | BOLD:AAF8900 | 6 | >1 | 1 | |||
| Sphyraena putnamae | BOLD:AAB2694 | 1 | >1 | 1 | |||
| Sphyraena qenie | BOLD:AAD8414 | 1 | 1 | 1 | 1 | ||
| Clupeiformes | |||||||
| Chirocentridae | |||||||
| Chirocentrus cf. dorab [BOLD:AAC2273] | BOLD:AAC2273 | 2 | 1 | >1 | Y | A | 1 |
| Clupeidae | |||||||
| Herklotsichthys [BOLD:AEQ8868] | BOLD:AEQ8868 | 1 | new | 1 | |||
| Dussumieria cf. elopsoides [BOLD:AEP5161] | BOLD:AEP5161 | 2 | new | Y | A | 2 | |
| Herklotsichthys quadrimaculatus | BOLD:AAC2887 | 8 | >1 | 3 | |||
| Spratelloides cf. delicatulus [BOLD:ACV7999] | BOLD:ACV7999 | 6 | 1 | >1 | Y | A | 1 |
| Spratelloides cf. gracilis | 0 | 1 | |||||
| Engraulidae | |||||||
| Stolephorus belaerius | BOLD:AAG4825 | 5 | 1 | >1 | 1 | ||
| Thryssa baelama | BOLD:ABU9831 | 1 | >1 | 1 | |||
| Elopiformes | |||||||
| Megalopidae | |||||||
| Megalops cyprinoides | BOLD:AAC4501 | 1 | 1 | >1 | 1 | ||
| Gobiiformes | |||||||
| Eleotridae | |||||||
| Eleotris klunzingerii | BOLD:ACV7471 | 1 | 1 | 1 | 1 | ||
| Gobiidae | |||||||
| Amblygobius nocturnus | 0 | 1 | |||||
| Amblygobius semicinctus | BOLD:AAB8727 | 10 | >1 | 1 | |||
| Amblygobius sphynx | BOLD:AAJ2321 | 5 | 1 | >1 | 1 | ||
| Asterropteryx [BOLD:AEU6891] | BOLD:AEU6891 | 1 | new | 1 | |||
| Asterropteryx cf. semipunctata [BOLD:AAC0108] | BOLD:AAC0108 | 12 | 1 | >1 | Y | A | 1 |
| Bathygobius [BOLD:AAF8787] | BOLD:AAF8787 | 1 | NA | 1 | |||
| Bathygobius cyclopterus | BOLD:AAB9729 | 1 | >1 | 1 | |||
| Callogobius flavobrunneus | BOLD:ACV9382 | 2 | 1 | 1 | 1 | ||
| Cryptocentrus [BOLD:ADM8879] | BOLD:ADM8879 | 1 | NA | 1 | |||
| Cryptocentrus [BOLD:AEP3749] | BOLD:AEP3749 | 4 | new | 1 | |||
| Cryptocentrus [BOLD:AFC5965] | BOLD:AFC5965 | 1 | new | 1 | |||
| Cryptocentrus cryptocentrus | BOLD:AAM4607 | 1 | 1 | 1 | 1 | ||
| Drombus key | BOLD:AEF3691 | 1 | new | 0 | |||
| Favonigobius cf. melanobranchus [BOLD:AAL8921] | BOLD:AAL8921 | 1 | >1 | Y | A | 1 | |
| Favonigobius reichei | BOLD:AAY4455 | 1 | 1 | 1 | 1 | ||
| Gunnellichthys copleyi | BOLD:ADM8209 | 1 | 1 | 1 | 0 | ||
| Istigobius cf. decoratus [BOLD:ADM7236] | BOLD:ADM7236 | 3 | NA | Y | A | 1 | |
| Istigobius cf. ornatus [BOLD:AAD7940] | BOLD:AAD7940 | 1 | 1 | >1 | Y | A | 1 |
| Oplopomus [BOLD:AEX8266] | BOLD:AEX8266 | 1 | NA | 1 | |||
| Oplopomus cf. oplopomus [BOLD:AAI3352] | BOLD:AAI3352 | 13 | 1 | >1 | Y | A | 2 |
| Palutrus reticularis | BOLD:AAL7906 | 1 | >1 | 1 | |||
| Psammogobius biocellatus | BOLD:AAC2888 | 8 | >1 | 1 | |||
| Ptereleotris evides | BOLD:AAD9105 | 1 | 1 | 1 | 1 | ||
| Valenciennea cf. puellaris [BOLD:AAC4124] | BOLD:AAC4124 | 1 | >1 | Y | A | 1 | |
| Valenciennea helsdingenii | BOLD:AAD4704 | 1 | 1 | >1 | Y | A | 1 |
| Valenciennea sexguttata | BOLD:AAC5611 | 6 | 1 | 1 | 1 | ||
| Vanderhorstia ornatissima | BOLD:AAF0288 | 4 | 1 | >1 | Y | A | 1 |
| Yongeichthys audax | BOLD:AAJ2911 | 1 | 1 | >1 | Y | A | 0 |
| Yongeichthys nebulosus | BOLD:AAC3655 | 2 | >1 | 0 | |||
| Yongeichthys signatus | BOLD:ACX9631 | 3 | NA | 1 | |||
| Oxudercidae | |||||||
| Gnatholepis cf. anjerensis [BOLD:AAI5395] | BOLD:AAI5395 | 10 | >1 | Y | A | 1 | |
| Oxyurichthys papuensis | BOLD:ACM4179 | 3 | 1 | 1 | 2 | ||
| Holocentriformes | |||||||
| Holocentridae | |||||||
| Myripristis [BOLD:AAA9764] | BOLD:AAA9764 | 1 | >1 | 0 | |||
| Myripristis berndti | BOLD:AAA9763 | 2 | >1 | 1 | |||
| Myripristis kuntee | BOLD:AAA9765 | 2 | >1 | 1 | |||
| Neoniphon sammara | BOLD:AAC8278 | 7 | 1 | >1 | Y | A | 2 |
| Sargocentron caudimaculatum | BOLD:AEZ5902 | 2 | >1 | 1 | |||
| Sargocentron diadema | BOLD:AAB3424 | 9 | 1 | >1 | Y | A | 1 |
| Sargocentron praslin | BOLD:AAC4647 | 3 | >1 | 1 | |||
| Kurtiformes | |||||||
| Apogonidae | |||||||
| Apogon [BOLD:AAF8427] | BOLD:AAF8427 | 5 | >1 | 1 | |||
| Apogon [BOLD:AAJ8751] | BOLD:AAJ8751 | 4 | 1 | >1 | 2 | ||
| Apogon [BOLD:ACY1702] | BOLD:ACY1702 | 3 | NA | 1 | |||
| Apogon cf. crassiceps [BOLD:ACW9154] | BOLD:ACW9154 | 3 | 1 | >1 | Y | A | 1 |
| Apogon erythrosoma | BOLD:ACC5690 | 6 | >1 | 1 | |||
| Apogon semiornatus | BOLD:AAD2206 | 4 | 1 | >1 | 1 | ||
| Apogonichthyoides timorensis | BOLD:ACV6948 | 19 | 1 | >1 | 1 | ||
| Apogonichthys [BOLD:ACW8182] | BOLD:ACW8182 | 2 | NA | 1 | |||
| Apogonichthys cf. ocellatus [BOLD:AAL6796] | BOLD:AAL6796 | 1 | 1 | >1 | Y | A | 0 |
| Cheilodipterus cf. quinquelineatus [BOLD:ABU8169] | BOLD:ABU8169 | 5 | 1 | >1 | Y | A | 1 |
| Cheilodipterus macrodon | BOLD:AAB7874 | 1 | 1 | >1 | Y | A | 1 |
| Foa cf. fo [BOLD:ABU8857] | BOLD:ABU8857 | 20 | 1 | >1 | Y | A | 1 |
| Foa fo | BOLD:ABU8856 | 6 | 1 | 1 | 1 | ||
| Fowleria cf. marmorata [BOLD:AAU0944] | BOLD:AAU0944 | 1 | 1 | >1 | Y | A | 0 |
| Fowleria cf. vaiulae [BOLD:AAD1017] | BOLD:AAD1017 | 1 | 1 | >1 | Y | A | 1 |
| Fowleria variegata | BOLD:AAD8726 | 15 | 1 | >1 | Y | A | 2 |
| Jaydia novaeguineae | BOLD:ACM4616 | 1 | 1 | 1 | 1 | ||
| Neamia octospina | BOLD:ACY8539 | 1 | >1 | 1 | |||
| Nectamia cf. fusca [BOLD:AAL9262] | BOLD:AAL9262 | 10 | 1 | >1 | Y | A | 1 |
| Nectamia cf. savayensis [BOLD:AAD9453] | BOLD:AAD9453 | 3 | >1 | Y | A | 1 | |
| Ostorhinchus [BOLD:AAJ1260] | BOLD:AAJ1260 | 4 | 1 | >1 | 1 | ||
| Ostorhinchus [BOLD:ADI1552] | BOLD:ADI1552 | 3 | NA | 1 | |||
| Ostorhinchus aureus | BOLD:ACE9301 | 1 | 1 | >1 | Y | A | 1 |
| Ostorhinchus cf. angustatus [BOLD:AAD5116] | BOLD:AAD5116 | 2 | 1 | >1 | Y | A | 2 |
| Ostorhinchus cf. apogonoides [BOLD:AAD5125] | BOLD:AAD5125 | 1 | >1 | Y | A | 1 | |
| Ostorhinchus cf. cooki [BOLD:AAC2084] | BOLD:AAC2084 | 16 | 1 | >1 | Y | A | 1 |
| Ostorhinchus cf. cyanosoma [BOLD:ACV9601] | BOLD:ACV9601 | 14 | NA | Y | A | 3 | |
| Ostorhinchus cf. fasciatus [BOLD:AAC1243] | BOLD:AAC1243 | 1 | 1 | >1 | Y | A | 1 |
| Ostorhinchus cf. gularis [BOLD:ACS5956] | BOLD:ACS5956 | 2 | 1 | >1 | Y | A | 1 |
| Ostorhinchus cf. taeniophorus [BOLD:AAD8453] | BOLD:AAD8453 | 1 | >1 | Y | S | 1 | |
| Ostorhinchus cf. taeniophorus [BOLD:AAD8454] | BOLD:AAD8454 | 7 | 1 | >1 | Y | S | 1 |
| Ostorhinchus flagelliferus | BOLD:AAJ1254 | 2 | 1 | 1 | 1 | ||
| Ostorhinchus fleurieu | BOLD:AAD5600 | 5 | >1 | 1 | |||
| Pristiapogon cf. fraenatus [BOLD:AAJ1264] | BOLD:AAJ1264 | 3 | 1 | >1 | Y | A | 1 |
| Pristiapogon kallopterus | BOLD:AAB4082 | 5 | 1 | >1 | Y | A | 1 |
| Pseudamia [BOLD:AFB6766] | BOLD:AFB6766 | 3 | new | 2 | |||
| Siphamia [BOLD:ADO4802] | BOLD:ADO4802 | 1 | NA | 0 | |||
| Siphamia mossambica | BOLD:AEF3435 | 1 | NA | 0 | |||
| Taeniamia cf. fucata [BOLD:AAB8394] | BOLD:AAB8394 | 10 | >1 | Y | A | 1 | |
| Taeniamia flavofasciata | BOLD:AAU1539 | 5 | 1 | 1 | 1 | ||
| Zoramia cf. leptacanthus [BOLD:AAU1535] | BOLD:AAU1535 | 4 | >1 | Y | A | 1 | |
| Lophiiformes | |||||||
| Antennariidae | |||||||
| Antennariidae | 0 | 1 | |||||
| Antennarius cf. striatus [BOLD:AAO6018] | BOLD:AAO6018 | 3 | 1 | >1 | Y | A | 0 |
| Moroniformes | |||||||
| Ephippidae | |||||||
| Platax orbicularis | BOLD:AAC6496 | 6 | 1 | 1 | 1 | ||
| Platax teira | BOLD:AAC5812 | 1 | 1 | >1 | 1 | ||
| Mugiliformes | |||||||
| Mugilidae | |||||||
| Crenimugil buchanani | BOLD:AAE3561 | 1 | >1 | 0 | |||
| Moolgarda crenilabis | BOLD:AAG6597 | 3 | >1 | 2 | |||
| Mugil cf. cephalus [BOLD:AAA7833] | BOLD:AAA7833 | 8 | >1 | Y | A | 1 | |
| Ovalentaria | |||||||
| Ambassidae | |||||||
| Ambassis dussumieri | BOLD:AAJ2348 | 2 | >1 | 1 | |||
| Plesiopidae | |||||||
| Plesiops coeruleolineatus | BOLD:AAE4785 | 5 | 1 | >1 | 1 | ||
| Pomacentridae | |||||||
| Abudefduf sparoides | BOLD:AAD7433 | 2 | 1 | 1 | 1 | ||
| Abudefduf vaigiensis | BOLD:ACK8109 | 6 | >1 | 1 | |||
| Amblyglyphidodon [BOLD:ACF1984] | BOLD:ACF1984 | 2 | 1 | >1 | 1 | ||
| Amblypomacentrus annulatus | BOLD:AAF2723 | 12 | 1 | 1 | 1 | ||
| Amphiprion allardi | BOLD:AAC4980 | 2 | 1 | 1 | 1 | ||
| Chromis [BOLD:ACF0042] | BOLD:ACF0042 | 4 | >1 | 1 | |||
| Chromis atripectoralis | BOLD:AAB9018 | 1 | 1 | 1 | 0 | ||
| Chromis cf. ternatensis [BOLD:AAC6972] | BOLD:AAC6972 | 1 | 1 | >1 | Y | A | 0 |
| Chromis fieldi | BOLD:AAC0458 | 1 | >1 | 1 | |||
| Chromis ternatensis | BOLD:AAF3462 | 1 | 1 | >1 | Y | A | 1 |
| Chromis viridis | BOLD:AAB4985 | 7 | 1 | 1 | 1 | ||
| Chrysiptera cf. brownriggii [BOLD:AAB6234] | BOLD:AAB6234 | 2 | >1 | Y | A | 0 | |
| Dascyllus abudafur | BOLD:AAB4091 | 6 | >1 | 1 | |||
| Dascyllus carneus | BOLD:AAD7114 | 5 | 1 | >1 | Y | A | 0 |
| Dascyllus trimaculatus | BOLD:AAB2685 | 13 | >1 | 1 | |||
| Neoglyphidodon melas | BOLD:AAC5328 | 1 | 1 | 1 | 1 | ||
| Neopomacentrus cyanomos | BOLD:ABX6100 | 1 | 1 | >1 | Y | A | 1 |
| Neopomacentrus fuliginosus | BOLD:AAI3160 | 9 | >1 | 0 | |||
| Neopomacentrus sororius | BOLD:AAC8318 | 6 | >1 | 1 | |||
| Plectroglyphidodon cf. lacrymatus [BOLD:AAB6988] | BOLD:AAB6988 | 8 | 1 | >1 | Y | A | 1 |
| Plectroglyphidodon dickii | BOLD:ACC1132 | 1 | 1 | 1 | 1 | ||
| Pomacentrus aquilus | BOLD:AAY3550 | 33 | >1 | 1 | |||
| Pomacentrus baenschi | BOLD:AAC9672 | 1 | 1 | 1 | 0 | ||
| Pomacentrus caeruleus | BOLD:AAB9539 | 5 | >1 | 1 | |||
| Pomacentrus sulfureus | BOLD:AAD2952 | 2 | 1 | 1 | 0 | ||
| Pycnochromis nigrurus | BOLD:AAD5022 | 1 | 1 | 1 | 1 | ||
| Pseudochromidae | |||||||
| Halidesmus [BOLD:AEU2441] | BOLD:AEU2441 | 1 | new | 1 | |||
| Halidesmus [BOLD:AAU1500] | BOLD:AAU1500 | 1 | NA | 0 | |||
| Pseudochromis cf. kristinae [BOLD:ADI5215] | BOLD:ADI5215 | 11 | NA | Y | A | 2 | |
| Pseudochromis madagascariensis | BOLD:AAU3299 | 3 | 1 | 1 | |||
| Perciformes | |||||||
| Caesionidae | |||||||
| Caesio cf. caerulaurea [BOLD:AAB4823] | BOLD:AAB4823 | 12 | >1 | Y | S | 2 | |
| Caesio cf. caerulaurea [BOLD:AAB4822] | BOLD:AAB4822 | 2 | >1 | Y | S | 1 | |
| Caesio lunaris | BOLD:AAJ8622 | 1 | 1 | 1 | 0 | ||
| Caesio xanthonota | BOLD:AAE8330 | 1 | 1 | 1 | 0 | ||
| Dipterygonotus balteatus | BOLD:AAD3666 | 1 | 1 | 1 | 1 | ||
| Pterocaesio chrysozona | BOLD:AAE8474 | 1 | >1 | 0 | |||
| Pterocaesio marri | BOLD:AAC2204 | 3 | >1 | 1 | |||
| Pterocaesio trilineata | BOLD:AAE8473 | 1 | >1 | 1 | |||
| Chaetodontidae | |||||||
| Chaetodon auriga | BOLD:AAB1540 | 7 | 1 | 1 | 2 | ||
| Chaetodon blackburnii | BOLD:AAE1178 | 3 | 1 | 1 | 1 | ||
| Chaetodon cf. bennetti [BOLD:ACE8647] | BOLD:ACE8647 | 1 | 1 | >1 | Y | A | 1 |
| Chaetodon guttatissimus | BOLD:AAD3872 | 1 | >1 | 1 | |||
| Chaetodon kleinii | BOLD:AAC2841 | 3 | >1 | 1 | |||
| Chaetodon lineolatus | BOLD:ABZ0931 | 1 | 1 | 1 | 1 | ||
| Chaetodon lunula | BOLD:AAB6339 | 2 | >1 | 1 | |||
| Chaetodon madagaskariensis | BOLD:AAC2634 | 2 | >1 | 1 | |||
| Chaetodon melannotus | BOLD:AAC2848 | 5 | >1 | 1 | |||
| Chaetodon trifasciatus | BOLD:AAB7102 | 5 | >1 | 2 | |||
| Chaetodon ulietensis | BOLD:AAC8636 | 1 | >1 | 1 | |||
| Chaetodon vagabundus | BOLD:AAB3198 | 8 | 1 | 1 | 2 | ||
| Chaetodon xanthocephalus | BOLD:AAE1213 | 5 | 1 | 1 | 1 | ||
| Chaetodon zanzibarensis | BOLD:AAE9260 | 1 | 1 | 1 | 1 | ||
| Heniochus cf. acuminatus [BOLD:AAB5716] | BOLD:AAB5716 | 4 | >1 | Y | A | 1 | |
| Heniochus diphreutes | BOLD:AAB5717 | 1 | >1 | 1 | |||
| Heniochus monoceros | BOLD:AAC7417 | 1 | 1 | 1 | 1 | ||
| Cirrhitidae | |||||||
| Cirrhitus cf. pinnulatus [BOLD:AAC5875] | BOLD:AAC5875 | 2 | >1 | Y | A | 2 | |
| Cyprinocirrhites cf. polyactis [BOLD:AAF8813] | BOLD:AAF8813 | 1 | 1 | >1 | Y | A | 1 |
| Paracirrhites arcatus | BOLD:AAC6007 | 2 | 1 | 1 | 1 | ||
| Paracirrhites forsteri | BOLD:AAC5873 | 1 | >1 | 1 | |||
| Gerreidae | |||||||
| Gerres cf. filamentosus [BOLD:AAC0377] | BOLD:AAC0377 | 3 | >1 | Y | A | 0 | |
| Gerres filamentosus | BOLD:AAC0380 | 2 | >1 | 1 | |||
| Gerres longirostris | BOLD:AAE6359 | 8 | 1 | >1 | Y | A | 3 |
| Gerres oblongus | BOLD:AAE6346 | 2 | 1 | 1 | 1 | ||
| Gerres oyena | BOLD:AAC1291 | 2 | 1 | >1 | Y | A | 0 |
| Haemulidae | |||||||
| Diagramma [BOLD:AAD4477] | BOLD:AAD4477 | 12 | >1 | 2 | |||
| Plectorhinchus cf. vittatus [BOLD:AAE4709] | BOLD:AAE4709 | 1 | 1 | >1 | Y | A | 1 |
| Plectorhinchus [BOLD:AAF8797] | BOLD:AAF8797 | 1 | >1 | 1 | |||
| Plectorhinchus flavomaculatus | BOLD:AAC4020 | 10 | 1 | 1 | 1 | ||
| Plectorhinchus gaterinus | BOLD:AAH9156 | 12 | 1 | 1 | 1 | ||
| Plectorhinchus plagiodesmus | BOLD:ACC0439 | 1 | 1 | 1 | 0 | ||
| Pomadasys cf. furcatus [BOLD:AAD1382] | BOLD:AAD1382 | 1 | 1 | >1 | Y | A | 1 |
| Kyphosidae | |||||||
| Kyphosus cinerascens | BOLD:ABX5727 | 1 | >1 | 1 | |||
| Kyphosus vaigiensis | BOLD:AAC3456 | 2 | >1 | 1 | |||
| Labridae | |||||||
| Anampses caeruleopunctatus | BOLD:AAB9969 | 1 | >1 | 0 | |||
| Bodianus cf. perditio [BOLD:AAC7631] | BOLD:AAC7631 | 1 | 1 | >1 | Y | A | 1 |
| Cheilinus cf. chlorourus [BOLD:AAB4186] | BOLD:AAB4186 | 8 | >1 | Y | A | 1 | |
| Cheilinus oxycephalus | BOLD:AAB4187 | 3 | >1 | 1 | |||
| Cheilinus trilobatus | BOLD:AAB4188 | 5 | >1 | 1 | |||
| Cheilinus undulatus | BOLD:AAF3078 | 1 | 1 | 1 | 1 | ||
| Cheilio inermis | BOLD:AAA6101 | 5 | 1 | 1 | 3 | ||
| Coris caudimacula | BOLD:AAC2101 | 4 | 1 | 1 | 1 | ||
| Cymolutes cf. torquatus [BOLD:ADM8951] | BOLD:ADM8951 | 1 | 1 | >1 | Y | A | 1 |
| Cymolutes praetextatus | BOLD:AAF7751 | 7 | 1 | >1 | 1 | ||
| Cymolutes torquatus | BOLD:AAI9445 | 3 | >1 | 1 | |||
| Epibulus cf. insidiator [BOLD:AAB8858] | BOLD:AAB8858 | 8 | 1 | >1 | Y | S | 1 |
| Epibulus cf. insidiator [BOLD:ADU6797] | BOLD:ADU6797 | 1 | 1 | >1 | Y | S | 1 |
| Gomphosus caeruleus | BOLD:AAD5965 | 2 | 1 | 1 | 2 | ||
| Halichoeres [BOLD:AAT9960] | BOLD:AAT9960 | 1 | >1 | 1 | |||
| Halichoeres cf. hortulanus [BOLD:AAB3085] | BOLD:AAB3085 | 2 | 1 | >1 | Y | A | 1 |
| Halichoeres cf. nebulosus [BOLD:AAC7896] | BOLD:AAC7896 | 3 | 1 | >1 | Y | A | 1 |
| Halichoeres cf. zeylonicus [BOLD:AAF7654] | BOLD:AAF7654 | 5 | 1 | >1 | Y | A | 1 |
| Halichoeres lapillus | BOLD:AAE3021 | 1 | 1 | 1 | 1 | ||
| Halichoeres scapularis | BOLD:AAC0139 | 6 | 1 | >1 | Y | A | 1 |
| Halichoeres timorensis | BOLD:AAU1265 | 1 | >1 | 1 | |||
| Hemigymnus cf. fasciatus [BOLD:ACE5917] | BOLD:ACE5917 | 1 | 1 | >1 | Y | A | 0 |
| Hemigymnus melapterus | BOLD:AAB5264 | 1 | 1 | 1 | 1 | ||
| Hologymnosus doliatus | BOLD:AAD1381 | 1 | >1 | 1 | |||
| Iniistius bimaculatus | BOLD:ACT0048 | 2 | 1 | 1 | 1 | ||
| Iniistius naevus | BOLD:ACY5613 | 1 | new | 0 | |||
| Iniistius pavo | BOLD:AAD8586 | 1 | 1 | >1 | 0 | ||
| Novaculichthys taeniourus | BOLD:AAB3259 | 3 | 1 | 1 | 1 | ||
| Novaculoides macrolepidotus | BOLD:AAD8577 | 15 | 1 | 1 | 1 | ||
| Oxycheilinus cf. bimaculatus [BOLD:AAC3195] | BOLD:AAC3195 | 26 | >1 | Y | A | 1 | |
| Oxycheilinus digramma | BOLD:AAC0639 | 1 | 1 | 1 | 1 | ||
| Pseudojuloides argyreogaster | BOLD:ADI3805 | 6 | 1 | 1 | 1 | ||
| Pteragogus flagellifer | BOLD:AAZ4093 | 11 | >1 | 1 | |||
| Pteragogus taeniops | BOLD:AAV2736 | 1 | 1 | 1 | 1 | ||
| Pteragogus trispilus | BOLD:ADI3844 | 2 | 1 | 1 | 1 | ||
| Stethojulis albovittata | BOLD:AAD4824 | 8 | 1 | 1 | 1 | ||
| Stethojulis interrupta | BOLD:AAC2336 | 7 | >1 | 1 | |||
| Stethojulis strigiventer | BOLD:AAE2186 | 12 | 1 | 1 | 3 | ||
| Stethojulis | 0 | 1 | |||||
| Thalassoma amblycephalum | BOLD:ADI4249 | 1 | >1 | 1 | |||
| Thalassoma hardwicke | BOLD:AAB0061 | 2 | 1 | 1 | 1 | ||
| Thalassoma hebraicum | BOLD:AAC2788 | 2 | 1 | 1 | 1 | ||
| Thalassoma lunare | BOLD:AAB0673 | 4 | 1 | 1 | 2 | ||
| Thalassoma trilobatum | BOLD:AAC2785 | 1 | >1 | 1 | |||
| Leiognathidae | |||||||
| Aurigequula fasciata | BOLD:AAB2489 | 1 | >1 | 0 | |||
| Deveximentum cf. insidiator [BOLD:ACF0053] | BOLD:ACF0053 | 1 | 1 | >1 | Y | A | 1 |
| Equulites klunzingeri | BOLD:AAC0238 | 1 | >1 | 0 | |||
| Equulites [BOLD:AAX8534] | BOLD:AAX8534 | 1 | NA | 0 | |||
| Gazza [BOLD:AAB7096] | BOLD:AAB7096 | 2 | >1 | 0 | |||
| Lutjanidae | |||||||
| Aprion virescens | BOLD:AAB8692 | 1 | 1 | 1 | 1 | ||
| Lutjanus argentimaculatus | BOLD:AAB2440 | 3 | 1 | 1 | 1 | ||
| Lutjanus bengalensis | BOLD:AAB7901 | 1 | >1 | 1 | |||
| Lutjanus bohar | BOLD:AAB4501 | 2 | >1 | 0 | |||
| Lutjanus cf. fulvus [BOLD:AAB7015] | BOLD:AAB7015 | 2 | 1 | >1 | Y | A | 1 |
| Lutjanus cf. kasmira [BOLD:ACC0930] | BOLD:ACC0930 | 4 | >1 | Y | A | 1 | |
| Lutjanus cf. lutjanus [BOLD:AAA8168] | BOLD:AAA8168 | 4 | >1 | Y | A | 2 | |
| Lutjanus fulviflamma | BOLD:ADF5681 | 18 | >1 | 2 | |||
| Lutjanus gibbus | BOLD:AAB3276 | 10 | >1 | 1 | |||
| Lutjanus monostigma | BOLD:AAB2907 | 1 | >1 | 1 | |||
| Lutjanus notatus | BOLD:AAF7731 | 6 | >1 | 1 | |||
| Lutjanus rivulatus | BOLD:AAB7684 | 1 | 1 | >1 | 1 | ||
| Lutjanus sebae | BOLD:AAB5388 | 1 | 1 | 1 | 1 | ||
| Monodactylidae | |||||||
| Monodactylus argenteus | BOLD:AAA9698 | 3 | 1 | >1 | Y | A | 1 |
| Mullidae | |||||||
| Mulloidichthys flavolineatus | BOLD:AAB2592 | 3 | >1 | 2 | |||
| Parupeneus [BOLD:AAB2590] | BOLD:AAB2590 | 15 | >1 | 1 | |||
| Parupeneus [BOLD:ADM7122] | BOLD:ADM7122 | 2 | NA | 1 | |||
| Parupeneus cf. barberinus [BOLD:AAB5978] | BOLD:AAB5978 | 7 | >1 | Y | S | 1 | |
| Parupeneus cf. barberinus [BOLD:AAB5980] | BOLD:AAB5980 | 6 | >1 | Y | S | 2 | |
| Parupeneus cyclostomus | BOLD:AAD1433 | 2 | >1 | 0 | |||
| Parupeneus fraserorum | BOLD:AAF8776 | 2 | 1 | 1 | 1 | ||
| Parupeneus indicus | BOLD:AAB0334 | 5 | 1 | 1 | 1 | ||
| Parupeneus macronemus | BOLD:ACF0223 | 7 | >1 | 1 | |||
| Parupeneus pleurostigma | BOLD:AAD6266 | 6 | 1 | 1 | 1 | ||
| Parupeneus rubescens | BOLD:AAC1405 | 6 | 1 | 1 | 1 | ||
| Parupeneus trifasciatus | BOLD:AAI4266 | 2 | 1 | 1 | 1 | ||
| Upeneus cf. margarethae [BOLD:AAB9714] | BOLD:AAB9714 | 3 | >1 | Y | A | 1 | |
| Upeneus guttatus | BOLD:AAH7551 | 1 | >1 | 1 | |||
| Upeneus moluccensis | BOLD:AAB6469 | 4 | >1 | 1 | |||
| Upeneus pori | BOLD:AAC1406 | 4 | >1 | 1 | |||
| Upeneus supravittatus | BOLD:ABZ7416 | 3 | >1 | 1 | |||
| Upeneus tragula | BOLD:AAB9327 | 1 | >1 | 1 | |||
| Upeneus vittatus | BOLD:ACV4665 | 3 | >1 | 1 | |||
| Pempheridae | |||||||
| Parapriacanthus [BOLD:AAC7599] | BOLD:AAC7599 | 3 | >1 | 1 | |||
| Pempheris connelli | BOLD:AAC6084 | 1 | 1 | 1 | 1 | ||
| Pempheris ibo | BOLD:AAF8820 | 3 | >1 | 0 | |||
| Pempheris mangula | BOLD:AAD1777 | 3 | >1 | 1 | |||
| Polynemidae | |||||||
| Polydactylus plebeius | BOLD:AAC6244 | 4 | 1 | >1 | 0 | ||
| Polydactylus sextarius | BOLD:AAB7311 | 1 | >1 | 0 | |||
| Pomacanthidae | |||||||
| Centropyge multispinis | BOLD:AAD3135 | 2 | 1 | 1 | 1 | ||
| Pomacanthus chrysurus | BOLD:AAL9941 | 1 | 1 | 1 | 1 | ||
| Pomacanthus semicirculatus | BOLD:ACK8181 | 5 | 1 | 1 | 1 | ||
| Priacanthidae | |||||||
| Heteropriacanthus carolinus | BOLD:AAB1874 | 2 | >1 | 1 | |||
| Priacanthus cf. arenatus [BOLD:AAB1642] | BOLD:AAB1642 | 2 | >1 | Y | A | 1 | |
| Priacanthus hamrur | BOLD:AAB1643 | 10 | >1 | 1 | |||
| Scaridae | |||||||
| Calotomus carolinus | BOLD:ADI4703 | 9 | >1 | 1 | |||
| Calotomus spinidens | BOLD:AAD4765 | 23 | 1 | 1 | 1 | ||
| Chlorurus atrilunula | BOLD:AAE8961 | 2 | >1 | 1 | |||
| Chlorurus cyanescens | BOLD:AEW3945 | 2 | 1 | 1 | 1 | ||
| Leptoscarus vaigiensis | 0 | 3 | |||||
| Scarus [BOLD:AAD0849] | BOLD:AAD0849 | 18 | >1 | 2 | |||
| Scarus cf. ghobban [BOLD:ABY4451] | BOLD:ABY4451 | 12 | 1 | >1 | Y | A | 2 |
| Scarus psittacus | BOLD:AAB8901 | 6 | 1 | 1 | 1 | ||
| Scarus scaber | BOLD:AAE9524 | 1 | >1 | 1 | |||
| Serranidae | |||||||
| Aethaloperca rogaa | BOLD:AAD9665 | 3 | 1 | >1 | Y | A | 1 |
| Cephalopholis argus | BOLD:AAC4474 | 1 | 1 | >1 | 1 | ||
| Cephalopholis cf. sonnerati [BOLD:AAB5431] | BOLD:AAB5431 | 1 | 1 | >1 | Y | A | 1 |
| Cephalopholis miniata | BOLD:AAC0216 | 2 | 1 | 1 | 1 | ||
| Cephalopholis nigripinnis | BOLD:AAC4202 | 1 | >1 | 1 | |||
| Epinephelus areolatus | BOLD:AAA9822 | 2 | >1 | 1 | |||
| Epinephelus coeruleopunctatus | BOLD:ADL0994 | 2 | >1 | 1 | |||
| Epinephelus fasciatus | BOLD:AAB1334 | 1 | 1 | >1 | Y | A | 1 |
| Epinephelus flavocaeruleus | BOLD:AAD1767 | 2 | >1 | 1 | |||
| Epinephelus longispinis | BOLD:AAD8800 | 1 | >1 | 1 | |||
| Epinephelus macrospilos | BOLD:AAE1882 | 2 | 1 | >1 | Y | A | 1 |
| Epinephelus merra | BOLD:AAB8387 | 1 | >1 | 1 | |||
| Epinephelus rivulatus | BOLD:ACZ9919 | 5 | 1 | >1 | Y | A | 1 |
| Epinephelus spilotoceps | BOLD:AAC3590 | 1 | 1 | >1 | 1 | ||
| Plectropomus punctatus | BOLD:AAN4283 | 3 | 1 | 1 | 1 | ||
| Variola louti | BOLD:AAC5719 | 1 | 1 | >1 | Y | A | 1 |
| Siganidae | |||||||
| Siganus argenteus | BOLD:AAC6461 | 2 | 1 | >1 | 2 | ||
| Siganus luridus | BOLD:AAL9467 | 2 | >1 | 0 | |||
| Siganus stellatus | BOLD:AAB2341 | 4 | >1 | 1 | |||
| Siganus sutor | BOLD:AAB6556 | 20 | >1 | 1 | |||
| Terapontidae | |||||||
| Pelates quadrilineatus | BOLD:AAA9700 | 2 | >1 | 1 | |||
| Terapon [BOLD:ACV8977] | BOLD:ACV8977 | 1 | >1 | 1 | |||
| Terapon jarbua | BOLD:AAA9351 | 1 | 1 | >1 | Y | A | 1 |
| Pleuronectiformes | |||||||
| Bothidae | |||||||
| Bothus cf. pantherinus [BOLD:AAC9155] | BOLD:AAC9155 | 13 | >1 | Y | A | 1 | |
| Crossorhombus valderostratus | BOLD:AAF8808 | 1 | 1 | 1 | 1 | ||
| Engyprosopon [BOLD:AD1616] | BOLD:ADI1616 | 5 | NA | 1 | |||
| Engyprosopon [BOLD:ADM7915] | BOLD:ADM7915 | 3 | NA | 1 | |||
| Engyprosopon [BOLD:AFI7142] | BOLD:AFI7142 | 1 | new | 0 | |||
| Cynoglossidae | |||||||
| Cynoglossus [BOLD:AFB7676] | BOLD:AFB7676 | 1 | new | 1 | |||
| Soleidae | |||||||
| Pardachirus marmoratus | BOLD:AAI5984 | 1 | 1 | 1 | 0 | ||
| Scombriformes | |||||||
| Nomeidae | |||||||
| Psenes cyanophrys | BOLD:AAE0701 | 1 | >1 | 1 | |||
| Scombridae | |||||||
| Auxis thazard | BOLD:AAB0928 | 1 | >1 | 1 | |||
| Rastrelliger kanagurta | BOLD:AAA9666 | 1 | >1 | 1 | |||
| Trichiuridae | |||||||
| Trichiurus cf. lepturus [BOLD:AAB0165] | BOLD:AAB0165 | 2 | >1 | Y | A | 2 | |
| Scorpaeniformes | |||||||
| Platycephalidae | |||||||
| Papilloculiceps longiceps | BOLD:AAI6160 | 20 | 1 | >1 | 2 | ||
| Platycephalus [BOLD:AAB2371] | BOLD:AAB2371 | 5 | 1 | >1 | 1 | ||
| Rogadius cf. pristiger [BOLD:ACY6212] | BOLD:ACY6212 | 10 | 1 | >1 | Y | A | 1 |
| Sunagocia otaitensis | BOLD:AAD2134 | 1 | 1 | 1 | 1 | ||
| Scorpaenidae | |||||||
| Ablabys binotatus | BOLD:AAF8834 | 2 | 1 | 1 | 0 | ||
| Dendrochirus cf. brachypterus [BOLD:AAC9564] | BOLD:AAC9564 | 6 | 1 | >1 | Y | A | 1 |
| Parascorpaena [BOLD:AAU1214] | BOLD:AAU1214 | 7 | NA | 1 | |||
| Pterois miles | BOLD:AAB8146 | 5 | >1 | 2 | |||
| Scorpaenopsis cf. possi [BOLD:AAD4142] | BOLD:AAD4142 | 1 | 1 | >1 | Y | A | 1 |
| Scorpaenopsis diabolus | BOLD:AAE1598 | 3 | 1 | 1 | 1 | ||
| Scorpaenopsis longispina | BOLD:AAD7048 | 2 | >1 | 1 | |||
| Scorpaenopsis venosa | BOLD:AAD9168 | 3 | 1 | 1 | 1 | ||
| Sebastapistes cf. strongia [BOLD:AAC4542] | BOLD:AAC4542 | 14 | 1 | >1 | Y | S | 1 |
| Sebastapistes cf. strongia [BOLD:AAC4543] | BOLD:AAC4543 | 41 | 1 | >1 | Y | S | 1 |
| Synanceia verrucosa | BOLD:AAE2821 | 3 | >1 | 1 | |||
| Siluriformes | |||||||
| Plotosidae | |||||||
| Plotosus cf. lineatus [BOLD:ACF3921] | BOLD:ACF3921 | 8 | 1 | >1 | Y | A | 2 |
| Spariformes | |||||||
| Lethrinidae | |||||||
| Lethrinus | 0 | 1 | |||||
| Gnathodentex aureolineatus | BOLD:AAC8927 | 1 | 1 | 1 | 1 | ||
| Gymnocranius cf. microdon [BOLD:AAB5193] | BOLD:AAB5193 | 1 | 1 | >1 | Y | A | 1 |
| Gymnocranius elongatus | BOLD:AAB5194 | 3 | >1 | 1 | |||
| Lethrinus [BOLD:AAC1547] | BOLD:AAC1547 | 5 | NA | 2 | |||
| Lethrinus [BOLD:AAC8078] | BOLD:AAC8078 | 19 | >1 | 1 | |||
| Lethrinus borbonicus | BOLD:AAB0511 | 50 | >1 | 3 | |||
| Lethrinus cf. lentjan [BOLD:ABZ0131] | BOLD:ABZ0131 | 11 | >1 | Y | A | 1 | |
| Lethrinus cf. nebulosus [BOLD:ABY6363] | BOLD:ABY6363 | 12 | 1 | >1 | Y | A | 0 |
| Lethrinus harak | BOLD:AAC1521 | 34 | 1 | >1 | Y | A | 2 |
| Lethrinus mahsena | BOLD:AAB6438 | 24 | >1 | 1 | |||
| Lethrinus obsoletus | BOLD:AAC1522 | 1 | >1 | 1 | |||
| Lethrinus rubrioperculatus | BOLD:AAB6439 | 2 | >1 | 1 | |||
| Lethrinus xanthochilus | BOLD:AAC1546 | 4 | 1 | >1 | 1 | ||
| Monotaxis grandoculis | BOLD:ABZ0166 | 1 | 1 | >1 | Y | A | 1 |
| Lobotidae | |||||||
| Lobotes surinamensis | BOLD:AAC1878 | 1 | 1 | 1 | 0 | ||
| Nemipteridae | |||||||
| Nemipterus bipunctatus | BOLD:AAF2507 | 1 | >1 | 1 | |||
| Nemipterus elaine | BOLD:AEH3406 | 2 | NA | 1 | |||
| Nemipterus zysron | BOLD:AAD1868 | 1 | >1 | 0 | |||
| Scolopsis [BOLD:AAC3574] | BOLD:AAC3574 | 1 | >1 | 0 | |||
| Scolopsis bimaculata | BOLD:AAD6249 | 4 | 1 | 1 | 0 | ||
| Scolopsis ghanam | BOLD:AAC4767 | 5 | 1 | 1 | 0 | ||
| Sillaginidae | |||||||
| Sillago cf. sihama [BOLD:AAA7598] | BOLD:AAA7598 | 1 | 1 | >1 | Y | A | 1 |
| Sparidae | |||||||
| Argyrops spinifer | BOLD:AAB3720 | 1 | 1 | >1 | 1 | ||
| Crenidens cf. crenidens [BOLD:AAE4408] | BOLD:AAE4408 | 4 | 1 | >1 | Y | A | 1 |
| Syngnathiformes | |||||||
| Aulostomidae | |||||||
| Aulostomus chinensis | BOLD:AAB9194 | 1 | 1 | 1 | 0 | ||
| Callionymidae | |||||||
| Callionymus [BOLD:AAI6959] | BOLD:AAI6959 | 2 | >1 | 1 | |||
| Callionymus [BOLD:ADN1799] | BOLD:ADN1799 | 2 | NA | 1 | |||
| Callionymus cf. filamentosus [BOLD:ACZ1215] | BOLD:ACZ1215 | 4 | 1 | >1 | Y | A | 1 |
| Callionymus | 0 | 2 | |||||
| Diplogrammus infulatus | BOLD:AAN1422 | 4 | 1 | 1 | 2 | ||
| Centriscidae | |||||||
| Aeoliscus punctulatus | BOLD:AAD3585 | 4 | 1 | 1 | 1 | ||
| Dactylopteridae | |||||||
| Dactyloptena orientalis | BOLD:AAB5966 | 9 | >1 | 1 | |||
| Fistulariidae | |||||||
| Fistularia commersonii | BOLD:AAB5992 | 7 | 1 | 1 | 1 | ||
| Pegasidae | |||||||
| Eurypegasus draconis | 0 | 1 | |||||
| Solenostomidae | |||||||
| Solenostomus cf. cyanopterus [BOLD:ACG8874] | BOLD:ACG8874 | 4 | 1 | >1 | Y | A | 1 |
| Syngnathidae | |||||||
| Acentronura [BOLD:ADI1740] | BOLD:ADI1740 | 7 | NA | 1 | |||
| Corythoichthys cf. conspicillatus [BOLD:AAI8860] | BOLD:AAI8860 | 7 | >1 | Y | A | 1 | |
| Corythoichthys haematopterus | BOLD:AAF0628 | 8 | >1 | 2 | |||
| Hippichthys [BOLD:AAE5342] | BOLD:AAE5342 | 5 | >1 | 2 | |||
| Hippichthys cf. cyanospilos [BOLD:AAK6079] | BOLD:AAK6079 | 11 | 1 | >1 | Y | A | 3 |
| Hippocampus [BOLD:ACE6993] | BOLD:ACE6993 | 8 | >1 | 1 | |||
| Hippocampus camelopardalis | BOLD:AAX0875 | 20 | 1 | 1 | 3 | ||
| Hippocampus cf. histrix [BOLD:AAE5356] | BOLD:AAE5356 | 1 | 1 | >1 | Y | A | 1 |
| Syngnathidae [BOLD:AEF4074] | BOLD:AEF4074 | 1 | new | 0 | |||
| Syngnathoides biaculeatus | BOLD:AAD7359 | 2 | >1 | 3 | |||
| Trachyrhamphus cf. bicoarctatus [BOLD:ADG5410] | BOLD:ADG5410 | 2 | NA | Y | A | 1 | |
| Tetraodontiformes | |||||||
| Balistidae | |||||||
| Balistapus undulatus | BOLD:AAC2755 | 2 | 1 | 1 | 2 | ||
| Balistoides viridescens | BOLD:AAD0474 | 8 | >1 | 1 | |||
| Pseudobalistes fuscus | BOLD:ACZ6203 | 5 | 1 | 1 | 1 | ||
| Rhinecanthus aculeatus | BOLD:AAB6992 | 2 | 1 | 1 | 1 | ||
| Rhinecanthus rectangulus | BOLD:AAD6918 | 1 | 1 | 1 | 1 | ||
| Sufflamen chrysopterum | BOLD:AAB1339 | 3 | >1 | 1 | |||
| Diodontidae | |||||||
| Lophodiodon calori | BOLD:AEW6267 | 1 | new | NA | 0 | ||
| Monacanthidae | |||||||
| Amanses cf. scopas [BOLD:ADX8121] | BOLD:ADX8121 | 1 | 1 | >1 | Y | A | 1 |
| Cantherhines pardalis | BOLD:AAB9564 | 4 | >1 | 1 | |||
| Paraluteres prionurus | BOLD:AAC3185 | 1 | 1 | 1 | |||
| Paramonacanthus frenatus | BOLD:AAI6357 | 14 | 1 | 1 | 1 | ||
| Paramonacanthus pusillus | BOLD:AAV6227 | 2 | 1 | >1 | Y | A | 1 |
| Pervagor melanocephalus | BOLD:AAD6159 | 2 | >1 | 1 | |||
| Pseudalutarius cf. nasicornis [BOLD:AAI4531] | BOLD:AAI4531 | 9 | >1 | Y | A | 0 | |
| Ostraciidae | |||||||
| Lactoria cornuta | BOLD:AAB2988 | 8 | 1 | 1 | 1 | ||
| Lactoria fornasini | BOLD:AAF2668 | 2 | >1 | 1 | |||
| Ostracion cubicus | BOLD:AAC2246 | 5 | >1 | 1 | |||
| Tetrosomus concatenatus | BOLD:AAD3999 | 1 | >1 | 1 | |||
| Tetraodontidae | |||||||
| Arothron hispidus | BOLD:AAB9202 | 18 | >1 | 3 | |||
| Arothron immaculatus | BOLD:AAD2857 | 2 | >1 | 1 | |||
| Arothron stellatus | BOLD:AAC8066 | 2 | 1 | 1 | 1 | ||
| Canthigaster bennetti | BOLD:AAC6218 | 3 | 1 | 1 | 2 | ||
| Canthigaster petersii | BOLD:AAD2222 | 7 | >1 | 1 | |||
| Canthigaster punctatissima | BOLD:ACC1190 | 2 | >1 | 1 | |||
| Canthigaster valentini | BOLD:AAC9721 | 1 | 1 | 1 | 0 | ||
| Lagocephalus [BOLD:AAC5565] | BOLD:AAC5565 | 1 | >1 | 1 | |||
| Torquigener flavimaculosus | BOLD:AAG3690 | 5 | >1 | 1 | |||
| Torquigener hypselogeneion | BOLD:ADC5749 | 1 | 1 | 1 | 0 | ||
| Trachiniformes | |||||||
| Pinguipedidae | |||||||
| Parapercis hexophtalma | BOLD:AAD6753 | 3 | 1 | 1 | 1 | ||
| Parapercis maculata | BOLD:AAE3727 | 3 | 1 | >1 | 2 | ||
| Trichonotidae | |||||||
| Trichonotus [BOLD:ACG8296] | BOLD:ACG8296 | 1 | NA | 0 | |||
| Uranoscopidae | |||||||
| Uranoscopus guttatus | BOLD:ACX9882 | 1 | NA | 1 | |||
| Elasmobranchii | |||||||
| Myliobatiformes | |||||||
| Dasyatidae | |||||||
| Neotrygon indica | BOLD:AAA5611 | 2 | >1 | 1 | |||
| Pristiformes | |||||||
| Rhinobatidae | |||||||
| Acroteriobatus andysabini | BOLD:AAG4398 | 4 | >1 | 2 | |||
| Torpediniformes | |||||||
| Torpedinidae | |||||||
| Torpedo [BOLD:AAU1234] | BOLD:AAU1234 | 3 | 1 | >1 | 1 | ||
| Total | 2146 | 524 | |||||
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Béné, C.; Macfadayen, G.; Allison, E.H. Increasing the Contribution of Small-Scale Fisheries to Poverty Alleviation and Food Security; FAO: Rome, Italy, 2007; p. 141. [Google Scholar]
- Pomeroy, R.; Andrew, N. Small-Scale Fisheries Management: Frameworks and Approaches for the Developing World; CABI: Oxford, UK, 2011. [Google Scholar] [CrossRef]
- Barnes-Mauthe, M.; Oleson, K.L.L.; Zafindrasilivonona, B. The Total Economic Value of Small-Scale Fisheries with a Characterization of Post-Landing Trends: An Application in Madagascar with Global Relevance. Fish. Res. 2013, 147, 175–185. [Google Scholar] [CrossRef]
- Mahafina, J. Perception et comportement des pêcheurs pour une gestion durable de la biodiversité et de la pêcherie récifale: Application au niveau des réserves marines temporaires du Sud Ouest de Madagascar. Ph.D. Thesis, Thèse en co-tutelle. Université de La Réunion, France et Université de Toliara, Madagascar, Toliara, Madagascar, 2011. [Google Scholar]
- Vasseur, P. Écosystèmes Côtiers En Danger Dans La Région de Tuléar. Analyse Des Agressions Humaines et Problèmes de Gestion. In Milieux et Sociétés Dans Le Sud-Ouest de Madagascar; Îles et Archipels, 23; Centre de Recherches sur les Espaces Tropicaux (Université Michel de Montaigne, Bordeaux 3): Talence, France, 1997; pp. 97–120. [Google Scholar]
- Behivoke, F. Caractérisation spatio-temporelle de la pêche aux poissons récifo-lagonaires par trajectométrie dans la baie de Toliara (sud-ouest de Madagascar). Ph.D. Thesis, Université de Toliara, Toliara, Madagascar, 2022. [Google Scholar]
- Ranaivomanana, H.S.; Jaquemet, S.; Ponton, D.; Behivoke, F.; Randriatsara, R.M.; Mahafina, J.; Léopold, M. Intense Pressure on Small and Juvenile Coral Reef Fishes Threatens Fishery Production in Madagascar. Fish. Manag. Ecol. 2023, 30, 494–506. [Google Scholar] [CrossRef]
- Tsoupas, A.; Papavasileiou, S.; Minoudi, S.; Gkagkavouzis, K.; Petriki, O.; Bobori, D.; Sapounidis, A.; Koutrakis, E.; Leonardos, I.; Karaiskou, N.; et al. DNA Barcoding Identification of Greek Freshwater Fishes. PLoS ONE 2022, 17, e0263118. [Google Scholar] [CrossRef] [PubMed]
- Jacquemot, L.; Hunt, B.P.V.; Li, S.; Schulze, A.D.; Deeg, C.M.; Sutherland, B.J.G.; Tabata, A.; Lovejoy, C.; Miller, K.M. Mapping Biodiversity Coast-to-Coast-to-Coast Across Canada’s Three Oceans Using eDNA Metabarcoding. Environ. DNA 2024, 6, e70028. [Google Scholar] [CrossRef]
- Sahu, A.; Singh, M.; Amin, A.; Malik, M.M.; Qadri, S.N.; Abubakr, A.; Teja, S.S.; Dar, S.A.; Ahmad, I. A Systematic Review on Environmental DNA (eDNA) Science: An Eco-Friendly Survey Method for Conservation and Restoration of Fragile Ecosystems. Ecol. Indic. 2025, 173, 113441. [Google Scholar] [CrossRef]
- Wang, S.; Yan, Z.; Hänfling, B.; Zheng, X.; Wang, P.; Fan, J.; Li, J. Methodology of Fish eDNA and Its Applications in Ecology and Environment. Sci. Total Environ. 2021, 755, 142622. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.A.; Turner, C.R. The Ecology of Environmental DNA and Implications for Conservation Genetics. Conserv. Genet. 2016, 17, 1–17. [Google Scholar] [CrossRef]
- DiBattista, J.D.; Coker, D.J.; Sinclair-Taylor, T.H.; Stat, M.; Berumen, M.L.; Bunce, M. Assessing the Utility of eDNA as a Tool to Survey Reef-Fish Communities in the Red Sea. Coral Reefs 2017, 36, 1245–1252. [Google Scholar] [CrossRef]
- West, K.; Travers, M.J.; Stat, M.; Harvey, E.S.; Richards, Z.T.; DiBattista, J.D.; Newman, S.J.; Harry, A.; Skepper, C.L.; Heydenrych, M.; et al. Large-scale eDNA Metabarcoding Survey Reveals Marine Biogeographic Break and Transitions over Tropical North-western Australia. Divers. Distrib. 2021, 27, 1942–1957. [Google Scholar] [CrossRef]
- Cantera, I.; Cilleros, K.; Valentini, A.; Cerdan, A.; Dejean, T.; Iribar, A.; Taberlet, P.; Vigouroux, R.; Brosse, S. Optimizing Environmental DNA Sampling Effort for Fish Inventories in Tropical Streams and Rivers. Sci. Rep. 2019, 9, 3085. [Google Scholar] [CrossRef]
- Fediajevaite, J.; Priestley, V.; Arnold, R.; Savolainen, V. Meta-analysis Shows That Environmental DNA Outperforms Traditional Surveys, but Warrants Better Reporting Standards. Ecol. Evol. 2021, 11, 4803–4815. [Google Scholar] [CrossRef]
- Watson, D.L.; Harvey, E.S.; Anderson, M.J.; Kendrick, G.A. A Comparison of Temperate Reef Fish Assemblages Recorded by Three Underwater Stereo-Video Techniques. Mar. Biol. 2005, 148, 415–425. [Google Scholar] [CrossRef]
- Marques, V.; Milhau, T.; Albouy, C.; Dejean, T.; Manel, S.; Mouillot, D.; Juhel, J. GAPeDNA: Assessing and Mapping Global Species Gaps in Genetic Databases for eDNA Metabarcoding. Divers. Distrib. 2021, 27, 1880–1892. [Google Scholar] [CrossRef]
- Andújar, C.; Arribas, P.; Yu, D.W.; Vogler, A.P.; Emerson, B.C. Why the COI Barcode Should Be the Community DNA Metabarcode for the Metazoa. Mol. Ecol. 2018, 27, 3968–3975. [Google Scholar] [CrossRef] [PubMed]
- Deagle, B.E.; Jarman, S.N.; Coissac, E.; Pompanon, F.; Taberlet, P. DNA Metabarcoding and the Cytochrome c Oxidase Subunit I Marker: Not a Perfect Match. Biol. Lett. 2014, 10, 20140562. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An Emerging Tool in Conservation for Monitoring Past and Present Biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Yao, M. A Comprehensive and Comparative Evaluation of Primers for Metabarcoding eDNA from Fish. Methods Ecol. Evol. 2020, 11, 1609–1625. [Google Scholar] [CrossRef]
- Collins, R.A.; Bakker, J.; Wangensteen, O.S.; Soto, A.Z.; Corrigan, L.; Sims, D.W.; Genner, M.J.; Mariani, S. Non-specific Amplification Compromises Environmental DNA Metabarcoding with COI. Methods Ecol. Evol. 2019, 10, 1985–2001. [Google Scholar] [CrossRef]
- Miya, M.; Sato, Y.; Fukunaga, T.; Sado, T.; Poulsen, J.Y.; Sato, K.; Minamoto, T.; Yamamoto, S.; Yamanaka, H.; Araki, H.; et al. MiFish, a Set of Universal PCR Primers for Metabarcoding Environmental DNA from Fishes: Detection of More than 230 Subtropical Marine Species. R. Soc. Open Sci. 2015, 2, 150088. [Google Scholar] [CrossRef]
- Chavez, F.; Min, M.; Pitz, K.; Truelove, N.; Baker, J.; LaScala-Grunewald, D.; Blum, M.; Walz, K.; Nye, C.; Djurhuus, A.; et al. Observing Life in the Sea Using Environmental DNA. Oceanography 2021, 34, 102–119. [Google Scholar] [CrossRef]
- Jaonalison, H.; Durand, J.-D.; Mahafina, J.; Valade, P.; Collet, A.; Cerqueira, F.; Ponton, D. Application of DNA Barcoding for Monitoring Madagascar Fish Biodiversity in Coastal Areas. Diversity 2022, 14, 377. [Google Scholar] [CrossRef]
- Vences, M.; Stützer, D.; Rasoamampionona Raminosoa, N.; Ziegler, T. Towards a DNA Barcode Library for Madagascar’s Threatened Ichthyofauna. PLoS ONE 2022, 17, e0271400. [Google Scholar] [CrossRef] [PubMed]
- Fricke, R.; Mahafina, J.; Behivoke, F.; Jaonalison, H.; Léopold, M. Annotated Checklist of the Fishes of Madagascar, Southwestern Indian Ocean, with 158 New Records. Fish Taxa 2018, 3, 1–432. [Google Scholar]
- Blackman, R.C.; Walser, J.; Rüber, L.; Brantschen, J.; Villalba, S.; Brodersen, J.; Seehausen, O.; Altermatt, F. General Principles for Assignments of Communities from EDNA: Open versus Closed Taxonomic Databases. Environ. DNA 2023, 5, 326–342. [Google Scholar] [CrossRef]
- Pentinsaari, M.; Ratnasingham, S.; Miller, S.E.; Hebert, P.D.N. BOLD and GenBank Revisited—Do Identification Errors Arise in the Lab or in the Sequence Libraries? PLoS ONE 2020, 15, e0231814. [Google Scholar] [CrossRef]
- Collet, A.; Ponton, D.; Salager, B.; Yven, C.; Pierre, V. SLEEP COLOR Développement et Test d’un Système Lumineux Electronique Evolutif (Multi Spectral) Pour l’échantillonnage Des Post-Larves et Juvéniles de Poissons de Récifs. Rapport de Phase 3: Calibration Spatio-Temporelle de La Stratégie d’échantillonnage; Organisme Consultant en Environnement Aquatique: Saint Pierre, La Réunion, 2022. [Google Scholar] [CrossRef]
- Collet, A.; Durand, J.-D.; Desmarais, E.; Cerqueira, F.; Cantinelli, T.; Valade, P.; Ponton, D. DNA Barcoding Post-Larvae Can Improve the Knowledge about Fish Biodiversity: An Example from La Reunion, SW Indian Ocean. Mitochondrial DNA Part A 2018, 29, 905–918. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA Barcoding Australia’s Fish Species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Xia, R.; Durand, J.-D.; Fu, C. Multilocus Resolution of Mugilidae Phylogeny (Teleostei: Mugiliformes): Implications for the Family’s Taxonomy. Mol. Phylogenetics Evol. 2016, 96, 161–177. [Google Scholar] [CrossRef]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F.; et al. Next-generation Monitoring of Aquatic Biodiversity Using Environmental DNA Metabarcoding. Mol. Ecol. 2016, 25, 929–942. [Google Scholar] [CrossRef]
- Pham, M.H.; Hoang, D.H.; Panfili, J.; Ponton, D.; Durand, J.-D. Diversity of Fishes Collected with Light Traps in the Oldest Marine Protected Area in Vietnam Revealed by DNA Barcoding. Mar. Biodivers. 2022, 52, 30. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-Based Registry for All Animal Species: The Barcode Index Number (BIN) System. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; Van-Der-Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. 2025. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 15 April 2025).
- Ruiz, E.; Lamy, T.; Mouillot, D.; Durand, J.-D. Not One Threshold to Rule Them All! Benchmarking the Taxonomic Resolution of Fish eDNA Metabarcodes Against COI Barcodes. Mol. Ecol. Resour. 2025, submitted.
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Laroche, J.; Ramananarivo, N. A Preliminary Survey of the Artisanal Fishery on Coral Reefs of the Tulear Region (Southwest Madagascar). Coral Reefs 1995, 14, 193–200. [Google Scholar] [CrossRef]
- Laroche, J.; Razanoelisoa, J.; Fauroux, E.; Rabenevanana, M.W. The Reef Fisheries Surrounding the South-west Coastal Cities of Madagascar. Fish. Manag. Ecol. 1997, 4, 285–299. [Google Scholar] [CrossRef]
- Attingli, A.H.; Ahouansou Montcho, S.; Vissin, E.W.; Zinsou, L.H.; Laleye, P.A. Influence des engins et techniques de pêche sur l’abondance relative des espèces dans la Basse Vallée de l’Ouémé au Bénin. Afr. Crop Sci. J. 2017, 25, 47. [Google Scholar] [CrossRef]
- Zemlak, T.S.; Ward, R.D.; Connell, A.D.; Holmes, B.H.; Hebert, P.D.N. DNA Barcoding Reveals Overlooked Marine Fishes. Mol. Ecol. Resour. 2009, 9, 237–242. [Google Scholar] [CrossRef]
- Cawthorn, D.-M.; Steinman, H.A.; Witthuhn, R.C. Evaluation of the 16S and 12S rRNA Genes as Universal Markers for the Identification of Commercial Fish Species in South Africa. Gene 2012, 491, 40–48. [Google Scholar] [CrossRef]
- Steinke, D.; Connell, A.D.; Hebert, P.D.N. Linking Adults and Immatures of South African Marine Fishes. Genome 2016, 59, 959–967. [Google Scholar] [CrossRef]
- Muhala, V.; Guimarães-Costa, A.; Macate, I.E.; Rabelo, L.P.; Bessa-Silva, A.R.; Watanabe, L.; Dos Santos, G.D.; Sambora, L.; Vallinoto, M.; Sampaio, I. DNA Barcoding for the Assessment of Marine and Coastal Fish Diversity from the Coast of Mozambique. PLoS ONE 2024, 19, e0293345. [Google Scholar] [CrossRef]
- Coker, D.J.; DiBattista, J.D.; Sinclair-Taylor, T.H.; Berumen, M.L. Spatial Patterns of Cryptobenthic Coral-Reef Fishes in the Red Sea. Coral Reefs 2018, 37, 193–199. [Google Scholar] [CrossRef]
- Asgharian, H.; Sahafi, H.H.; Ardalan, A.A.; Shekarriz, S.; Elahi, E. Cytochrome c Oxidase Subunit 1 Barcode Data of Fish of the Nayband National Park in the Persian Gulf and Analysis Using Meta-data Flag Several Cryptic Species. Mol. Ecol. Resour. 2011, 11, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Rabaoui, L.; Yacoubi, L.; Sanna, D.; Casu, M.; Scarpa, F.; Lin, Y.; Shen, K.; Clardy, T.R.; Arculeo, M.; Qurban, M.A. DNA Barcoding of Marine Fishes from Saudi Arabian Waters of the Gulf. J. Fish Biol. 2019, 95, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Duchene, D.; Klanten, S.O.; Munday, P.L.; Herler, J.; Van Herwerden, L. Phylogenetic Evidence for Recent Diversification of Obligate Coral-Dwelling Gobies Compared with Their Host Corals. Mol. Phylogenet. Evol. 2013, 69, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Winterbottom, R. Trimma Tevegae and T. Caudomaculatum Revisited and Redescribed (Acanthopterygii, Gobiidae), with Descriptions of Three New Similar Species from the Western Pacific. Zootaxa 2016, 4144, 1–53. [Google Scholar] [CrossRef]
- Troyer, E.M.; Coker, D.J.; Berumen, M.L. Comparison of Cryptobenthic Reef Fish Communities among Microhabitats in the Red Sea. PeerJ 2018, 6, e5014. [Google Scholar] [CrossRef]
- Tornabene, L.; Ahmadia, G.N.; Berumen, M.L.; Smith, D.J.; Jompa, J.; Pezold, F. Evolution of Microhabitat Association and Morphology in a Diverse Group of Cryptobenthic Coral Reef Fishes (Teleostei: Gobiidae: Eviota). Mol. Phylogenet. Evol. 2013, 66, 391–400. [Google Scholar] [CrossRef]
- Luehrmann, M.; Cortesi, F.; Cheney, K.L.; De Busserolles, F.; Marshall, N.J. Microhabitat Partitioning Correlates with Opsin Gene Expression in Coral Reef Cardinalfishes (Apogonidae). Funct. Ecol. 2020, 34, 1041–1052. [Google Scholar] [CrossRef]
- Doll, P.; Munday, P.; Bonin, M.; Jones, G. Habitat Specialisation and Overlap in Coral Reef Gobies of the Genus Eviota (Teleostei: Gobiidae). Mar. Ecol. Prog. Ser. 2021, 677, 81–94. [Google Scholar] [CrossRef]
- Hubert, N.; Meyer, C.P.; Bruggemann, H.J.; Guérin, F.; Komeno, R.J.L.; Espiau, B.; Causse, R.; Williams, J.T.; Planes, S. Cryptic Diversity in Indo-Pacific Coral-Reef Fishes Revealed by DNA-Barcoding Provides New Support to the Centre-of-Overlap Hypothesis. PLoS ONE 2012, 7, e28987. [Google Scholar] [CrossRef]
- Hubert, N.; Dettai, A.; Pruvost, P.; Cruaud, C.; Kulbicki, M.; Myers, R.; Borsa, P. Geography and Life History Traits Account for the Accumulation of Cryptic Diversity among Indo-West Pacific Coral Reef Fishes. Mar. Ecol. Prog. Ser. 2017, 583, 179–193. [Google Scholar] [CrossRef]
- Durand, J.-D.; Borsa, P. Mitochondrial Phylogeny of Grey Mullets (Acanthopterygii: Mugilidae) Suggests High Proportion of Cryptic Species. C. R. Biol. 2015, 338, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.A.; Cruickshank, R.H. The Seven Deadly Sins of DNA Barcoding. Mol. Ecol. Resour. 2013, 13, 969–975. [Google Scholar] [CrossRef]
- Hanner, R.; Becker, S.; Ivanova, N.V.; Steinke, D. FISH-BOL and Seafood Identification: Geographically Dispersed Case Studies Reveal Systemic Market Substitution across Canada. Mitochondrial DNA 2011, 22, 106–122. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Altermatt, F.; Zhang, X.; Cai, Y.; Yang, Z. Gap Analysis for DNA-Based Biomonitoring of Aquatic Ecosystems in China. Ecol. Indic. 2022, 137, 108732. [Google Scholar] [CrossRef]
| Project Name in MADFI Container | Sampling Period | Sampling Gear | Sampling Areas | Number of Samples | Reference |
|---|---|---|---|---|---|
| CALMA | March 2019 to May 2019 | light traps | Great barrier reef of Toliara and Anakao reef | 60 | Collet et al. [31] |
| COMAD | October 2014 to March 2015 | mosquito seine nets and light traps | Great barrier reef of Toliara and Anakao reef | 92 | Jaonalison et al. [26] |
| RFSIO | November 2016 to April 2017, and November 2017 to April 2018 | light traps | Great barrier reef of Toliara and Anakao reef | 200 | Jaonalison et al. [26] |
| IFBIO | November 2016 to April 2017, and November 2017 to April 2018 | mosquito seine nets and light traps | Fishing landings in Toliara Bay | 78 | Jaonalison et al. [26] |
| GEOFI | May 2018 to April 2019 | beach seine, mosquito trawl net, gillnet, handline, speargun | Fish landings in the eight villages along Ranobe Bay | 252 | present study |
| ARMSR | October 2021 to November 2022 | beach seine, mosquito trawl net, gillnet, handline, speargun | Fish landings in the 12 villages along Ranobe Bay | 597 | present study |
| SAROD | March 2022 to April 2023 | gillnet, mosquito trawl net | Seagrass beds and along the mangrove edges of Sarodrano | 130 | present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volanandiana, J.J.A.; Ponton, D.; Ruiz, E.; Fiadanamiarinjato, A.E.M.; Rieuvilleneuve, F.; Raberinary, D.; Collet, A.; Behivoke, F.; Jaonalison, H.; Ranaivomanana, S.; et al. Building a DNA Reference for Madagascar’s Marine Fishes: Expanding the COI Barcode Library and Establishing the First 12S Dataset for eDNA Monitoring. Diversity 2025, 17, 495. https://doi.org/10.3390/d17070495
Volanandiana JJA, Ponton D, Ruiz E, Fiadanamiarinjato AEM, Rieuvilleneuve F, Raberinary D, Collet A, Behivoke F, Jaonalison H, Ranaivomanana S, et al. Building a DNA Reference for Madagascar’s Marine Fishes: Expanding the COI Barcode Library and Establishing the First 12S Dataset for eDNA Monitoring. Diversity. 2025; 17(7):495. https://doi.org/10.3390/d17070495
Chicago/Turabian StyleVolanandiana, Jean Jubrice Anissa, Dominique Ponton, Eliot Ruiz, Andriamahazosoa Elisé Marcel Fiadanamiarinjato, Fabien Rieuvilleneuve, Daniel Raberinary, Adeline Collet, Faustinato Behivoke, Henitsoa Jaonalison, Sandra Ranaivomanana, and et al. 2025. "Building a DNA Reference for Madagascar’s Marine Fishes: Expanding the COI Barcode Library and Establishing the First 12S Dataset for eDNA Monitoring" Diversity 17, no. 7: 495. https://doi.org/10.3390/d17070495
APA StyleVolanandiana, J. J. A., Ponton, D., Ruiz, E., Fiadanamiarinjato, A. E. M., Rieuvilleneuve, F., Raberinary, D., Collet, A., Behivoke, F., Jaonalison, H., Ranaivomanana, S., Leopold, M., Randriatsara, R. M., Mbony, J., Mahafina, J., Hartmann, A., Todinanahary, G., & Durand, J.-D. (2025). Building a DNA Reference for Madagascar’s Marine Fishes: Expanding the COI Barcode Library and Establishing the First 12S Dataset for eDNA Monitoring. Diversity, 17(7), 495. https://doi.org/10.3390/d17070495








