Abstract
Understanding the biodiversity of aquatic communities and the underlying mechanisms that shape biodiversity patterns and community dynamics is crucial for the effective conservation and management of freshwater ecosystems. However, traditional survey methods often fail to comprehensively capture species diversity, particularly for low-abundance taxa. Moreover, studies integrating both metazoan and fish communities at fine spatial scales remain limited. To address these gaps, we employed a multi-marker eDNA metabarcoding approach, targeting both the 12S and 18S rRNA gene regions, to comprehensively investigate the composition of metazoan and fish communities in the Yujiang River. A total of 12 metazoan orders were detected, encompassing 15 families, 21 genera, and 19 species. For the fish community, 32 species were identified, belonging to 25 genera, 10 families, and 7 orders. Among these, Adula falcatoides and Coptodon zillii were identified as the most prevalent and abundant metazoan and fish species, respectively. Notably, the most prevalent fish species, C. zillii and Oreochromis niloticus, are both recognized as invasive species. The Bray–Curtis distance of metazoa (average: 0.464) was significantly lower than that of fish communities (average: 0.797), suggesting higher community heterogeneity among fish assemblages. Beta-diversity decomposition indicated that variations in the metazoan and fish communities were predominantly driven by species replacement (turnover) (65.4% and 70.9% for metazoa and fish, respectively) rather than nestedness. Mantel tests further revealed that species turnover in metazoan communities was most strongly influenced by water temperature, while fish community turnover was primarily affected by water transparency, likely reflecting the physiological sensitivity of metazoans to thermal gradients and the dependence of fish on visual cues for foraging and habitat selection. In addition, a co-occurrence network of metazoan and fish species was constructed, highlighting potential predator-prey interactions between native species and Corbicula fluminea, which emerged as a potential keystone species. Overall, this study demonstrates the utility of multi-marker eDNA metabarcoding in characterizing aquatic community structures and provides new insights into the spatial dynamics and species interactions within river ecosystems.
1. Introduction
River ecosystems support diverse biological communities that function as vital indicators of ecological health and biodiversity [1]. Among these, fish play pivotal roles in maintaining food web structure and contributing to aquatic biodiversity [2,3]. Healthy fish populations often reflect well-functioning ecosystems capable of supporting other species and ecological processes. Due to their long lifespan and mobility, fish can integrate environmental changes over time, making them excellent indicators of cumulative and long-term ecological shifts [4]. Metazoans, broadly defined as all multicellular animals from Porifera to Chordata, play vital roles in ecosystem functioning through their contributions to nutrient cycling, secondary production, and sediment processing [5,6]. Changes in metazoan communities can provide early warning signals of ecosystem stress or degradation, allowing for timely management interventions [7]. Consequently, the monitoring of fish and metazoa is instrumental for evaluating the overall biological integrity and aquatic biodiversity, supporting sustainable management and conservation of river ecosystems [8].
The effective conservation and rehabilitation of river ecosystems necessitates a comprehensive understanding of biological communities [9]. Repeated and consistent monitoring can reveal trends in species composition, abundance, and biomass, thereby providing insights into ecosystem health and changes [10]. eDNA has emerged as a powerful tool to facilitate the identification of species presence and biodiversity through the process of sequencing DNA fragments extracted from water samples [11]. This method demonstrates high sensitivity, even for elusive species [12], and has been validated through comparison with traditional survey methods in subtropical rivers [13]. Furthermore, eDNA sampling has been shown to minimise ecosystem disturbance in comparison to conventional methods such as electrofishing or benthic grabs, particularly in sensitive habitats such as hyporheic zones [14]. eDNA metabarcoding reduces reliance on manual specimen collection and taxonomic expertise, enabling frequent, large-scale monitoring [15]. Nonetheless, broader integration of eDNA metabarcoding into routine biomonitoring programmes is contingent upon standardisation, a prerequisite for enhancing the predictive capabilities of ecosystem response models to stressors [16].
Analyzing the structure and dynamics of biological communities provides critical insights into community assembly processes and the mechanisms that shape biodiversity and ecosystem function [17]. Beta diversity, which describes the variation in species composition among communities within a region [18], is a key metric for understanding these patterns. Its decomposition helps ecologists disentangle the processes driving community differences [19]. The most widely utilised framework subdivides beta-diversity into two primary components: turnover (replacement) and nestedness (richness difference) [20]. Turnover is indicative of the replacement of some species by others between sites, while nestedness captures differences in species richness among sites, where one community is essentially a subset of another [7]. Furthermore, trophic interactions, such as predation and competition, are fundamental drivers of community structure in river ecosystems. For example, fish often prey on other metazoan species, shaping both prey and predator populations. Understanding these interactions requires comprehensive biodiversity assessments across multiple taxonomic levels. Multi-marker eDNA metabarcoding, which simultaneously targets different organism groups using specific genetic regions, offers an effective approach to capture the complexity of these diverse communities. By integrating data from multiple markers, researchers can better characterize species diversity, resolve food web structures, and identify keystone species that play disproportionate roles in maintaining ecosystem stability [21].
In this study, we used the Yujiang River as a case study to explore the community structure of metazoan and fish assemblages based on eDNA metabarcoding. By simultaneously applying two genetic markers (12S and 18S rRNA genes), we aimed to achieve a comprehensive assessment of aquatic biodiversity and community dynamics. Specifically, we examined the relative contributions of species turnover and nestedness to beta diversity, identified key environmental drivers shaping community composition, and investigated potential trophic relationships between taxonomic groups.
2. Materials and Methods
2.1. Study Area and Sample Collection
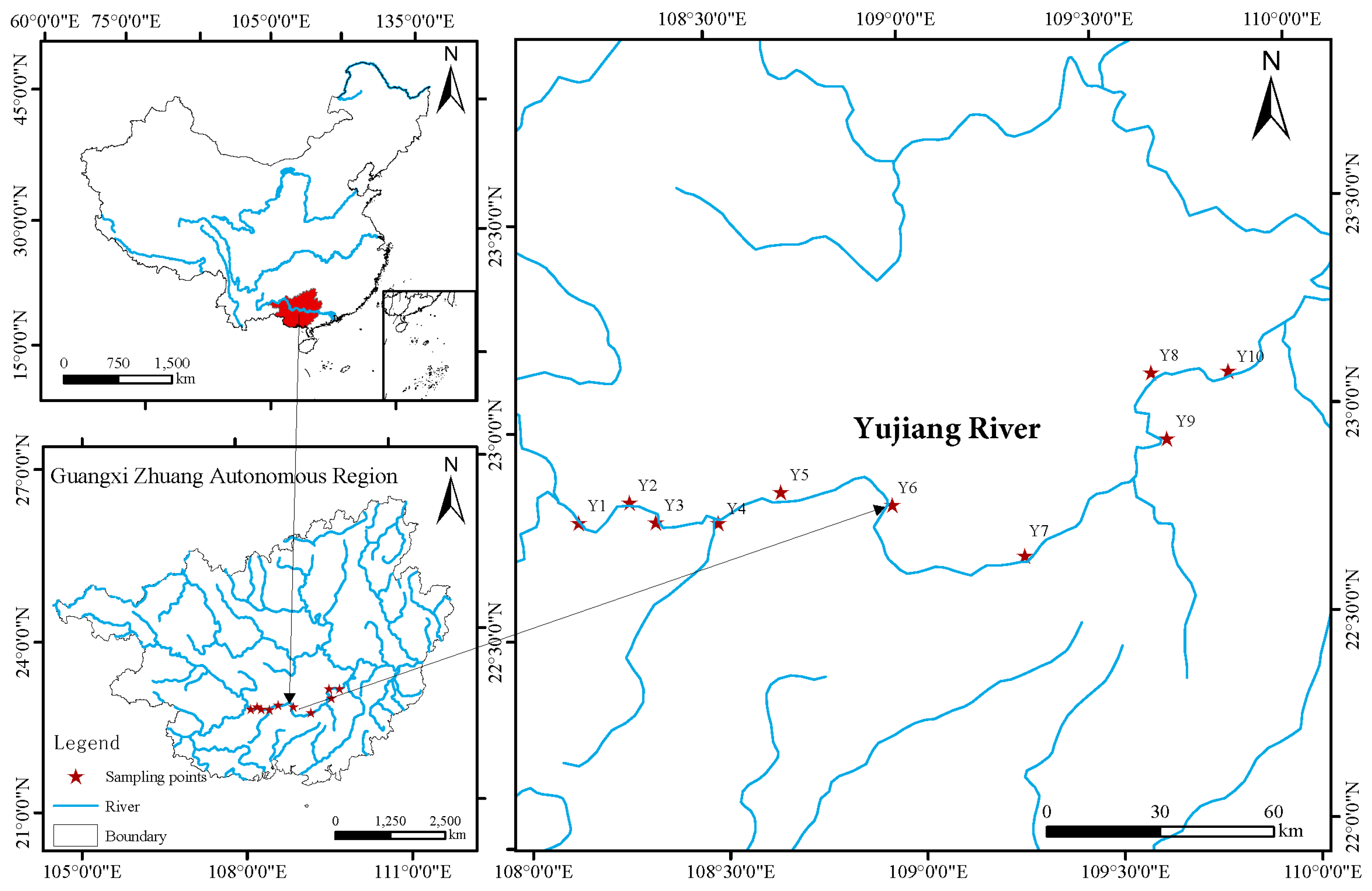
The Yujiang River is the largest tributary of the Xijiang River system within the Pearl River Basin and serves as the boundary between the Qianjiang and Xunjiang sections of the Xijiang River. The mainstem section from Songcun to Guiping is approximately 426 km long, with a total elevation drop of 42 m and an average gradient of 0.1‰. The river flows through hilly plains with wide valleys and a gentle current. The surrounding region is densely populated, economically developed, and intensively cultivated. This section of the river passes through the central urban districts of Nanning City, Heng County, Guigang City, and Guiping City. The basin is located in a subtropical monsoon climate zone, characterized by distinct wet and dry seasons. Long-term hydrological data from the Guigang hydrological station indicate that the mean annual runoff is approximately 47.9 × 109 m3. Of this, around 40 × 109 m3 (or 83.6%) occurs during the flood season from May to October. The annual average sediment concentration is 0.34 kg/m3.
A total of 10 sites along the mainstem of the Yujiang River were selected for the collection of water samples (see Figure 1). To minimize potential temporal variation among sampling sites, all water samples were collected within a short time frame—specifically, on 24 and 25 October 2023. Therefore, the samples can be considered as having been collected almost simultaneously under comparable environmental conditions. In each station, approximately 2 L of water was collected using a 2.5 L water sampler (Wuhan Shuitiandi Instruments, Wuhan, China). Firstly, 1 L of water was immediately filtered through 50 mm-diameter, 0.22 μm pore-size polycarbonate membranes using a vacuum filtration pump (Model GM-0.5A, Jinteng, Tianjin, China). The filter membranes were subjected to rapid freezing in liquid nitrogen, following which they were stored at −80 °C until DNA extraction. A further 1 L of water samples were stored temporarily at 4 °C for the purpose of conducting total phosphorus (TP) analysis.
Figure 1.
Map of sampling.
2.2. Measurements of Environmental Factors
Water temperature, dissolved oxygen (DO), specific conductance (SPC), total dissolved solids (TDS), pH, oxidation–reduction potential (ORP), ammonia nitrogen, and nitrate nitrogen were measured in situ using YSI ProPlus (YSI Inc., Yellow Springs, OH, USA). Water transparency was determined using a Secchi disk. Total phosphorus (TP) was measured using the spectrophotometric method, following the national standard method GB 11893-89 [22].
2.3. DNA Extraction and High-Throughput Sequencing
Total genomic DNA of water samples was extracted from the filters using a DNeasy PowerWater Kit (QIAGEN, Germantown, MD, USA) following the manufacturer’s instructions. Agarose gel electrophoresis (1% concentration) was used to detect the success of DNA extraction. DNA quality was measured using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The V4 region of the eukaryotic 18S rRNA gene and the 12S rRNA gene were amplified by the primers of TAReuk454FWD1F-TAReukREV3R [23] and MiFish-U [24], respectively, to measure the metazoan and fish communities. The purified PCR products were then sequenced on the Illumina PE250 platform by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
2.4. Data Processing
The bioinformatics analysis of the amplicon sequencing data was performed following our previous workflow [25,26]. Briefly, paired-end reads were merged, quality-filtered, and dereplicated using the --fastq_mergepairs, --fastx_filter, and --derep_fulllength commands in VSEARCH v2.28.1 [27,28,29]. Subsequently, non-redundant sequences were denoised into amplicon sequence variants (ASVs) using the –unoise3 command in USEARCH [30]. Taxonomic assignment of ASVs was conducted using the sintax algorithm with a confidence cutoff of 0.6 in USEARCH. For the ASVs of the eukaryotic 18S rRNA gene, they were annotated by the PR2 database [31], and then ASVs related to metazoa were selected based on the keyword “Metazoa”. Taxonomic assignments for fish ASVs were made using the MIDORI2 database [32].
2.5. Statistical Analysis
The biodiversity of metazoan and fish communities was evaluated using four alpha diversity indices (Chao1, Pd faith, Shannon, and Pielou J) and one gamma diversity index (Chao). These indices were calculated using the “vegan” package. The Bray–Curtis distance was also estimated for metazoan and fish communities between each of the two samples, respectively, with the “vegan” package being utilised for this purpose. The Wilcoxon rank-sum test was employed to analyse the variation in the Bray–Curtis index between the metazoan and fish communities. The effects of species replacement and richness difference on community composition were investigated using the “agricolae” and “adespatial” packages, and visualising the results using the “ggtern” and “vcd” packages [33]. Furthermore, linear regression was employed to evaluate the relationships between community similarity (as measured by the 1-Bray–Curtis distance) and the shared ASV number in each of two samples: one for metazoan communities and the other for fish communities.
A co-occurrence network of metazoa and fish in the Yujiang River was built using the ASV table. ASVs present in at least 60% of samples and with an average relative abundance above 0.05% were selected for Spearman correlation analysis. Significant correlations were defined as those with |r| > 0.6 and Benjamini–Hochberg adjusted p < 0.05 [34]. The network roles of nodes were determined based on within-module connectivity (Zi) and among-module connectivity (Pi), using thresholds of 2.5 and 0.62, respectively [35].
3. Results
3.1. Biodiversity of Metazoan and Fish Communities
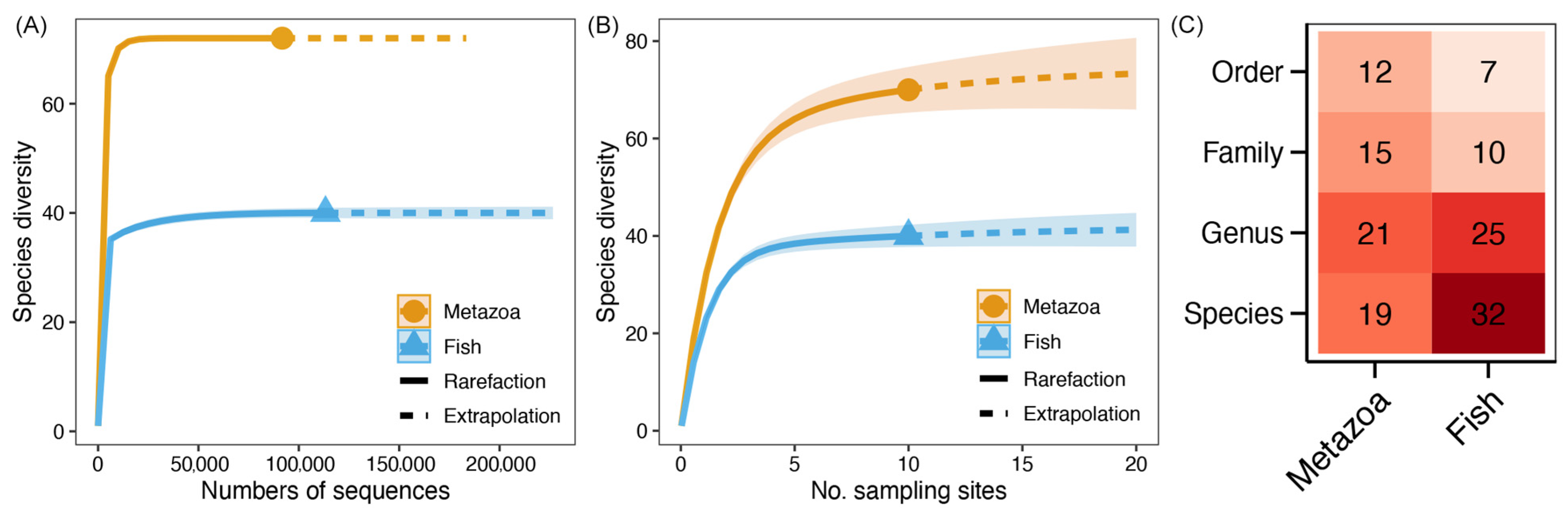
As indicated by the sequencing of 18S rRNA and 12S rRNA genes, a total of 917,010 (ranging from 2530 to 371,120) and 1,113,011 (ranging from 76,623 to 204,415) sequences for metazoa and fishes were obtained, respectively. The rarefaction and species accumulation curves exhibited a horizontal state (Figure 2A,B), thereby indicating that the sequencing depth and sample size represented the intact communities. As demonstrated in Figure 2C, the taxonomic annotation revealed the presence of 12 metazoan orders, encompassing a total of 15 families, 21 genera, and 19 species (Table S1). A total of 32 species were identified within the fish community, which belonged to 25 genera, 10 families, and 7 orders (Figure 2C; Table S2). The diversity indices of metazoan and fish communities are shown in Table 1. It was found that the diversity indices of Chao1 and Pd faith of the metazoan community were higher than those of the fish community. Conversely, the Pielou J and Chao (gamma)_ indices were found to be lower.
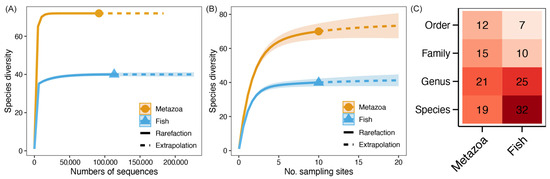
Figure 2.
Rarefaction curves (A), species accumulation curves (B), and detected taxonomic numbers (C) of metazoan and fish commmunities, respectively. Points in curves represent the actual values of sequencing depth and sample size. The dotted line represents the computative curve with the increase of sequencing depth or sample size. Color of each block in heatmap represents the taxonomic number, and the actual value is also labelled in the corresponding block.

Table 1.
Diversity of metazoan and fish communities in the Yujing River.
3.2. Compositions of Metazoan and Fish Communities
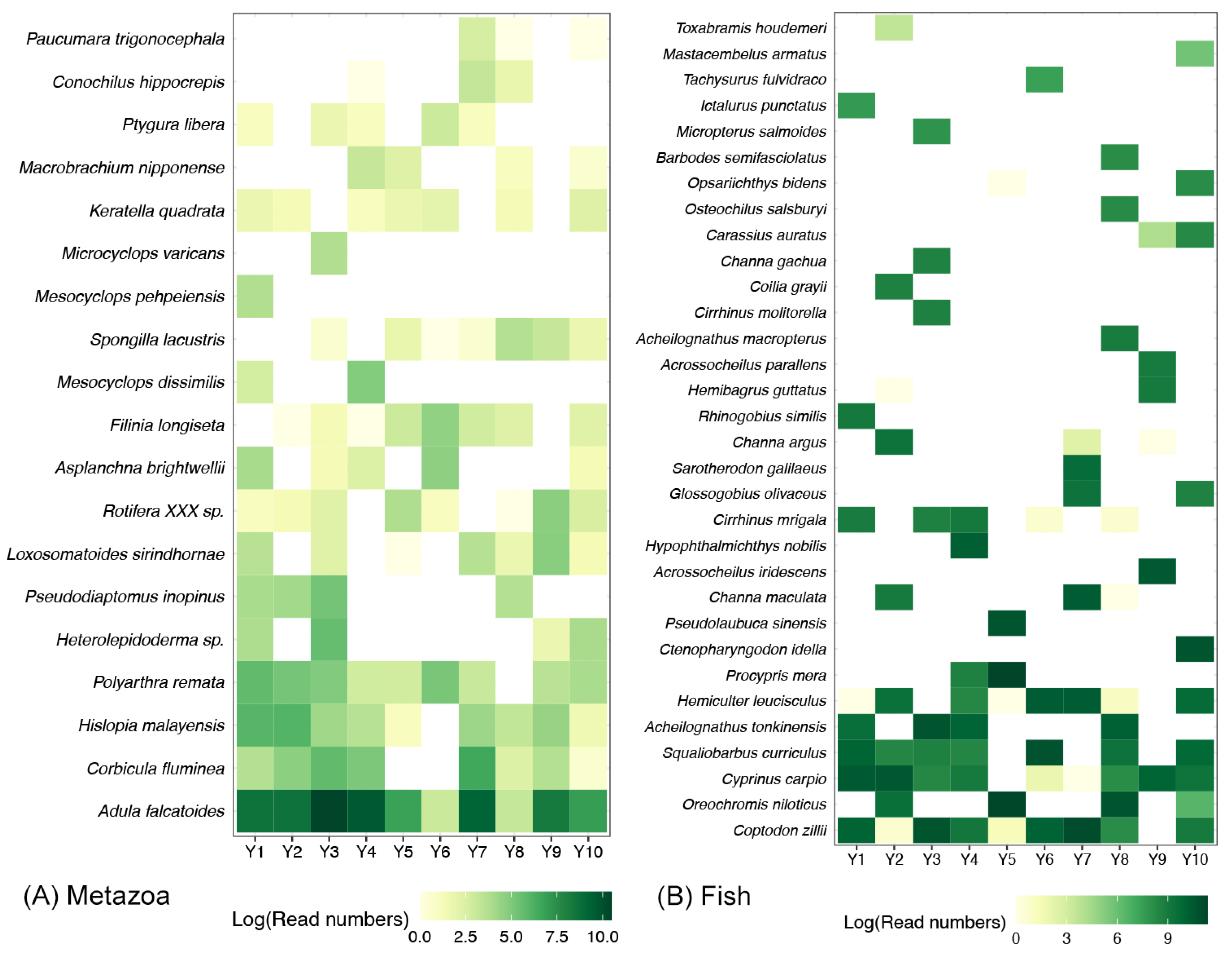
Among the 19 metazoan species detected in the Yujiang River, more than half of them had a detection rate exceeding 50% (Figure 3A). Of the species observed, Adula falcatoides was the only one to be present in all samples, and it was the most abundant (Figure 3A). The other main metazoan species included Corbicula fluminea, Hislopia_malayensis, and Polyarthra remata (Figure 3A). With regard to the fish community, the majority of species were observed in individual samples, while a mere five species exhibited a detection rate exceeding 50% (Figure 3B). Coptodon zillii was identified as the fish species with the widest distribution and highest abundance (Figure 2B). The other main fish species contained Oreochromis niloticus, Cyprinus carpio, Squaliobarbus curriculus, Acheilognathus tonkinensis, and Hemiculter leucisculus (Figure 3B).
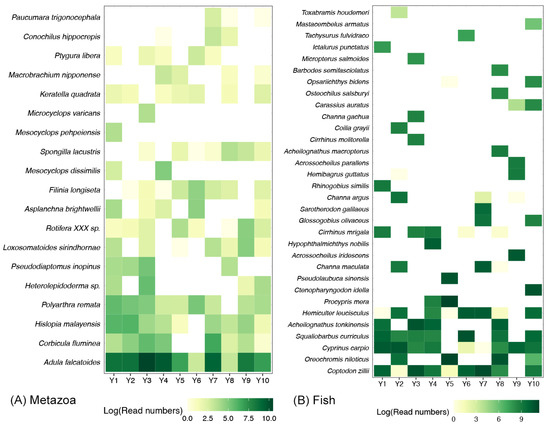
Figure 3.
Heatmaps revealing the detected ratio of metazoan (A) and fish species (B) in Yujiang River. The white block represents that the corresponding species was not detected in the sample. Colors filled in other blocks represent the sequence numbers of corresponding species.
3.3. Beta Diversity Partitioning and Drivers of Metazoan and Fish Communities
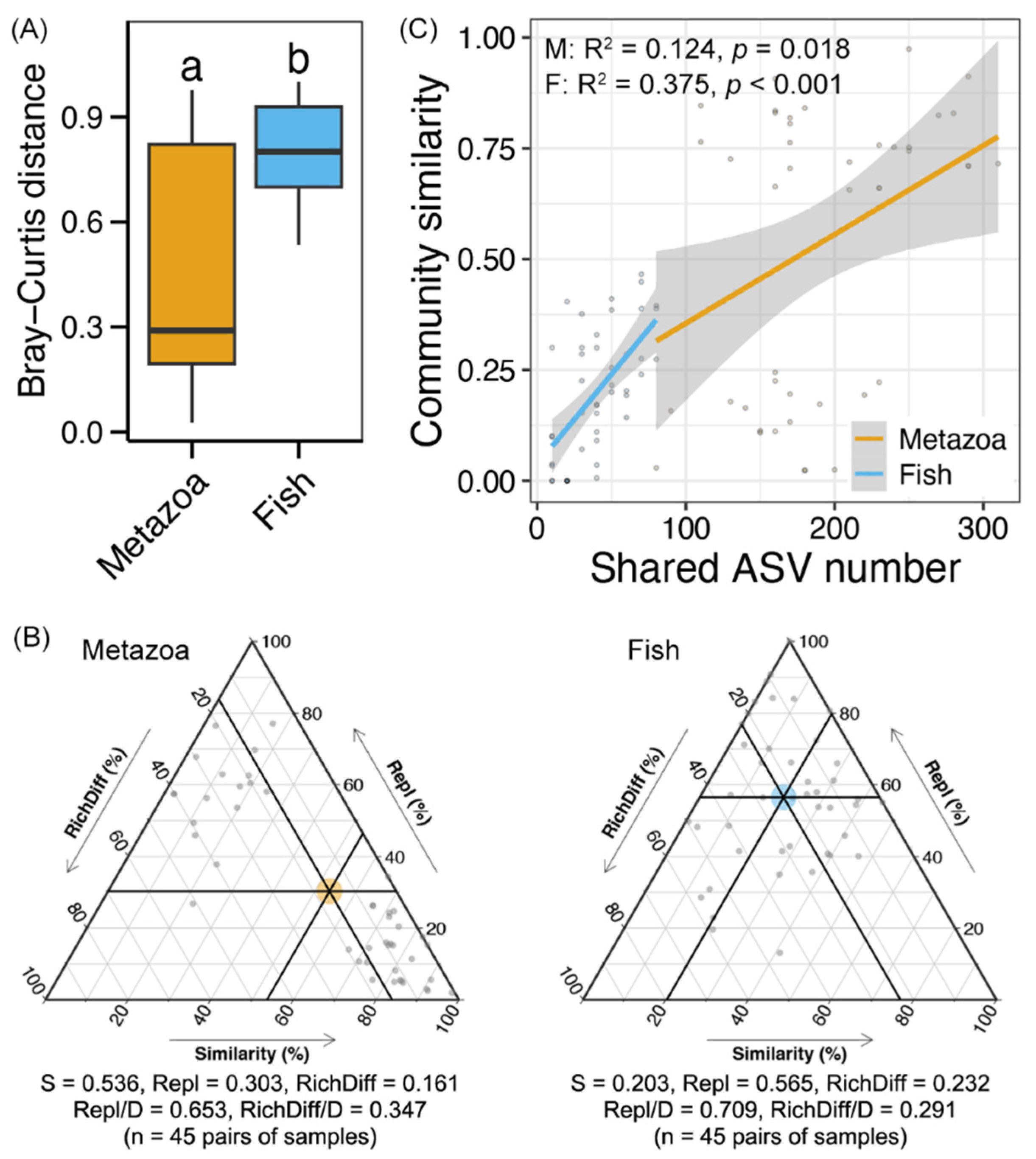
A significantly lower Bray–Curtis distance was found in the metazoan communities compared to the fish communities (Wilcoxon rank-sum test, p < 0.05, Figure 4A), indicating a high degree of variability in the fish communities along the Yujiang River. Beta-diversity decomposition showed that turnover was higher in fish communities (70.9%) compared to metazoan communities (65.3%), while the contribution of replacement processes was relatively greater in metazoans (34.7%) than in fish (29.1%) (Figure 4B). Furthermore, a significant correlation was identified between the shared ASV number of each paired sample and the corresponding variation in community composition (Linear regression, p < 0.05, Figure 4C). The correlation was stronger for fish (0.375) compared to metazoans (0.124). The results indicated a higher rate of turnover of fish species along the Yujiang River, which is indicative of significant variability in community composition.
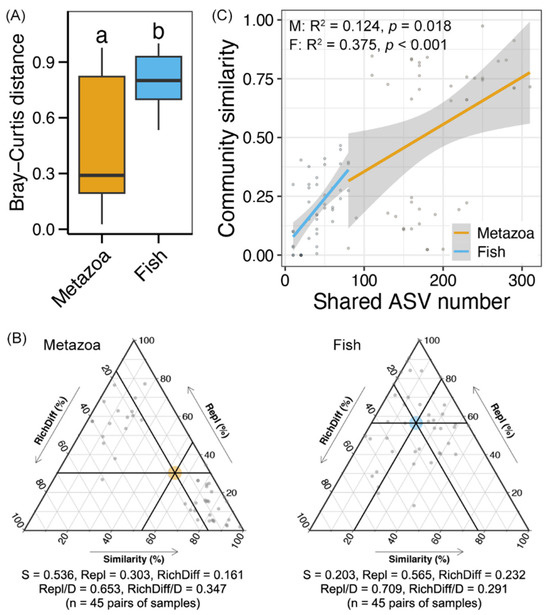
Figure 4.
(A) Bray–Curtis distance between the metazoan and fish communities along the Yujiang River. (B) Ternary diagram showing community ecological processes (species replacement and richness difference) of metazoan and fish communities along the Yujiang River. (C) Linear regression between the shared ASV number and community similarity (1-Bray–Curtis distance) of each pair of samples for metazoan and fish communities, respectively.
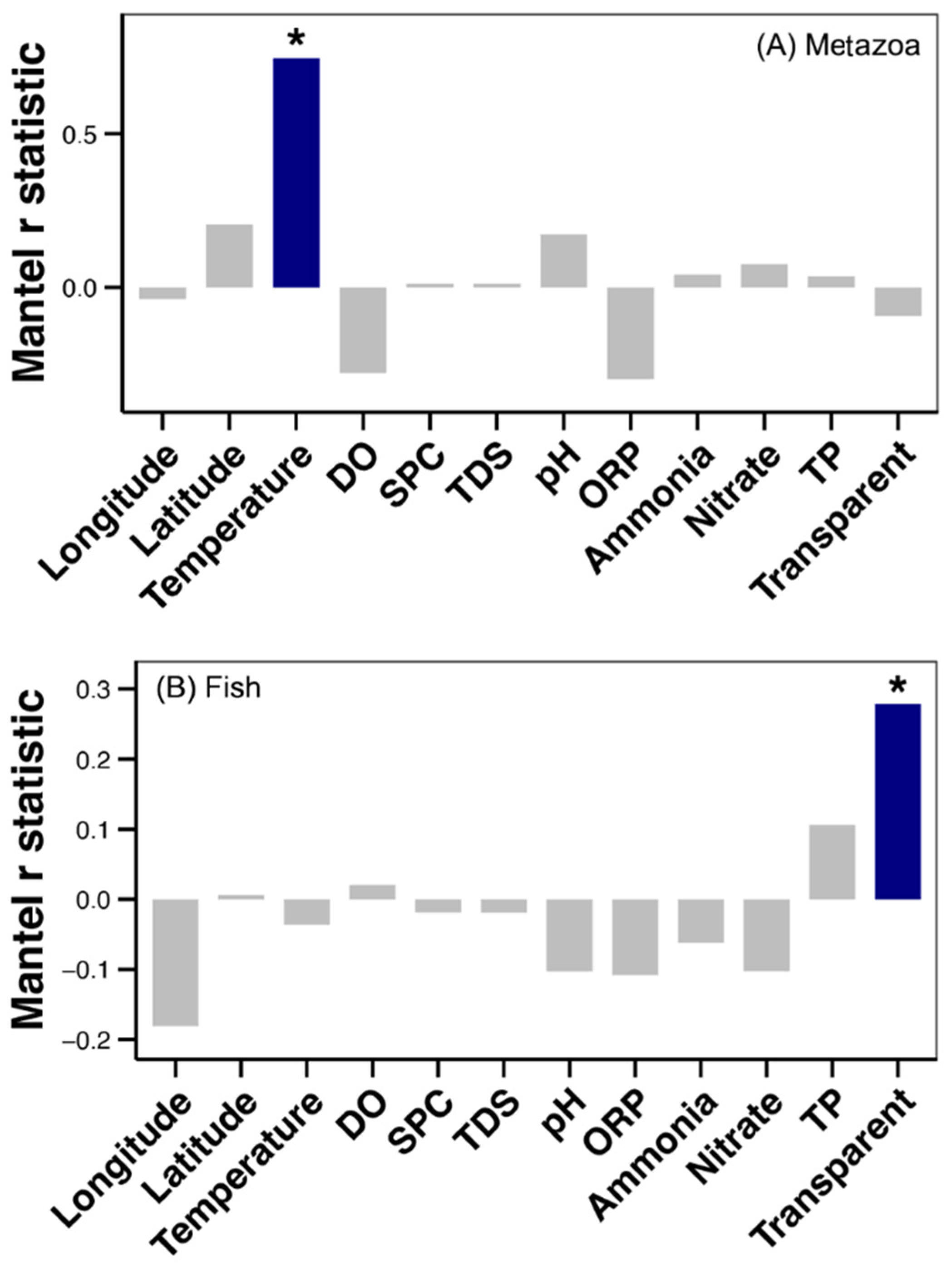
Further analysis was conducted to ascertain the correlation between the beta diversity of metazoan and fish communities with spatial and environmental factors along the Yujiang River. A significant relationship was observed between water temperature and the metazoan community (Mantel test, p < 0.05, Figure 5). A significant relationship was also observed between water transparency and the fish community (Mantel test, p < 0.05, Figure 5).
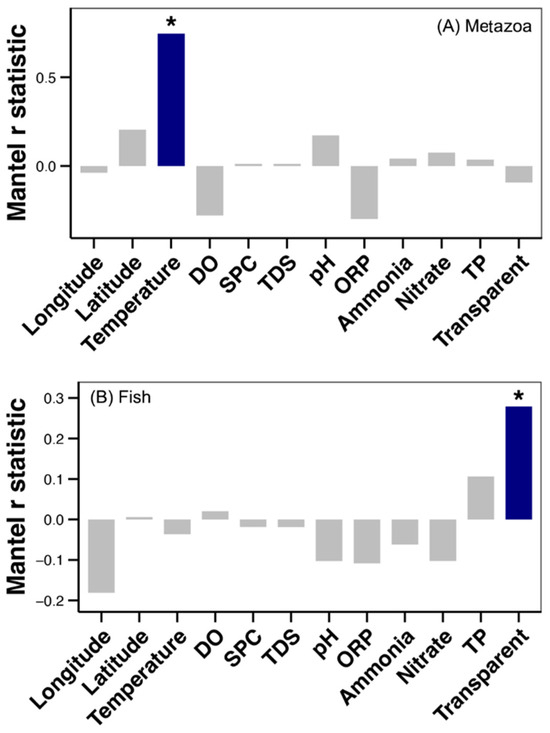
Figure 5.
Results of the Mantel test between the Bray–Curtis distance of metazoan (A) and fish (B) communities with spatial and environmental factors along the Yujiang River. Blue bar and the asterisk above it represent significant correlation (Mantel test, p < 0.05).
3.4. Co-Occurrence Network of Metazoa and Fish
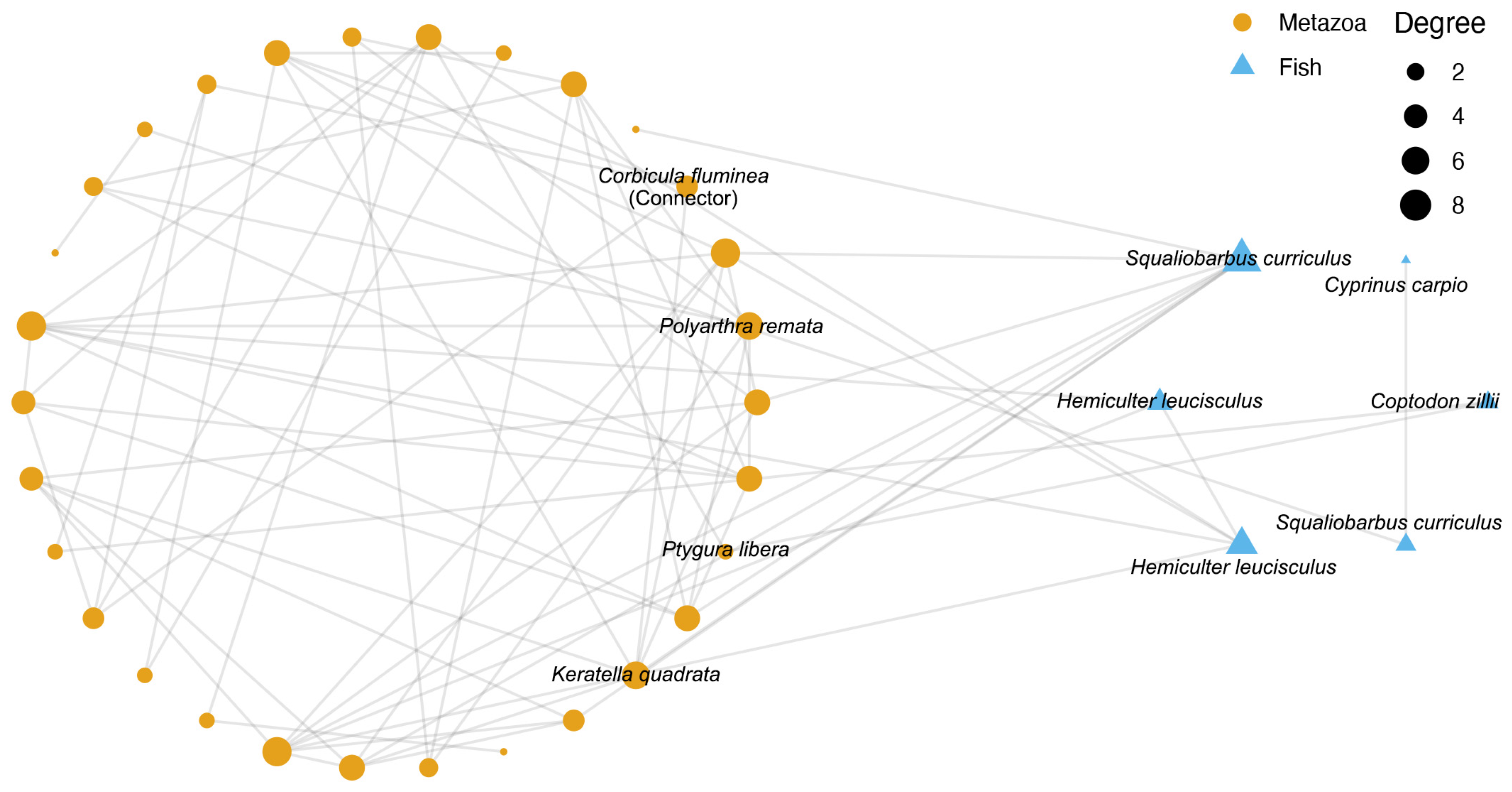
The co-occurrence network of metazoa and fish along the Yujing River at the ASV level was constructed (Figure 6; Table S3). A total of 30 metazoan ASVs were presented in the network; however, only 4 ASVs had a taxonomic annotation at the species level. Of particular interest was the recognition of Corbicula fluminea as a connector of the network, with three additional metazoan species (Polyarthra remata, Ptygura libera, and Keratella quadrata) exhibiting a high degree of connectivity with the fish ASVs. For Fish, six ASVs in the network belonged to four species (Cyprinus carpio, Coptodon zillii, Squaliobarbus curriculus, and Hemiculter leucisculus). Of these, three species (Coptodon zillii, Squaliobarbus curriculus, and Hemiculter leucisculus) exhibited a high degree of similarity to metazoan ASVs, while Cyprinus carpio was exclusively associated with Squaliobarbus curriculus.
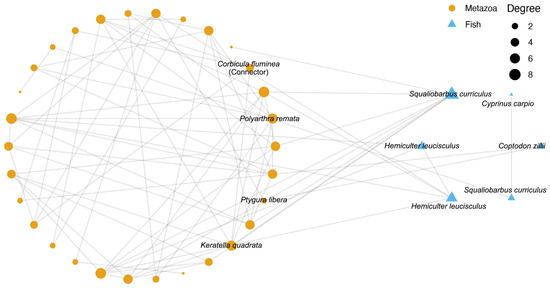
Figure 6.
Co-occurrence network of metazoa and fish along the Yujiang River. Different colors and shapes of points represent metazoan and fish ASVs, respectively. The size of point represents the degree of each node.
4. Discussion
The significance of fish for local economies, recreational activities, and cultural values is well-documented. The monitoring of fish assemblages is imperative in ensuring the sustained delivery of these benefits, which is achieved by maintaining healthy fish populations and water quality [36]. The biodiversity of fish species in Chinese rivers is considerable; however, this is being jeopardised by factors such as habitat degradation, pollution, and overfishing. The Yangtze River basin, which is renowned for its exceptional aquatic biodiversity, has undergone a substantial decline in fish resources. A recent survey documented an 85% reduction in fish species since the 1960s, with only 323 species being recorded [37]. Initiatives such as the “10-year fishing ban,” which was implemented in 2021, are designed to restore fish stocks and biodiversity. Preliminary indications suggest a recovery in fish catch per unit effort and fish fry abundance [38]. Notably, only 32 fish species were detected in the Yujiang River, markedly fewer than the 74 species historically recorded there [39]. While this limited detection may partly result from constraints in survey depth and temporal coverage, it nonetheless indicates a significant decline in fish diversity. Similar declines in fish species richness have been observed in other rivers of comparable size to the Yujiang River. Recent surveys identified 91, 42, and 50 fish species in the Ganjiang River [40], Daqing River [41], and Taizi River [42], respectively—figures that are all lower than their historical records. Environmental factors such as habitat diversity, water quality, and land use play significant roles in shaping fish community richness and composition in Chinese rivers [43]. Encouragingly, habitat enhancement efforts in nearby rivers such as the Youjiang River—a tributary of the Yujiang River—have shown positive impacts on fish diversity [44,45], indicating that similar strategies could be successfully applied to the Yujiang River.
Monitoring fish populations is essential not only for assessing conservation efforts and fisheries management but also for understanding changes in community composition, including the spread of invasive species. This study revealed that C. zillii and O. niloticus are the fish species with the widest distribution and abundance in the Yujiang River. C. zillii (Redbelly tilapia) and O. niloticus (Nile tilapia) are both fish species in the Cichlidae family, native to Africa and the Middle East. Nile tilapia is one of the most extensively cultivated fish species on a global scale and represents a pivotal species in the domain of aquaculture [46]. Although smaller and more challenging to capture than Nile tilapia, redbelly tilapia possesses advantageous traits for aquaculture, such as feeding at lower trophic levels, high fecundity, and tolerance to salinity and low temperatures [47]. As non-native species, both tilapias have exerted negative impacts on local ecosystems and biodiversity [48]. Redbelly tilapia, in particular, disrupt freshwater habitats by reducing submerged aquatic macrophytes, which are vital for water quality, shelter, and spawning sites for native species [49]. It is regarded as one of the most destructive fish species due to its ability to eliminate submerged vegetation, thereby degrading habitats essential for native aquatic organisms [50]. Similarly, Nile tilapia influences phytoplankton communities and other aquatic biota, affecting ecosystem productivity and nutrient cycling [51]. The substantial presence and invasiveness of these two species in the Yujiang River underscore the necessity of ongoing monitoring. Such efforts are essential to inform adaptive management strategies aimed at mitigating their ecological and socio-economic impacts [52].
Across diverse taxa and ecosystems, turnover often predominates in driving beta diversity in environments with strong environmental gradients or high habitat heterogeneity, as these conditions favor species replacement adapted to different niches [53]. Conversely, the significance of nestedness is more pronounced in systems where species loss or gain (rather than replacement) is the predominant process. This is exemplified in stressed habitats, where specialist species are lost and communities become subsets of more diverse communities [54]. The balance between turnover and nestedness is influenced by environmental gradients, habitat connectivity, and species’ ecological traits [55]. Fish exhibited higher species turnover than metazoans, likely due to their superior swimming ability and mobility, which enables them to actively select habitats and respond rapidly to environmental heterogeneity such as flow regimes and hydraulic gradients [56,57]. Conversely, many metazoans—including benthic invertebrates and parasites—are less mobile and more dependent on local environmental conditions and habitat structure, which can lead to lower turnover but potentially higher nestedness in response to habitat filtering or stress [58]. This distinction highlights the need for targeted and adaptive conservation strategies: preserving habitat connectivity and environmental heterogeneity is essential for sustaining dynamic fish communities, while mitigating local stressors is critical for maintaining metazoan diversity in the Yujiang River. Additionally, it should be noted that some environmental factors, particularly temperature and turbidity, may influence the degradation rate and detectability of environmental DNA. Although we standardized the sampling and filtration procedures to reduce such variability, differences in local water temperature (24.3–30.4 °C; Table S4) could still affect eDNA persistence and potentially introduce subtle biases in the observed diversity patterns. This limitation should be considered when interpreting correlations between environmental variables and community structure.
Identifying key species that link fish and invertebrate communities is fundamental to designing effective conservation and river health monitoring strategies. Among them, C. fluminea (Asian clam) stands out as a central node in the co-occurrence network linking metazoan and fish communities. Native to China, this small freshwater bivalve functions as a filter feeder and contributes substantially to nutrient cycling and water purification [59,60]. However, it is also highly sensitive to environmental pollutants such as microplastics, pharmaceuticals, and cyanobacterial toxins, which impair its physiological functions [61,62]. C. fluminea is recognized as an ecologically and regionally important species, commonly used in environmental monitoring due to its sensitivity to pollutants and role in nutrient cycling [63]. Our findings reinforce this view and suggest its utility as an indicator species for river health assessments in the Yujiang River. In addition, three rotifer species were also tightly associated with fish species in the co-occurrence network. As primary consumers and prey for juvenile fish and small invertebrates, rotifers play a crucial role in connecting basal and higher trophic levels and reflect active predator–prey dynamics. Interestingly, the fish species linked to these rotifers were primarily native species such as Saurogobio curriculus and Hemiculter leucisculus. These findings suggest that effective conservation should integrate invasive species control with the protection of native species that play key roles in maintaining trophic interactions and food web structure.
5. Conclusions
In conclusion, this study revealed the diversity of metazoan and fish communities in the Yujiang River using multi-marker eDNA metabarcoding targeting both 12S and 18S rRNA genes. Although fish exhibited lower alpha diversity, their gamma diversity was higher than that of metazoa due to the contribution of rare species. Species turnover was the main driver of community variation, with fish communities showing greater spatial heterogeneity than metazoan communities. Community changes were significantly associated with water temperature for metazoa and water transparency for fish. The dominance of invasive fish species and the key ecological roles of native metazoan species highlight the need for continuous biological monitoring to assess potential ecological risks and community shifts in river ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17070488/s1, Table S1: ASV table of metazoa. Table S2: ASV table of fish. Table S3. Node in the co-occurrence network of metazoa and fish along the Yujiang River. Table S4: Environmental factors measured at each sampling site in the Yujiang River.
Author Contributions
Conceptualization, Y.L. and D.W.; methodology, Y.H., J.S., W.W., C.Y., S.N. and C.G.; writing—original draft preparation, Y.L. and D.W.; writing—review and editing, W.C. and L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the open fund of China (Guangxi)-ASEAN Key Laboratory of Comprehensive Exploitation and Utilization of Aquatic Germplasm Resources, Ministry of Agriculture and Rural Affairs; Key Laboratory of Aquaculture genetic and Breeding and Healthy Aquaculture of Guangxi Academy of Fishery Sciences, Nanning 530021, China (No. GXKEYLA-2023-01-1); Project of Financial Funds of Ministry of Agriculture and Ruaral Affairs: Investigation of Fishery Resources and Habitat in the Pearl River Basin (No. ZJZX-04); State Key Laboratory of Breeding Biotechnology and Sustainable Aquaculture (No. 2025BBSA12).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, P.; Li, D.; Sun, X.; Chu, Z.; Xia, T.; Zheng, B. Application of Ecological Restoration Technologies for the Improvement of Biodiversity and Ecosystem in the River. Water 2022, 14, 1402. [Google Scholar] [CrossRef]
- Blackman, R.C.; Ho, H.-C.; Walser, J.-C.; Altermatt, F. Spatio-Temporal Patterns of Multi-Trophic Biodiversity and Food-Web Characteristics Uncovered across a River Catchment Using Environmental DNA. Commun. Biol. 2022, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Pinna, M.; Zangaro, F.; Saccomanno, B.; Scalone, C.; Bozzeda, F.; Fanini, L.; Specchia, V. An Overview of Ecological Indicators of Fish to Evaluate the Anthropogenic Pressures in Aquatic Ecosystems: From Traditional to Innovative DNA-Based Approaches. Water 2023, 15, 949. [Google Scholar] [CrossRef]
- Tesfaye, G.C.; Souza, A.T.; Bartoň, D.; Blabolil, P.; Čech, M.; Draštík, V.; Frouzová, J.; Holubová, M.; Kočvara, L.; Kolařík, T.; et al. Long-Term Monitoring of Fish in a Freshwater Reservoir: Different Ways of Weighting Complex Spatial Samples. Front. Environ. Sci. 2022, 10, 1000087. [Google Scholar] [CrossRef]
- Fonseca, V.G.; Carvalho, G.R.; Sung, W.; Johnson, H.F.; Power, D.M.; Neill, S.P.; Packer, M.; Blaxter, M.L.; Lambshead, P.J.D.; Thomas, W.K.; et al. Second-Generation Environmental Sequencing Unmasks Marine Metazoan Biodiversity. Nat. Commun. 2010, 1, 98. [Google Scholar] [CrossRef] [PubMed]
- Talluto, L.; del Campo, R.; Estévez, E.; Altermatt, F.; Datry, T.; Singer, G. Towards (Better) Fluvial Meta-Ecosystem Ecology: A Research Perspective. NPJ Biodivers. 2024, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Z.; Yang, G.; Feng, G. Dominated Taxonomic and Phylogenetic Turnover but Functional Nestedness of Wetland Bird Beta Diversity in North China. Land 2022, 11, 1090. [Google Scholar] [CrossRef]
- Kuehne, L.M.; Dickens, C.; Tickner, D.; Messager, M.L.; Olden, J.D.; O’Brien, G.; Lehner, B.; Eriyagama, N. The Future of Global River Health Monitoring. PLoS Water 2023, 2, e0000101. [Google Scholar] [CrossRef]
- Kaufmann, P.R.; Hughes, R.M.; Paulsen, S.G.; Peck, D.V.; Seeliger, C.W.; Weber, M.H.; Mitchell, R.M. Physical Habitat in Conterminous US Streams and Rivers, Part 1: Geoclimatic Controls and Anthropogenic Alteration. Ecol. Indic. 2022, 141, 109046. [Google Scholar] [CrossRef] [PubMed]
- Stenekes, S.; Parlee, B.; Seixas, C. Culturally Driven Monitoring: The Importance of Traditional Ecological Knowledge Indicators in Understanding Aquatic Ecosystem Change in the Northwest Territories’ Dehcho Region. Sustainability 2020, 12, 7923. [Google Scholar] [CrossRef]
- Fueyo, Á.; Sánchez, O.; Carleos, C.; Escudero, A.; Cordón, J.; Granero-Castro, J.; Borrell, Y.J. Unlocking Rivers’ Hidden Diversity and Ecological Status Using DNA Metabarcoding in Northwest Spain. Ecol. Evol. 2024, 14, e70110. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.N.; Shen, E.W.; Seemann, J.; Correa, A.M.S.; O’Donnell, J.L.; Altieri, A.H.; Knowlton, N.; Crandall, K.A.; Egan, S.P.; McMillan, W.O.; et al. Environmental DNA Survey Captures Patterns of Fish and Invertebrate Diversity across a Tropical Seascape. Sci. Rep. 2020, 10, 6729. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, D.-H.; Song, Y.-D.; Wang, T.-T.; Fan, S.-D.; Wu, E.-N.; Chen, N.-L.; Xia, W.-T.; Xu, M.N.; Chen, Z.-B.; et al. Application of Environmental DNA Metabarcoding to Identify Fish Community Characteristics in Subtropical River Systems. Ecol. Evol. 2024, 14, e11214. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.M.; Macher, J.-N. A DNA Metabarcoding Protocol for Hyporheic Freshwater Meiofauna: Evaluating Highly Degenerate COI Primers and Replication Strategy. Metabarcoding Metagenomics 2018, 2, e26869. [Google Scholar] [CrossRef]
- Lim, N.K.M.; Tay, Y.C.; Srivathsan, A.; Tan, J.W.T.; Kwik, J.T.B.; Baloğlu, B.; Meier, R.; Yeo, D.C.J. Next-Generation Freshwater Bioassessment: EDNA Metabarcoding with a Conserved Metazoan Primer Reveals Species-Rich and Reservoir-Specific Communities. R. Soc. Open Sci. 2016, 3, 160635. [Google Scholar] [CrossRef] [PubMed]
- Buchner, D.; Macher, T.-H.; Beermann, A.J.; Werner, M.-T.; Leese, F. Standardized High-Throughput Biomonitoring Using DNA Metabarcoding: Strategies for the Adoption of Automated Liquid Handlers. Environ. Sci. Ecotechnol. 2021, 8, 100122. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.P.; Delahooke, K.M.; Barnes, N.; Rideout, B.W.T.; Kenchington, C.G.; Manica, A.; Mitchell, E.G. Morphology Shapes Community Dynamics in Early Animal Ecosystems. Nat. Ecol. Evol. 2024, 8, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Miranda, J.R.; Hernandez, D.F.; Mouillot, D. Low Functional β-Diversity despite High Taxonomic β-Diversity among Tropical Estuarine Fish Communities. PLoS ONE 2012, 7, e40679. [Google Scholar] [CrossRef] [PubMed]
- Lamy, T.; Legendre, P.; Chancerelle, Y.; Siu, G.; Claudet, J. Understanding the Spatio-Temporal Response of Coral Reef Fish Communities to Natural Disturbances: Insights from Beta-Diversity Decomposition. PLoS ONE 2015, 10, e0138696. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Fan, C.; Zhang, C.; Zhao, X.; Gadow, K. von Decomposing Spatial β-Diversity in the Temperate Forests of Northeastern China. Ecol. Evol. 2021, 11, 11362–11372. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Xu, D.; Ren, L.; Zhao, D. Using a Topological Approach to Identify Keystone Species of Fish in Eutrophic Lake Ecosystems: A Case of Zhushan Bay, Taihu Lake. Fish. Manag. Ecol. 2023, 30, 89–98. [Google Scholar] [CrossRef]
- GB 11893-89; Water Quality—Determination of Total Phosphorus—Ammonium Molybdate Spectrophotometric Method. State Bureau of Technical Supervision: Beijing, China, 1989.
- Stoeck, T.; Bass, D.; Nebel, M.; Christen, R.; Jones, M.D.M.; Breiner, H.-W.; Richards, T.A. Multiple Marker Parallel Tag Environmental DNA Sequencing Reveals a Highly Complex Eukaryotic Community in Marine Anoxic Water. Mol. Ecol. 2010, 19, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Miya, M.; Sato, Y.; Fukunaga, T.; Sado, T.; Poulsen, J.Y.; Sato, K.; Minamoto, T.; Yamamoto, S.; Yamanaka, H.; Araki, H.; et al. MiFish, a Set of Universal PCR Primers for Metabarcoding Environmental DNA from Fishes: Detection of More than 230 Subtropical Marine Species. R. Soc. Open Sci. 2015, 2, 150088. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, J.; Zhao, Y.; He, Y.; Chen, J.; Dong, C.; Liu, L.; Wang, J.; Zhou, L. Contrasting Pollution Responses of Native and Non-Native Fish Communities in Anthropogenically Disturbed Estuaries Unveiled by eDNA Metabarcoding. J. Hazard. Mater. 2024, 480, 136323. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Wang, R.; Xu, S.; Li, Z.; Liu, L.; Li, M.; Zhou, L. Changes in Protist Communities in Drainages across the Pearl River Delta under Anthropogenic Influence. Water Res. 2021, 200, 117294. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-Filtering Vastly Improves Diversity Estimates from Illumina Amplicon Sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference Database (PR2): A Catalog of Unicellular Eukaryote Small Sub-Unit rRNA Sequences with Curated Taxonomy. Nucleic Acids Res. 2013, 41, D597–D604. [Google Scholar] [CrossRef] [PubMed]
- Leray, M.; Knowlton, N.; Machida, R.J. MIDORI2: A Collection of Quality Controlled, Preformatted, and Regularly Updated Reference Databases for Taxonomic Assignment of Eukaryotic Mitochondrial Sequences. Environ. DNA 2022, 4, 894–907. [Google Scholar] [CrossRef]
- Wan, W.; Grossart, H.; He, D.; Liu, W.; Wang, S.; Yang, Y. Differentiation Strategies for Planktonic Bacteria and Eukaryotes in Response to Aggravated Algal Blooms in Urban Lakes. Imeta 2023, 2, e84. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, D.; Krall, L.; Müssig, C.; Büssis, D.; Usadel, B. Correlation Networks. In Analysis of Biological Networks; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 305–333. ISBN 978-0-470-25348-9. [Google Scholar]
- Zhao, Z.; Li, H.; Sun, Y.; Zhan, A.; Lan, W.; Woo, S.P.; Shau-Hwai, A.T.; Fan, J. Bacteria versus Fungi for Predicting Anthropogenic Pollution in Subtropical Coastal Sediments: Assembly Process and Environmental Response. Ecol. Indic. 2022, 134, 108484. [Google Scholar] [CrossRef]
- Chen, W.; Liu, L.; Wang, J.; Zhou, L. Threatened Freshwater Fish Need Protection. Science 2021, 374, 164. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, P.; Xu, C.; Sun, Y.; Shi, L.; Zhou, L.; Jeppesen, E.; Chen, J.; Xie, P. Can the “10-Year Fishing Ban” Rescue Biodiversity of the Yangtze River? Innovation 2022, 3, 100235. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Wang, P.; Jeppesen, E.; Xie, P. How to Manage Fish within and after the 10-Year Fishing Ban. Innovation 2024, 5, 100694. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Jin, Z.; Yuan, Z.; Liu, Q.; Bowler, P.A. Biodiversity and Conservation of Freshwater Fishes in the Yujiang River, China. Pak. J. Zool. 2024, 56, 2501–3000. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, X.; Ao, X.; Qin, J.; Wu, X.; Ouyang, S. Fish Diversity in the Middle and Lower Reaches of the Ganjiang River of China: Threats and Conservation. PLoS ONE 2018, 13, e0205116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Y.; He, J.; Hou, Y.; Wang, F. The Native Fish Diversity with Environmental Influencing Factors in the Daqing River Basin, China. Front. Environ. Sci. 2024, 12, 1450903. [Google Scholar] [CrossRef]
- Wang, C.; Shao, J.; Ma, B.; Xie, J.; Li, D.; Liu, X.; Huo, B. Longitudinal Patterns in Fish Assemblages after Long-Term Ecological Rehabilitation in the Taizi River, Northeastern China. Sustainability 2022, 14, 14973. [Google Scholar] [CrossRef]
- Li, S.; Huang, Y.; Li, F.; Liu, Y.; Ma, H.; Zhang, X.; Wang, X.; Chen, W.; Cui, G.; Wang, T. Functional Alpha and Beta Diversity of Fish Communities and Their Relationship with Environmental Factors in the Huanghe River (Yellow River) Estuary and Adjacent Seas, China. Fishes 2024, 9, 222. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, D.; Zeng, L.; Xu, P.; Tang, Q.; Chen, Z.; Zhu, Q.; Wang, G.; Chen, Q.; Chen, L.; et al. The Structuring Role of Artificial Structure on Fish Assemblages in a Dammed River of the Pearl River in China. Aquat. Living Resour. 2018, 31, 15. [Google Scholar] [CrossRef]
- Guo, D.; Zhou, L.; Wang, G.; Lai, H.; Bi, S.; Chen, X.; Zhao, X.; Liu, S.; Luo, Y.; Li, G. Use of Artificial Structures to Enhance Fish Diversity in the Youjiang River, a Dammed River of the Pearl River in China. Ecol. Evol. 2020, 10, 13439–13450. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.S.; Batool, A.I.; Rehman, M.F.U.; Naz, S. Comparative Analysis of the Haemato-Biochemical Parameters and Growth Characteristics of Oreochromis Niloticus (Nile tilapia) Cultured under Different Feed and Habitats (Biofloc Technology and Earthen Pond System). Aquac. Res. 2022, 53, 6184–6192. [Google Scholar] [CrossRef]
- Geletu, T.T.; Tang, S.; Xing, Y.; Zhao, J. Ecological Niche and Life-History Traits of Redbelly Tilapia (Coptodon Zillii, Gervais 1848) in Its Native and Introduced Ranges. Aquat. Living Resour. 2024, 37, 2. [Google Scholar] [CrossRef]
- Radkhah, A.R.; Eagderi, S. Ecological Consequences of Tilapia Species on Fish Biodiversity of Iran and Challenges Arising from Their Introduction. Iran. J. Ichthyol. 2021, 8, 342–350. [Google Scholar] [CrossRef]
- Feng, S.; Pan, X.; Wang, J.; Liu, W.; Hui, Y.; Wang, G.; Liu, K.; Li, J.; Xu, H.; Lin, L.; et al. Risk Screening of the Non-Native Fish in the Jiulong River Basin of Southeast China. Animals 2025, 15, 461. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xing, Y.; Geletu, T.T.; Zhao, J. Trophic Plasticity of the Invasive Redbelly Tilapia (Coptodon zillii) in China Inferred from DNA Metabarcoding Analysis. Ecol. Evol. 2025, 15, e71118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mei, X.; Tang, Y.; Razlutskij, V.; Peterka, J.; Taylor, W.D.; Naselli-Flores, L.; Liu, Z.; Tong, C.; Zhang, X. Effects of Nile Tilapia (Oreochromis niloticus) on Phytoplankton Community Structure and Water Quality: A Short-Term Mesocosm Study. Knowl. Manag. Aquat. Ecosyst. 2022, 423, 11. [Google Scholar] [CrossRef]
- Chaianunporn, T.; Panthum, T.; Singchat, W.; Chaianunporn, K.; Suksavate, W.; Chaiyes, A.; Muangmai, N.; Marod, D.; Duengkae, P.; Srikulnath, K. Sustainable Ecosystem Management Strategies for Tackling the Invasion of Blackchin Tilapia (Sarotherodon melanotheron) in Thailand: Guidelines and Considerations. Animals 2024, 14, 3292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, S.; Xu, S.; Xiong, P.; Cao, Y.; Chen, Z.; Li, M. Comparison of Environmental DNA Metabarcoding and Bottom Trawling for Detecting Seasonal Fish Communities and Habitat Preference in a Highly Disturbed Estuary. Ecol. Indic. 2023, 146, 109754. [Google Scholar] [CrossRef]
- Erős, T.; Funk, A.; Pont, D.; Hein, T.; Meulenbroek, P.; Preiszner, B.; Valentini, A.; Czeglédi, I. eDNA Metabarcoding Reveals the Role of Habitat Specialization and Spatial and Environmental Variability in Shaping Diversity Patterns of Fish Metacommunities. PLoS ONE 2024, 19, e0296310. [Google Scholar] [CrossRef] [PubMed]
- Victorero, L.; Robert, K.; Robinson, L.F.; Taylor, M.L.; Huvenne, V.A.I. Species Replacement Dominates Megabenthos Beta Diversity in a Remote Seamount Setting. Sci. Rep. 2018, 8, 4152. [Google Scholar] [CrossRef] [PubMed]
- Ohira, M.; Fukuda, S. Exploring Functional Flow Heterogeneity in Regulated Flow Regime: Fish Species Turnover along Hydraulic Gradients in an Artificial Waterway Network. River Res. Appl. 2023, 39, 1961–1971. [Google Scholar] [CrossRef]
- Borges, S.H.; D’Aquino, D.D.; da Cruz, M.V.; de Souza, R.F. Turnover in Fish Species Composition Is Related to Water Colour of Amazonian Rivers. J. Trop. Ecol. 2023, 39, e7. [Google Scholar] [CrossRef]
- Rutt, C.L.; Cooper, W.J.; Andretti, C.B.; Costa, T.V.V.; Stouffer, P.C.; Vargas, C.F.; Luther, D.A.; Cohn-Haft, M. Low Species Turnover of Upland Amazonian Birds in the Absence of Physical Barriers. Divers. Distrib. 2023, 29, 466–477. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, X.; Hu, J.; Chang, X.; Qian, Y. Adverse Effects of Microcystis Aeruginosa Exudates on the Filtration, Digestion, and Reproduction Organs of Benthic Bivalve Corbicula fluminea. Sci. Rep. 2024, 14, 10934. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Damborenea, C.; Penchaszadeh, P.E.; Darrigran, G. Gonadal Cycle of Corbicula fluminea (Bivalvia: Corbiculidae) in Pampean Streams (Southern Neotropical Region). PLoS ONE 2017, 12, e0186850. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, C.; Pang, W.; Tian, C.; Zhao, Y. Nanoplastic-Induced Genotoxicity and Intestinal Damage in Freshwater Benthic Clams (Corbicula fluminea): Comparison with Microplastics. ACS Nano 2021, 15, 9469–9481. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Ding, J.; Wu, J.; Bao, E.; Chu, Y.; Hu, F. A Multibiomarker Approach to Assess the Ecotoxicological Effects of Diclofenac on Asian Clam Corbicula fluminea (O. F. Müller, 1774). Environ. Sci. Pollut. Res. 2023, 30, 88598–88611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yin, J.; Tang, S.; Li, D.; Gu, X.; Zhang, S.; Suo, W.; Liu, X.; Liu, Y.; Jiang, Q.; et al. Dissecting the Chromosome-Level Genome of the Asian Clam (Corbicula fluminea). Sci. Rep. 2021, 11, 15021. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).