Abstract
Recent updates to the reconstructions of Cenozoic environmental changes (global sea level, temperature, and atmospheric carbon dioxide content) have made it intriguing to compare them to paleontological records for original interpretations. Paleogene brachiopods have remained in the shadow of their Paleozoic–Mesozoic predecessors, and the reactions of their diversity to the Earth’s dramatic changes are poorly understood. The present work aims to fill this gap via a comparison of several diversity and paleoenvironmental curves. The generic diversity was established by stages with two essentially different paleontological datasets, and several fresh paleoenvironmental reconstructions were adopted. It was observed that neither Paleogene eustatic fluctuations nor changes in the atmospheric carbon dioxide content correspond well to the generic diversity dynamics of brachiopods. The changes in the total number of genera and the global temperatures demonstrate similarity at the Danian–Ypresian interval, but not later. The fluctuations in the brachiopod diversity are near the same level during the Eocene–Oligocene, despite strong paleoenvironmental changes, implying the intrinsic resistivity of these organisms to external influences. Additionally, the Cretaceous/Paleogene mass extinction, the Paleocene–Eocene thermal maximum, and the Early Eocene optimum could enhance the diversity dynamics together with the long-term temperature changes. In contrast, the influences of the Late Danian warming event and the Oi-1 glaciation were not observed.
1. Introduction
Geological knowledge is regularly updated, and the frequency and the depth of such updates accelerate together with the rise of the researchers’ interest in particular topics. A typical example is the almost simultaneous appearance of the alternative, new-generation global sea-level curves [1,2], which have changed the vision of the Cenozoic eustasy. Such updates require full reconsideration of the previous, now outdated interpretations of various geological and paleontological phenomena. Alternatively, the knowledge of them becomes more and more inconsistent and chaotic [3], and this can be complicated by the thematic distortion of the research activity on long-term scales [4]. The same, even if old-fashioned approaches as used in the initial interpretations should be followed to realize what has really changed after the current updates. These theoretical thoughts imply that the sustainable growth of geoscience should be based on not only conventional research projects utilizing novel data and/or methods, but also investigations aimed at the reinterpretation of the earlier ideas in the light of fresh developments.
Brachiopods remained important marine invertebrates throughout the Phanerozoic [5,6]. These organisms boasted significant and fluctuated diversity, which made them highly-demanded in the studies of biotic crises (also mass extinctions) and paleoenvironmental factors of the sea life’s evolution. Various examples of such investigations can be found in works by Chen et al. [7], Copper [8], García Joral et al. [9], Mukherjee [10], Patzkowsky [11], Powell et al. [12], Rong and Harper [13], Ruban [14], Shi and Huang [15], and Vörös and Szives [16]. However, this research focused mainly on the Paleozoic–middle Mesozoic intervals, when brachiopods were significantly diverse. Less attention was paid to the Cenozoic and, particularly, Paleogene diversity dynamics of these organisms. Apparently, the most authoritative synthesis of the related information was provided by Curry and Brunton [6], but it is short and attached to 20-year-old geological knowledge. This synthesis demonstrated that Paleogene brachiopods experienced a reduction in the number of taxa relative to the Mesozoic and lost their importance in marine ecosystems, but remained diverse on the generic and suprageneric levels [6]. Recently, Guo et al. [17] suggested that brachiopods played a marginal role in Mesozoic–Cenozoic seas and lost diversity, even if they were able to demonstrate morphological innovations. Indeed, it would be very interesting to learn which factors influenced these organisms during their prolonged “stagnation”. The appearance of several fresh curves representing Cenozoic paleoenvironmental changes has made it urgent and reasonable to compare the available information on the brachiopod diversity dynamics with such curves. The related interpretations will enrich the understanding of these dynamics.
An important question is whether the information on Paleogene brachiopods is enough to address their diversity dynamics. The work by Curry and Brunton [6] demonstrated that the number of their genera in this period was not so small, which is an indirect sign of the sufficient amount of data. Moreover, no paleontological data can be “ideal”, and, thus, the only solution is to deal with them in their present state taking into account the degree of uncertainty. Alternatively, waiting for the accumulation of larger amounts of data may take years and decades without any progress in paleobiological knowledge. This is why each study addressing the diversity dynamics of brachiopods in this particular geological time slice is an important step toward obtaining a better understanding, although it is one of many steps to be taken.
The objective of the present paper is to provide a comparison of the generic diversity dynamics of Paleogene brachiopods with the long-term changes in global sea-level, temperature, and atmospheric carbon dioxide content. These aspects of paleoenvironmental changes were selected due to the availability of their fresh reconstructions (see below) and their potential but still unclear importance to the evolution of the considered group of fossils. The previous studies implied that brachiopods were either sensitive or not (and directly or indirectly) to sea level [14,18,19], temperature [20,21,22], and carbon dioxide concentration [23] at the different intervals of their development. This is why the comparison of the recently updated knowledge of these aspects of paleoenvironmental changes with the brachiopod diversity dynamics is reasonable. This work deals only with the Paleogene Period because a consideration of the entire Cenozoic Era would be too challenging due to the serious differences of the global environments between the periods. It should be added that the present work does not pretend to draw ultimate conclusions: paleontological and geological knowledge grows and experiences regular changes, and, therefore, the related interpretations will change in the future. Nonetheless, the availability of the different curves showing brachiopod diversity and the paleoenvironmental fluctuations enables us to make tentative interpretations and raises new questions and/or hypotheses for further investigations. In other words, the present paper offers an analysis of the knowledge in that shape, which is fixed in contemporary science.
2. Materials and Methods
Stratigraphic ranges of taxa are essential to document the diversity dynamics of a given fossil group at a particular interval of geological time. The information about brachiopods was obtained from two main sources. The first of them is the work by Curry and Brunton [6], who employed the stratigraphic ranges of brachiopod genera to calculate their diversity per stage of all periods, including the Paleogene. A significant advantage of the related diversity reconstruction is that it originated from a homogenous source of paleontological data prepared by brachiopodologists. Although published >15 years ago and reflecting the paleontological knowledge as it was at the beginning of the 21st century, this work retains its importance due to its completeness (note that the information of Paleogene brachiopods has not grown actively in the past years, and only a few new taxa have been reported [24,25,26]). The second source is the Paleobiology Database [27], which is updated regularly and aims to synthesize paleontological information from across the globe. An advantage is that this database often deals with original publications and pays close attention to the spatiotemporal aspects of paleontological data. However, this may also be a kind of disadvantage due to the inevitable heterogeneity of the information compiled in this manner. Although such a database cannot be complete by definition and it does not avoid certain biases [28,29], its strong features are its regular updates and employment of the modern version of the geological time scale. Such relatively new genera as Danian Basiliocostella Dulai et al. [25], mid-Eocene Meznericsia Bitner et al. [24], and Chattian Germanoplatidia Dulai and Von Der Hocht [26], are present in the Paleobiology Database. The latter focuses on fossil occurrences, but it also has powerful tools for extracting stratigraphic ranges of taxa and measurements of generic diversity in geological time units. The utility of the Paleobiology Database [27] to the studies of brachiopod diversity was proven recently by Guo et al. [17]. Additionally, there is a third precious source of information, namely the famous compilation by Sepkoski [30]. It is not too outdated and may potentially contain a few data absent from the two other sources. However, it cannot be used for the purpose of the present study because of the limited and, thus, insufficient resolution of the Paleogene time scale preferred in that work.
The two sources mentioned above [6,27] were used to document the Paleogene diversity dynamics of brachiopods with two curves. Curry and Brunton [6] explained the procedures of how they estimated diversity. The Paleobiology Database [27] offers automatic algorithm for the up-to-date diversity estimates. The resolution of the curves is limited to genera and stages. The curves were plotted along the present version of the geological time scale as established by the International Commission on Stratigraphy [31,32], if even needing some improvements [33]. The diversity curves are justified against the numerical time scale. The two curves refer to the differently compiled paleontological data, and, thus, they seem to be of equal importance, whereas differences between them should be understood as an indication of uncertainties of the present-day diversity estimates. These uncertainties can be related not only to possible data incompleteness, but also to taxonomic and stratigraphic deficiencies, which are inevitable in all kinds of paleontological compilations. This is why two curves should be considered simultaneously in the comparisons to the other information. It should be added that these curves (as well as the majority of other diversity curves constructed and interpreted in the contemporary paleobiological studies) cannot be understood as “final” reconstructions. Sampling biases, uncertainties in taxonomic interpretations, and possible stratigraphic errors always matter and cannot be avoided. This is why the interpretations presented in this analysis can be only provisional, and they will need updates in the future, i.e., together with the improvement of the paleontological knowledge.
It is very enigmatic as to why the curve based on the Paleobiology Database [27] shows a slightly lower generic diversity than the curve by Curry and Brunton [6], although the former is fresher than the latter and takes into account new genera reported after the latter was published (see above). The possible explanations are related to occasional losses of some portion of the paleontological information, synonymization of taxa, and/or corrections of stratigraphic ranges of taxa. Nonetheless, the noted enigma is generally unimportant in the present comparative analysis, which focuses not on absolute numbers of taxa but on long-term trends of diversity dynamics, i.e., on relative changes, which are more or less similar for the considered curves (see below). In other words, the differences in the diversity curves in detail is minimized via their generalization.
The changes in global sea-level, temperature, and atmospheric carbon dioxide content during the Paleogene are reflected by several curves, as follows. Two global sea-level curves appeared very recently. One of them was published by Haq and Ogg [1], who updated the earlier, well-known curve proposed by Haq et al. [34] and then modified by Haq and Al-Qahtani [35]. The other curve was published by Miller et al. [2], who updated the previous developments by the same research team [36,37]. Principally, these curves can provisionally be called eustatic, although Miller et al. [2] coined the term “global mean geocentric sea level estimate”. Two curves can be considered to depict Paleogene temperature changes (although the latter may also be referred to as climate changes, one should note that climate is a wider term and also includes precipitation, wind direction and speed, and some other patterns). The first of them was proposed by Scotese et al. [38], and the second was a development by Westerhold et al. [39]. The former established the changes in global average temperatures, and the latter documented the mean temperature differences from today. Finally, two fresh curves showing changes in the content of atmospheric carbon dioxide were employed. One of them can be found in the abovementioned work by Miller et al. [2], and the other reconstruction was carried out by Hönisch et al. [40]. The temperature and carbon dioxide curves update the famous reconstructions by Zachos et al. [41], which remained in demand for about a quarter of century. In fact, there are some other notable curves reflecting Cenozoic paleoenvironmental changes (for instance, the global sea-level reconstruction by Young et al. [42] or the paleotemperature curve by Grossman and Joachimski [43]). However, these developments are not considered in this study, chiefly due to their lower resolution.
Collecting the abovementioned curves is not enough to compare them with the brachiopod diversity dynamics. First of all, the curves should be modified slightly to become more or less compatible with one another, and long-term changes should be shaped better. The work by Haq and Ogg [1] gives an example of how relatively short- and long-term curves can be differentiated. For this purpose, the selected curves were smoothed, i.e., simplified and generalized. Moreover, period-level tendencies of the changes were outlined, and they are the primary objects of interest in this analysis. Then, the modified curves were rescaled. They were justified against the contemporary version of the geological time scale [32]. Then, the pairs of the curves needed to be plotted against the same reference scales. This became possible only in the case of the global sea-level change curves. The temperature and carbon dioxide curves either reflect different parameters of the same pattern or are arranged differently. Additionally, the curves were modified, considering the magnitude of the changes, to make the latter more visible.
After the noted modifications, the curves were compared qualitatively (graphically) to the changes in the total number of brachiopod genera. Attention was paid to their similarities and differences in the long-term perspective, i.e., as established at the level of epochs. The general direction and the relative magnitude of the changes were especially important to take into account. These interpretations constitute the original findings of the present study and, thus, determine its novelty. Each of three pairs of paleoenvironmental curves were thought to be alternative reconstructions, the mutual importance of which should be considered in the interpretations.
3. Results
3.1. Diversity Dynamics
The generic diversity of brachiopods changed throughout the Paleogene (Figure 1). Both curves demonstrate a significant (at several times) decrease in the total number of genera in the beginning of this period, with the negative peak being in the second half of the Paleocene Epoch. A recovery started weakly in the Thanetian and culminated in the Ypresian, but the Maastrichtian, i.e., pre-Paleogene level, was not reached. For the younger intervals, the curves differ in their details. One of them demonstrates a significant stability throughout the Eocene–Oligocene, whereas the other curve implies a decrease in the number of genera in the Lutetian and a smaller diversity drop in the Rupelian.
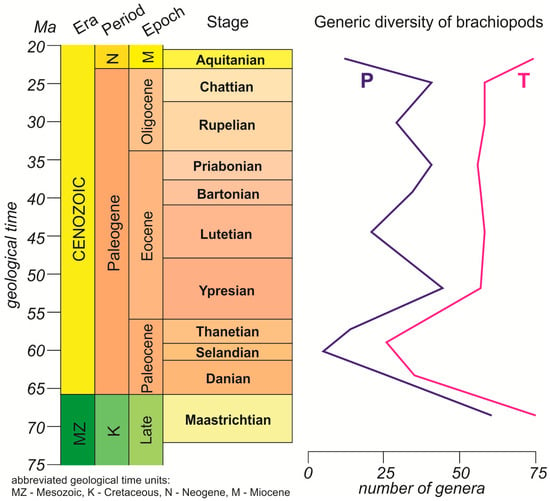
Figure 1.
Generic diversity dynamics of Paleogene brachiopods: T—on the basis of the information from Curry and Brunton [6]; P—on the basis of the information from the Paleobiology Database [27]. The geological time scale follows the developments of the International Commission on Stratigraphy [32], and the color codes of the time units follow the recommendations by Gradstein et al. [31]. For general reference, diversity is also shown for the Maastrichtian and the Aquitanian, which are not the Paleogene stages; a significant difference between the curves in the Aquitanian is notable, but it is out of scope of the present analysis.
The differences in the curves are expected due to the difference in the sources of the related paleontological information (see above for methodological explanations). Nonetheless, this work focuses on the general, long-term trends, i.e., the most principal diversity changes. In this aspect, both curves imply a decrease–increase cycle in the Danian–Ypresian interval (Figure 1). As for the Eocene–Oligocene interval, both curves show diversity fluctuations around the same level (only the magnitude of these fluctuations differed), and, thus, they registered a comparable long-term trend. Taken together, these lines of evidence mean that brachiopods experienced significant and long-term stress in the Paleocene, diversified quickly in the beginning of the Eocene, and then remained relatively diverse until the end of the period.
3.2. Comparisons to Selected Paleoenvironmental Changes
The history of the Paleogene global sea-level changes seems to be uncertain to some degree (Figure 2). One curve shows a tendency to sea-level fall in the Paleocene, whereas the other, in contrast, suggests a tendency for sea levels to have risen. However, both curves depict a highpoint in the Ypresian and a gradual fall in the Lutetian–Chattian. The former coincided with the peak in generic diversity, while the latter did not correspond to any steady long-term decline or rise in the number of genera (Figure 2). One should also note that the global sea level rose in the Priabonian, but this stage was marked by only a moderate increase in the number of genera. Generally, the correspondence between the long-term changes in the generic diversity dynamics and the global sea level was rather weak. The previous interpretations for the other intervals of the brachiopod evolution [14,18,19] implied that such a correspondence can exist, although it does not necessarily exist.
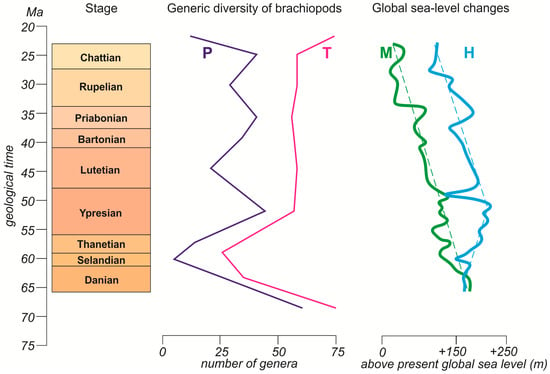
Figure 2.
Alternative curves of Paleogene eustatic fluctuations (bold solid lines) and their long-term trends (thin dashed lines): H—modified from Haq and Ogg [1]; M—modified from Miller et al. [2]. Note that these are not the original curves from the cited works, but rather their smoothed and rescaled versions, which should not be utilized for solving research tasks other than those addressed in the present study; the H curve is not the long-term curve shown by Haq and Ogg [1], but a reconstruction based on their short-term curve. See Figure 1 for explanations of the diversity curves and the time scale.
The Paleogene temperature changes are known with better certainty, as evidenced by the similarity in the related curves (Figure 3). Both show a cooling tendency in the Danian–Selandian, active warming in the Thanetian–Ypresian, gradual cooling in the Lutetian–Priabonian, and fluctuations around more or less the same level in the Oligocene. The Danian–Selandian cooling and the Thanetian–Ypresian warming corresponded well to the strong diversity decline and rise, respectively (Figure 3). However, the long-term tendency of the changes in the number of genera during the Eocene–Oligocene contrasted significantly with the temperature fluctuations. The evident increase in the frequency and magnitude of fluctuations either in the late Eocene, as evidenced by Westerhold et al. [39], or in the Oligocene, as implied by the information from Scotese et al. [38], did not apparently affect the diversity patterns.
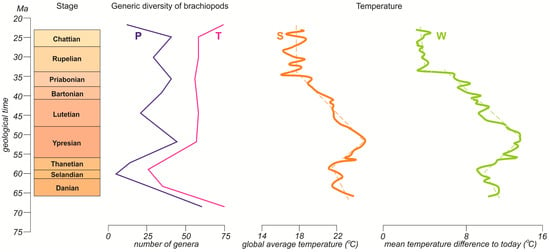
Figure 3.
Alternative curves of Paleogene temperature fluctuations (bold solid lines) and their long-term trends (thin dashed lines): S—modified from Scotese et al. [38]; W—modified from Westerhold et al. [39]. Note that these are not the original curves from the cited works, but rather their smoothed and rescaled versions, which should not be utilized for solving research tasks other than those addressed in the present study. See Figure 1 for explanations of the diversity curves and the time scale.
The considered reconstructions of the content of atmospheric carbon dioxide in the Paleogene are also similar (Figure 4). The concentrations were relatively low in the Paleocene, rose strikingly in the Ypresian, actively decreased in the Lutetian–Chattian, and then reached levels lower than those of the Paleocene. Although the Ypresian diversity peak corresponded to the same peak in the atmospheric carbon dioxide content, no direct and strong relationship can be found for the entire Paleogene (Figure 4).
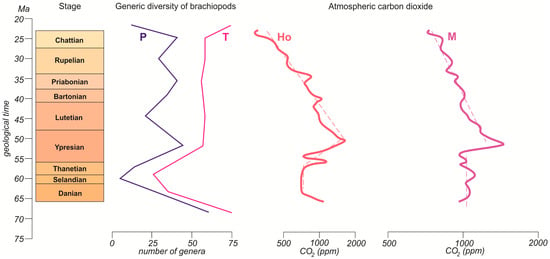
Figure 4.
Alternative curves of Paleogene changes in atmospheric carbon dioxide content (bold solid lines) and their long-term trends (thin dashed lines) Ho—modified from Hönisch et al. [40]; M—modified from Miller et al. [2]. Note that these are not the original curves from the cited works, but rather their smoothed and rescaled versions, which should not be utilized for solving research tasks other than those addressed in the present study. See Figure 1 for explanations of the diversity curves and the time scale.
4. Discussion
4.1. Summarized Findings and Their Interpretations
The comparisons of the curves imply that from the three aspects of the global paleoenvironmental changes, only temperature can be proposed as a factor of the brachiopod diversity dynamics and only for the first half of the Paleogene. Eustatic and carbon dioxide controls are not evident. These are interesting and important observations, although not too unexpected considering the ambiguous evidence of the eustatic and other environmental controls of brachiopod evolution from the different time intervals [14,18,22]. Indeed, these observations are valid relative to the only present state of paleontological and geological knowledge. Moreover, the possible influences of the long-term temperature changes are registered only too generally, and further investigations are needed to check some related details (e.g., whether the diversity changed differently between “warm” and “cool” domains).
Nonetheless, the abovementioned observations become surprising (even enigmatic) if we take into account the fact that Cenozoic brachiopods “stagnated” [17] while all of the considered paleoenvironmental changes remained strong throughout the Eocene–Oligocene (Figure 3 and Figure 4). One can propose two hypothetical explanations for further verification. First, the evident and prolonged brachiopod crisis that lasted during the Danian–Selandian resulted in the survival of the most tolerant taxa, which remained more or less unaffected by even strong paleoenvironmental changes after the Eocene diversification. Second, Paleogene brachiopods retained their ability to adapt to the changed environments via the quick population of narrow but suitable ecological niches, as known from the pre-Cenozoic period [44]. If so, the diversity effects of the paleoenvironmental changes were short-term and cannot be registered with the resolution of the present analysis. It cannot be excluded that both explanations are valid. Irrespective of the validity of the explanation(s), it appears that intrinsic (pure biological) forces were able to sustain the long-term tendency of the generic diversity dynamics of brachiopods in the Eocene–Oligocene. Of course, these inference is valid considering the present state of the paleontological data.
4.2. Long-Term Versus Short-Term Factors
In their explanation of the diversity patterns, Curry and Brunton [6] paid attention to the possible effects of the mass extinction that happened at the Cretaceous/Paleogene boundary. This is known as one of the biggest biotic crises [45,46,47] which stressed brachiopod communities [48,49]. Both diversity curves demonstrate a striking reduction in the brachiopod diversity in the Danian relative to the Maastrichtian (Figure 1). Moreover, the Selandian diversity was even lower, and the Paleobiology Database [27] registers only five genera of this stage. At first glance, it appears to be a very logical way to explain the Paleocene diversity decline (Figure 2), i.e., as a result of the direct devastative influence of the Cretaceous/Paleogene catastrophe. However, the Danian and Selandian stages lasted >5 Ma; thus, how possible is it that the negative influences of the mass extinction were so prolonged? Although this was principally possible (depending on the brachiopods’ ability to repopulate devastated environments), it has been argued that the post-extinction recovery of macrobenthos started without a lengthy delay [50]. Moreover, the evidence from the other intervals of the geological time implies the ability of brachiopods to restore their communities very quickly [44]. A more plausible explanation seems to be a combination of post-extinction stress with the long-term temperature decrease in the Paleocene (Figure 3). Their joint actions could cause the low generic diversity of brachiopods and their delayed recovery.
The abovementioned mass extinction was not the only major event in the considered geological time span (Figure 5). The other events were either short-term, such as the Late Danian warming [39,51,52], the Paleocene-Eocene thermal maximum (PETM) [39,41,53,54] and the associated extinction [55,56,57,58,59], and the Oi-1 glaciation [39,41,60,61,62] and the associated extinction at the Eocene–Oligocene transition [63,64,65,66,67], or relatively long-term events, such as the Early Eocene optimum [39,41,68]. From these events, the Early Eocene optimum coincided with the peak diversity of brachiopods, and PETM happened when the total number of genera actively increased (Figure 5). Apparently, these climatic changes established favorable conditions for the recovery of brachiopods after their Danian–Selandian decline. In contrast, the Late Danian warming did not interrupt the tendency towards a drop in diversity, but this was a relatively small-scale and short-term event in light of the present paleotemperature data [38,39]. As for the Oi-1 glaciation, it is known that this major event was preceded by a long-term cooling trend (Figure 3), and that the glaciation itself was, probably, less abrupt than imagined [69]; thus, brachiopods had enough time for gradual adaptation. The long-term tendency was not interrupted, although the moderate diversity decrease in the Rupelian [27] can be related to the effects of the Oi-1 glaciation and associated biotic perturbations.
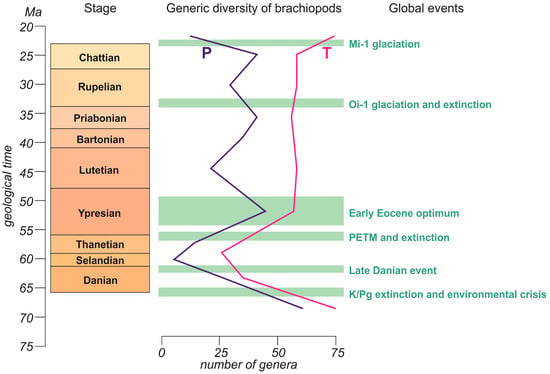
Figure 5.
Major Paleogene events plotted against the brachiopod generic diversity dynamics. See Figure 1 for explanations of the diversity curves and the time scale.
Summarizing the lines of evidence presented above, it appears that both long- and short-term processes and events influenced the generic diversity dynamics of brachiopods in the Paleogene. However, the importance of these external influences relative to the intrinsic mechanisms should not be overestimated on the long-term scale. For instance, one can hypothesize that it was the overall Thanetian–Ypresian warming that enhanced the recovery of the brachiopods, which, thus, would have diversified even without PETM and the Early Eocene optimum. Vice versa, it cannot be excluded that the brachiopod diversity would not have declined in the Paleocene without the Cretaceous/Paleogene mass extinction, which made these organisms more vulnerable to temperature changes.
5. Conclusions
The comparative analysis of the generic diversity dynamics of Paleogene brachiopods to several aspects of the global paleoenvironmental changes in the light of fresh developments in the literature permits the drawing of three general (if preliminary) conclusions.
First, the generic diversity of brachiopods changed dramatically in the Paleocene under the influences of climatic changes (temperature decrease) coupled with the effects from the Cretaceous/Paleogene mass extinctions.
Second, the generic diversity of brachiopods does not show a long-term tendency to increase or decrease during the Eocene–Oligocene despite the both long- and short-term paleoenvironmental changes and events. The hypothetic explanations are related to the intrinsic mechanisms.
Third, the Paleogene eustatic fluctuations and changes in the atmospheric carbon dioxide content did not correspond clearly to the long-term changes in the generic diversity of brachiopods, which seem to have been resistant to them.
These conclusions are provisional and reflect the present state of the paleontological and geological knowledge. Indeed, they may change in the future, i.e., together with the expansion and the improvement of this knowledge. Nonetheless, the offered interpretations indicate some important questions and hypotheses that require further study.
The main limitation of this study is the attention to only the total number of genera at the level of stages. Shifting the focus to the rates of appearance (origination) and disappearance (extinction) and the level of substages is an important but highly ambitious task, which cannot be solved presently due to the paleontological data restrictions. However, an in-depth reexamination of the brachiopod records can lead to its successful solution in the future. Other perspectives include the verification of the hypotheses and other interpretations presented in this work, as well as the extension of the comparative analysis to the Neogene. A broader circle of paleoenvironmental changes can be considered, together with the appearance of new/updated reconstructions and their related curves.
The present paper attempts to analyze several similarly styled curves. However, the relationship between Paleogene brachiopod diversity and paleoenvironmental changes is a much broader research problem. For instance, one can address the paleoecological peculiarities of brachiopod taxa, their habitats, and paleogeographical patterns (climate zones, bathymetry, bottom sediments, etc.). The completion of such tasks will require special investigations using much larger datasets. Apparently, these investigations cannot be accomplished in a single research projects, and, thus, the present work should be considered as only the first, initial step in this direction. Nonetheless, its outcomes ensure that further steps should be taken, because Paleogene brachiopods are, at least, no less interesting to study than Ordovician, Carboniferous, and Jurassic representatives of this fossil group.
Funding
This research was funded by the subsidy allocated to Kazan Federal University for the state assignment project no. FZSM-2023-0023 in the sphere of scientific activities.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Haq, B.U.; Ogg, J.G. Retraversing the Highs and Lows of Cenozoic Sea Levels. GSA Today 2024, 34, 4–11. [Google Scholar] [CrossRef]
- Miller, K.G.; Schmelz, W.J.; Browning, J.V.; Rosenthal, Y.; Hess, V.A.; Kopp, R.E.; Wright, J.D. Global Mean and Relative Sea-Level Changes Over the Past 66 Myr: Implications for Early Eocene Ice Sheets. Earth Sci. Syst. Soc. 2024, 3, 10091. [Google Scholar] [CrossRef]
- Ruban, D.A. Unawareness and Theorizing in Modern Geology: Two Examples Based on Citation Analysis. Earth 2020, 1, 1–14. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Garzanti, E.; Qi, J.; Kang, H.; Nong, H.; Wu, L.; Ding, J.; Wang, X.; Wang, C. A century of knowledge growth in sedimentology. Gondwana Res. 2025, 145, 49–56. [Google Scholar] [CrossRef]
- Carlson, S.J. The Evolution of Brachiopoda. Annu. Rev. Earth Planet. Sci. 2016, 44, 409–438. [Google Scholar] [CrossRef]
- Curry, G.B.; Brunton, C.H.C. Stratigraphic distribution of brachiopods. In Treatise on Invertebrate Paleontology; Part H. Brachiopoda. Revised; Selden, P.A., Ed.; Geological Society of America, University of Kansas: Boulder, KS, USA, 2007; Volume 6, pp. 2901–3081. [Google Scholar]
- Chen, Z.-Q.; Kaiho, K.; George, A.D. Early Triassic recovery of the brachiopod faunas from the end-Permian mass extinction: A global review. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 224, 270–290. [Google Scholar] [CrossRef]
- Copper, P. Evaluating the Frasnian-Famennian mass extinction: Comparing brachiopod faunas. Acta Palaeontol. Pol. 1998, 43, 137–154. [Google Scholar]
- García Joral, F.; Gómez, J.J.; Goy, A. Mass extinction and recovery of the Early Toarcian (Early Jurassic) brachiopods linked to climate change in Northern and Central Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 302, 367–380. [Google Scholar] [CrossRef]
- Mukherjee, D. Brachiopod migration and palaeosea temperatures: A case study from the Jurassic of Western India. Indian J. Geosci. 2016, 70–71, 269–276. [Google Scholar]
- Patzkowsky, M.E. Gradient analysis of Middle Ordovician brachiopod biofacies: Biostratigraphic, biogeographic, and macroevolutionary implications. Palaios 1995, 10, 154–179. [Google Scholar] [CrossRef]
- Powell, M.G.; Moore, B.R.; Smith, T.J. Origination, extinction, invasion, and extirpation components of the brachiopod latitudinal biodiversity gradient through the Phanerozoic Eon. Paleobiology 2015, 41, 330–341. [Google Scholar] [CrossRef]
- Rong, J.-Y.; Harper, D.A.T. Brachiopod survival and recovery from the latest Ordovician mass extinctions in South China. Geol. J. 1999, 34, 321–348. [Google Scholar] [CrossRef]
- Ruban, D.A. Do the available data permit clarifcation of the possible dependence of Palaeozoic brachiopod generic diversity dynamics on global sea-level changes? A viewpoint. Geologos 2014, 20, 215–221. [Google Scholar] [CrossRef]
- Shi, K.; Huang, B. Is there synchronicity between brachiopod diversity changes and palaeobiogeographical shifts across the Late Ordovician mass extinction? Palaeontology 2024, 67, e12730. [Google Scholar] [CrossRef]
- Vörös, A.; Szives, O. Role of Oceanic Anoxic Events in regulating the Jurassic–Early Cretaceous taxonomic diversity of Mediterranean brachiopods. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2025, 663, 112788. [Google Scholar] [CrossRef]
- Guo, Z.; Benton, M.J.; Stubbs, T.L.; Chen, Z.-Q. Morphological innovation did not drive diversification in Mesozoic–Cenozoic brachiopods. Nat. Ecol. Evol. 2024, 8, 1948–1958. [Google Scholar] [CrossRef]
- Afanasjeva, G.A. Diversity and Distribution of the Carboniferous Brachiopods of the Order Chonetida. Paleontol. J. 2022, 56, 487–495. [Google Scholar] [CrossRef]
- Corrêa, L.F.A.; Ramos, M.I.F. Relationships between brachiopod fauna (Lochkovian–Frasnian) from northwest Gondwana (Amazonas Basin) and environmental changes during the Devonian. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 629, 111803. [Google Scholar] [CrossRef]
- Congreve, C.R.; Patzkowsky, M.E.; Wagner, P.J. An early burst in brachiopod evolution corresponding with significant climatic shifts during the Great Ordovician Biodiversification Event. Proc. R. Soc. B Biol. Sci. 2021, 288, 20211450. [Google Scholar]
- Sterren, A.F.; Cisterna, G.A. Bivalves and brachiopods in the Carboniferous—Early Permian of Argentine Precordillera: Diversification and faunal turnover in Southwestern Gondwana. Geol. Acta 2010, 8, 501–517. [Google Scholar]
- Ye, F.; Bitner, M.A. Exploring the association between temperature and multiple ecomorphological traits of biocalcifiers (Brachiopoda). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2025, 667, 112883. [Google Scholar] [CrossRef]
- Foster, W.J.; Martindale, R.C.; Lehrmann, D.J.; Yu, M.; Ji, L. Persistent Environmental Stress Delayed the Recovery of Marine Communities in the Aftermath of the Latest Permian Mass Extinction. Paleoceanogr. Paleoclimatol. 2018, 33, 338–353. [Google Scholar] [CrossRef]
- Bitner, M.A.; Dulai, A.; Galácz, A. Middle Eocene brachiopods from the Szoc Limestone Formation (Bakony Mountains, Hungary), with a description of a new genus. Neues Jahrb. Geol. Palaontol. Abh. 2011, 259, 113–128. [Google Scholar] [CrossRef]
- Dulai, A.; Bitner, M.A.; Müller, P.Á.L. A monospecific assemblage of a new rhynchonellide brachiopod from the Paleocene of Austria. Foss. Strat. 2008, 54, 193–201. [Google Scholar]
- Dulai, A.; Von Der Hocht, F. Upper Oligocene brachiopods from NW Germany, with description of a new Platidiinae genus, Germanoplatidia n. gen. Riv. Ital. Paleontol. Stratigr. 2020, 126, 223–248. [Google Scholar]
- The Paleobiology Database. Available online: https://paleobiodb.org/ (accessed on 14 June 2025).
- Guenser, P.; Lefebvre, B.; El Hariri, K.; Jalil, N.-E. Historical bias in palaeontological collections: Stylophora (Echinodermata) as a case study. Swiss J. Palaeontol. 2025, 144, 6. [Google Scholar] [CrossRef]
- Raja, N.B.; Dunne, E.M.; Matiwane, A.; Khan, T.M.; Nätscher, P.S.; Ghilardi, A.M.; Chattopadhyay, D. Colonial history and global economics distort our understanding of deep-time biodiversity. Nat. Ecol. Evol. 2022, 6, 145–154. [Google Scholar] [CrossRef]
- Sepkoski, J.J., Jr. A compendium of fossil marine animal genera. Bull. Am. Paleontol. 2002, 363, 1–560. [Google Scholar]
- Gradstein, F.M.; Ogg, J.G.; Schmitz, M.D.; Ogg, G.M. (Eds.) Geologic Time Scale 2020; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- International Commission on Stratigraphy. Available online: https://stratigraphy.org/ (accessed on 16 June 2025).
- Aubry, M.-P.; Piller, W.E.; Van Couvering, J.A.; Berggren, W.A.; Flynn, J.J.; Head, M.J.; Hilgen, F.; Jun, T.; Kent, D.V.; Miller, K.G. Unifying Cenozoic chronostratigraphy and geochronology: Applying the rules. Newsl. Stratigr. 2024, 57, 25–36. [Google Scholar] [CrossRef]
- Haq, B.U.; Hardenbol, J.; Vail, P.R. Chronology of fluctuating sea levels since the Triassic. Science 1987, 235, 1156–1167. [Google Scholar] [CrossRef]
- Haq, B.U.; Al-Qahtani, A.M. Phanerozoic cycles of sea-level change on the Arabian platform. GeoArabia 2005, 10, 127–160. [Google Scholar] [CrossRef]
- Miller, K.G.; Kominz, M.A.; Browning, J.V.; Wright, J.D.; Mountain, G.S.; Katz, M.E.; Sugarman, P.J.; Cramer, B.S.; Christie-Blick, N.; Pekar, S.F. The Phanerozoic record of global sea-level change. Science 2005, 310, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Kominz, M.A.; Browning, J.V.; Miller, K.G.; Sugarman, P.J.; Mizintseva, S.; Scotese, C.R. Late Cretaceous to Miocene sea-level estimates from the New Jersey and Delaware coastal plain coreholes: An error analysis. Basin Res. 2008, 20, 211–226. [Google Scholar] [CrossRef]
- Scotese, C.R.; Song, H.; Mills, B.J.W.; van der Meer, D.G. Phanerozoic paleotemperatures: The earth’s changing climate during the last 540 million years. Earth-Sci. Rev. 2021, 215, 103503. [Google Scholar] [CrossRef]
- Westerhold, T.; Marwan, N.; Drury, A.J.; Liebrand, D.; Agnini, C.; Anagnostou, E.; Barnet, J.S.K.; Bohaty, S.M.; De Vleeschouwer, D.; Florindo, F.; et al. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 2020, 369, 1383–1388. [Google Scholar] [CrossRef]
- Hönisch, B.; Royer, D.L.; Breecker, D.O.; Polissar, P.J.; Bowen, G.J.; Henehan, M.J.; Cui, Y.; Steinthorsdottir, M.; McElwain, J.C.; Kohn, M.J.; et al. Toward a Cenozoic history of atmospheric CO2. Science 2023, 382, eadi5177. [Google Scholar]
- Zachos, J.; Pagani, H.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Young, A.; Flament, N.; Williams, S.E.; Merdith, A.; Cao, X.; Müller, R.D. Long-term Phanerozoic sea level change from solid Earth processes. Earth Planet. Sci. Lett. 2022, 584, 117451. [Google Scholar] [CrossRef]
- Grossman, E.L.; Joachimski, M.M. Ocean temperatures through the Phanerozoic reassessed. Sci. Rep. 2022, 12, 8938. [Google Scholar] [CrossRef]
- Prosorovskaya, E.L. Facies control of Jurassic brachiopods: Examples from Central Asia. In Proceedings of the Brachiopods—3d International Brachiopod Congress, Sudbery, ON, Canada, 2–5 September 1995; Copper, P., Jin, J., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1996; pp. 215–220. [Google Scholar]
- Bambach, R.K.; Knoll, A.H.; Wang, S.C. Origination, extinction, and mass depletions of marine diversity. Paleobiology 2004, 30, 522–542. [Google Scholar] [CrossRef]
- Edie, S.M.; Collins, K.S.; Jablonski, D. The end-Cretaceous mass extinction restructured functional diversity but failed to configure the modern marine biota. Sci. Adv. 2025, 11, eadv1171. [Google Scholar] [CrossRef]
- Schulte, P.; Alegret, L.; Arenillas, I.; Arz, J.A.; Barton, P.J.; Bown, P.R.; Bralower, T.J.; Christeson, G.L.; Claeys, P.; Cockell, C.S.; et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 2010, 327, 1214–1218. [Google Scholar] [CrossRef]
- Johansen, M.B. Background extinction and mass extinction of the brachiopods from the chalk of northwest Europe. Palaios 1989, 4, 243–250. [Google Scholar] [CrossRef]
- Surlyk, F.; Johansen, M.B. End-Cretaceous brachiopod extinctions in the Chalk of Denmark. Science 1984, 223, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tovar, F.J.; Lowery, C.M.; Gulick, S.P.S.; Bralower, T.J.; Jones, H.L. Rapid macrobenthic diversification and stabilization after the end-Cretaceous mass extinction event. Geology 2020, 48, 1048–1052. [Google Scholar] [CrossRef]
- Deprez, A.; Speijer, R.P.; Jehle, S.; Bornemann, A. Pronounced biotic and environmental change across the latest Danian warming event (LDE) at Shatsky Rise, Pacific Ocean (ODP Site 1210). Mar. Micropaleontol. 2017, 137, 31–45. [Google Scholar] [CrossRef]
- Miniati, F.; Monechi, S.; Cappelli, C. The Late Danian Event at Site 1209: A rapid diversification of calcareous nannofossils. Rend. Online Soc. Geol. Ital. 2014, 31, 149–150. [Google Scholar] [CrossRef]
- McInerney, F.A.; Wing, S.L. The Paleocene-Eocene thermal maximum: A perturbation of carbon cycle, climate, and biosphere with implications for the future. Annu. Rev. Earth Planet. Sci. 2011, 39, 489–516. [Google Scholar] [CrossRef]
- Piedrahita, V.A.; Heslop, D.; Roberts, A.P.; Rohling, E.J.; Galeotti, S.; Florindo, F.; Li, J. Assessing the Duration of the Paleocene-Eocene Thermal Maximum. Geophys. Res. Lett. 2025, 52, e2024GL113117. [Google Scholar] [CrossRef]
- Alegret, L.; Ortiz, S. Global extinction event in benthic foraminifera across the Paleocene/Eocene boundary at the Dababiya Stratotype section. Micropaleontology 2006, 52, 433–447. [Google Scholar] [CrossRef]
- Arcila, D.; Tyler, J.C. Mass extinction in tetraodontiform fishes linked to the Palaeocene-Eocene thermal maximum. Proc. R. Soc. B: Biol. Sci. 2017, 284, 20171771. [Google Scholar] [CrossRef]
- Keller, G.; Mateo, P.; Punekar, J.; Khozyem, H.; Gertsch, B.; Spangenberg, J.; Bitchong, A.M.; Adatte, T. Environmental changes during the Cretaceous-Paleogene mass extinction and Paleocene-Eocene Thermal Maximum: Implications for the Anthropocene. Gondwana Res. 2018, 56, 69–89. [Google Scholar] [CrossRef]
- Ortiz, N. Differential patterns of benthic foraminiferal extinctions near the Paleocene/Eocene boundary in the North Atlantic and the western Tethys. Mar. Micropaleontol. 1995, 26, 341–359. [Google Scholar] [CrossRef]
- Winguth, A.M.E.; Thomas, E.; Winguth, C. Global decline in ocean ventilation, oxygenation, and productivity during the Paleocene-Eocene Thermal Maximum: Implications for the benthic extinction. Geology 2012, 40, 263–266. [Google Scholar] [CrossRef]
- DeConto, R.M.; Pollard, D.; Wilson, P.A.; Pälike, H.; Lear, C.H.; Pagani, M. Thresholds for Cenozoic bipolar glaciation. Nature 2008, 455, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.J.P.; van Mourik, C.A.; Montanari, A.; Coccioni, R.; Brinkhuis, H. The Eocene-Oligocene transition: Changes in sea level, temperature or both? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 335–336, 75–83. [Google Scholar] [CrossRef]
- Tang, H.; Cui, H.; Li, S.-F.; Spicer, R.A.; Li, S.-H.; Su, T.; Zhou, Z.-K.; Witkowski, C.R.; Lauretano, V.; Wei, G.-J. Orbital-paced silicate weathering intensity and climate evolution across the Eocene-Oligocene transition in the southeastern margin of the Tibetan Plateau. Glob. Planet. Change 2024, 234, 104388. [Google Scholar] [CrossRef]
- Cotton, L.J.; Pearson, P.N. Extinction of larger benthic foraminifera at the Eocene/Oligocene boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 311, 281–296. [Google Scholar] [CrossRef]
- Hansen, T.A. Extinction of Late Eocene to Oligocene molluscs: Relationship to shelf area, temperature changes, and impact events. Palaios 1987, 2, 69–75. [Google Scholar] [CrossRef]
- Ivany, L.C.; Patterson, W.P.; Lohmann, K.C. Cooler winters as a possible cause of mass extinctions at the Eocene/Oligocene boundary. Nature 2000, 407, 887–890. [Google Scholar] [CrossRef]
- Keller, G. Stepwise mass extinctions and impact events: Late Eocene to early Oligocene. Mar. Micropaleontol. 1986, 10, 267–293. [Google Scholar] [CrossRef]
- Prothero, D.R. The late Eocene-Oligocene extinctions. Annu. Rev. Earth Planet. Sci. 1994, 22, 145–165. [Google Scholar] [CrossRef]
- Filippi, G.; Luciani, V. Planktic foraminiferal response to the Early Eocene Climatic Optimum (EECO) from southern mid-to-high latitudes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2025, 659, 112660. [Google Scholar] [CrossRef]
- Van Breedam, J.; Huybrechts, P.; Crucifix, M. Modelling evidence for late Eocene Antarctic glaciations. Earth Planet. Sci. Lett. 2022, 586, 117532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).