Abstract
The formation and development of mushrooms depend on suitable conditions of humidity, substrate, and temperature. These environmental factors are directly influenced by ongoing climate change, which may alter fungal growth patterns, distribution, and morphology. However, these attributes remain inadequately investigated in Antarctic mushrooms. In this study, we examined 334 basidiomes of Arrhenia antarctica, discovered on Livingston Island in 2023. The morphological characteristics of these basidiomes were analyzed to explore how recent variations may be linked to and explained by climatic changes. Comparing the original description from over 60 years ago with the recent literature on the subject, we observed a larger pileus diameter (47.3% of basidiomes with diameters exceeding 23 mm, up to 75 mm) than previously documented (reported as up to 23 mm). Additionally, there were changes in pileus morphology, with not all of them exhibiting an umbilicate form, contrary to the references. We propose that these morphological variations may be attributed to climatic changes. The basidiomata were also found in association with pure Antarctic grass banks, prompting the question of whether Arrhenia antarctica is indeed a moss parasite. The information presented in this study aims to support ongoing research on the taxonomy and diversity of Agaricales fungi in Antarctica.
1. Introduction
Antarctica, which covers approximately 14 million km2 south of 60°S, is predominantly ice-covered and is recognized as Earth’s most inhospitable continent [1,2]. Since the 1950s, the Antarctic Peninsula and the South Shetland Islands have been among the fastest-warming regions on the planet, with increasing trends in both temperature and precipitation [3,4,5,6,7]. These environmental changes have significant implications for Antarctic biodiversity, influencing the survival and distribution of organisms adapted to extreme conditions.
Analyzing biological diversity in such inhospitable environments yields crucial insights into the adaptation and survival strategies of organisms under extreme conditions. Research centered on Antarctic organisms plays a pivotal role in shaping evidence-based conservation strategies, thereby contributing to biodiversity protection at both local and global scales [8,9]. For instance, biodiversity surveys have contributed to the designation of Antarctic Specially Protected Areas (ASPAs), such as Byers Peninsula (ASPA No. 126), which is recognized for its rich biological diversity and unique terrestrial and freshwater ecosystems [10]. Moreover, microbial communities, including fungi, have been increasingly acknowledged in conservation efforts; specific ASPAs, such as Linneaus Terrace (ASPA No. 138), are designated to protect these unique microbial habitats [11].
Fungi are notable for their resilience in extreme environments [12]. However, most research has focused on isolating fungi from environmental substrates, whereas studies on the diversity, distribution, and ecological roles of macroscopic, mushroom-forming fungi in Antarctica remain scarce [13,14]. Additional research is required to analyze the morphological variation and distribution of species, aiming to comprehend their plasticity and adaptation to extreme conditions.
Mushroom development is typically influenced by substrate availability and optimal temperature and humidity conditions [15]. In Antarctica, suitable organic substrates are largely restricted to moss fields, lichens, and two native angiosperms. Among these, bryophytes and Antarctic hair grass (Deschampsia antarctica É. Desv.) are recognized as the primary hosts for most known mushroom-forming fungi [1,16], providing essential structural and nutritional support for fungal growth. A representative example of this ecological association is the species Arrhenia antarctica (Singer) Redhead, Lutzoni, Moncalvo, and Vilgalys, wich is frequently found growing on bryophyte mats. Notably, A. antarctica holds both historical and taxonomic significance as the first native macromycete formally described from maritime Antarctica. Originally classified as Omphalina antarctica Sing., the species was later reassigned to the genus Arrhenia based on studies that supported its reclassification [17,18].
Among all of the mushroom-forming fungi recorded in Antarctica, A. antarctica is the most commonly reported species, with the widest documented distribution across both maritime and continental Antarctica. In the South Shetland Islands, the species is reported to be present on King George (Collins Bay; Admiralty Bay; Barton Peninsula), Robert (Coppermine Peninsula), Deception (Punta La Descubierta; Whalers Bay), Elephant, and Livingston Islands (Byers Peninsula; South Bay region) [16,19,20,21,22]. Considering the extensive distribution of A. antarctica in Antarctica, conducting studies to assess the bionomy of this fungus is essential for elucidating how climatic phenomena may be influencing its distribution.
Despite the abundance of documented macroscopic fungi in Antarctica, morphological variation within individual species remains unexamined. Understanding phenotypic plasticity and cold-adaptation mechanisms is crucial for comparing populations and correlating them with environmental influences, including those driven by climate change [23]. While collecting macrofungi in the South Shetland Islands, we identified a substantial population of A. antarctica on Byers Peninsula, Livingston Island. A detailed study revealed previously unreported variations in basidiome morphology, prompting a reevaluation of the traits considered typical for this species.
This study provides a comprehensive morphological reassessment of A. antarctica, an endemic Antarctic fungus. Although it is widely distributed across maritime and continental Antarctica, existing descriptions of its morphology are incomplete or outdated. We address two questions: How does a detailed morphological reassessment refine our understanding of A. antarctica? And what taxonomic or ecological implications arise from this updated characterization? Our precise description addresses a critical knowledge gap regarding Antarctic fungi. This characterization provides a reference for future taxonomic revisions, biodiversity assessments, and ecological studies in extreme environments, advancing understanding of fungal diversity and ecosystem roles in Antarctica.
2. Materials and Methods
The fieldwork was conducted during the austral summer of 2022/2023 as part of the XLI Brazilian Antarctic Operation in Byers Peninsula, Livingston Island, South Shetland Archipelago, Antarctica (62°38′19.90″ S and 61°08′19.48″ W) (Figure 1). The study focused on various moss formations located on each side of Laager Point (Figure 1).
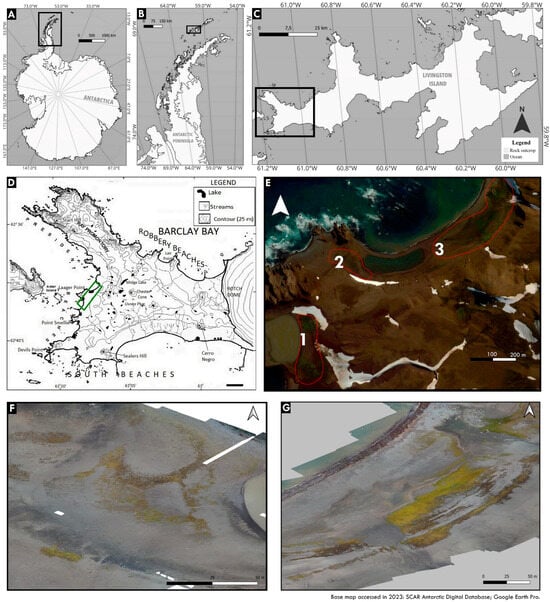
Figure 1.
(A) Antarctica. (B) Antarctic Peninsula. (C) Livingston Island. (D) Byers Peninsula, featuring the sampled area (green rectangle). (E) Laager Point, indicating the studied moss fields (red outline). (F) Detailed images of sampling area two. (G) Detailed images of sampling area three. Data from the Google Earth Pro and SCAR Antarctic Digital Database, 2023.
Random free walks [15] were conducted in moss fields across the Byers Peninsula, specifically targeting the basidiomes of A. antarctica. The sampling method involved searching for individual basidiomes rather than following transects or standardized plot areas, a decision made to maximize coverage and flexibility in a heterogeneous terrain. While this approach was effective in capturing a broad range of morphological variability, it may limit the ability to make precise quantitative inferences about the abundance or density of A. antarctica basidiomes.
Encountered specimens were photographed (Xiaomi, Redmi Note 10, Beijing, China; Apple, iPhone X, Cupertino, CA, USA), and their morphological characteristics were measured using calipers. Samples were then collected for further analysis at a field camp laboratory, with selection criteria prioritizing individuals representing the observed morphological diversity. This included specimens at different developmental stages or exhibiting distinct morphological traits. Additionally, to minimize environmental impact and preserve the fungal population in this protected area, only a subset of the encountered basidiomes was collected.
The study site was revisited after 30 days to assess whether new basidiomes had emerged and to verify the persistence of previously recorded individuals. The surrounding vegetation was identified on-site, with small samples taken to the laboratory for more detailed identification. Photographs taken in the field supported the morphological identification process, and GPS coordinates were recorded for each collection site.
Morphological variations were identified using definitions from Putzke and Putzke [15], and comparisons were made with the original description by Singer [17], as well as descriptions from other authors [16,22] to establish variations. Diameter classes were set with an interval of 5 mm, starting from 15 mm. After analysis, the samples were transported to Brazil at −20 °C, dehydrated in an oven at 40 °C in the Laboratório de Taxonomia de Fungos at the Universidade Federal do Pampa, and deposited in the Bruno Edgar Irgang Herbarium (HBEI).
Morphological traits and color assessments were primarily performed in the field under natural light conditions to ensure accurate documentation of fresh basidiome characteristics. Additionally, to improve measurement precision and enable subsequent verification, digital photographs of the specimens were analyzed using ImageJ software (https://imagej.net/ij/ accessed one 14 July 2025), particularly for determining pileus diameter. This dual approach allowed for both in situ evaluation and post-fieldwork validation of key morphological parameters, thereby enhancing the robustness and reproducibility of the dataset. For data tabulation, percentage calculation, graph generation, and image design, R 4.3.2, Microsoft Excel 16.0, and PhotoFiltre Studio X 10.12.1 were employed.
3. Results and Discussion
During the collection period, a total of 334 basidiomes of A. antarctica were discovered in Laager Point, Byers Peninsula, Livingston Island, South Shetland Archipelago, Antarctica. These were classified into 11 size classes and five pileus forms (Figure 2A–D). This represents the highest number of basidiomata documented in a single published Antarctic season. The recent new occurrence records reported in Antarctica may suggest an increase in sexual reproduction [16], and the substantial number of basidiomata found in this study could further support this hypothesis.
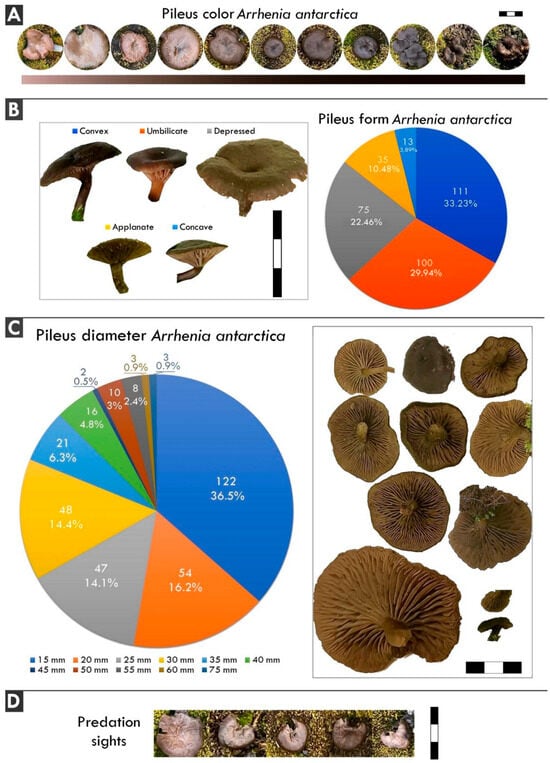
Figure 2.
Morphological characteristics of Arrhenia antarctica. (A) Pileus color. (B) Pileus form. (C) Pileus diameter. (D) Predation sights in A. antarctica. Scale: 3 cm.
Recent studies have unveiled that macroscopic fungi successfully colonized Antarctica during the Pleistocene period [24]. However, records of macroscopic fungi occurrences in Antarctica remain scarce, encompassing only a limited number of species and individuals [14,16,19,21,22,24]. In this context, the abundance of basidiomata observed in this study is particularly significant. Although long-term quantitative data on macroscopic fungal diversity in Antarctica remain limited, experienced Antarctic researchers have reported that such a high density of macroscopic fungi is unprecedented in their field observations. Notably, one of the authors of this study, with over 20 years of research experience in Antarctica, has never encountered such a substantial occurrence of basidiomata.
While our observations indicate an unusually high abundance of A. antarctica in the study area, we acknowledge that establishing a direct link between climate change and fungal proliferation requires long-term monitoring and broader geographic sampling. The logistical constraints of Antarctic expeditions often limit the temporal and spatial scope of fungal surveys, making historical comparisons challenging. However, our findings underscore the need for further systematic studies to assess whether recent environmental changes are influencing macroscopic fungal communities in Antarctica.
It is worth noting that the warming of polar regions may contribute to increased activity among fungal communities involved in decomposition, nutrient cycling, and parasitism of other organisms. This further highlights the complex interplay between climate change and fungal dynamics in these unique habitats [16]. Furthermore, global warming accelerates the melting of glaciers and permafrost, resulting in the release of vast numbers of dormant microorganisms—including potential human pathogens—into polar and subpolar ecosystems. This microbial resurgence not only poses risks to human and animal health but also has significant implications for microbial and fungal diversity in these rapidly changing environments [25].
Polar ecosystems demonstrate a high capacity to respond to global climate change [26]. It is estimated that the diversity of the Antarctic mycobiota will increase by 20–27%, according to predictions of the impacts of climate change [27]. In this context, assessing the occurrence and elevated morphological development of organisms rarely described in the area can serve as a foundation for evaluating the impact of warming on the biodiversity of the Antarctic continent and contribute to future studies monitoring communities and morphological variability. Therefore, an emendation to the description is needed.
Arrhenia antarctica (Singer) Redhead, Lutzoni, Moncalvo and Vilgalys, Mycotaxon 83: 46 (2002).
Basionym: Omphalina antarctica Singer, Beih. Sydowia 1: 16 (1957) [1956].
Pileus deep fuscous brown to dark fuscous or fuliginous, becoming paler with age, smooth or with short sulculate or short–striate margin, eventually sometimes somewhat uneven but never finely grooved–streaked or squamulose, neither velutinous nor pruinate when young, convex–umbilicate, then almost flat and umbilicate or convex-to-applanate, depressed-to-concave, with a greasy shine when fresh, (15)17–35(75) mm in diameter.
3.1. Pileus Color
The color of Arrhenia antarctica is described as ‘brown when fresh … and becoming paler’ [17], or completely fuliginous [22] to dark gray–brown with a greasy shine when fresh [16]. Our observations revealed colors ranging from fuliginous to completely black (Figure 2A), with older specimens appearing paler, consistent with the original description [17]. The greasy shine when fresh was noted as very frequent. Mushroom assemblages in colder areas, based on the evaluation of European mushrooms, tend to be significantly darker. However, colors play a more complex ecological role, including functions beyond thermal adaptation [28].
The soil microbial community in Antarctica is predominantly composed of fungi with dark septate hyphae [29]. The melanins present in their structures may play a protective role against extreme temperatures, contributing to their survival in cold environments [30,31]. The blackish color of A. antarctica may regulate mushroom temperature, facilitating growth and development. Additionally, a variety of colors were observed in relation to the vegetation associated with the occurrence of A. antarctica. The mushroom specimens in moss fields exhibited a fuliginous coloration, while those in moss fields dominated by Deschampsia antarctica É. Desv. presented a completely black color. Although studies have investigated the impact of various factors on fungal color variation [32], this study represents the first investigation of color diversity in a mushroom species influenced by associated vegetation in Antarctica.
3.2. Pileus Form
The most common pileus form was convex (111), followed by umbilicate (100), depressed (75), applanate (35), and concave (13) (Figure 2B). In the original description, the form is referred to as convex–umbilicate, then almost flat and umbilicate [17]. In a recent paper, the form was described as plane–convex with a depressed center or umbilicate [16]. The umbilicate pileus was found in our survey, but almost half of the mushrooms (46.7%) were not umbilicate, including another 22.4% identified as only depressed (total = 60.1%) (Figure 2B). This observation likely represents an addition to the forms expressed by A. antarctica.
3.3. Pileus Diameter
The pileus diameter of the mushrooms ranged from 15 mm to 75 mm, with a mean of 25.0 mm, a median of 20.0 mm, and a standard deviation of 11.74 mm. The distribution across the various diameter classes was as follows: 15 mm (122 mushrooms—36.5%), 20 mm (54 mushrooms—16.2%), 25 mm (47 mushrooms—14.1%), 30 mm (48 mushrooms—14.4%), 35 mm (21 mushrooms—6.3%), 40 mm (16 mushrooms—4.8%), 45 mm (two mushrooms—0.6%), 50 mm (10 mushrooms—3.0%), 55 mm (eight mushrooms—2.4%), 60 mm (three mushrooms—0.9%), and 75 mm (three mushrooms—0.9%) (Figure 2C).
Previous reports documented the species with a maximum diameter of up to 20 mm [22], or up to 30 mm [16], despite the original description by Singer citing a range of 17–23 mm [17]. Our findings indicate that 47.3% of the basidiomes exceeded the originally described maximum of 23 mm, suggesting that the species may reach larger diameters or may have done so in recent years. Notably, other studies have reported basidiomata up to 30 mm in diameter [16]
The current variation in size may be influenced by higher temperatures and/or increased rainfall. The decline in rainfall has been linked to a reduction in mushroom occurrence in natural environments, with precipitation playing a crucial role in the dynamics of mushroom populations in forest ecosystems [33]. While these authors did not confirm the impact of temperature on the number of wild mushrooms foraged, precipitation is consistently cited as the most significant factor. Precipitation in Antarctica is generally challenging to measure due to its solid state and the area’s high wind speeds, leading to imprecise automatic records [7]. However, recent studies suggest an increasing trend in Antarctic precipitation [5], which may contribute to the observed augmentation in mushroom diameter, although precise measurements for our study site remain scarce.
Studies evaluating the size of basidiomes have not yet been conducted in nature, particularly in the Antarctic continent. However, research has been carried out in controlled environments with edible mushrooms, revealing morphological differences depending on the substrates used for cultivation [34,35]. These data indicate that the nutritional composition of the substrate can significantly influence fungal morphology, emphasizing the need for future soil analyses in studies. Soil analyses can determine whether the nutritional composition of Antarctic soils influences and relates to the development and morphological aspects of macroscopic fungi.
3.4. Possible Signs of Predation
Approximately 56 mushrooms (16.8%) exhibited missing parts of the pileus, which could indicate the potential predation of mushrooms by birds in Antarctica (Figure 2D). This phenomenon requires further investigation, as there are no known reports of birds feeding on mushrooms in the Antarctic. Mycophagy is documented in other continents, and in Antarctica, it could potentially provide additional nutrients to regional birds, as observed in other regions [36,37,38,39]. Additionally, Elliot and Marshall [40] highlight that fungi can represent a seasonally significant portion of the avian diet, as they may occur during periods of limited food availability, as reported by Andreev [41], who found that Siberian Jays (Perisoreus infaustus, Linnaeus, 1758) relied significantly on the nutritional value provided by fungi during the cold winters of northern Eurasia.
Given the constrained food supply for birds resulting from the inhospitable conditions in Antarctica, the potential signs of predation observed on A. antarctica specimens in this study may suggest previously unreported bird–fungus mycophagic interactions specific to the region. Although the evidence remains inconclusive, the apparently low rate of predation may be ecologically significant. In polar environments, where potential consumers are scarce, reduced mycophagy may allow basidiomata to persist longer in the field, thereby increasing the window for effective spore release and dispersal. This suggests that the stability and visibility of A. antarctica basidiomata may be influenced not only by climatic conditions but also by the absence of fungal predators. Such factors could be critical for the reproductive success of this species in extreme habitats. Nevertheless, these interpretations remain preliminary. Additional field observations, dietary studies, and molecular analyses are needed to confirm whether mycophagy occurs in Antarctic ecosystems. To date, this ecological interaction remains virtually unexplored in the region.
3.5. Grass/Mosses/Lichens Associated to the Basidiomata
Although mycelium can be widespread in the substrate, the mosses surrounding the basidiomata are not necessarily the substrate used. However, the mosses surrounding the mushroom can provide the humidity needed for its development and maintenance. Therefore, assessing the immediate 10 cm of moss/lichen formation around the basidiome can provide insights into the influence of plant communities on mushroom development. In our assessment of 226 basiodiomes, we identified seven primary plant formations (Table 1). Moss fields solely composed of Sanionia uncinata (Hedw.) Loeske stood out as the most abundant in the A. antarctica basidiomes, with 93 basidiomata, representing 40.9% of the total analyzed (Table 1).

Table 1.
Distribution of Arrhenia antarctica basidiomes among different plant formations.
The species is typically reported to grow on the moss Sanionia uncinata. However, Palfner et al. [16] also documented its occurrence on Bryum sp. and, notably, on the Antarctic hairgrass Deschampsia antarctica. The present study confirmed these associations, with three specimens found exclusively within a D. antarctica formation and 9.2% associated with both grass and mosses (Table 1). These findings suggest that Arrhenia antarctica may interact with a broader range of substrates beyond mosses alone. Additionally, the species was frequently observed among S. uncinata (40.9%) and in mixed patches of S. uncinata and Syntrichia spp. (26.9%) (Table 1), reinforcing its strong affinity for bryophyte-dominated environments.
A particularly novel finding was the association of A. antarctica with patches containing the lichen Leptogium puberulum Hue, observed in 18.5% of the specimens. Although Leptogium is not considered a primary substrate, its frequent co-occurrence may indicate the ability of A. antarctica to colonize complex microhabitats where bryophytes and lichens coexist, potentially benefiting from the microclimatic and structural conditions they provide. Interestingly, L. puberulum has been observed in direct association with D. antarctica, adhering to its roots and leaves, suggesting a dynamic and influential role in vascular plant–lichen interactions in Antarctic ecosystems [42]. This observation broadens our understanding of the species’ ecological plasticity and implies that its substrate preferences may be more flexible than previously assumed, extending to composite cryptogamic mats composed of mosses, lichens, and vascular plants.
These findings indicate that A. antarctica exhibits broader ecological plasticity than previously recognized, occurring in association with diverse bryophyte and lichen-dominated substrates. While the species has been primarily reported in moss-dominated environments, its occurrence on D. antarctica and in mixed formations suggests that its ecological role may extend beyond strict bryophyte dependency. Moreover, the widespread presence of basidiomata within moss carpets, without visible signs of degradation, raises critical questions about its trophic mode.
Despite the absence of detailed ecological studies on Arrhenia in Antarctica, research elsewhere indicates diverse trophic strategies within the genus. Recent phylogenetic analyses [43] categorize Arrhenia sensu lato into ecologically distinct clades, including obligate sphagnophiles, bryotrophs, and potentially biotrophic or lignicolous species [43]. This diversity is reflected in specific interactions: some species are obligate bryophiles (e.g., A. retiruga), others form associations with lichenized fungi (e.g., A. peltigerina), while members of the acerosa complex exhibit broader substrate preferences [43,44,45]. Furthermore, parasitic behavior, documented in species like A. bryophthora and A. peltigerina, demonstrates that Arrhenia’s ecological roles encompass a continuum from saprotrophy and mutualism to parasitism [43,44,45,46].
Given this context and the unresolved nature of its trophic strategy in Antarctic ecosystems, further investigations are warranted. Specifically, molecular and anatomical analyses of potential root or rhizoid associations in A. antarctica could clarify whether this species engages in facultative mutualistic interactions or primarily utilizes available organic matter as a saprotroph. This study thus highlights the need for targeted research to determine the functional ecology of A. antarctica within its unique habitat.
4. Conclusions
The data presented in this study contribute novel insights to our understanding of Agaricales fungi in Antarctica. We offer new morphological descriptions for A. antarctica and provide novel information regarding its associations with the vegetation of the continent. These findings are intended to facilitate the identification of future collections and support the research efforts of other scientists in the field. Furthermore, by examining fragments of collected and observed mushrooms, this study explores the potential mycophagic relationship between birds and fungi, a topic that has not been previously discussed in the context of the Antarctic continent.
Furthermore, the morphological diversity observed in A. antarctica, documented for the first time in this study, emphasizes the necessity for heightened attention to the bionomics and distribution of fungi on the Antarctic continent—a region that had not been previously investigated. The diverse array of structural forms and size variations exhibited by this species exemplifies its adaptability and diversity in inhospitable survival conditions. Therefore, the information provided herein is intended to contribute to the study of the taxonomy and diversity of Agaricales fungi in Antarctica, while also encouraging research efforts related to the impact of climate change on the diversity of these organisms.
Author Contributions
Conceptualization, F.A.B.-S., J.P., M.T.L.P. and C.E.G.R.S.; methodology, F.A.B.-S. and J.P.; investigation, F.A.B.-S. and J.P.; resources, J.P. and C.E.G.R.S.; data curation, F.A.B.-S. and J.P.; writing—original draft preparation, F.A.B.-S. and J.P.; writing—review and editing, F.A.B.-S., J.P., J.L.M., M.T.L.P. and C.E.G.R.S.; supervision, J.P.; project administration, J.P. and C.E.G.R.S.; funding acquisition, J.P. and C.E.G.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; grant number 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant number 442703/2018-0).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
The Marinha do Brasil for its pivotal role in providing vital logistical support for the fieldwork carried out during OPERANTAR XLI (41st Brazilian Antarctic Operation). The Brazilian Antarctic Program (PROANTAR) for its support. To Conselho Nacional de Pesquisa for financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ochyra, R.; Lewis-Smith, R.I.; Bednarek-Ochyra, H. The Illustrated Moss Flora of Antarctica, 1st ed.; Cambridge University Press: Cambridge, UK, 2008; p. 704. [Google Scholar]
- Black, M.; Sacks, B.J.; Dortmans, P.; Yeung, J.; Savitz, S.; Stephenson, S.R.; Tingstad, A.; Pezard, S.; Jouan, N.; Black, J. Antarctica at Risk: Geostrategic Manoeuvring and the Future of the Antarctic Treaty System; RAND Corporation: Santa Monica, CA, USA, 2023; p. 88. [Google Scholar]
- Carrasco, J.F.; Bozkurt, D.; Cordero, R.R. A review of the observed air temperature in the Antarctic Peninsula. Did the warming trend come back after the early 21st hiatus? Polar Sci. 2021, 28, 100653. [Google Scholar] [CrossRef]
- Kerr, R.; Mata, M.M.; Mendes, C.R.B.; Secchi, E.R. Northern Antarctic Peninsula: A marine climate hotspot of rapid changes on ecosystems and ocean dynamics. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 149, 4–9. [Google Scholar] [CrossRef]
- Vignon, É.; Roussel, M.L.; Gorodetskaya, I.V.; Genthon, C.; Berne, A. Present and future of rainfall in Antarctica. Geophys. Res. Lett. 2021, 48, e2020GL092281. [Google Scholar] [CrossRef]
- Carrasco, J.F.; Cordero, R.R. Analyzing precipitation changes in the northern tip of the Antarctic Peninsula during the 1970–2019 period. Atmosphere 2020, 11, 1270. [Google Scholar] [CrossRef]
- Bañón, M.; Justel, A.; Velázquez, D.; Quesada, A. Regional weather survey on Byers Peninsula, Livingston Island, South Shetland Islands, Antarctica. Antarct. Sci. 2013, 25, 146–156. [Google Scholar] [CrossRef]
- Convey, P.; Chown, S.L.; Clarke, A.; Barnes, D.K.A.; Bokhorst, S.; Cummings, V.; Ducklow, H.W.; Frati, F.; Green, T.G.A.; Gordon, S.; et al. The spatial structure of Antarctic biodiversity. Ecol. Monogr. 2014, 84, 203–244. [Google Scholar] [CrossRef]
- Kennicutt, M.C.; Chown, S.L.; Cassano, J.J.; Liggett, D.; Massom, R.; Peck, L.S.; Rintoul, S.R.; Storey, J.W.V.; Vaughan, D.G.; Wilson, T.J.; et al. Polar research: Six priorities for Antarctic science. Nature 2014, 512, 23–25. [Google Scholar] [CrossRef]
- Benayas, J.; Pertierra, L.; Tejedo, P.; Lara, F.; Bermudez, O.; Hughes, K.A.; Quesada, A. A review of scientific research trends within ASPA No. 126 Byers Peninsula, South Shetland Islands, Antarctica. Antarct. Sci. 2013, 25, 128–145. [Google Scholar] [CrossRef]
- Hughes, K.A.; Cowan, D.A.; Wilmotte, A. Protection of Antarctic microbial communities—‘Out of sight, out of mind’. Front. Microbiol. 2015, 6, 151. [Google Scholar] [CrossRef]
- Coleine, C.; Stajich, J.E.; Selbmann, L. Fungi are key players in extreme ecosystems. Trends Ecol. Evol. 2022, 37, 517–528. [Google Scholar] [CrossRef]
- Tsuji, M. Survey on fungi in Antarctica and High Arctic regions, and their impact on climate change. Climate 2023, 11, 195. [Google Scholar] [CrossRef]
- Bertazzo-Silva, F.A.; Putzke, J.; Furlan-Lopes, C.; D’Ávila, M.F.; Costa, A.L.; Carvalho, E.L.; Zorzi, A.F.; Schaefer, C.E.G.R. Expanding geographic distribution knowledge of Galerina marginata (Batsch) Kühner (Agaricales, Hymenogastraceae) with a novel Antarctic record. Biodivers. Data J. 2024, 12, e125727. [Google Scholar] [CrossRef] [PubMed]
- Putzke, J.; Putzke, M. Cogumelos-Fungos Agaricales no Brasil. Famílias Agaricaceae, Amanitaceae, Bolbitaceae, Entolomataceae, Coprinaceae/Psathyrellaceae, Crepidotaceae e Hygrophoraceae, 1st ed.; LupaGraf: Santa Cruz do Sul, Brazil, 2018; p. 518. [Google Scholar]
- Palfner, G.; Binimelis-Salazar, J.; Alarcón, S.T.; Torres-Mellado, G.; Gallegos, G.; Peña-Cortés, F.; Casanova-Katny, A. Do new records of macrofungi indicate warming of their habitats in terrestrial Antarctic ecosystems? Czech Polar Rep. 2020, 10, 281–296. [Google Scholar] [CrossRef]
- Singer, R. A fungus collected in the Antarctic. Sydowia-Beih. 1956, 1, 16–23. [Google Scholar]
- Redhead, S.A. Phylogeny of agarics: Partial systematics solutions for core omphalinoid genera in the Agaricales (euagarics). Mycotaxon 2002, 83, 19–57. [Google Scholar]
- Pegler, D.N.; Spooner, B.M.; Smith, R.I.L. Higher fungi of Antarctica, the Subantarctic zone and Falkland Islands. Kew Bull. 1980, 35, 499–562. [Google Scholar] [CrossRef]
- Horak, E. Agaricales in Antarctica and Subantarctica: Distribution, ecology, and taxonomy. In Arctic and Alpine Mycology; Laursen, G.A., Ammirati, J.F., Eds.; University of Washington Press: Seattle, WA, USA, 1982; pp. 82–118. [Google Scholar]
- Putzke, J.; Pereira, A.B. Macroscopic fungi from the South Shetland Islands, Antarctica. Ser. Cient. INACH 1996, 46, 31–39. [Google Scholar]
- Gumińska, B.; Heinrich, Z.; Olech, M. Macromycetes of the South Shetland Islands (Antarctica). Pol. Polar Res. 1994, 15, 103–109. [Google Scholar]
- Gutt, J.; Isla, E.; Xavier, J.C.; Adams, B.J.; Ahn, I.-Y.; Cheng, C.-H.C.; Colesie, C.; Cummings, V.J.; Di Prisco, G.; Griffiths, H.; et al. Antarctic ecosystems in transition—Life between stresses and opportunities. Biol. Rev. 2021, 96, 798–821. [Google Scholar] [CrossRef]
- Garrido-Benavent, I.; Blanchette, R.A.; de los Ríos, A. Deadly mushrooms of the genus Galerina found in Antarctica colonized the continent as early as the Pleistocene. Antarct. Sci. 2023, 35, 345–358. [Google Scholar] [CrossRef]
- Yarzábal, L.A.; Salazar, L.M.B.; Batista-García, R.A. Climate change, melting cryosphere and frozen pathogens: Should we worry…? Environ. Sustain. 2021, 4, 489–501. [Google Scholar] [CrossRef]
- Rosa, L.H.; Zani, C.L.; Cantrell, C.L.; Duke, S.O.; Van Dijck, P.; Desideri, A.; Rosa, C.A. Fungi in Antarctica: Diversity, ecology, effects of climate change, and bioprospection for bioactive compounds. In Fungi of Antarctica; Rosa, L.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 1–17. [Google Scholar]
- Newsham, K.K.; Garnett, M.H.; Robinson, C.H.; Cox, F. Discrete taxa of saprotrophic fungi respire different ages of carbon from Antarctic soils. Sci. Rep. 2018, 8, 7866. [Google Scholar] [CrossRef] [PubMed]
- Krah, F.S.; Büntgen, U.; Schaefer, H.; Müller, J.; Andrew, C.; Boddy, L.; Diez, J.; Egli, S.; Freckleton, R.; Gange, A.C.; et al. European mushroom assemblages are darker in cold climates. Nat. Commun. 2019, 10, 2890. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 2nd ed.; Academic Press Ltd.: London, UK, 1997; p. 605. [Google Scholar]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.H. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001, 151, 341–353. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X. Changes in color, antioxidant, and free radical scavenging enzyme activity of mushrooms under high oxygen modified atmospheres. Postharvest Biol. Technol. 2012, 69, 1–6. [Google Scholar] [CrossRef]
- Procházka, P.; Jana, S.; Karel, T.; Mullen, K.J.; Čabelková, I. Climatic factors affecting wild mushroom foraging in Central Europe. Forests 2023, 14, 382. [Google Scholar] [CrossRef]
- Royse, D.J.; Rhodes, T.W.; Ohga, S.; Sanchez, J.E. Yield, mushroom size and time to production of Pleurotus cornucopiae (oyster mushroom) grown on switch grass substrate spawned and supplemented at various rates. Bioresour. Technol. 2004, 91, 85–91. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Li, M.; Li, X.; Sun, Z. Yield, size, nutritional value, and antioxidant activity of oyster mushrooms grown on perilla stalks. Saudi J. Biol. Sci. 2017, 24, 347–354. [Google Scholar] [CrossRef]
- Elliott, T.F.; Jusino, M.A.; Trappe, J.M.; Lepp, H.; Ballard, G.A.; Bruhl, J.J.; Vernes, K. A global review of the ecological significance of symbiotic associations between birds and fungi. Fungal Divers. 2019, 98, 161–194. [Google Scholar] [CrossRef]
- Costa, A.L.; Lopes, C.F.; Heberle, M.A.; Putzke, J. The bird shiny cowbirds (Molothrus bonariensis) in a relationship interesting of mycophagy with the mushroom Macrolepiota bonaerensis in the Brazilian pampa biome. Stud. Multidiscip. Rev. 2022, 3, 153–167. [Google Scholar] [CrossRef]
- Costa, A.L.; Mendes, M.F.; Furlan-Lopes, C.; Bertazzo-Silva, F.A.; Köhler, A.; Putzke, J. First report of Zygothrica candens Burla, 1956 (Diptera, Drosophilidae) in mycophagic association with the mushroom Oudemansiella cubensis (Berk. and M.A. Curtis) R.H. Petersen, 2010 (Agaricales, Physalacriaceae) in southern Brazil. Braz. J. Biol. 2023, 82, e267871. [Google Scholar] [CrossRef]
- Santamaria, B.; Verbeken, A.; Haelewaters, D. Mycophagy: A global review of interactions between invertebrates and fungi. J. Fungi 2023, 9, 163. [Google Scholar] [CrossRef]
- Elliot, T.F.; Marshall, P.A. Animal-fungal interactions 1: Notes on bowerbird’s use of fungi. Aust. Zool. 2016, 38, 59–61. [Google Scholar] [CrossRef]
- Andreev, A.V. Winter energy balance and hypothermia of the Siberian jay. Sov. J. Ecol. 1978, 9, 352–357. [Google Scholar]
- Bertazzo-Silva, F.A.; Putzke, J.; Pereira, A.B.; Furlan-Lopes, C.; Costa, A.L.; Ferraz, K.R.; Klotz-Neves, A.L.; Schaefer, C.E.G.R. Novel insights into the association between Leptogium puberulum (Ascomycota) and Deschampsia antarctica (Poaceae): Implications for plant–lichen dynamics in Antarctica. Polar Biol. 2025, 48, 54. [Google Scholar] [CrossRef]
- Karich, A.; Jarling, R.; Ullrich, R.; Demski, D.; Bubner, B.; Hofrichter, M. Two new Agaricomycetes related to post-fire mosses. Mycol. Prog. 2024, 23, 28. [Google Scholar] [CrossRef]
- Voitk, A.; Saar, I.; Lücking, R.; Moreau, P.-A.; Corriol, G.; Krisai-Greilhuber, I.; Thorn, R.G.; Hay, C.R.J.; Moncada, B.; Gulden, G. Surprising morphological, ecological and ITS sequence diversity in the Arrhenia acerosa complex (Basidiomycota: Agaricales: Hygrophoraceae). Sydowia 2020, 73, 133–162. [Google Scholar]
- Voitk, A.; Saar, I.; Moncada, B.; Lickey, E.B. Circumscription and typification of sphagnicolous omphalinoid species of Arrhenia (Hygrophoraceae) in Newfoundland and Labrador: Three obligate and one facultative species. Mycol. Prog. 2022, 21, 57. [Google Scholar] [CrossRef]
- Diederich, P.; Millanes, A.M.; Wedin, M.; Lawrey, J.D. Flora of Lichenicolous Fungi, Vol. 1; National Museum of Natural History: Luxembourg, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).