Effect of Temperature on Allelopathic Interactions Between Copepods (Copepoda) and Rotifers (Rotifera)
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Growth Study
2.2. Morphometric Data
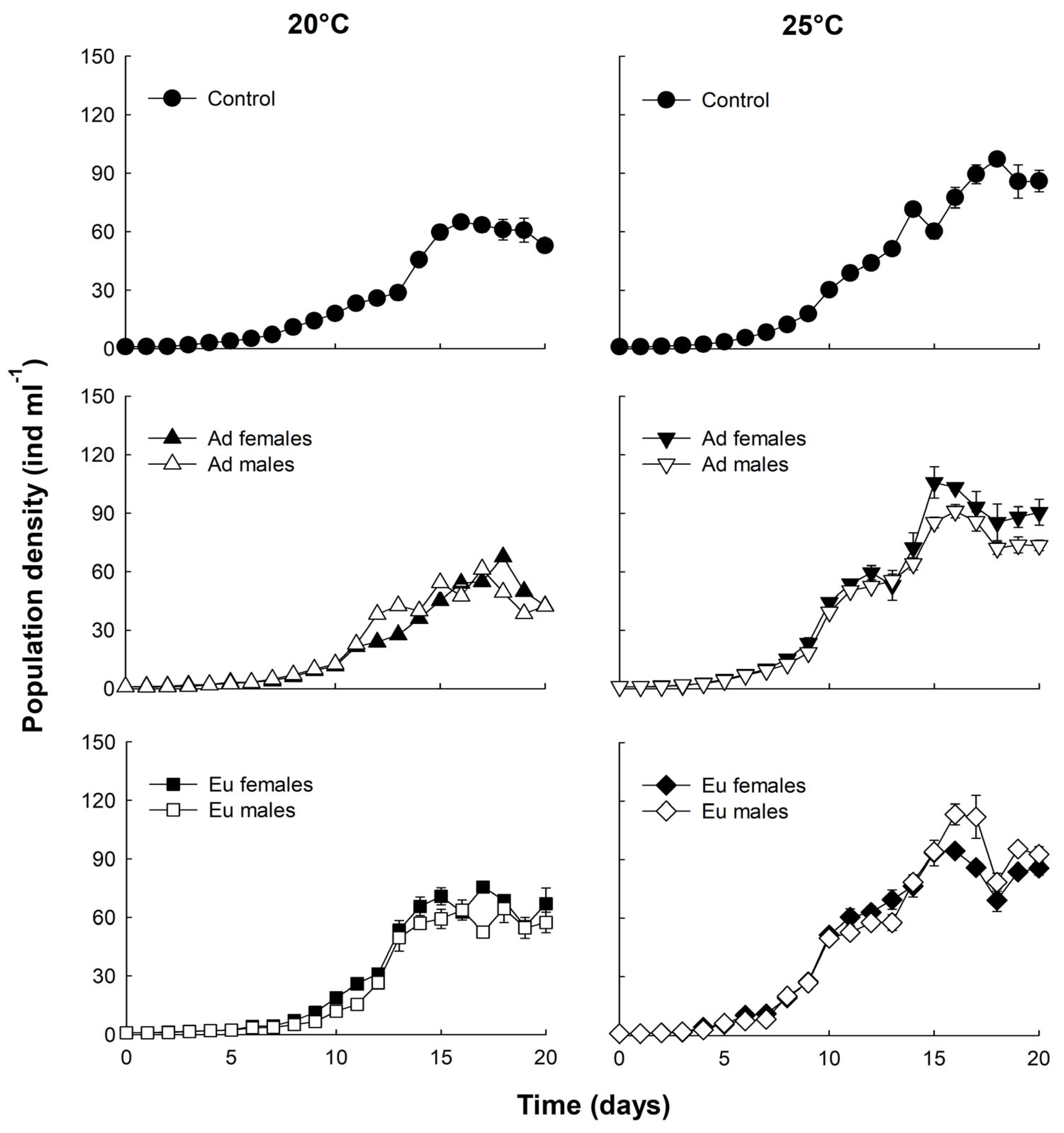
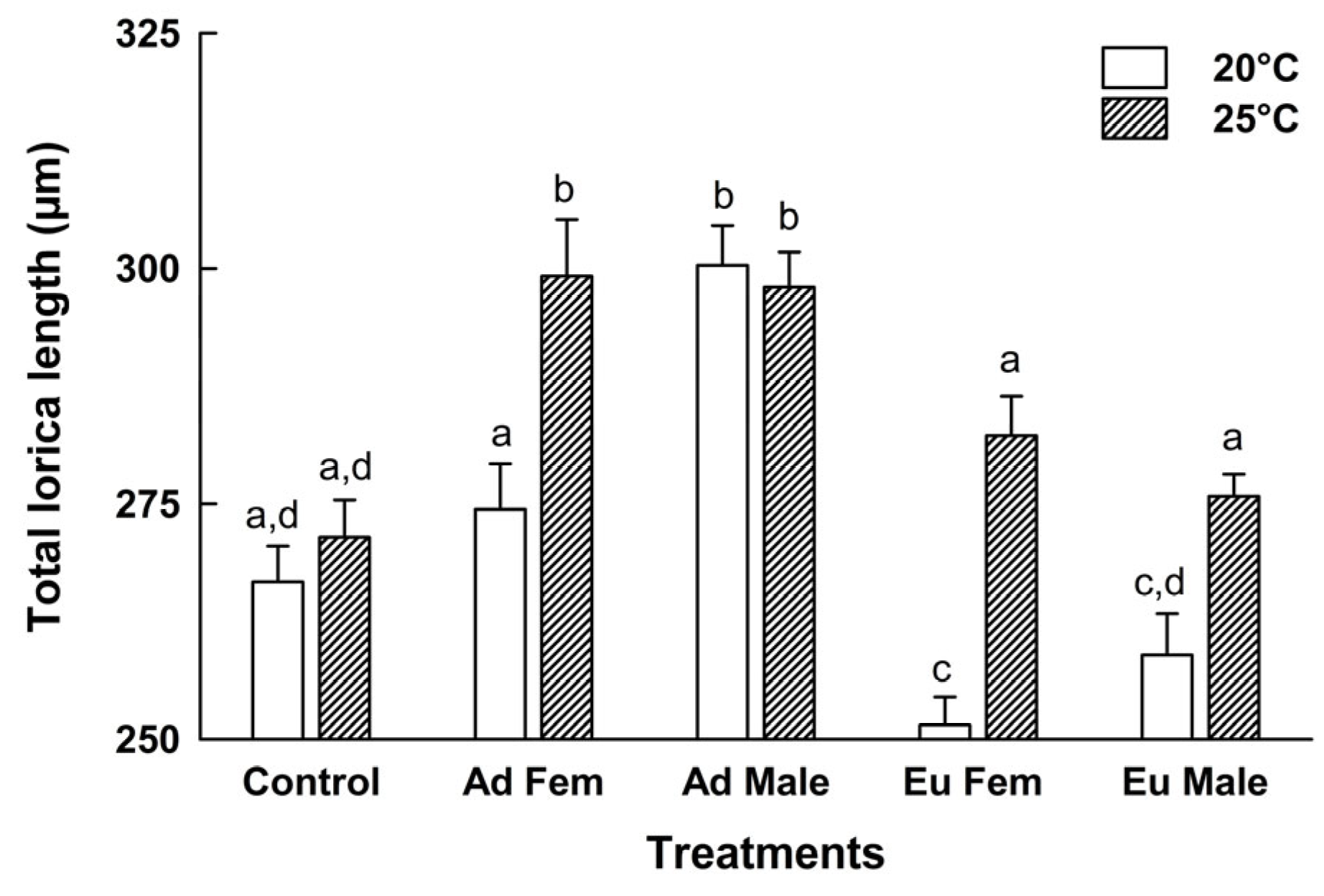
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, J.L.; Dodson, S.I. Predation, Body Size, and Composition of Plankton. Science (1979) 1965, 150, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Heuschele, J.; Selander, E. The Chemical Ecology of Copepods. J. Plankton Res. 2014, 36, 895–913. [Google Scholar] [CrossRef]
- Valencia-Vargas, M.A.; Nandini, S.; Castellanos Páez, M.E.; Sarma, S.S.S. Impact of Native and Invasive Cyclopoid Predators in Relation to the Diversity of the Zooplankton Community. J. Plankton Res. 2022, 44, 1000–1013. [Google Scholar] [CrossRef]
- Snell, T.W. Analysis of Proteins in Conditioned Medium That Trigger Monogonont Rotifer Mictic Reproduction. Hydrobiologia 2017, 796, 245–253. [Google Scholar] [CrossRef]
- Aránguiz-Acuña, A.; Ramos-Jiliberto, R.; Sarma, N.; Sarma, S.S.S.; Bustamante, R.O.; Toledo, V. Benefits, Costs and Reactivity of Inducible Defences: An Experimental Test with Rotifers. Freshw. Biol. 2010, 55, 2114–2122. [Google Scholar] [CrossRef]
- Gilbert, J.J. Kairomone-Induced Morphological Defenses in Rotifers. In The Ecology and Evolution of Inducible Defenses; Tollrian, R., Harvell, C.D., Eds.; Princeton University Press: Princeton, NJ, USA, 1998; pp. 127–141. ISBN 9780691004945. [Google Scholar]
- Lazzaretto, I.; Salvato, B.; Libertini, A. Evidence of Chemical Signalling in Tigriopus fulvus (Copepoda, Harpacticoida). Crustaceana 1990, 59, 171–179. [Google Scholar] [CrossRef]
- Procter, J.; Hopkins, F.E.; Fileman, E.S.; Lindeque, P.K. Smells Good Enough to Eat: Dimethyl Sulfide (DMS) Enhances Copepod Ingestion of Microplastics. Mar. Pollut. Bull. 2019, 138, 1–6. [Google Scholar] [CrossRef]
- López-Rocha, A.N.; Sarma, S.S.S.; Nandini, S. Allelopathic Effects of Male and Female Calanoids and Cyclopoids (Copepoda) on the Demographic Response of Brachionus havanaensis (Rotifera). Aquat. Ecol. 2022, 56, 399–407. [Google Scholar] [CrossRef]
- Heinle, D.R. Temperature and Zooplankton. Chesap. Sci. 1969, 10, 186–209. [Google Scholar] [CrossRef]
- Ratnarajah, L.; Abu-Alhaija, R.; Atkinson, A.; Batten, S.; Bax, N.J.; Bernard, K.S.; Canonico, G.; Cornils, A.; Everett, J.D.; Grigoratou, M.; et al. Monitoring and Modelling Marine Zooplankton in a Changing Climate. Nat. Commun. 2023, 14, 564. [Google Scholar] [CrossRef]
- Calbet, A. The Effects of Global Change on Plankton in Tropical Regions. In Plankton in a Changing World: The Impact of Global Change on Marine Ecosystems; Calbet, A., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 195–198. ISBN 978-3-031-76121-8. [Google Scholar]
- Panja, P.; Kar, T.; Jana, D.K. Impacts of Global Warming on Phytoplankton—Zooplankton Dynamics: A Modelling Study. Environ. Dev. Sustain. 2024, 26, 13495–13513. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Ross, C.; Paul, V.J. Elevated Temperature and Allelopathy Impact Coral Recruitment. PLoS ONE 2016, 11, e0166581. [Google Scholar] [CrossRef] [PubMed]
- Więski, K.; Ślusarczyk, M. On the Different Role of Alarm Substances and Fish Kairomones in Diapause Induction in a Freshwater Planktonic Crustacean. J. Plankton Res. 2022, 44, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.E. Cyclomorphosis of Daphnia pulex Spined Morphs. Limnol. Oceanogr. 1985, 30, 853–861. [Google Scholar] [CrossRef]
- Slusarczyk, M.; Rybicka, B. Role of Temperature in Diapause Response to Fish Kairomones in Crustacean Daphnia. J. Insect Physiol. 2011, 57, 676–680. [Google Scholar] [CrossRef]
- Gilbert, J.J. Temperature, Kairomones, and Phenotypic Plasticity in the Rotifer Keratella tropica (Apstein, 1907). Hydrobiologia 2011, 678, 179–190. [Google Scholar] [CrossRef]
- Sarma, S.S.S.; Resendiz, R.A.L.; Nandini, S. Morphometric and Demographic Responses of Brachionid Prey (Brachionus calyciflorus Pallas and Plationus macracanthus (Daday)) in the Presence of Different Densities of the Predator Asplanchna brightwellii (Rotifera: Asplanchnidae). Hydrobiologia 2011, 662, 179–187. [Google Scholar] [CrossRef]
- Soto, C.S.; Sarma, S.S.S. Morphometric Changes in Lecane stokesii (Pell, 1890) (Rotifera: Lecanidae) Induced by Allelochemicals from the Predator Asplanchnopus multiceps (Schrank, 1793). Allelopath. J. 2009, 44, 3. [Google Scholar]
- Nandini, S.; Zúñiga-Juárez, F.S.; Sarma, S.S.S. Direct and Indirect Effects of Invertebrate Predators on Population Level Responses of the Rotifer Brachionus havanaensis (Rotifera). Int. Rev. Hydrobiol. 2014, 99, 107–116. [Google Scholar] [CrossRef]
- Zhang, H.; Hollander, J.; Hansson, L.-A. Bi-Directional Plasticity: Rotifer Prey Adjust Spine Length to Different Predator Regimes. Sci. Rep. 2017, 7, 10254. [Google Scholar] [CrossRef]
- Weber, C.I. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1991. [Google Scholar]
- Borowitzka, M.A.; Borowitzka, L.J. Micro-Algal Biotechnology; Borowitzka, M., Borowitzka, L., Eds.; Cambridge University Press: London, UK, 1988. [Google Scholar]
- Loose, C.J.; von Elert, E.; Dawidowicz, P. Chemically-Induced Diel Vertical Migration in Daphnia: A New Bioassay for Kairomones Exuded by Fish. Arch. Hydrobiol. 1993, 126, 329–337. [Google Scholar] [CrossRef]
- Bowen, K.L. Methods for the Determination of Zooplankton Density, Biomass and Secondary Production; Fisheries and Oceans Canada: Burlington, ON, Canada, 2017; 50p, ISBN 978-0-660-08553-1. [Google Scholar]
- Princée, F.P.G. Ecological Models. In Exploring Studbooks for Wildlife Management and Conservation; Springer: Cham, Switzerland, 2016; Volume 17, pp. 83–88. [Google Scholar]
- Lass, S.; Spaak, P. Temperature Effects on Chemical Signalling in a Predator-Prey System. Freshw. Biol. 2003, 48, 669–677. [Google Scholar] [CrossRef]
- Enríquez-García, C.E.; Chaparro-Herrera, D.D.; Nandini, S.; Sarma, S.S.S. Life-History Strategies of Brachionus havanaensis Subject to Kairomones of Vertebrate and Invertebrate Predators. Chem. Ecol. 2007, 23, 303–313. [Google Scholar] [CrossRef]
- Stelzer, C.-P. Population Growth in Planktonic Rotifers. Does Temperature Shift the Competitive Advantage for Different Species? Hydrobiologia 2008, 387, 349–353. [Google Scholar] [CrossRef]
- Wenjie, L.; Binxia, L.; Cuijuan, N. Effects of Temperature on Life History Strategy of the Rotifer Euchlanis dilatata. Zool. Sci. 2019, 36, 52–57. [Google Scholar] [CrossRef]
- Qin, S.; Ma, L.; Li, D.; Huang, J.; Zhang, L.; Sun, Y.; Yang, Z. Rising Temperature Accelerates the Responses of Inducible Anti-Predator Morphological Defenses of Ceriodaphnia cornuta but Decreases the Responsive Intensity. Ecol. Indic. 2021, 120, 106919. [Google Scholar] [CrossRef]
- Wallace, R.L.; Snell, T.W.; Ricci, C.; Nogrady, T. Rotifera Part 1: Biology, Ecology and Systematics. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Kenobi Productions: Gent, Belgium, 2006; p. 299. [Google Scholar]
- Lürling, M. Investigation of a Rotifer (Brachionus calyciflorus)-Green Alga (Scenedesmus pectinatus) Interaction under non- and Nutrient-Limited Conditions. Ann. Limnol.-Int. J. Limnol. 2006, 42, 9–17. [Google Scholar] [CrossRef]
- Kotani, T.; Hagiwara, A.; Snell, T.; Serra, M. Euryhaline Brachionus Strains (Rotifera) from Tropical Habitats: Morphology and Allozyme Patterns. Hydrobiologia 2005, 546, 161–167. [Google Scholar] [CrossRef]
- Snell, T.W. Intraspecific Competition and Population Structure in Rotifers. Ecology 1979, 60, 494–502. [Google Scholar] [CrossRef]


| Treatments | Peak Density (ind. mL−1) | Growth Rate (r) Day−1 | ||
|---|---|---|---|---|
| 20 °C | 25 °C | 20 °C | 25 °C | |
| Control | 70 ± 0.74 a | 100 ± 2.21 c | 0.268 ± 0.006 a | 0.289 ± 0.002 c |
| Ad F | 78 ± 0.68 a | 114 ± 2.4 d | 0.259 ± 0.001 a | 0.343 ± 0.004 d |
| Ad M | 70 ± 2.49 a | 94 ± 6 c | 0.240 ± 0.005 a,b | 0.324 ± 0.004 d |
| Eu F | 68 ± 2.1 a,b | 100 ± 2.3 c | 0.287 ± 0.012 a | 0.321 ± 0.006 d |
| Eu M | 61 ± 1.34 a | 122 ± 5.84 d | 0.282 ± 0.003 a | 0.342 ± 0.0052 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Rocha, A.N.; Sarma, S.S.S.; Nandini, S. Effect of Temperature on Allelopathic Interactions Between Copepods (Copepoda) and Rotifers (Rotifera). Diversity 2025, 17, 455. https://doi.org/10.3390/d17070455
López-Rocha AN, Sarma SSS, Nandini S. Effect of Temperature on Allelopathic Interactions Between Copepods (Copepoda) and Rotifers (Rotifera). Diversity. 2025; 17(7):455. https://doi.org/10.3390/d17070455
Chicago/Turabian StyleLópez-Rocha, Ana Nayeli, S. S. S. Sarma, and S. Nandini. 2025. "Effect of Temperature on Allelopathic Interactions Between Copepods (Copepoda) and Rotifers (Rotifera)" Diversity 17, no. 7: 455. https://doi.org/10.3390/d17070455
APA StyleLópez-Rocha, A. N., Sarma, S. S. S., & Nandini, S. (2025). Effect of Temperature on Allelopathic Interactions Between Copepods (Copepoda) and Rotifers (Rotifera). Diversity, 17(7), 455. https://doi.org/10.3390/d17070455








