Abstract
Background: Microbial communities of insects have distinct roles for their respective hosts. For the black fly (Diptera: Simuliidae), an important vector and ecological indicator, the representative microbiota from the different body regions are not known. Here, we investigated the microbial composition and diversity of the head, thorax, and abdomen of wild-caught Simulium vanluni. Methods: Adult Simulium vanluni were surface-sterilized and dissected into head, thorax, and abdomen. For each body region, 20 individuals were pooled into one sample with six replicates per region. DNA was extracted and sequenced using the 16S rRNA amplification method to assess for possible microbial diversity. Data were analyzed using MicrobiomeAnalyst, where we calculated alpha diversity, beta diversity, and tested compositional differences using PERMANOVA. Results: Across 17 pooled samples, three core genera, Wolbachia (78.33%), Rickettsia (9.74%), and Acinetobacter (9.20%), accounted for more than 97% of the 16S rRNA sequencing reads. Head communities were compositionally distinct compared to the thorax and abdomen (PERMANOVA, p < 0.05). Heads were nearly monodominated by Wolbachia (95–97%), exhibiting significantly lower diversity and evenness compared to other body regions. In contrast, the thoracic and abdominal communities were more even, where thoraces were enriched with Acinetobacter (19.16%) relative to Rickettsia (10.85%), while abdomens harbored higher Rickettsia (10.96%) than Acinetobacter (5.68%). Collectively, the near-monodominance of Wolbachia in heads and inverse abundances of Acinetobacter and Rickettsia in thoraces and abdomens suggest possible anatomical niche partitioning or competition exclusion of microbiota across body regions. Conclusions: Our findings reveal fine-scale anatomical niche partitioning in S. vanluni microbiota, with the heads being almost exclusively colonized by Wolbachia, while the thoracic and abdominal niche regions exhibit distinct enrichment patterns for Acinetobacter and Rickettsia. These spatially distinct microbial distributions suggest potential functional specialization across anatomical regions of S. vanluni.
1. Introduction
Insects are among the most diverse groups of living organisms on Earth, with species richness estimated at more than 20 million [1]. Although insects are widespread and ecologically important, we still know very little about their relationship with microbes, even though microbes can make up to 10% of an insect’s biomass [2]. Microbes are ubiquitous and can interact with insects as hosts in commensal, mutualistic, parasitic, or incidental relationships. These symbionts can provide direct or indirect nutritional support, boost immunity, influence development, and even manipulate host reproduction [3,4,5,6]. Moreover, symbiont interactions can be shaped not only by host-driven microevolution but also by co-evolution, where both parties evolve in response to each other’s adaptation, resulting in a unique dynamic relationship. In recent years, studies have shown that insect microbiomes vary not only across developmental stages but also among organs and different body parts, specifically in agriculturally and medically important species [7,8,9]. Much of this work, however, has focused on gut microbiota, given the gut’s central role in digestion and physiology [6,10,11], and salivary glands, which mediate pathogen transmission in vectors such as mosquitoes and ticks [12,13]. Over time, there has been increasing recognition that different body parts harbor distinct microbial communities that could contribute to the overall microbial profile of the insect. Studying these region-specific microbiomes is therefore key to understanding host–microbe interaction, potentially revealing new mechanisms of vector adaptation, immunity, or ecological fitness.
Black flies (Simulium spp.) are particularly important due to their hematophagous nature and role as vectors of pathogens infecting farm animals [14,15] and humans [15,16,17,18]. They are known as vectors for the transmission of Onchocerca volvulus, the causative agent of onchocerciasis, or river blindness, mainly reported in Africa [18,19], with additional reports in Latin America [20,21] and regions of the Middle East [22]. Black flies are known to breed exclusively in clean running water; hence, they serve as an indicator of water quality. Despite more than 2400 black fly species being documented [15], the microbial communities associated with only a few studies explore the microbiome in laboratory-reared adults emerging from the wild-caught pupae [23], identifying culturable bacteria communities in one of the most common species in Malaysia [24] and wild-caught adults in Cameroon [25,26].
More than 90 species of black flies have been documented in Malaysia [15,27], but none are known as vectors for human diseases. Although in general, most black fly species are hematophagous, only one species, Simulium asakoae, has been confirmed to bite humans in Malaysia [28]. Despite the lack of reported disease transmission to date, understanding microbial diversity in local black fly species is crucial for elucidating their ecological roles and potential influence on vector fitness and capacities for pathogen transmission. Among the documented species, Simulium vanluni, a unique cryptic black fly species found exclusively in Peninsular Malaysia, diverged from Simulium nobile [29], was selected due to its high prevalence in terrestrial ecosystems and its potential ecological significance [29,30,31]. S. vanluni inhabits lowland forests, with immature stages developing in medium to fast, clean, flowing streams [29,32,33], while the adults forage at temperatures ranging from 28 °C to 32 °C and under normal humidity conditions [30]. Since microbial diversity in insects is not homogenous across different body parts, understanding the key differences could provide invaluable insight into the specific interactions between microbial communities with their host at the different regions of the body and the surrounding environment. Therefore, in the current study, we aim to reveal at finer anatomical scales the microbiota of S. vanluni and establish the foundation for understanding symbiont roles in black fly ecology.
2. Methods
2.1. Sampling and Species Identification
Wild black fly adults were trapped at Sungkai Wildlife Conservation Centre, Sungkai, Perak (Figure 1), an animal sanctuary managed by the Department of Wildlife and National Parks (PERHILITAN), Peninsular Malaysia. The trappings were carried out using four malaise traps (width, 110 cm; length, 180 cm; height, 150 cm) that were deployed for 24 h at different animal enclosures. All collected samples were sorted and preserved in 80% ethanol and transported to the Tropical Infectious Diseases Research and Education Centre (TIDREC), Universiti Malaya. All flies were identified morphologically using identification keys applicable to the species in Malaysia [27,34] and confirmed using DNA barcoding based on the COI gene as previously described [35,36]. At the study site, two black fly species were captured: Simulium roslihashimi and Simulium vanluni. Only S. vanluni, which was the more abundant, was chosen for microbial analysis to ensure adequate replication.
Figure 1.
Location of sampling site situated at Sungkai, Perak, Malaysia.
2.2. Sample Preparation and DNA Extraction
Prior to the dissection, all adult black flies underwent surface sterilization (1% bleach, 30 s; 95% ethanol, 30 s; 70% ethanol, 30 s; nuclease-free water, 3 min) as previously described [37]. Subsequently, dissections were performed to separate each fly into three anatomical regions—head, thorax, and abdomen. For each anatomical region, tissues from 20 adults were combined into one pooled sample. The pooling process was repeated six times for each body part, yielding 18 pooled samples in total. DNA extraction was performed using a QIAamp DNA micro kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. DNA concentration was measured using an Invitrogen Qubit 4 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The extracted DNA samples were stored at −80 °C for further analysis.
2.3. 16S rRNA Amplification and Sequencing
Two primer sets targeting V2-4-8 and V3-6, 7-9 were used to amplify seven hypervariable regions of the 16S rRNA gene in separate reactions with the Ion 16S™ Metagenomics Kit (Thermo Fisher Scientific, Carlsbad, CA, USA) following the manufacturer’s protocol. A negative extraction control (buffer only) was included to monitor for potential contamination. Amplicons from both reactions were pooled in equal volumes and then purified using Agencourt AMPure XP beads (Beckman Coulter Inc., Brea, CA, USA). The concentration of amplicons was quantified and then converted into sequencing libraries using the Ion Plus Fragment Library Kit (Thermo Fisher Scientific, Carlsbad, CA, USA). Library preparation steps, including end repair, adaptor and barcode ligation, nick repair, and library amplification, were performed according to the manufacturer’s protocol. The final libraries were purified and templated using the Ion 520™ and Ion 530™ Kit-Chef (Thermo Fisher Scientific, Carlsbad, CA, USA) on the Ion Chef instrument (Thermo Fisher Scientific, Carlsbad, CA, USA), followed by loading onto the Ion 530 Chip (Thermo Fisher Scientific, Carlsbad, CA, USA). Sequencing was performed using the Ion GeneStudio S5 platform (Thermo Fisher Scientific, Carlsbad, CA, USA).
2.4. Bioinformatics and Data Analysis
Base-calling and demultiplexing were performed by Torrent Suite version 5.18.1 (Thermo Fisher Scientific, Carlsbad, CA, USA). Subsequently, the obtained sequence reads were analyzed using the 16S rRNA profiling workflow as implemented in the Ion ReporterTM software ver. 5.20.2.0. During the analysis, primer sequences were trimmed from low-quality reads, and reads shorter than 150 bp were removed. Only unique sequences with ≥10 copies were mapped to the curated MicroSEQ® 16S Library v.2013.1 and Greengenes v13.5 references in the Ion Torrent ReporterTM software for taxonomic assignment following the Clinical and Laboratory Standards Institute (CLSI) guidelines, where genus-level identification was accepted at >97%. The resulting ASV table and metadata file were imported into MicrobiomeAnalyst 2.0 [38,39,40]. Data filtering was performed using the default setting, which included a low-count filter (prevalence <10% across samples) and a low-variance filter based on the inter-quantile range. Data were then normalized using the trimmed mean of M-values (TMM). The alpha diversity indices (Chao1, Shannon, and Simpson) were calculated, and relative taxonomic abundance was generated. The processed dataset was subsequently used for beta diversity, which was estimated using Bray–Curtis distance and visualized by Principal Coordinates Analysis (PCoA), followed by Permutational Multivariate Analysis of Dispersion (PERMDISP) to assess dispersion and permutational multivariate analysis of variance (PERMANOVA) for compositional differences. Shared and unique genera across different anatomical regions were visualized using InteractiVenn [41]. The differentially abundant genera were identified by Linear Discriminant Analysis (LDA) Effect Size (LEfSe) with LDA threshold > 2 [42].
3. Results
3.1. 16S rRNA Gene Sequencing Results
A total of 18 samples, representing six replicates each for the head, thorax, and abdomen of S. vanluni, alongside negative extraction controls, were sequenced. After data processing, the negative control yielded only 155 reads, exhibited a distinct, low diversity profile, and was excluded from downstream analysis. After quality filtering, 4,754,021 clean reads remained across 17 body parts (252,563 and 317,751 reads per sample; Table 1), as one head replicate (P2H2) yielded a low number of reads and was excluded from further analysis.

Table 1.
Summary of sequencing reads for 17 S. vanluni anatomical region libraries.
3.2. Taxonomic Composition by Anatomical Regions
The bacterial communities across all samples were predominantly composed of Proteobacteria, which accounted for 99.57% of the reads (Figure 2A). Three genera, Wolbachia (78.33%), Rickettsia (9.74%), and Acinetobacter (9.20%), dominated across all samples (Figure 2B).
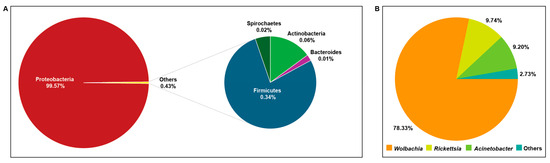
Figure 2.
Microbial community composition in S. vanluni. (A) Microbiota at the phylum level. (B) Microbiota at the genus level.
In the region of the S. vanluni heads, Wolbachia accounted for 91.19% of the sequencing reads; Rickettsia and Acinetobacter comprised 7.05% and 1.35%, respectively (Figure 3). In the thoraces, Wolbachia accounted for 68.37%, followed by Acinetobacter (19.16%), then Rickettsia (10.85%). In abdomens, Wolbachia comprised 77.42%, followed by Rickettsia (10.96%), Acinetobacter (5.68%), Enterobacter (2.51%), and Citrobacter (1.13%). One abdomen replicate (A3), however, diverged markedly with Wolbachia composition and fell below 50% of the total reads, followed by Enterobacter (21.55%), Citrobacter (9.68%), Rickettsia (7.61%), and Trabulsiella (6.65%), indicating occasional enrichment of non-core taxa (Figure 2).
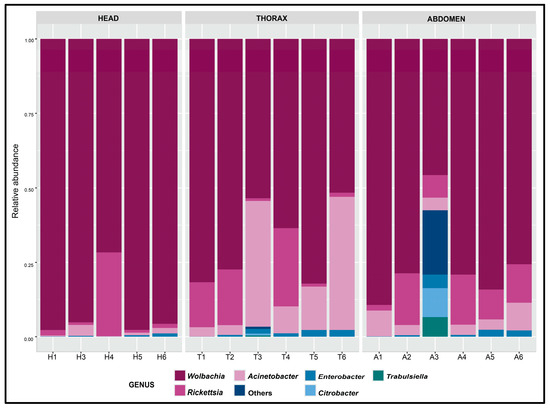
Figure 3.
Relative abundance across replicates of head, thorax, and abdomen of S. vanluni.
3.3. Alpha Diversity
The Chao 1 (richness) did not show significant differences in microbial diversity between heads, thoraces, and abdomens (Figure 4A), suggesting similar taxonomic richness across different anatomical regions. In contrast, the Shannon index (diversity) showed significant differences (Kruskal–Wallis, p = 0.0121, Figure 4B) across different anatomical regions. Pairwise comparisons revealed that the Shannon index was significantly lower in the head compared to the thorax and abdomen, while no significant difference was observed between the thorax and abdomen. The Simpson index (evenness) varied among different anatomical regions, where the heads showed reduced evenness relative to thoraces and abdomens, and this trend approached statistical significance (Kruskal–Wallis, p = 0.0537, Figure 4C). Collectively, the results suggested that the head of S. vanluni harbors a much less microbial diversity, whereas the thorax and abdomen show more diverse and evenly distributed microbial populations.

Figure 4.
Alpha diversity indices across anatomical regions of S. vanluni. Boxplots show (A) Chao 1 richness, (B) Shannon index, and (C) Simpson index. The different alphabet in each figure indicates pairwise significant differences between body parts.
3.4. Beta Diversity
In the Bray–Curtis distance-based PCoA visualization, PCoA1 (Axis 1) accounted for 50.5% of the total variance, and PCoA2 (Axis 2) accounted for 30.8%, together explaining 81.3% of the total variation in the bacterial community structure in S. vanluni (Figure 5). The PCoA plot revealed a clear separation of head samples from thorax and abdomen samples. PERMANOVA demonstrated significant differences in microbial community composition across anatomical regions (R2 = 0.2644; p < 0.05). Pairwise PERMANOVA comparison revealed head–thorax (R2 = 0.3066; p < 0.05) and head–abdomen (R2 = 0.2097; p < 0.05) differences, while there were no significant differences between thorax and abdomen (R2 = 0.1344; p > 0.05). PERMDISP showed no significant differences in dispersion among groups (p = 0.3050). Collectively, these results suggest that the observed differences were due to actual variation in microbial community composition rather than differences in within-group dispersion.
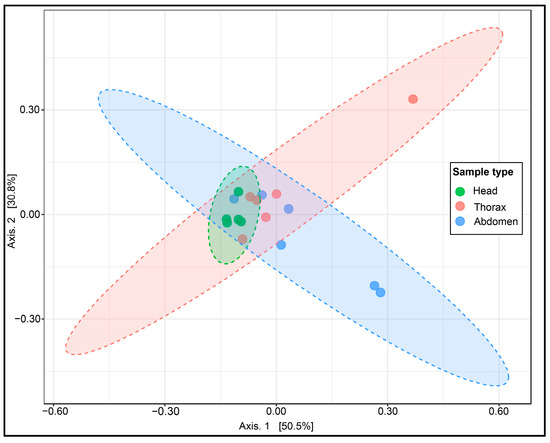
Figure 5.
PCoA of Bray–Curtis distances for microbial communities in S. vanluni anatomical regions. The dashed ellipses represent the confidence intervals for each sample type. Minimal overlap between ellipses indicates distinct bacterial communities for each anatomical region.
3.5. Core and Unique Microbiome Across Different Body Parts
Analysis of the microbial community composition revealed that 19 genera were common across all anatomical regions (head, thorax, and abdomen), suggesting they established stable associations throughout the adult black flies (Figure 6). These stable core genera include Wolbachia, Enterobacter, Enterococcus, Bacillus, Staphylococcus, Pseudomonas, and Rickettsia. However, 7 genera, including Corynebacterium and Streptococcus, were found exclusively in both the head and thorax but were absent from the abdomen. A total of 17 genera, including Proteus, were found in both the thorax and abdomen but not in the head. Despite the head and abdomen being anatomically distant, 2 shared genera were found (Micrococcus and Phaseolibacter). In addition to that, each region harbored unique genera, where the head contained 31 unique genera, including Bartonella and Actinomyces. The thorax harbored 8 unique genera, while the abdomen had 11 unique genera.
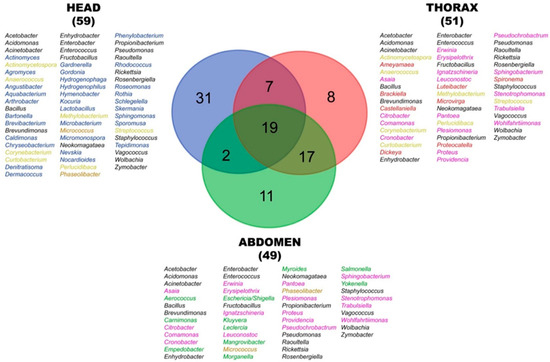
Figure 6.
Venn diagram of shared and unique bacterial genera across different anatomical regions. Numbers indicate the count for each overlap or exclusive genera. Colors of text listing specific genera correspond to each anatomical and shared anatomical regions. Head (blue), thorax (red), abdomen (green), head–thorax (light yellow), head–abdomen (brown), thorax–abdomen (pink), head–thorax–abdomen (black).
3.6. Differential Abundance Analysis
LEfSe was used to identify genera whose relative abundances differed significantly across the anatomical regions (LDA > 2). At the more stringent threshold (p = 0.05), Acinetobacter was significantly enriched in thoraces. No other genus reached LDA > 2 at p < 0.05 in heads or abdomens. At a more relaxed threshold (p < 0.10), two genera, Acinetobacter in the thorax and Wolbachia in the abdomen, were obtained.
4. Discussion
Overall, our study characterized and compared the microbiota in three anatomical regions (head, thorax, abdomen) of wild-caught adult S. vanluni using 16S rRNA amplicon sequencing. Our region-specific microbiota profiling of S. vanluni revealed the presence of a core set of bacterial taxa shared across all three anatomical regions, alongside distinct and region-specific microbial taxa that are unique to each anatomical region. Similar stable-core and fluctuating community patterns have been observed in the gut microbiome survey of Simulium damnosum [43], but our findings further demonstrated that these patterns manifest distinctively across different anatomical regions (head, thorax, abdomen). Generally, most of the microbes recorded in this study belonged to the phylum Proteobacteria, while the remaining microbes, which constituted less than 1%, were from other phyla. Most blood-feeding insects have restricted feeding behaviors, relying on blood-hosts and nectar as energy sources, and thus depend on specific microbes, such as a proteobacterial symbiont, to supply essential nutrients [44]. This may affect the bacterial composition in hematophagous insects compared to others, leading to selective microbial associations [6], nutritional specialization [45], and even the vertical transmission of microbes [46].
The head region exhibited low diversity, with over 90% of the reads being Wolbachia, compared to the more heterogeneous bacterial communities in the thorax and abdomen. Although high Wolbachia prevalence has been documented in the gut microbiota study of other black fly species [25,43], the present study is the first to report head-specific monodominance. Beta diversity (Bray–Curtis PCoA) and PERMANOVA concurred that the head bacterial communities are compositionally distinct from both the thorax and abdomen. Although most samples clustered closely, one thorax and two abdomen samples appeared to be distinct from their respective cluster. These outliers may reflect biological variation among wild-caught black flies, which could have acquired microbes from different environments or food sources. However, we cannot rule out that the small number of outliers may have contributed to the apparent beta-diversity differences. To further confirm these patterns, future work should include a larger sample size and biological replicates to better capture the microbial structure in different anatomical regions. Despite similar richness across all three anatomical regions, the extreme unevenness of the microbiota (near-monodominance of Wolbachia) in the S. vanluni heads implies a highly specialized niche rather than a general lack of microbial colonizers. Nevertheless, low evenness and diversity can also be a signal of ecological disturbance [47]. In our wild-caught flies, however, there is no evidence of recent pesticide or sudden diet shifts. In fact, the overwhelmingly Proteobacteria-dominated community that is anchored by vertically transmitted Wolbachia suggests a stable and host-driven microbiota. Repeated feeding cycles and insect immune-mediated clearance likely impose recurrent bottlenecks that eliminate transient taxa and favor a less diverse and endosymbiont-rich microbiome [6]. Further studies comparing flies from natural and human-impacted sites will help distinguish the true disturbance from long-term adaptive symbiosis.
Anatomically, the black fly’s head primarily specializes in sensory perception and feeding. The antennae serve as an essential organ for mechanosensation and thermal detection, and they also mediate chemosensation and olfaction, which are crucial in detecting the blood-host cues, such as carbon dioxide [48,49,50,51]. Meanwhile, the mouthparts play an important role in feeding, mainly in liquid form for the adult stage. During the feeding process, direct contact with various food sources and the surrounding environment may explain the presence of transient, low-abundance bacteria detected in our study. Similar observations were also observed in other insects, such as mosquitoes, lice, and ants, where the higher species richness in the head was associated with the interaction between the mouthparts and food sources [52,53,54]. Although feeding exposes the heads to transient microbes, including bacteria, antimicrobial secretions from the salivary glands may create selective microhabitats that are permissive to support the existence of selected dominant taxa [55], and any newly incoming bacteria into the head regions are either cleared by these defenses or outcompeted by Wolbachia [56,57,58]. Wolbachia is one of the most common endosymbiotic bacteria that can be found in about 70% of insects [5,59,60]. In many hosts, Wolbachia is known for its ability to manipulate host reproduction by inducing feminization, male killing, cytoplasmic incompatibility, and parthenogenesis [61,62,63,64]. Moreover, interactions between Wolbachia and some insects have been reported that are mutualistic, where Wolbachia is a nutritional symbiont providing essential nutrients to the host [62,65]. In addition, Wolbachia can also prime the insect immune system for defense against pests, insects, and pathogenic microbes [66,67,68]. Wolbachia is predominantly passed via transovarial transmission [69,70,71], with only sporadic horizontal transfers documented in other insects [72,73]. Consistent with a vertical-inheritance model, wild Simulium populations in Cameroon retain high Wolbachia prevalence, even when associated with Onchocerca volvulus [25,43], whereas laboratory-reared flies often lose detectable infection [23], indicating that vertical transmission can be one of the principal drivers of Wolbachia colonization. Together, it is plausible that the low-abundance bacteria in the head were acquired horizontally through food- or blood-feeding activities, while some may represent transient environmental taxa, or others could have functional associations with the host. The limited microbial diversity (near-monodominance) in the S. vanluni’s head reflects anatomical specialization and efficient maternal transmission.
In contrast to the head, the thorax and abdomen of S. vanluni harbor a more diverse microbial composition with greater evenness. Although Wolbachia remained the dominant taxa in both the thorax and abdomen, the secondary taxa varied by regions. The thoracic hemocoel and flight muscle microenvironment is enriched in Acinetobacter, which is known for environmental versatility and potential roles in nutrient recycling [74]. While the abdomen region hosts the gut and reproductive tissue, it may provide a more suitable habitat for intracellular Rickettsia with the capacity for vertical transmission and host reproductive manipulation [75]. Notably, samples with high Acinetobacter show lower Rickettsia and vice versa, suggesting that niche partitioning or potential microbial competition may drive this inverse relationship. The partitioning could have arisen due to differences in niche specialization [76,77], resource utilization [78], and even potential antagonistic interactions between specific bacterial groups [79,80].
The spatial segregation of the head versus body-region microbiota in S. vanluni suggests these communities could influence how black flies interact with environmental microbes. Therefore, understanding these region-specific microbial landscapes could provide crucial insight into black fly ecology and reveal finer-scale microbial–host interactions than gut-focused surveys. As the feeding status of collected black flies was undetermined, future work should therefore document adult feeding sources and evaluate how these non-hematophagous diets influence the microbial composition. In particular, examining how different diets can affect the Acinetobacter and Rickettsia balance will facilitate the understanding of the intrinsic tissue niche and diet-driven shift of microbiota. Furthermore, future work can also focus on examining tissue-specific factors, such as antennal and mouthpart anatomy or salivary gland antimicrobial secretions, in shaping these region-specific microbiomes. Longitudinal tracking of Wolbachia across developmental stages in black flies will reveal the potential role of vertical inheritance and environmental acquisition in maintaining black fly symbiont communities. Lastly, we used pools of 20 flies per region to ensure sufficient DNA yield and focus on core community patterns; however, this approach may mask the inter-individual variability in the microbiota composition. Future studies should include single-fly analysis alongside pooled samples to capture population-level trends and individual heterogeneity.
5. Conclusions
In summary, our findings revealed that adult S. vanluni harbor a shared core microbiota that is dominated by Wolbachia alongside distinct and region-specific microbial communities in the head, thorax, and abdomen. The head region exhibited near-monodominance by Wolbachia, with significantly lower diversity and evenness than the thorax and abdomen. Although Wolbachia remained the most abundant taxon in all regions, the thorax and abdomen supported more even microbiota enriched in Acinetobacter and Rickettsia, respectively, indicating fine-scale anatomical niche partitioning. These data establish a spatial framework for the black fly microbiome and provide a clear baseline for future studies of black fly microbial function and transmission. Furthermore, untargeted metagenomic and metabolomic analyses will be essential next steps to uncover the functional roles of these bacteria.
Author Contributions
Conceptualization, N.I.-A., Z.Y., V.L.L. and K.-K.T.; Methodology, N.I.-A., Z.Y., V.L.L. and K.-K.T.; Formal analysis, N.I.-A., Z.Y. and K.-K.T.; Data curation, N.I.-A.; Writing—original draft, N.I.-A., Z.Y. and K.-K.T.; Writing—review & editing, N.I.-A., V.L.L., Z.Y., S.A. and K.-K.T.; Funding acquisition, Z.Y., S.A. and K.-K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Ministry of Higher Education, Malaysia, under Dana Langganan SUKUK Pakej Rangsangan Ekonomi Prihatin Rakyat (SUKUK PRIHATIN)—Fasa 2 (MO002-2021) and the Higher Institution Centre of Excellence (HICoE) niche area vector and vector-borne diseases (MO002-2019 and TIDREC-2023).
Institutional Review Board Statement
Ethical review and approval were waived for this study because it did not involve human participants or vertebrate animals. Field sampling of Simulium vanluni in protected area (Sungkai Wildlife Conservation Centre) was conducted under research permits from the Department of Wildlife and Nature Parks (DWNP), Peninsular Malaysia JPHL/TN(IP):100-34/1.24 Jld. 17(35).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors acknowledge DWNP for providing the necessary facilities and assistance and express gratitude to all parties involved, either directly or indirectly, in making this study a success.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, X.; Wiens, J.J. Estimating Global Biodiversity: The Role of Cryptic Insect Species. Syst. Biol. 2023, 72, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Andongma, A.A.; Wan, L.; Dong, X.P.; Akami, M.; He, J.; Clarke, A.R.; Niu, C.Y. The impact of nutritional quality and gut bacteria on the fitness of Bactrocera minax (Diptera: Tephritidae). R. Soc. Open Sci. 2018, 5, 180237. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Habineza, P.; Ji, T.; Hou, Y.; Shi, Z. Intestinal Microbiota Confer Protection by Priming the Immune System of Red Palm Weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Front. Physiol. 2019, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.J.; Kim, M.S.; Park, D.S.; Yoon, C.; Nam, Y.D.; Kim, Y.J.; Choi, J.H.; et al. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Shan, H.W.; Chen, J.P.; Wu, W. Diversity and Dynamics of Bacterial Communities in the Digestive and Excretory Systems across the Life Cycle of Leafhopper, Recilia dorsalis. Insects 2023, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.Y.; Lv, Q.B.; Wang, C.R.; Ju, H.; Luo, C.F.; Liu, S.S.; Na, M.H.; Chang, Q.C.; Jiang, J.F. Microbiota profile in organs of the horseflies (Diptera: Tabanidae) in Northeastern China. Front. Microbiol. 2024, 15, 1467875. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.V.; Damiani, C.; Accoti, A.; Tallarita, M.; Nunzi, E.; Cappelli, A.; Bozic, J.; Catanzani, R.; Rossi, P.; Valzano, M.; et al. Estimating bacteria diversity in different organs of nine species of mosquito by next generation sequencing. BMC Microbiol. 2018, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An insight into diversity and functionalities of gut microbiota in insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.K.; Xiao, H.; Yang, Z.B.; Yang, D.S.; Yang, Y.H. Shotgun metagenomics reveals the gut microbial diversity and functions in Vespa mandarinia (Hymenoptera: Vespidae) at multiple life stages. Front. Microbiol. 2024, 15, 1288051. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Carvalho, V.R.; Souza-Neto, J.A.; Alonso, D.P.; Ribolla, P.E.M.; Medeiros, J.F.; Araujo, M.D.S. Bacterial Microbiota from Lab-Reared and Field-Captured Anopheles darlingi Midgut and Salivary Gland. Microorganisms 2023, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- Kazimirova, M.; Stibraniova, I. Tick salivary compounds: Their role in modulation of host defences and pathogen transmission. Front. Cell Infect. Microbiol. 2013, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Pramual, P.; Thaijarern, J.; Tangkawanit, U.; Wongpakam, K. Molecular identification of blood meal sources in black flies (Diptera: Simuliidae) suspected as leucocytozoon vectors. Acta Trop. 2020, 205, 105383. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.H. World Blackflies (Diptera: Simuliidae): A Comprehensive Revision of the Taxonomic and Geographical Inventory. 2025. Available online: https://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf (accessed on 15 April 2025).
- Blacklock, D. The development of Onchocerca volvulus in Simulium damnosum. Ann. Trop. Med. Parasitol. 1926, 20, 1–48. [Google Scholar] [CrossRef]
- Shelley, A.J.; Coscaron, S. Simuliid blackflies (Diptera: Simuliidae) and ceratopogonid midges (Diptera: Ceratopogonidae) as vectors of Mansonella ozzardi (Nematoda: Onchocercidae) in northern Argentina. Mem. Inst. Oswaldo Cruz. 2001, 96, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.; Kruger, A.; Pfarr, K.; De Witte, J.; Kibweja, A.; Mwingira, U.; Dujardin, J.C.; Post, R.; Colebunders, R.; O’Neill, S.; et al. The blackfly vectors and transmission of Onchocerca volvulus in Mahenge, south eastern Tanzania. Acta Trop. 2018, 181, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.A.; Cromwell, E.A.; Hill, E.; Donkers, K.M.; Schipp, M.F.; Johnson, K.B.; Pigott, D.M.; LBD 2019 Neglected Tropical Diseases Collaborators; Hay, S.I. The prevalence of onchocerciasis in Africa and Yemen, 2000–2018: A geospatial analysis. BMC Med. 2022, 20, 293. [Google Scholar] [CrossRef] [PubMed]
- Botto, C.; Basanez, M.G.; Escalona, M.; Villamizar, N.J.; Noya-Alarcon, O.; Cortez, J.; Vivas-Martinez, S.; Coronel, P.; Frontado, H.; Flores, J.; et al. Evidence of suppression of onchocerciasis transmission in the Venezuelan Amazonian focus. Parasit. Vectors 2016, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, M.A.; Fernandez-Santos, N.A.; Orozco-Algarra, M.E.; Rodriguez-Atanacio, J.A.; Dominguez-Vazquez, A.; Rodriguez-Morales, K.B.; Real-Najarro, O.; Prado-Velasco, F.G.; Cupp, E.W.; Richards, F.O., Jr.; et al. Elimination of Onchocerciasis from Mexico. PLoS. Negl. Trop. Dis. 2015, 9, e0003922. [Google Scholar] [CrossRef] [PubMed]
- Gebrezgabiher, G.; Mekonnen, Z.; Yewhalaw, D.; Hailu, A. Reaching the last mile: Main challenges relating to and recommendations to accelerate onchocerciasis elimination in Africa. Infect. Dis. Poverty 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Adler, P.H.; Vogel, H.; Ping, L. Gender-specific bacterial composition of black flies (Diptera: Simuliidae). FEMS Microbiol. Ecol. 2012, 80, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Loong, S.K.; Ya’cob, Z.; Low, V.L.; Teoh, B.T.; Ahmad-Nasrah, S.N.; Yap, P.C.; Sofian-Azirun, M.; Takaoka, H.; AbuBakar, S.; et al. Culturable bacteria in adults of a Southeast Asian black fly, Simulium tani (Diptera:Simuliidae). Acta Trop. 2021, 219, 105923. [Google Scholar] [CrossRef] [PubMed]
- Efon Ekangouo, A.; Nana Djeunga, H.C.; Sempere, G.; Kamgno, J.; Njiokou, F.; Moundipa Fewou, P.; Geiger, A. Bacteriome Diversity of Blackflies’ Gut and Association with Onchocerca volvulus, the Causative Agent of Onchocerciasis in Mbam Valley (Center Region, Cameroon). Pathogens 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, L.; Hadermann, A.; Ingletto, L.; Colebunders, R.; Gamnsi Njamnshi, K.; Njamnshi, A.K.; Mokili, J.L.; Siewe Fodjo, J.N.; Matthijnssens, J. Cameroonian blackflies (Diptera: Simuliidae) harbour a plethora of RNA viruses. Virus Evol. 2025, 11, veaf024. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, H.; Ya’cob, Z.; Sofian-Azirun, M. Classification, annotated list and keys for the black flies (Diptera: Simuliidae) of Peninsular Malaysia. Zootaxa 2018, 4498, 1–65. [Google Scholar] [CrossRef] [PubMed]
- Izwan-Anas, N.; Ya’cob, Z.; Low, V.L.; Lourdes, E.Y.; Teoh, B.T.; Mansor, M.S.; Ramli, R.; Pramual, P.; Adler, P.H.; Takaoka, H.; et al. First blood-meal record of Simulium asakoae (Diptera: Simuliidae) in Malaysia, with notes on its distribution in Asia and status as a potential vector. Trop. Biomed. 2024, 41, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Ya’cob, Z.; Takaoka, H.; Low, V.L.; Sofian-Azirun, M. First description of a new cryptic species, Simulium vanluni from Peninsular Malaysia: An integrated morpho-taxonomical and genetic approach for naming cryptic species in the family Simuliidae. Acta Trop. 2017, 167, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Izwan-Anas, N.; Halim, M.R.A.; Low, V.L.; Adler, P.H.; Ya’cob, Z. Wild-caught adult black flies (Diptera: Simuliidae) from various ecological landscapes in Malaysia. Acta Trop. 2024, 259, 107374. [Google Scholar] [CrossRef] [PubMed]
- Pavitra, S.P.; Ya’cob, Z.; Tan, T.K.; Lim, Y.A.L.; Low, V.L. Genetic diversity and differentiation in the blackflies Simulium cheongi, Simulium jeffreyi and Simulium vanluni (Diptera: Simuliidae) in Peninsular Malaysia. Acta Trop. 2020, 205, 105415. [Google Scholar] [CrossRef] [PubMed]
- Ya’cob, Z.; Takaoka, H.; Pramual, P.; Low, V.L.; Sofian-Azirun, M. Breeding habitat preference of preimaginal black flies (Diptera: Simuliidae) in Peninsular Malaysia. Acta Trop. 2016, 153, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ya’cob, Z.; Takaoka, H.; Pramual, P.; Low, V.L.; Sofian-Azirun, M. Distribution pattern of black fly (Diptera: Simuliidae) assemblages along an altitudinal gradient in Peninsular Malaysia. Parasit. Vectors 2016, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, H.; Davies, D.M. The Black Flies (Diptera: Simuliidae) of West Malaysia; Kyushu University Press: Fukuoka, Japan, 1995. [Google Scholar]
- Izwan-Anas, N.; Low, V.L.; Ya’cob, Z.; Lourdes, E.Y.; Halim, M.R.A.; Sofian-Azirun, M.; Takaoka, H.; Adler, P.H. DNA barcodes and species boundaries of black flies (Diptera: Simuliidae) in Malaysia. Arthropod. Syst. Phylogeny 2023, 81, 931–943. [Google Scholar] [CrossRef]
- Low, V.L.; Takaoka, H.; Adler, P.H.; Ya’cob, Z.; Norma-Rashid, Y.; Chen, C.D.; Sofian-Azirun, M. A multi-locus approach resolves the phylogenetic relationships of the Simulium asakoae and Simulium ceylonicum species groups in Malaysia: Evidence for distinct evolutionary lineages. Med. Vet. Entomol. 2015, 29, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Binetruy, F.; Dupraz, M.; Buysse, M.; Duron, O. Surface sterilization methods impact measures of internal microbial diversity in ticks. Parasit. Vectors 2019, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Efon-Ekangouo, A.; Nana-Djeunga, H.C.; Nwane, P.B.; Nzune-Toche, N.; Sondi-Dissake, J.C.; Sempere, G.; Domche, A.; Njiokou, F.; Kamgno, J.; Moundipa-Fewou, P.; et al. Spatial and temporal diversity of Simulium damnosum s.l. gut microbiota and association with Onchocerca volvulus infection in Cameroon. Infect. Genet. Evol. 2024, 125, 105683. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Aksoy, S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011, 27, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E.; Stewart, P.E. Microbiomes of blood-feeding arthropods: Genes coding for essential nutrients and relation to vector fitness and pathogenic infections. A review. Microorganisms 2021, 9, 2433. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: NonpathogeniciInteractions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.L.; Berga, M.; Burgmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B.; et al. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kulkarni, N.; Lei, H.H.; Lai, K.; Nematova, O.; Wei, K.; Lei, H. Experimental and theoretical probe on mechano- and chemosensory integration in the insect antennal lobe. Front. Physiol. 2022, 13, 1004124. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Srisuka, W.; Aupalee, K.; Yasanga, T.; Phuackchantuck, R.; Pitasawat, B.; Junkum, A.; Limsopatham, K.; Sanit, S.; Saingamsook, J.; et al. Ultrastructure of sensilla on the antennae and maxillary palpi of the human-biting black flies, Simulium nigrogilvum and Simulium umphangense, (Diptera: Simuliidae) in Thailand. Acta Trop. 2022, 232, 106494. [Google Scholar] [CrossRef] [PubMed]
- Shipp, J.; Grace, B.; Janzen, H. Influence of temperature and water vapour pressure on the flight activity of Simulium arcticum Malloch (Diptera: Simuliidae). Int. J. Biometeorol. 1988, 32, 242–246. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Wong, A.M.; Axel, R. An olfactory sensory map in the fly brain. Cell 2000, 102, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, S.; Maurya, R.K.; Das De, T.; Thomas, T.; Lata, S.; Singh, N.; Pandey, K.C.; Valecha, N.; Dixit, R. Salivary glands harbor more diverse microbial communities than gut in Anopheles culicifacies. Parasit. Vectors 2014, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Agany, D.D.M.; Potts, R.; Hernandez, J.L.G.; Gnimpieba, E.Z.; Pietri, J.E. Microbiome Differences Between Human Head and Body Lice Ecotypes Revealed by 16S RRNA Gene Amplicon Sequencing. J. Parasitol. 2020, 106, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhao, M.; Zhang, Z.; Hu, X.; Xu, Y.; Wei, C.; He, H. Lactic Acid Bacteria Are Prevalent in the Infrabuccal Pockets and Crops of Ants That Prefer Aphid Honeydew. Front. Microbiol. 2021, 12, 785016. [Google Scholar] [CrossRef] [PubMed]
- Lachat, J.; Lextrait, G.; Jouan, R.; Boukherissa, A.; Yokota, A.; Jang, S.; Ishigami, K.; Futahashi, R.; Cossard, R.; Naquin, D.; et al. Hundreds of antimicrobial peptides create a selective barrier for insect gut symbionts. Proc. Natl. Acad. Sci. USA 2024, 121, e2401802121. [Google Scholar] [CrossRef] [PubMed]
- Ghoul, M.; Mitri, S. The Ecology and Evolution of Microbial Competition. Trends Microbiol. 2016, 24, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Jang, S.; Takeshita, K.; Ohbayashi, T.; Ohnishi, N.; Meng, X.Y.; Mitani, Y.; Kikuchi, Y. Host-symbiont specificity determined by microbe-microbe competition in an insect gut. Proc. Natl. Acad. Sci. USA 2019, 116, 22673–22682. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J.; Bouchon, D. Feminizing Wolbachia influence microbiota composition in the terrestrial isopod Armadillidium vulgare. Sci. Rep. 2018, 8, 6998. [Google Scholar] [CrossRef] [PubMed]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?–a statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.; Giordano, R.; Fialho, R.F. Male-killing, nematode infections, bacteriophage infection, and virulence of cytoplasmic bacteria in the genus Wolbachia. Annu. Rev. Ecol. Syst. 2001, 32, 519–545. [Google Scholar] [CrossRef]
- Giorgini, M.; Bernardo, U.; Monti, M.M.; Nappo, A.G.; Gebiola, M. Rickettsia Symbionts Cause Parthenogenetic Reproduction in the Parasitoid Wasp Pnigalio soemius (Hymenoptera: Eulophidae). Appl. Environ. Microbiol. 2010, 76, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Koga, R.; Kikuchi, Y.; Meng, X.-Y.; Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA 2010, 107, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.S.; Perlman, S.J.; Kelly, S.E. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Hurst, G.D.; Zhang, W.; Breeuwer, J.A.; Stouthamer, R.; Majerus, M.E. Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata). J. Bacteriol. 1994, 176, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Nikoh, N.; Hosokawa, T.; Moriyama, M.; Oshima, K.; Hattori, M.; Fukatsu, T. Evolutionary origin of insect—Wolbachia nutritional mutualism. Proc. Natl. Acad. Sci. USA 2014, 111, 10257–10262. [Google Scholar] [CrossRef] [PubMed]
- Berticat, C.; Rousset, F.; Raymond, M.; Berthomieu, A.; Weill, M. High Wolbachia density in insecticide-resistant mosquitoes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.J. Bugs in transition: The dynamic world of Wolbachia in insects. PLoS Genet. 2013, 9, e1004069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, A.; Kucharczyk, H.; Kucharczyk, M.; Kapusta, P.; Sell, J.; Zielinska, S. First insight into microbiome profile of fungivorous thrips Hoplothrips carpathicus (Insecta: Thysanoptera) at different developmental stages: Molecular evidence of Wolbachia endosymbiosis. Sci. Rep. 2018, 8, 14376. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.O.; Vieira, A.S.; Pereira, M.C.; Moreau, C.S.; Bueno, O.C. Transovarian Transmission of Blochmannia and Wolbachia Endosymbionts in the Neotropical Weaver Ant Camponotus textor (Hymenoptera, Formicidae). Curr. Microbiol. 2018, 75, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Heath, B.D.; Butcher, R.D.; Whitfield, W.G.; Hubbard, S.F. Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr. Biol. 1999, 9, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Li, S.J.; Xue, X.; Yin, X.J.; Ren, S.X.; Jiggins, F.M.; Greeff, J.M.; Qiu, B.L. The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathog. 2015, 10, e1004672. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Park, W. Acinetobacter species as model microorganisms in environmental microbiology: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2533–2548. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.L.; Gaff, H.D.; Sonenshine, D.E.; Hynes, W.L. Experimental vertical transmission of Rickettsia parkeri in the Gulf Coast tick, Amblyomma maculatum. Ticks Tick Borne Dis. 2015, 6, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pernas, P.; Arias-Cordero, E.; Novoselov, A.; Ebert, C.; Rybak, J.; Kaltenpoth, M.; Westermann, M.; Neugebauer, U.; Boland, W. Bacterial Community and PHB-Accumulating Bacteria Associated with the Wall and Specialized Niches of the Hindgut of the Forest Cockchafer (Melolontha hippocastani). Front. Microbiol. 2017, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Meng, X.Y.; Kamagata, Y.; Tsuchida, T. Subcellular Niche Segregation of Co-Obligate Symbionts in Whiteflies. Microbiol. Spectr. 2023, 11, e0468422. [Google Scholar] [CrossRef] [PubMed]
- Litchman, E.; Edwards, K.F.; Klausmeier, C.A. Microbial resource utilization traits and trade-offs: Implications for community structure, functioning, and biogeochemical impacts at present and in the future. Front. Microbiol. 2015, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS. Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).