Dual Influence of Rainfall and Water Temperature on Phytoplankton Diversity and Nutrient Dynamics in a Mountainous Riverine Reservoir
Abstract
1. Introduction
2. Materials and Methods
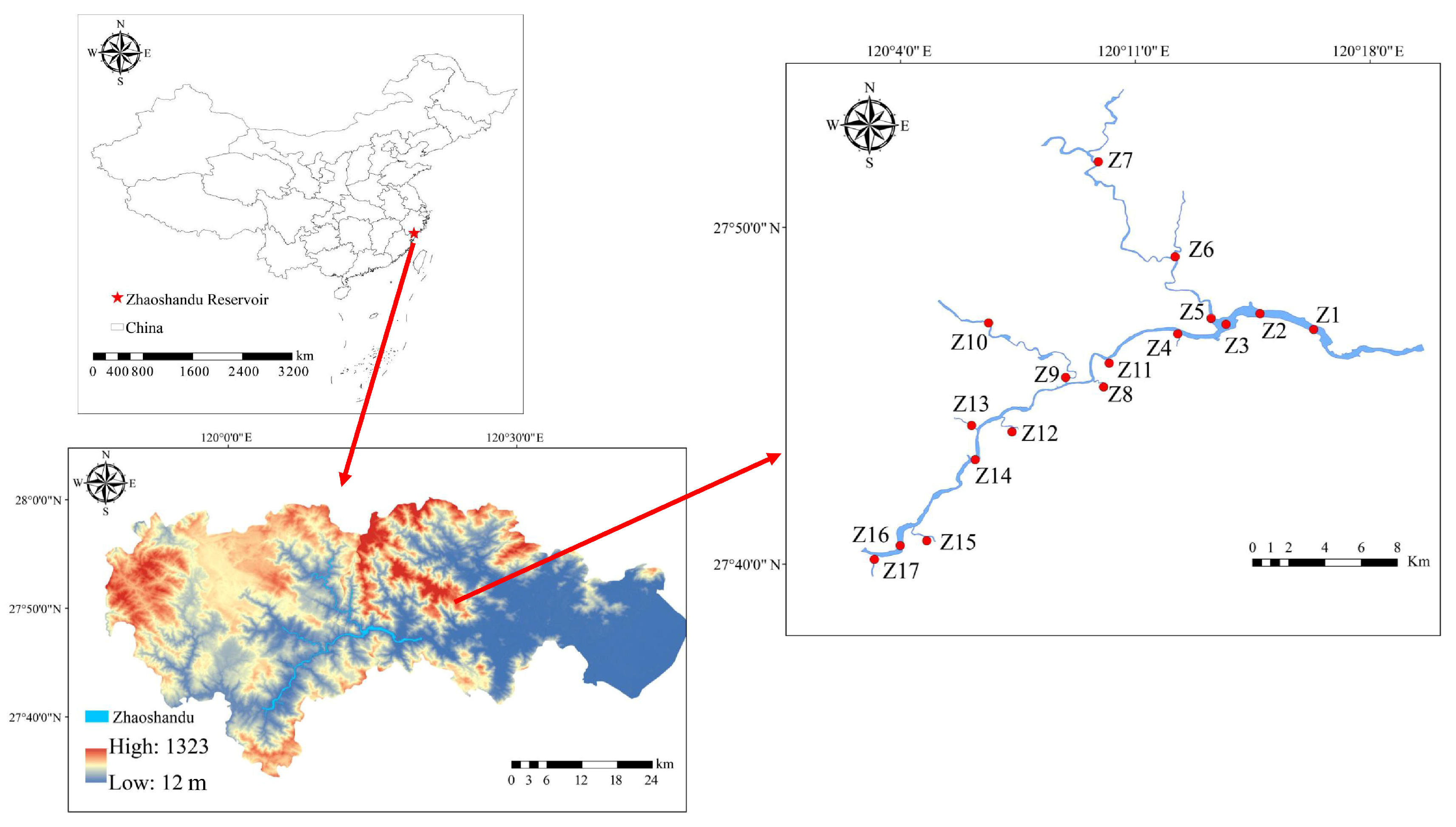
2.1. Study Area and Sampling
2.2. Data Collection
2.3. Sample Analysis
2.4. Statistical Analysis
3. Results
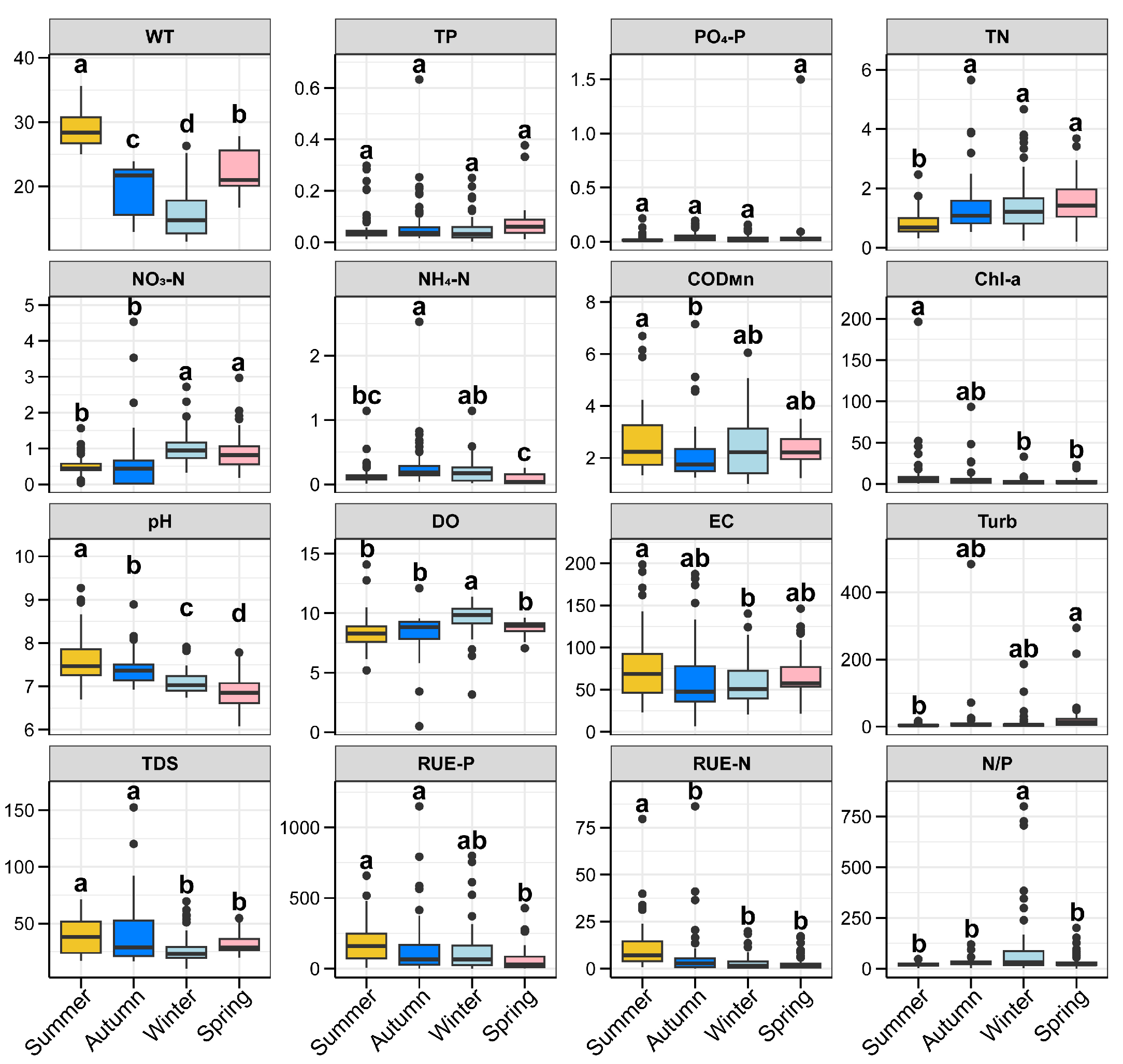
3.1. Physicochemical Characteristics of Zhaoshandu Reservoir
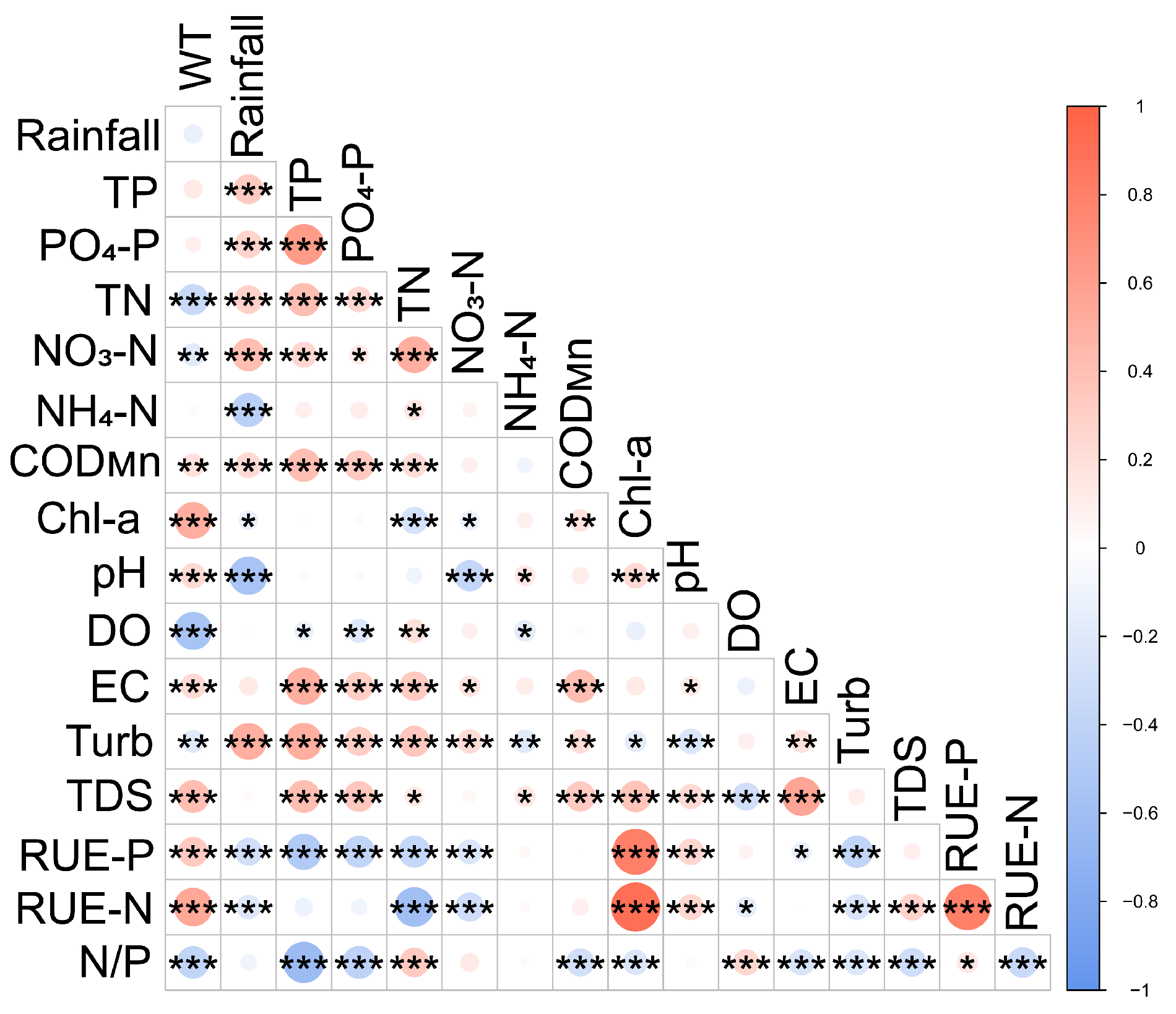
3.2. Relationship Between Rainfall and Water Quality
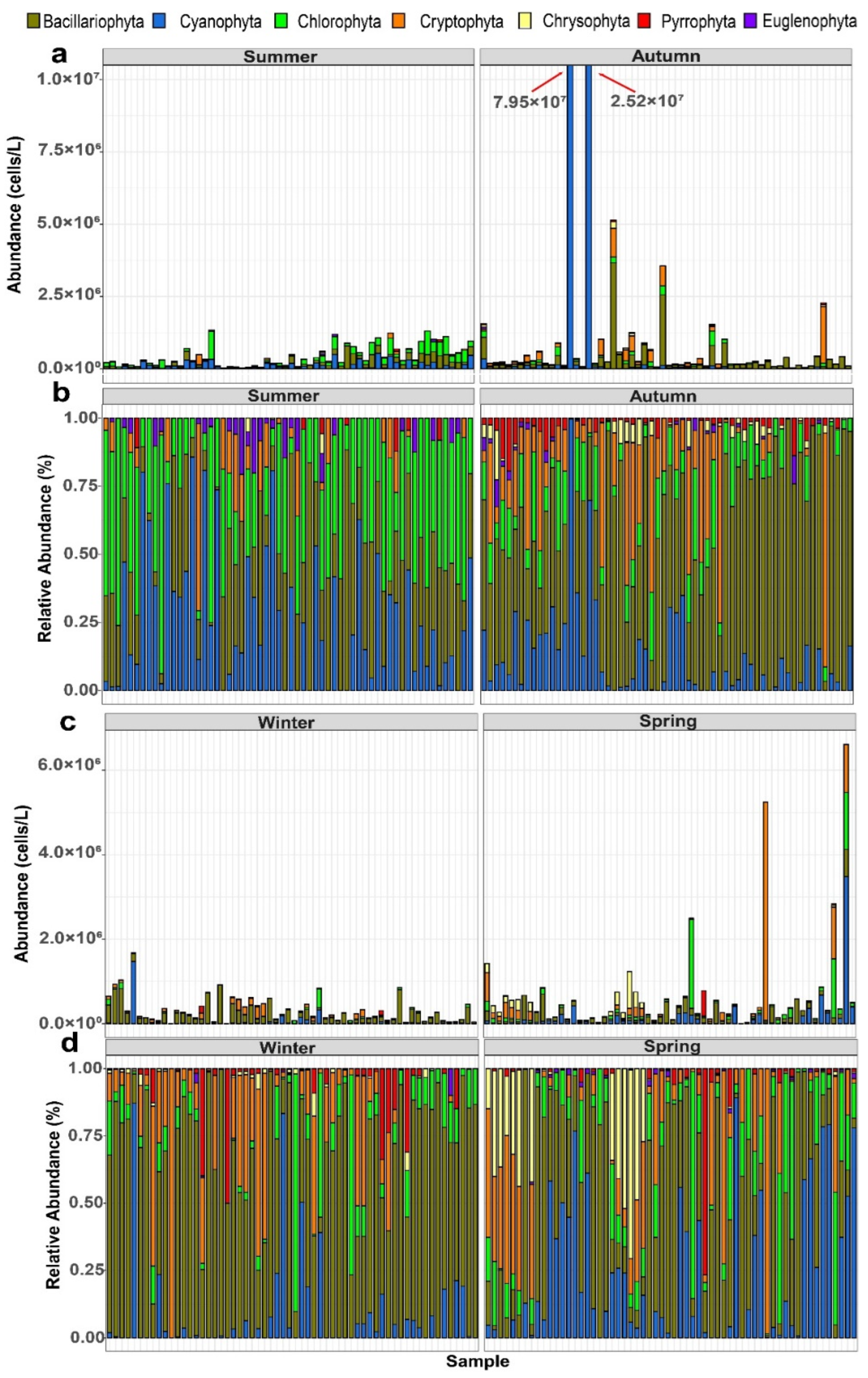
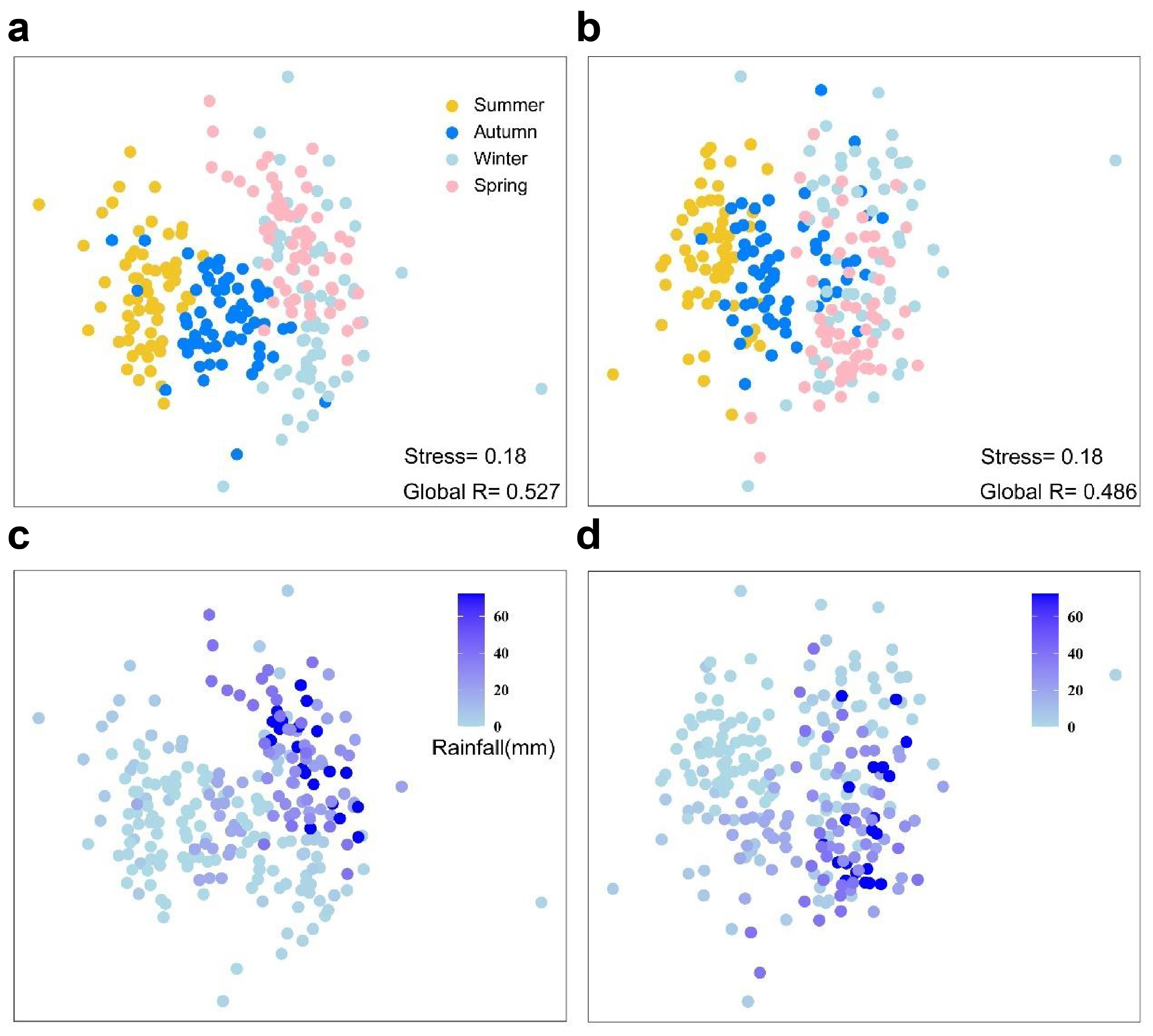
3.3. Dynamic Changes in Phytoplankton Species Composition in Zhaoshandu Reservoir
3.4. Dynamics of Phytoplankton Diversity and Niche Characteristics in Zhaoshandu Reservoir
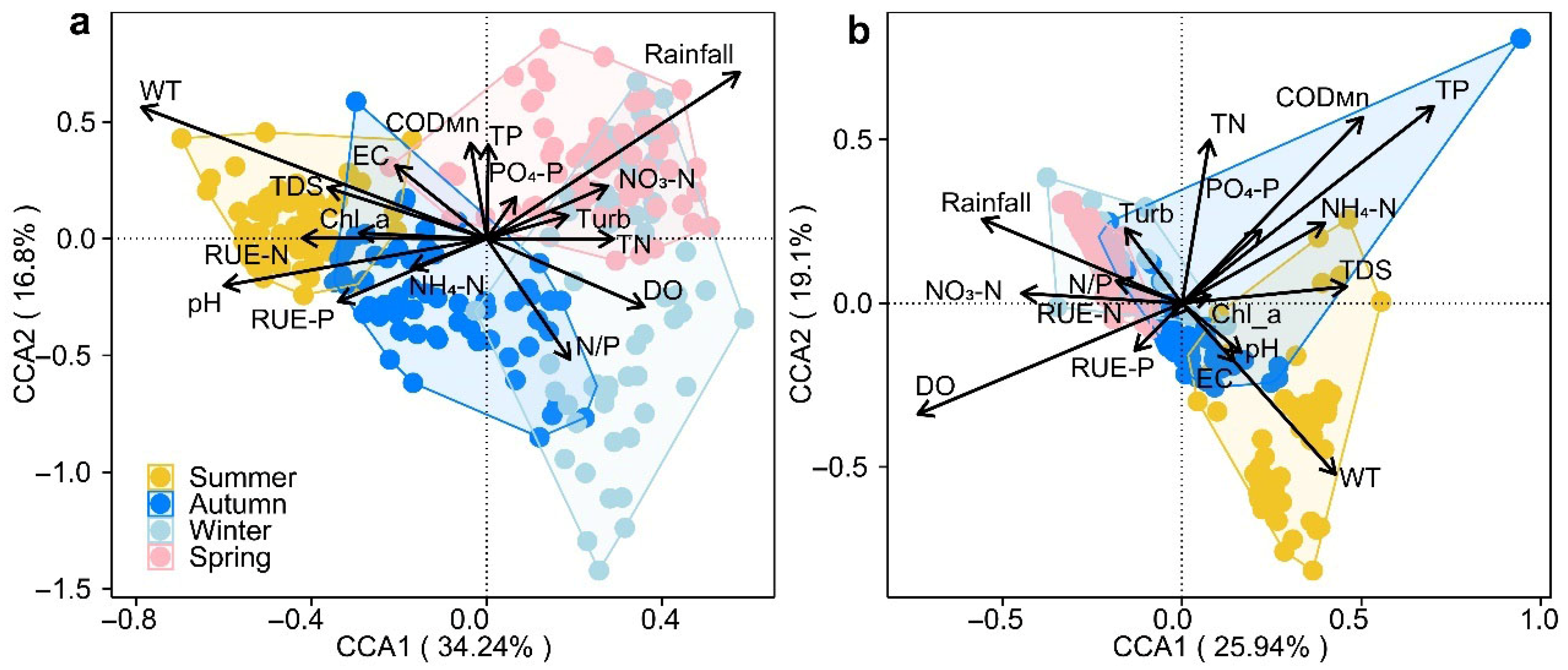
3.5. Key Drivers of Phytoplankton Community Dynamics
3.6. Responses of Phytoplankton Diversity and Niche Characteristics to WT and Rainfall
3.7. Structural Equation Modeling (SEM)
4. Discussion
4.1. Temporal Dynamics of Phytoplankton Communities
4.2. Rainfall and WT Jointly Affect Phytoplankton Diversity, Niche Characteristics, and Community Assembly
4.3. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, S.I.; Barton, A.D.; Clayton, S.; Dutkiewicz, S.; Rynearson, T.A. Marine phytoplankton functional types exhibit diverse responses to thermal change. Nat. Commun. 2021, 12, 6413. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, R.; Li, Q.; Lu, Q.; Chen, J. Bacterially mediated phosphorus cycling favors resource use efficiency of phytoplankton communities in a eutrophic plateau lake. Water Res. 2025, 277, 123300. [Google Scholar] [CrossRef]
- Kong, X.; Koelmans, A.A. Modeling decreased resilience of shallow lake ecosystems toward eutrophication due to microplastic ingestion across the food web. Environ. Sci. Technol. 2019, 53, 13822–13831. [Google Scholar] [CrossRef]
- Jia, J.; Gao, Y.; Wang, S.; Wu, F.; Lu, Y.; Ha, X. Feedbacks between phytoplankton and global changes in a riverine source-mainstem-estuary continuum. Water Res. 2025, 268, 122746. [Google Scholar] [CrossRef]
- Shao, N.F.; Yang, S.T.; Sun, Y.; Gai, Y.; Zhao, C.S.; Wang, F.; Dong, B. Assessing aquatic ecosystem health through the analysis of plankton biodiversity. Mar. Freshw. Res. 2019, 70, 647–655. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Li, L.; Lu, F.; Liu, Y.; Lu, X.; Fan, Y. Phytoplankton alpha diversity indices response the trophic state variation in hydrologically connected aquatic habitats in the Harbin Section of the Songhua River. Sci. Rep. 2020, 10, 21337. [Google Scholar] [CrossRef] [PubMed]
- Kleinteich, J.; Frassl, M.A.; Schulz, M.; Fischer, H. Climate change triggered planktonic cyanobacterial blooms in a regulated temperate river. Sci. Rep. 2024, 14, 16298. [Google Scholar] [CrossRef]
- Kim, H.G.; Cha, Y.; Cho, K.H. Projected climate change impact on cyanobacterial bloom phenology in temperate rivers based on temperature dependency. Water Res. 2024, 249, 120928. [Google Scholar] [CrossRef]
- Shi, P.; Zhu, M.; You, R.; Li, H.; Zou, W.; Xu, H.; Zhu, G. Rainstorm events trigger algal blooms in a large oligotrophic reservoir. J. Hydrol. 2023, 622, 129711. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Y.; Xia, J. Hydrological cycle and water resources in a changing world: A review. Geogr. Sustain. 2021, 2, 115–122. [Google Scholar] [CrossRef]
- Li, L.; Knapp, J.L.; Lintern, A.; Ng, G.H.C.; Perdrial, J.; Sullivan, P.L.; Zhi, W. River water quality shaped by land–river connectivity in a changing climate. Nat. Clim. Change 2024, 14, 225–237. [Google Scholar] [CrossRef]
- Seliger, C.; Zeiringer, B. River connectivity, habitat fragmentation and related restoration measures. In Riverine Ecosystem Management; Springer International Publishing: Cham, Switzerland, 2018; pp. 171–186. [Google Scholar]
- Alexander, L.C.; Autrey, B.; DeMeester, J.; Fritz, K.M.; Golden, H.E.; Goodrich, D.C.; Wigington, P.J. Connectivity of Streams and Wetlands to Downstream Waters: A Review and Synthesis of the Scientific Evidence; US Environmental Protection Agency: Washington, DC, USA, 2015.
- Liu, X.; Pan, B.; Liu, X.; He, H.; Zhao, X.; Huang, Z.; Li, M. Riverine microbial community assembly with watercourse distance–decay patterns in the north–south transitional zone of China. J. Hydrol. 2024, 628, 130603. [Google Scholar] [CrossRef]
- Kong, X.; He, Q.; Yang, B.; He, W.; Xu, F.; Janssen, A.B.; Mooij, W.M. Hydrological regulation drives regime shifts: Evidence from paleolimnology and ecosystem modeling of a large shallow Chinese lake. Glob. Change Biol. 2017, 23, 737–754. [Google Scholar] [CrossRef]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 138. [Google Scholar] [PubMed]
- Hao, L.; Zhang, Y.; Shen, Y.; Liu, Y.; Gao, H.; Guo, P. Driving mechanism of land use and landscape pattern to phytoplankton and zooplankton community and their trophic interactions in river ecosystems. J. Environ. Manag. 2024, 370, 122691. [Google Scholar] [CrossRef] [PubMed]
- Stappert, H.M.; Blanke, P.; Hering, D.; Kail, J. Mitigating the effects of extreme low-flow on stream macroinvertebrates: The role of woody riparian vegetation and groundwater. Hydrobiologia 2025, 1–18. [Google Scholar] [CrossRef]
- Ruan, Q.; Liu, H.; Dai, Z.; Wang, F.; Cao, W. Damming exacerbates the discontinuities of phytoplankton in a subtropical river in China. J. Environ. Manag. 2024, 351, 119832. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhao, X.; Kang, L.; Qiu, Y.; Paerl, H.; Zhu, G.; Li, H.; Zhu, M.; Qin, B.; et al. Rainfall impacts on nonpoint nitrogen and phosphorus dynamics in an agricultural river in subtropical montane reservoir region of southeast China. J. Environ. Manag. 2025, 149, 551–563. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Shi, K.; Zhang, Y.; Zhou, Y.; Zhu, M.; Liu, M. Effects of rainfall on thermal stratification and dissolved oxygen in a deep drinking water reservoir. Hydrol. Process. 2020, 34, 3387–3399. [Google Scholar] [CrossRef]
- Li, A.L.; Chen, H.T.; Liu, Y.Y.; Qiu, L.; Wang, W.C. Net Anthropogenic Nitrogen Input (NANI) Evolution and Total Nitrogen (TN) Concentration Response in Zhaoshandu Water Source. Nat. Environ. Pollut. Technol. 2020, 19, 1319–1325. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations (No. 2); Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Shipley, B. A new inferential test for path models based on directed acyclic graphs. Struct. Equ. Model. 2000, 7, 206–218. [Google Scholar] [CrossRef]
- Mamet, S.D.; Redlick, E.; Brabant, M.; Lamb, E.G.; Helgason, B.L.; Stanley, K.; Siciliano, S.D. Structural equation modeling of a winnowed soil microbiome identifies how invasive plants re-structure microbial networks. ISME J. 2019, 13, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepistö, L.; Rekolainen, S. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef]
- Hodapp, D.; Hillebrand, H.; Striebel, M. “Unifying” the concept of resource use efficiency in ecology. Front. Ecol. Evol. 2019, 6, 233. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, Z.; Fu, G.; Wu, S.; Meng, J.; Liu, W.; Zhou, Y. Distribution and dynamics of niche and interspecific association of dominant phytoplankton species in the Feiyun River basin, Zhejiang, China. J. Oceanol. Limnol. 2024, 42, 1157–1172. [Google Scholar] [CrossRef]
- Briddon, C.L.; Miclăuş, M.; Hegedüs, A.; Nicoară, M.; Chiriac, M.C.; Drugă, B. Long-term exposure to elevated temperature leads to altered gene expression in a common bloom-forming cyanobacterium. Limnol. Oceanogr. 2023, 68, 2654–2667. [Google Scholar] [CrossRef]
- Liang, Y.; Koester, J.A.; Liefer, J.D.; Irwin, A.J.; Finkel, Z.V. Molecular mechanisms of temperature acclimation and adaptation in marine diatoms. ISME J. 2019, 13, 2415–2425. [Google Scholar] [CrossRef]
- Mendes, C.R.B.; Costa, R.R.; Ferreira, A.; Jesus, B.; Tavano, V.M.; Dotto, T.S.; Secchi, E.R. Cryptophytes: An emerging algal group in the rapidly changing Antarctic Peninsula marine environments. Glob. Change Biol. 2023, 29, 1791–1808. [Google Scholar] [CrossRef]
- Luo, X.; Lan, W.L.; Li, T.S.; Li, M.M. Distribution of phytoplankton and its relationship with environmental factors in the Qinzhou Bay in spring and summer. Acta Ecol. Sin. 2019, 39, 2603–2613. [Google Scholar] [CrossRef]
- Wu, T.; Luo, L.; Cui, G.; Qin, B. Study on the Triggering Factors of Algal Bloom in Fuchunjiang Reservoir Based on a Vertically Integrated Hydrodynamic Model. In Advances in Water Resources and Hydraulic Engineering: Proceedings of 16th IAHR-APD Congress and 3rd Symposium of IAHR-ISHS; Springer: Berlin/Heidelberg, Germany, 2009; pp. 662–666. [Google Scholar]
- He, S.; Zhang, Y.; Li, N.; Shi, K.; Zhang, Y.; Qin, B.; Shao, K. Summer heatwaves promote harmful algal blooms in the Fuchunjiang Reservoir, an important drinking water source. J. Environ. Manag. 2024, 359, 121056. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.S.; Halsey, K.H. Phytoplankton community structuring and succession in a competition-neutral resource landscape. ISME Commun. 2021, 1, 12. [Google Scholar] [CrossRef]
- Yimin, L.; Xihao, S.; Chongying, G. Niche width and niche overlap: A method based on type-2 fuzzy sets. Ecol. Res. 2006, 21, 713–722. [Google Scholar] [CrossRef]
- Pastore, A.I.; Barabás, G.; Bimler, M.D.; Mayfield, M.M.; Miller, T.E. The evolution of niche overlap and competitive differences. Nat. Ecol. Evol. 2021, 5, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Dory, F.; Nava, V.; Spreafico, M.; Orlandi, V.; Soler, V.; Leoni, B. Interaction between temperature and nutrients: How does the phytoplankton community cope with climate change? Sci. Total Environ. 2024, 906, 167566. [Google Scholar] [CrossRef] [PubMed]
- Schabhüttl, S.; Hingsamer, P.; Weigelhofer, G.; Hein, T.; Weigert, A.; Striebel, M. Temperature and species richness effects in phytoplankton communities. Oecologia 2013, 171, 527–536. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, R. Precipitation input increases biodiversity of planktonic communities in the Qinghai-Tibet Plateau. Sci. Total Environ. 2024, 947, 174666. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, C.; Tarran, G.A.; Schuback, N.; Woodward, E.M.S.; Arístegui, J.; Marañón, E. Phytoplankton responses to changing temperature and nutrient availability are consistent across the tropical and subtropical Atlantic. Commun. Biol. 2022, 5, 1035. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, S.; Niu, X. Effect of water temperature on the dynamic behavior of phytoplankton–zooplankton model. Appl. Math. Comput. 2020, 378, 125211. [Google Scholar] [CrossRef]
- Weisse, T.; Gröschl, B.; Bergkemper, V. Phytoplankton response to short-term temperature and nutrient changes. Limnologica 2016, 59, 78–89. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Li, Y.; Jeppesen, E.; Zhang, M.; Chen, F. Nitrogen reduction causes shifts in winter and spring phytoplankton composition and resource use efficiency in a large subtropical lake in China. Ecosystems 2023, 26, 1640–1655. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wu, Y.; Yu, Y.; Sun, J.; Mao, D.; Zhang, G. Intensified effect of nitrogen forms on dominant phytoplankton species succession by climate change. Water Res. 2024, 264, 122214. [Google Scholar] [CrossRef]
- Mesquita, M.C.; Graco-Roza, C.; de Magalhães, L.; Ger, K.A.; Marinho, M.M. Environmental variables outpace biotic interactions in shaping a phytoplankton community. Diversity 2024, 16, 438. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, B.; Zhao, X.; Liu, X.; Liu, X.; Zhao, G. Hydrological disturbances enhance stochastic assembly processes and decrease network stability of algae communities in a highland floodplain system. Sci. Total Environ. 2023, 903, 166207. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Tu, W.; Luo, X.; Fan, M.; Chen, S.; Wang, B.; Yang, Y.; Li, S. Performance of ecological floating beds and microbial communities under different flow velocities. J. Water Process Eng. 2022, 48, 102876. [Google Scholar] [CrossRef]
- Eyring, S.; Reyes, M.; Merz, E.; Baity-Jesi, M.; Ntetsika, P.; Ebi, C.; Dennis, S.; Pomati, F. Five years of high-frequency data of phytoplankton zooplankton and limnology from a temperate eutrophic lake. Sci. Data 2025, 12, 653. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Hu, L.; Ren, X.; Hu, X.; Sun, T.; Zuo, J.; Xiao, P.; Zhang, H.; Zhang, R.; Li, R. Dual Influence of Rainfall and Water Temperature on Phytoplankton Diversity and Nutrient Dynamics in a Mountainous Riverine Reservoir. Diversity 2025, 17, 573. https://doi.org/10.3390/d17080573
Zhao Q, Hu L, Ren X, Hu X, Sun T, Zuo J, Xiao P, Zhang H, Zhang R, Li R. Dual Influence of Rainfall and Water Temperature on Phytoplankton Diversity and Nutrient Dynamics in a Mountainous Riverine Reservoir. Diversity. 2025; 17(8):573. https://doi.org/10.3390/d17080573
Chicago/Turabian StyleZhao, Qihang, Lian Hu, Xinyue Ren, Xiang Hu, Tianchi Sun, Jun Zuo, Peng Xiao, He Zhang, Rongzhen Zhang, and Renhui Li. 2025. "Dual Influence of Rainfall and Water Temperature on Phytoplankton Diversity and Nutrient Dynamics in a Mountainous Riverine Reservoir" Diversity 17, no. 8: 573. https://doi.org/10.3390/d17080573
APA StyleZhao, Q., Hu, L., Ren, X., Hu, X., Sun, T., Zuo, J., Xiao, P., Zhang, H., Zhang, R., & Li, R. (2025). Dual Influence of Rainfall and Water Temperature on Phytoplankton Diversity and Nutrient Dynamics in a Mountainous Riverine Reservoir. Diversity, 17(8), 573. https://doi.org/10.3390/d17080573







